Abstract
Patients with chronic kidney disease (CKD) have higher rates of fracture than the general population. Increased bone remodeling, leading to microarchitectural deterioration and increased fragility, may accompany declining kidney function, but there are no reliable methods to identify patients at increased risk for fracture. In this cross-sectional study of 82 patients with predialysis CKD, high-resolution imaging revealed that the 23 patients with current fractures had significantly lower areal density at the femoral neck; total, cortical, and trabecular volumetric bone density; cortical area and thickness; and trabecular thickness. Compared with levels in the lowest tertile, higher levels of osteocalcin, procollagen type-1 N-terminal propeptide, and tartrate-resistant acid phosphatase 5b were associated with higher odds of fracture, even after adjustment for femoral neck T-score. Discrimination of fracture prevalence was best with a femoral neck T-score of −2.0 or less and a value in the upper two tertiles for osteocalcin, procollagen type-1 N-terminal propeptide, or tartrate-resistant acid phosphatase 5b; these values corresponded to the upper half of the normal premenopausal reference range. In summary, these cross-sectional data suggest that measurement of bone turnover markers may increase the diagnostic accuracy of densitometry to identify patients with CKD at high risk for fracture.
Fracture rates are elevated in patients with predialysis chronic kidney disease (CKD).1–3 Cross-sectional data suggest fracture risk increases as kidney function declines and by CKD stage 4 approximates that for ESRD.2 Furthermore, fracture-related mortality for patients with predialysis CKD is approximately twofold higher than the general population.4 These data are alarming because CKD and osteoporosis are highly co-prevalent,5 and incidence and prevalence of predialysis CKD, osteoporosis, and fragility fracture are expected to increase exponentially as the population ages. Reliable methods to identify patients with CKD at risk for fracture are lacking.6 Thus, there is a critical need to develop diagnostic tests and assess their utility to detect increased fracture risk in the CKD population.
Bone strength is determined by the amount of bone present and the quality of that bone. In the clinic, bone mass is usually assessed by dual energy x-ray absorptiometry (DXA), which provides an estimate of areal bone mineral density (aBMD, g/cm2). Bone quality is related to other material properties of the skeleton, including bone microarchitecture and remodeling activity. Microarchitecture and remodeling are linked; low and high remodeling lead to loss of bone structural integrity and increased fracture risk.7–11
High-resolution peripheral computed tomography (HR-pQCT; XtremeCT; Scanco Medical AG, Brüttisellen, Switzerland; voxel size approximately 82 μm) is a new imaging technique that measures true volumetric bone mineral density (vBMD, g/cm3) and microarchitecture of the distal skeleton (radius and tibia). Bone remodeling can also be assessed with reasonable accuracy by measurement of bone turnover markers (BTMs) in patients with normal kidney function.12,13 Certain BTMs, including osteocalcin and the C-terminal telopeptides of type I collagen (CTX), are renally cleared. Others, such as bone-specific alkaline phosphatase (BSAP), procollagen type-1 N-terminal propeptide (P1NP), and tartrate-resistant acid phosphatase-5b (Trap-5b), are metabolized by nonrenal mechanisms. Regardless of whether they are renally excreted, BTMs are higher in patients with more severe CKD and are associated with lower aBMD by DXA.7 It is possible that nonrenally cleared BTMs provide more accurate assessments of fracture risk in CKD.
We previously reported that patients with predialysis CKD and a history of fragility fracture have lower aBMD by DXA and lower vBMD and abnormal cortical and trabecular microarchitecture by HR-pQCT compared with CKD patients without fracture.14 However, the diagnostic accuracy of DXA and HR-pQCT was not sufficiently high to identify reliably patients with prevalent fractures, except in those with longstanding (>7 years) CKD. We hypothesized that the microarchitectural abnormalities that we detected in CKD patients with fracture were related to abnormal bone remodeling. Furthermore, we hypothesized that calciotropic hormones and BTMs, particularly those that are not renally cleared, would discriminate fracture status independent of bone mass.
RESULTS
Subject Characteristics According to Fracture Status
We recruited participants from the nephrology clinics of Columbia University Medical Center (CUMC). Of 82 patients, 23 had prevalent fracture: 14 vertebral (18 mild, 3 moderate) and 14 nonvertebral (2 rib, 1 humerus, 5 forearm, 1 femur, 4 ankle, 1 metatarsal). Five patients had multiple nonvertebral fractures. Median time (interquartile range) from fracture was 7.9 (2.1 to 9.8) years. Groups with and without fracture (Table 1) were similar with respect to body mass index, kidney function, CKD duration, race, ethnicity, use of calcium and noncalcium phosphate binders, and parent and active forms of vitamin D. No patients took cinacalcet. Patients with fracture were more likely to be female, although this difference was NS, and were significantly older and less likely to have diabetes.
Table 1.
Baseline characteristics of patients with fracture ≥1 year in comparison to those without fracture
| Fracture | Nonfracture | Unadjusted P | Age-, Gender-, and Diabetes-Adjusted P | |
|---|---|---|---|---|
| n | 23 | 59 | ||
| eGFR, ml/min, median (IQR) | 25 (14 to 35) | 28 (19 to 41) | NS | |
| Age, years, median (IQR) | 78 (66 to 84) | 69 (62 to 76) | 0.006 | |
| BMI, median (IQR) | 27.1 (24.2 to 30.9) | 29.3 (26.1 to 33.8) | NS | |
| Years since nonspine fracture, median (IQR) | 7.9 (2.1 to 9.8) | NA | ||
| Gender, % female | 57 | 39 | NS | |
| Race, % | ||||
| white | 48 | 59 | NS | |
| black | 42 | 40 | NS | |
| ethnicity, % | ||||
| Hispanic | 52 | 58 | NS | |
| non-Hispanic | 48 | 52 | NS | |
| Diabetes, % | 39 | 68 | 0.02 | |
| Duration of CKD, years, median (IQR) | 5.0 (3.4 to 10.0) | 5.5 (3.4 to 9.3) | NS | |
| Laboratory parameters, median (IQR) | ||||
| serum calcium, mg/dl | 9.4 (8.9 to 9.8) | 9.3 (9.0 to 9.7) | NS | |
| serum phosphorus, mg/dl | 3.7 (3.3 to 4.7) | 3.6 (3.0 to 4.2) | NS | |
| serum bicarbonate, mM/L | 22 (21 to 26) | 23 (22 to 25) | NS | |
| Medications, % | ||||
| calcium-containing agents | 26 | 21 | NS | |
| noncalcium-containing phosphate binding agents | 17 | 18 | NS | |
| vitamin D—parent | 70 | 63 | NS | |
| vitamin D—active | 35 | 32 | NS | |
| Behaviors, % | ||||
| current alcohol use | 13 | 10 | NS | |
| current tobacco use | 9 | 7 | NS | |
| DXA measurements, median (IQR)a | ||||
| LS aBMD, g/cm2 | 0.938 (0.853 to 1.057) | 1.060 (0.957 to 1.216) | 0.006 | NS |
| LS T-score | −1.4 (−2.1; −0.2) | −0.1 (−1.1 to 1.2) | 0.01 | 0.04 |
| TH aBMD, g/cm2 | 0.783 (0.728 to 0.878) | 0.943 (0.805 to 1.069) | 0.004 | NS |
| TH T-score | −1.5 (−2.1; −0.6) | −0.6 (−1.6 to 0.3) | 0.007 | NS |
| FN aBMD, g/cm2 | 0.621 (0.569 to 0.666) | 0.747 (0.667 to 0.848) | 0.0009 | NS |
| FN T-score | −2.2 (−2.7; −1.4) | −1.4 (−2.1; −0.8) | 0.002 | 0.03 |
| 1/3R aBMD, g/cm2 | 0.630 (0.549 to 0.714) | 0.773 (0.634 to 0.805) | 0.001 | NS |
| 1/3R T-score | −1.8 (−3.1; −0.6) | −0.9 (−2.1 to 0.06) | 0.006 | 0.02 |
| UDR aBMD, g/cm2 | 0.340 (0.302 to 0.415) | 0.442 (0.369 to 0.503) | <0.0001 | 0.005 |
| UDR T-score | −2.2 (−3.4; −1.2) | −1.2 (−1.8; −0.5) | 0.009 | 0.007 |
| HR-pQCT measurements distal radius, median (IQR) | ||||
| cross-sectional area, mm2 | 278 (235 to 314) | 300 (236 to 357) | NS | NS |
| cortical area, mm2 | 40 (35 to 48) | 55 (46 to 72) | 0.0001 | 0.01 |
| cortical thickness, μm | 610 (480 to 710) | 750 (600 to 930) | <0.0001 | 0.02 |
| total density, mg HA/cm3 | 248 (209 to 299) | 308 (268 to 347) | <0.0001 | 0.006 |
| cortical density, mg HA/cm3 | 795 (724 to 850) | 842 (781 to 910) | 0.006 | NS |
| trabecular density, mg HA/cm3 | 114 (86 to 161) | 154 (130 to 189) | 0.002 | 0.02 |
| trabecular number, 1/mm | 1.7 (1.4 to 2.1) | 2.0 (1.8 to 2.3) | 0.04 | NS |
| trabecular thickness, μm | 59 (48 to 69) | 65 (60 to 68) | 0.02 | 0.03 |
| trabecular heterogeneity (μm) | 237 (165 to 359) | 178 (151 to 201) | 0.03 | NS |
| HR-pQCT measurements distal tibia, median (IQR) | ||||
| cross-sectional area, mm2 | 783 (624 to 866) | 782 (642 to 863) | NS | NS |
| cortical area, mm2 | 80 (56 to 89) | 113 (84 to 138) | <0.0001 | 0.01 |
| cortical thickness, μm | 690 (500 to 810) | 1005 (820 to 1210) | <0.0001 | 0.01 |
| total density, mg HA/cm3 | 222 (186 to 244) | 257 (224 to 304) | 0.0002 | 0.03 |
| cortical density, mg HA/cm3 | 718 (662 to 802) | 797 (752 to 846) | 0.002 | 0.04 |
| trabecular density, mg HA/cm3 | 150 (119 to 158) | 161 (131 to 186) | NS | NS |
| trabecular number, 1/mm | 1.7 (1.5 to 1.9) | 1.9 (1.7 to 2.2) | NS | NS |
| trabecular thickness, μm | 69 (60 to 73) | 69 (61 to 77) | NS | NS |
| trabecular heterogeneity (μm) | 245 (206 to 295) | 193 (161 to 264) | NS | NS |
BMI, body mass index; IQR, interquartile range.
aT-scores are only adjusted for age and diabetes status.
Consistent with our previous report,14 measures of aBMD by DXA and of vBMD, size, and microarchitecture by HR-pQCT were associated with fracture (Table 1). Fracture patients had significantly lower aBMD at the lumbar spine (LS), total hip (TH), femoral neck (FN), and 1/3 and ultradistal radius (1/3R and UDR, respectively). After adjustment for group differences, aBMD remained significantly lower only at the UDR site. Unadjusted T-scores at all sites were associated with fracture history. After adjustment for age and diabetes, only FN, 1/3R, and UDR T-scores were associated with fracture history.
At the radius, several HR-pQCT measures of vBMD, size, and trabecular microarchitecture were associated with fracture (Table 1). After adjustment for group differences, total and trabecular vBMD, cortical area, and trabecular thickness remained significantly lower in the fracture group. At the tibia, cortical parameters (vBMD, thickness, and area) were significantly lower in the fracture group and remained so after adjustment; trabecular parameters did not differ, possibly because of the mitigating effects of weight bearing.
Calciotropic Hormones, Fibroblast Growth Factor-23, and Biochemical Markers of Bone Turnover by Fracture Status
Median levels for intact parathyroid hormone (iPTH), fibroblast growth factor-23 (FGF-23), and both resorption markers were above the normal range for healthy premenopausal women (Table 2). Although large, the differences between fracture and nonfracture groups in median levels of iPTH and FGF-23 were NS, likely because of heterogeneity in measurement distribution or the relatively small number of subjects with fracture. BSAP levels did not differ. In contrast, osteocalcin and P1NP were significantly higher in patients with fracture, by 52% and 42% respectively; each SD increase in their levels corresponded with 2.6- and 3.2-fold increased odds of fracture, respectively. Trap-5b was 29% higher in the fracture group; each SD increase was associated with a 2.3-fold increased odds of fracture. CTX was 19% higher in the fracture group, but by univariate logistic regression the association was NS. Adjustment for age, gender, and diabetes did not materially change the results. However, the between-group difference in CTX achieved significance.
Table 2.
Calciotropic hormones and biochemical markers of bone turnover among patients with and without fracture
| Fracture | Nonfracture | Percent Difference between Means | Univariate Logistic Regression OR (95% CI) for each SD increase in BTM | Unadjusted P | Age-, Gender-, and Diabetes-Adjusted P | Normal Range for Premenopausal Women | |
|---|---|---|---|---|---|---|---|
| iPTH, pg/ml | 105 (40 to 249) | 68 (39 to 138) | 42 | 1.24 (0.77 to 2.00) | NS | NS | 14 to 66 |
| 25OHD, ng/ml | 29 (20 to 37) | 32 (22 to 38) | −4 | 0.96 (0.56 to 1.63) | NS | NS | >30 |
| FGF-23, RU/ml | 175 (115 to 578) | 162 (107 to 303) | 44 | 1.67 (0.74 to 3.84) | NS | NS | <100 |
| BSAP, U/L | 30.3 (24.9 to 45.4) | 29.6 (20.8 to 40.7) | 8 | 1.21 (0.72 to 2.05) | NS | 0.059 | 11.6 to 29.6 |
| Osteocalcin, ng/ml | 41.8 (24.1 to 66.0) | 24.2 (14.2 to 41.6) | 52 | 2.66 (1.27 to 5.54) | 0.006 | 0.003 | 8.4 to 33.9 |
| P1NP, μl/L | 79.5 (58.0 to 92.0) | 54.0 (39.0 to 79.0) | 42 | 3.25 (1.50 to 7.05) | 0.0008 | 0.0007 | 19 to 83 |
| CTX, ng/ml | 1.24 (0.77 to 1.47) | 0.87 (0.52 to 1.35) | 19 | 1.78 (0.94 to 3.38) | 0.047 | 0.03 | 0.11 to 0.74 |
| Trap-5b, U/L | 5.33 (4.01 to 7.47) | 4.41 (3.38 to 5.36) | 29 | 2.31 (1.21 to 4.42) | 0.01 | 0.006 | 1.03 to 4.15 |
Values presented as median (IQR).
Prevalence of Hyperparathyroidism and Relationships among Parathyroid Hormone and Calciotropic Hormones, FGF-23, and BTMs
On the basis of the Kidney Disease Outcomes Quality Initiative (KDOQI) and Kidney Disease Improving Global Outcomes (KDIGO) guidelines, 40% and 52%, respectively, of the subjects had hyperparathyroidism. Unadjusted serum iPTH levels correlated directly with 25-hydroxyvitamin D (25OHD), FGF-23, and BTMs. After estimated GFR (eGFR) adjustment, iPTH correlated inversely with 25OHD (r = −0.28, P = 0.01) and directly with BSAP (r = 0.30, P = 0.007), osteocalcin (r = 0.50, P < 0.0001), P1NP (r = 0.26, P = 0.02), CTX (r = 0.43, P < 0.0001), and Trap-5b (r = 0.31, P = 0.005). FGF-23 and iPTH did not correlate after eGFR adjustment.
Relationships between Calciotropic Hormones, FGF-23, and BTMs and Measures of Bone Size, Density, and Microarchitecture
To assess mechanisms associated with fracture, we investigated relationships between eGFR-adjusted biochemical parameters and aBMD, vBMD, bone size, and microstructure (Table 3). Higher iPTH levels were associated with lower aBMD by DXA at the TH, FN, and UDR and with lower trabecular vBMD by HR-pQCT at radius and tibia. Higher iPTH levels were also associated with trabecular microarchitectural deterioration—thinner trabeculae at the radius and a more widely separated, heterogeneous trabecular network at the tibia. There were no relationships between iPTH and cortical parameters.
Table 3.
Pearson correlations between BTMs and measures of bone mass, geometry, and microarchitecture adjusted for eGFR
| PTH | 25OHD | FGF-23 | BSAP | Osteocalcin | P1NP | CTX | Trap-5b | |
|---|---|---|---|---|---|---|---|---|
| Age | −0.1 | 0.17 | 0.18 | −0.33b | −0.004 | −0.02 | −0.08 | 0.03 |
| BMI | 0.1 | −0.18 | −0.12 | 0.12 | 0.004 | 0.03 | −0.03 | −0.04 |
| DXA | ||||||||
| LS | −0.21 | 0.0004 | 0.05 | 0.05 | −0.14 | −0.12 | −0.06 | −0.1 |
| TH | −0.26a | −0.01 | −0.22 | −0.14 | −0.36b | −0.21 | −0.26a | −0.14 |
| FN | −0.29b | 0.06 | −0.18 | −0.11 | −0.37c | −0.27a | −0.25a | −0.16 |
| 1/3R | −0.19 | 0.01 | −0.03 | −0.17 | −0.25a | −0.23a | −0.07 | −0.30b |
| UDR | −0.27a | −0.08 | −0.16 | −0.12 | −0.28a | −0.29a | −0.2 | −0.30b |
| Distal radius HR-pQCT | ||||||||
| cross-sectional area, mm2 | −0.001 | −0.01 | 0.03 | −0.05 | −0.06 | −0.03 | −0.06 | −0.04 |
| cortical area, mm2 | −0.11 | −0.12 | 0.02 | −0.03 | −0.08 | −0.19 | −0.01 | −0.15 |
| cortical thickness, μm | −0.15 | −0.13 | −0.01 | −0.02 | −0.1 | −0.2 | 0.002 | −0.18 |
| total density, mg HA/cm3 | −0.27a | −0.03 | −0.06 | −0.12 | −0.27a | −0.33b | −0.16 | −0.28a |
| cortical density, mg HA/cm3 | −0.14 | −0.11 | −0.04 | 0.05 | −0.06 | −0.13 | 0.09 | −0.17 |
| trabecular density, mg HA/cm3 | −0.27a | 0.05 | −0.07 | −0.17 | −0.36b | −0.33b | −0.30b | −0.25a |
| trabecular number, n/mm | −0.16 | 0.05 | −0.04 | −0.15 | −0.24a | −0.17 | −0.22 | −0.23a |
| trabecular thickness, μm | −0.29b | 0.04 | −0.08 | −0.15 | −0.34b | −0.36b | −0.27a | 0.2 |
| trabecular separation, μm | 0.17 | −0.07 | −0.08 | 0.12 | 0.27a | 0.19 | 0.21 | 0.18 |
| trabecular heterogeneity, μm | 0.16 | −0.06 | −0.06 | 0.09 | 0.25a | 0.19 | 0.22 | 0.27a |
| Distal tibia HR-pQCT | ||||||||
| cross-sectional area, mm2 | −0.04 | 0.03 | 0.11 | −0.07 | −0.004 | 0.08 | 0.03 | 0.006 |
| cortical area, mm2 | −0.03 | −0.11 | −0.07 | 0.01 | −0.14 | −0.25a | −0.07 | −0.14 |
| cortical thickness, μm | −0.01 | −0.11 | −0.1 | 0.02 | −0.16 | −0.28a | −0.09 | −0.15 |
| total density, mg HA/cm3 | −0.21 | −0.01 | −0.11 | −0.1 | −0.26a | −0.32b | −0.24a | −0.25a |
| cortical density, mg HA/cm3 | −0.2 | −0.06 | −0.09 | 0.03 | −0.17 | −0.26a | −0.05 | −0.17 |
| trabecular density, mg HA/cm3 | −0.34b | 0.08 | −0.05 | −0.16 | −0.27a | −0.23a | −0.28a | −0.23a |
| trabecular number, n/mm | −0.31b | 0.12 | 0.1 | −0.2 | −0.21 | −0.17 | −0.21 | −0.22 |
| trabecular thickness, μm | −0.18 | −0.02 | −0.26a | −0.01 | −0.15 | −0.14 | −0.19 | −0.12 |
| trabecular separation, μm | 0.34b | −0.17 | −0.06 | 0.21 | 0.23 | 0.2 | 0.26a | 0.27a |
| trabecular heterogeneity, μm | 0.34c | −0.24a | −0.01 | 0.17 | 0.18 | 0.17 | 0.19 | 0.26a |
OR, odds ratio; CI, confidence interval.
P value a<0.05,
b<0.01,
c<0.001;
d<0.0001.
Higher formation markers were associated with lower aBMD by DXA and vBMD and microarchitectural deterioration by HR-pQCT. Osteocalcin and P1NP correlated inversely and significantly with FN, 1/3R, and UDR aBMD; osteocalcin also correlated with TH aBMD. Osteocalcin and P1NP correlated inversely with total and trabecular vBMD at radius and tibia. In terms of microarchitecture, higher osteocalcin was associated with lower trabecular number and a more widely separated, heterogeneous trabecular network at the radius, but not at the tibia. P1NP was only associated with thinner trabeculae at the radius. Higher P1NP levels were also only associated with lower cortical area, density, and thickness at the tibia.
Higher resorption markers were also associated with lower aBMD, vBMD, and microarchitectural deterioration. Higher CTX was associated with lower TH and FN aBMD, whereas higher Trap-5b was associated with lower 1/3R and UDR aBMD. CTX and Trap-5b correlated inversely with trabecular vBMD at the radius and tibia. Although relationships with microarchitectural parameters were not consistently significant, in general, CTX and Trap-5b were associated with trabecular but not cortical microarchitectural deterioration.
Discrimination of Fracture Status by Biochemical Markers of Bone Turnover, Bone Mass, and Microarchitecture
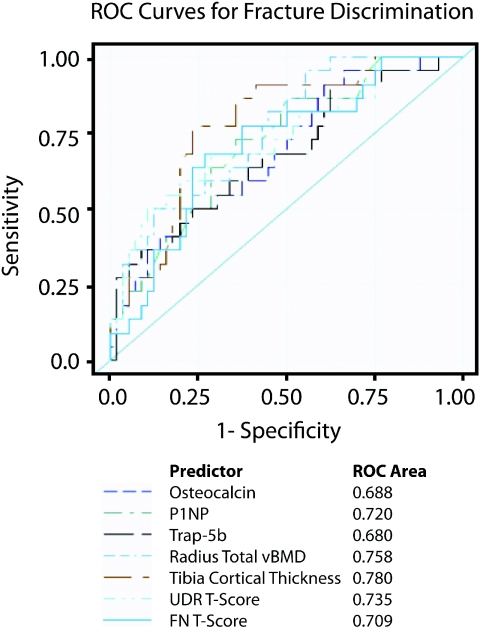
Although HR-pQCT measures were strongly associated with fracture, the XtremeCT is available at few academic centers. In contrast, DXA is widely available and FN aBMD and T-score are used in the World Health Organization (WHO) Fracture Risk Assessment Tool (FRAX) to predict absolute 10-year risk of hip and major osteoporotic fracture.15 We therefore investigated whether any DXA, HR-pQCT, or biochemical parameter provided better discrimination of fracture status than FN T-score using receiver-operator characteristic (ROC) curve analysis of the most robust predictors of fracture by univariate analyses. Specifically assessed were (1) osteocalcin, P1NP, and Trap-5b; (2) FN and UDR T-score; and (3) radius total vBMD and tibial cortical thickness by HR-pQCT. Areas under the curve (AUCs) ranged from 0.68 (Trap 5b) to 0.78 (tibia cortical thickness) (Figure 1), but differences were NS.
Figure 1.
In comparison to FN T-Score, there were no differences in AUCs for BTMs, aBMD by DXA, and vBMD and bone geometry by HR-pQCT.
Relationships among Fracture, Biochemical Markers of Bone Turnover, and Bone Mineral Density
To determine whether BTMs measured in the CKD subjects discriminated fracture status independent of bone mineral density (BMD), we performed multiple logistic regression analyses adjusting for age, diabetes, and FN T-score (Table 4). BTMs associated with fracture status by univariate analysis (osteocalcin, P1NP, and Trap-5b) were divided into tertiles. In unadjusted and adjusted analyses, the highest tertiles of all three BTMs were associated with fracture status. In addition, combining BTMs with FN T-score increased the R2 value, a measure of how much the data contribute to the variance of the outcome (fracture status), and the model-specific AUC, a measure of discrimination.
Table 4.
Association between tertiled levels of BTMs and fracture after adjustment for FN T-score
| FN T-Score Model | BTM Models | FN T-Score and BTM Models | |
|---|---|---|---|
| R2 0.28 | |||
| AUC 0.78 | |||
| FN T-score | 1.88 (1.02 to 3.49) | ||
| Osteocalcin | |||
| R2 0.33 | R2 0.38 | ||
| AUC 0.81 | AUC 0.83 | ||
| FN T-score | 1.84 (0.94 to 3.60) | ||
| OC tertiles | |||
| 7.4 to 20.6 | Reference | Reference | |
| 20.8 to 37.1 | 3.68 (0.78 to 17.34) | 3.51 (0.71 to 17.31) | |
| 38.7 to 138.1 | 6.69 (1.41 to 31.84) | 5.47 (1.11 to 27.05) | |
| P1NP | |||
| R2 0.35 | R2 0.39 | ||
| AUC 0.81 | AUC 0.82 | ||
| FN T-score | 1.85 (0.94 to 3.66) | ||
| P1NP tertiles | |||
| 22 to 46 | Reference | Reference | |
| 48 to 78 | 3.54 (0.71 to 17.61) | 3.66 (0.70 to 19.10) | |
| 79 to 176 | 8.17 (1.67 to 39.90) | 7.02 (1.36 to 36.28) | |
| Trap-5b | |||
| R2 0.31 | R2 0.37 | ||
| AUC 0.79 | AUC 0.83 | ||
| FN T-score | 1.98 (1.04 to 3.76) | ||
| Trap-5b tertiles | |||
| 1.8 to 3.7 | Reference | Reference | |
| 3.8 to 5.2 | 1.76 (0.40 to 7.79) | 1.96 (0.42 to 9.17) | |
| 5.3 to 10.7 | 4.93 (1.15 to 21.23) | 5.16 (1.10 to 24.17) |
All models adjusted for age and diabetes status.
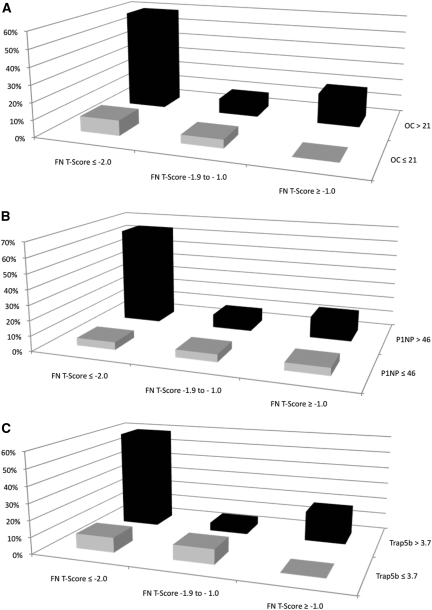
We next evaluated the co-prevalence of low FN T-scores and high BTMs among subjects with a history of prior fracture (Figure 2A through 2c). FN T-score was stratified as (1) −2.0 or less, (2) more than −2.0 to −1.0 or less, and (3) greater than −1.0 because these approximated the tertile cutoffs. We dichotomized osteocalcin, P1NP, and Trap-5b at the upper limit of the lowest tertile, which approximates the middle of the normal range for a healthy premenopausal reference population. We selected this cutoff because the clinical target for monitoring antiresorptive therapy is to reduce BTMs to the lower half to one third of the premenopausal reference range and no reference population has been established for men.16,17 Most patients (55% to 60%) with fractures had a FN T-score of −2.0 or less and serum BTMs (Figure 2a through 2c) in the upper two tertiles of the CKD subject range.
Figure 2.
Percent prevalence of patients with fracture (n = 23) according to BTM levels and FN T-scores. FN T-score is divided at −2.0 or less, −2 to −1.0, and more than −1. BTM levels are dichotomized at </> the first tertile. (A) osteocalcin, (B) P1NP, and (C) Trap-5b.
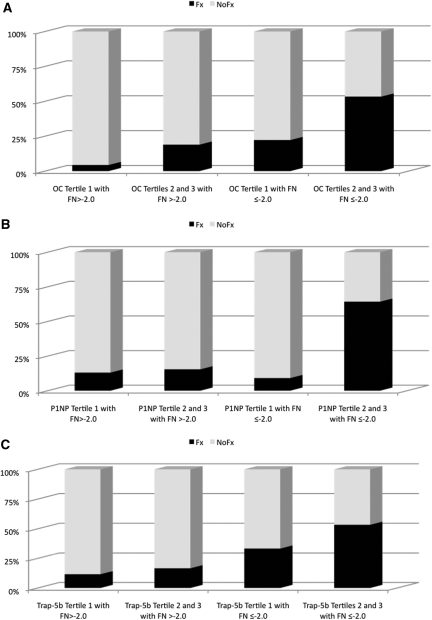
Finally, we determined the proportion of CKD patients, with and without fracture, according to whether FN T-score was less than or equal to or more than −2.0 and whether BTMs were in the lowest tertile or the upper two tertiles of the range of the CKD subject values, approximating the middle of the premenopausal normal range. For each BTM, the proportion of patients with fractures was markedly higher in those with FN T-scores of −2.0 or less and with serum osteocalcin, P1NP, or Trap-5b level in the upper two tertiles (Figure 3a to 3c, respectively). Although diagnostic test characteristics demonstrated poor sensitivity, there were moderate specificity, negative predictive values, and positive and negative likelihood ratios (Table 5). When we performed this analysis with tibia cortical thickness rather than FN T-score, the results were similar.
Figure 3.
Prevalence of patients with CKD with and without fracture according to whether the FN T-score is −2 or less or more than −2.0 and BTMs are in the upper two tertiles versus the lowest tertile. (A) osteocalcin tertile 1, 7.4 to 20.6; tertile 2, 20.8 to 37.1; and tertile 3, 38.7 to 138.1. (B) P1NP tertile 1, 22 to 46; tertile 2, 48 to 78; and tertile 3, 79 to 176. (C) Trap-5b tertile 1, 1.8 to 3.7; tertile 2, 3.8 to 5.2; and tertile 3, 5.3 to 10.7.
Table 5.
Diagnostic test characteristics for fracture: BTM levels in the upper two tertiles and a FN T-score of −2 or less versus other BTM and FN T-score groups
| Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Positive Likelihood Ratio (95% CI) | Negative Likelihood Ratio (95% CI) | |
|---|---|---|---|---|---|---|
| Osteocalcin ≥ 21 and FN T-score of −2 or less | 56 (37 to 79) | 81 (69 to 90) | 54 (33 to 74) | 84 (72 to 92) | 3.17 (1.68 to 5.99) | 0.50 (0.69 to 0.84) |
| P1NP ≥ 46 and FN T-score of −2 or less | 63 (41 to 82) | 86 (74 to 93) | 64 (41 to 82) | 86 (74 to 93) | 4.61 (2.25 to 9.45) | 0.42 (0.24 to 0.74) |
| Trap-5b ≥ 3.7 and FN T-score −2 or less | 59 (37 to 79) | 81 (68 to 90) | 54 (33 to 74) | 84 (71 to 92) | 3.12 (1.65 to 5.88) | 0.50 (0.30 to 0.84) |
PPV, positive predictive value; NPV, negative predictive value.
DISCUSSION
These results confirm our previous findings that CKD patients with prevalent fragility fractures have lower aBMD by DXA and lower vBMD and abnormal microarchitecture by HR-pQCT.14 They extend those findings by demonstrating that certain BTMs distinguish between patients with and without fracture. The highest tertile of formation (osteocalcin, P1NP) and resorption (Trap-5b) markers were independently associated with increased odds of prior fracture. Combining the highest tertile level of osteocalcin, P1NP, or Trap-5b with FN T-score improved discrimination of fracture history over measurement of FN T-score or BTMs alone. Fracture prevalence was highest in CKD patients with a FN T-score of −2.0 or less and either osteocalcin, P1NP, or Trap-5b in the highest two tertiles. Indeed, for any combination of elevated BTM and low FN BMD, specificity and negative predictive value for fracture were >80%. These diagnostic test characteristics exceed those of prostate-specific antigen testing for prostate cancer screening.18 These data support our hypothesis that predialysis CKD patients can be risk-stratified for fragility by combining BTMs and aBMD. Interestingly, whether BTMs were renally excreted did not affect fracture discrimination. In terms of pathogenesis, high iPTH and BTM levels were associated with low bone mass, smaller bone size, and a disrupted trabecular network, abnormalities we14,19 and others20–23 have found to be associated with fracture.
Contrary to our hypotheses, BSAP, a nonrenally cleared BTM, was not associated with bone mass, size, or microarchitecture or fracture. These findings may be explained by the cross-sectional design or small sample size of the study. They are in agreement with a cross-sectional study by Tsuchida et al.,24 who reported that BSAP did not correlate with cortical or trabecular vBMD measured by peripheral computed tomography in predialysis CKD. In prospective studies, high BSAP levels predicted abnormalities associated with fracture, such as bone loss,7,25–27 turnover, and mineralization defects,28–31 but have not been shown to predict fracture per se.
To our knowledge, these are the first data demonstrating utility for BTMs in discriminating fracture among predialysis CKD patients over age 50. Whether BTMs, measured a median of 7.9 years after fractures occurred, reflect remodeling activity at the time of fracture is unknown. Remodeling rates may change over time, particularly given likely deterioration of kidney function between time of fracture and BTM measurement. However, multiple prospective studies have shown that BTMs predict fracture in elderly women.32–35 In elderly women with a history of fragility fracture, elevated urinary CTX was superior to FN T-score for fracture prediction.34 BTMs also predicted fractures that occurred up to 6.5 years in the future.12 Moreover, a past history of fragility fracture is a marker of overall poor bone quality and an important risk factor for future fracture, as evidenced by inclusion of this historical feature in the WHO FRAX.15 Although our data suggest that a combination of BTMs, FN BMD, and a history of fracture may be useful to identify CKD patients who might benefit from fracture prevention strategies, prospective studies are needed to assess the utility of these parameters for fracture prediction in the CKD population.
We know of no data regarding FGF-23 and fracture in CKD. In children with ESRD, FGF-23 has recently been correlated with abnormal mineralization,36 which could lead to increased fragility. Vitamin D deficiency is a risk factor for fracture.37,38 However, our results did not detect any relationship between 25OHD and fracture risk, possibly because of equivalent vitamin D supplementation in each group. Furthermore, most subjects had an eGFR < 45 ml/min, a level at which serum 25OHD weakly correlates with hyperparathyroidism.39
In patients with ESRD, data regarding iPTH and fracture are conflicting. Some studies found no association between iPTH and fracture40,41 whereas others suggest that low42–44 or high44,45 iPTH levels are associated with fracture. Studies in patients with predialysis CKD demonstrated that higher iPTH is inversely associated with aBMD by DXA7,25,27,46 and total and cortical vBMD by HR-pQCT.47 We also found inverse relationships between iPTH and aBMD by DXA and trabecular vBMD and microarchitecture by HR-pQCT. Bone biopsy studies have demonstrated that very low or very high iPTH levels are moderately predictive of turnover abnormalities29–31; in turn, abnormal turnover may cause low BMD, microarchitectural deterioration, and decreased bone strength that could lead to fracture.21,48–53 However, in our study, the relationship between iPTH and fracture history was NS. There are several possible reasons for this: The number of patients with fracture was relatively small, iPTH levels were outside of the range shown to predict low or high turnover,29–31 and the iPTH assay used detects the intact (1-84) parathyroid hormone (PTH) molecule and PTH fragments that accumulate in kidney failure and may not accurately reflect bone turnover.54 PTH assays that measure only the intact (1-84) molecule may provide superior discrimination for fracture.
Although hyperparathyroidism is classically associated with cortical thinning and porosity, we noted more consistent, robust relationships between iPTH and BTMs and trabecular vBMD and microarchitecture than with cortical vBMD or thickness. This may be because hyperparathyroidism affects more metabolically active trabecular bone and causes cancellization of the cortical endosteal surface. In hyperparathyroid states, the Laplace–Hamming filter and threshold,55 used by HR-pQCT to delineate the trabecular-endosteal surface boundary, may not be reliable. Furthermore, the resolution of HR-pQCT is not sufficient to detect small cortical pores.
Measurement of FN BMD by DXA is widely available, strongly predicts fracture in postmenopausal women,56,57 and is the main driver of the WHO FRAX tool. FRAX has not been validated in CKD. It is not known if FRAX can be used to determine fracture risk or decide upon initiating fracture prevention treatments in CKD. Although more research is needed to develop a “CKD-specific” FRAX tool, our data are the first to suggest that fracture prediction is enhanced by combining certain BTMs with FN BMD in CKD patients.
Limitations of this study include its cross-sectional design, which prevents assessment of relationships between BTMs and incident fractures. In addition, BMD and BTMs were measured years after fractures occurred and may not reflect levels at the time of fracture. The small sample size may have limited the detection of relationships between fracture and BSAP and iPTH. Levels of PTH and FGF-23 are altered with declining kidney function because of accumulation of degradation products.54 The assays used for these biomarkers do not distinguish between various forms of these proteins. The fracture and nonfracture groups were not balanced for predictors of fragility; however, after adjustment for these differences, relationships remained significant between fracture and osteocalcin, P1NP, and Trap-5b. Bone biopsies, the gold standard to assess turnover, were not performed. We used the HR-pQCT manufacturer's algorithm to separate cortical from trabecular regions, which may affect the accuracy of cortical measures.
In conclusion, commercially available assays for osteocalcin, P1NP, and Trap-5b discriminated fracture status in predialysis CKD patients independent of BMD. Combining a BTM with FN BMD provided greater utility to identify CKD patients with fractures than either test alone. If confirmed by longitudinal studies with fracture outcomes, these data suggest that combining a BTM with measurement of FN BMD may prove useful for fracture risk screening in CKD patients. Longitudinal studies are needed to determine whether BTM assessment predicts incident fracture, whether measuring bone turnover and BMD improves fracture prediction, and mechanisms by which elevated bone turnover increases propensity for fracture in predialysis CKD.
CONCISE METHODS
Subjects
Predialysis CKD patients with eGFR < 90 ml/min, with and without a history of fracture, and enrolled in an ongoing longitudinal study of relationships between kidney function and bone structure and strength were included (n = 82). Patients with nonspine fracture were ≥1 year from fracture occurrence. Participants were recruited from the general nephrology clinics of CUMC between August 2006 and September 2010. All patients referred to the nephrology clinics and meeting study inclusion criteria were eligible. The CUMC nephrology clinics serve as a referral center for patients with CKD from the northern Manhattan, Bronx, Queens, southern New York State and Connecticut, and northern and central New Jersey areas. We did not exclude patients on the basis of the etiology of CKD. eGFR was determined by the Modification of Diet in Renal Disease short formula.58 Patients with a history of kidney transplantation; malignancy; bilateral lower extremity amputations; residing in a nursing home; requiring a wheelchair; and those taking bisphosphonates, gonadal steroids, aromatase inhibitors, and anticonvulsants that induce hepatic cytochrome P450 enzymes were excluded. Two patients had a remote history of glucocorticoid use (>1 year before the study visit) for treatment of IgA nephropathy and idiopathic focal segmental glomerulosclerosis. Therefore, the cohort included in this investigation represents the wide spectrum of ambulatory patients with CKD and is generalizable to those patients typically treated in a general nephrology clinic. The CUMC Institutional Review Board approved the study and all subjects provided written informed consent.
Laboratory Measurements
Routine laboratory parameters were measured by Quest diagnostics. Serum creatinine was determined by the Jaffe reaction and serum calcium, phosphorus, and bicarbonate were measured by spectrophotometry. Calciotropic hormones and BTMs were measured at CUMC in a specialized research laboratory. Serum 25OHD was measured by RIA (Diasorin, Stillwater, MN); intra- and interassay precision are 10.5% and 11.0%, respectively, and the normal range is ≥30 ng/ml. iPTH was measured by RIA (Scantibodies, Santee, CA); intra- and interassay precision are 4.8% and 6.8%, respectively, and the normal range is 14 to 66 pg/ml. C-terminal FGF-23 was measured by ELISA (Immutopics, San Clemente, CA); intra- and interassay precision are 2.4% and 4.7%, respectively, and normal values are <100 RU/ml. BSAP activity was measured by immunoassay (Metra BAP, Quidel Corporation, San Diego, CA); inter- and intra-assay variabilities are 7.6% and 3.9%, respectively, and the normal range is 11.6 to 29.6 U/L in premenopausal women. Osteocalcin was measured by ELISA (N-mid Osteocalcin, IDS, Ltd., Scottsdale, AZ); the inter- and intra-assay variability is 2.7% and 1.8%, respectively, and the normal range is 8.4 to 33.9 ng/ml in premenopausal women. P1NP was measured by RIA (IDS, Ltd., Fountain Hills, AZ); inter- and intra-assay variabilities are 8.3% and 6.5%, respectively, and the normal range is 19 to 83 pg/ml for premenopausal women. Serum CTX was measured by ELISA (IDS, Ltd., Scottsdale, AZ); the inter- and intra-assay variabilities are 9.75 and 1.7%, respectively, and the normal range is 0.112 to 0.738 ng/ml for premenopausal women. TRAP-5b was measured by ELISA (IDS, Ltd., Scottsdale AZ); intra- and interassay precisions are 4.7% and 9.0%, respectively, and the normal range is 1.03 to 4.15 U/L in premenopausal women.
Assessment of CKD, Fracture Status, Date of Fracture Occurrence, Factors Associated with BMD, and Definitions of Hyperparathyroidism
CKD duration was estimated for all patients by review of medical records and prior measurements of serum creatinine. The online medical records system at CUMC contains clinical and demographic data dating to 1990, and this system was utilized to determine CKD duration for patients seen within the CUMC system (approximately 95% of this cohort). The onset of CKD was determined from the earliest serum creatinine that consistently corresponded to an eGFR < 90 ml/min, in compliance with KDOQI guidelines.59 For patients lacking historical information (n = 7; 8%), duration of kidney function was based on the date of first presentation to a nephrologist. In patients with inadequate historical clinical data, the serum creatinine at nephrology presentation was uniformly abnormal (range 1.5 to 6.0 mg/dl). In patients when the earliest serum creatinine was abnormal, and no normal range of serum creatinines was available, duration of CKD was based on the date of the earliest abnormal serum creatinine. Etiology of kidney disease was grouped into three categories: (1) diabetic and hypertensive kidney disease, (2) glomerular causes of CKD (nephritis or nephrosis), and (3) other causes of CKD (polycystic kidney disease, tubularinterstitial, or unknown).
Fragility fracture was defined as a fracture associated with trauma equivalent to or less than a fall from a standing height; fractures associated with major trauma (e.g., motor vehicle accidents) and skull and digit fractures were excluded. Vertebral and nonvertebral fragility fractures were included. Nonvertebral fractures were ascertained by self-reported history during our standardized interview and confirmed by review of radiographs or radiology reports whenever possible. Nine patients had 14 nonvertebral fractures: 6 fractures were confirmed by radiographs and 8 were included based on patient report, which included being casted or having undergone radiographic procedures and being told they had a broken bone. All study subjects underwent lateral spine x-rays. Vertebral fractures were identified by a skeletal radiologist (R.B.S.) and graded by the semiquantitative method on spine radiographs performed according to the protocol of the Study of Osteoporotic Fractures.60 Because all vertebral fractures were identified at the study visit, it was not possible to determine their occurrence in relationship to the duration of CKD or the study visit. There were a total of 21 vertebral fractures in 14 patients: 8 patients with a single vertebral fracture, 5 patients with two vertebral fractures, and 1 patient with three vertebral fractures. Of these, 18 fractures were mild and 3 were moderate.
Current alcohol consumption was defined as one or more drinks per day. Current tobacco use was reported as having smoked tobacco in the 5 years before the study visit. Vitamin D supplementation was defined as any use of ergocalciferol (n = 25), cholecalciferol (n = 30), paricalcitol (n = 14), doxercalciferol (n = 8), or calcitriol (n = 4). Phosphate binders included calcium acetate (n = 2), sevelamer (n = 13), or lanthanum carbonate (n = 1). No patient was taking cinacalcet or aluminum-containing phosphate binding agents. Eight patients were taking calcium carbonate.
Hyperparathyroidism was defined by KDOQI and KDIGO guidelines. KDOQI defines hyperparathyroidism on the basis of CKD stage61: (1) patients with CKD stages 3 and 4 who have plasma levels of iPTH > 70 pg/ml (7.7 pmol/L; stage 3) or >110 pg/ml (12.1 pmol/L; stage 4) on more than two consecutive measurements, and (2) patients with CKD stage 5 who have elevated plasma levels of iPTH > 300 pg/ml (33.0 pmol/L). Because of the cross-sectional design of this investigation, a single iPTH measurement was used. KDIGO defines hyperparathyroidism as being above the upper limit of normal for the assay.62 The iPTH assay utilized in this investigation had an upper limit of normal of 66 pg/ml.
Measurement of aBMD by DXA
aBMD by DXA was measured at the total LS (L1 through L4), TH, FN, and nondominant 1/3R and UDR using a Hologic QDR 4500 densitometer (Hologic, Inc., Waltham, MA) in the array (fan beam) mode. In our laboratory, short-term, in vivo precision is 0.68% for the spine, 1.36% for the FN, and 0.70% for the radius. T-scores compared subjects to data from young-normal populations of the same race and sex provided by the manufacturer (spine and forearm) and by the National Health and Nutrition Examination Survey III (TH and FN).
HR-pQCT Imaging of the Radius and Tibia
HR-pQCT (XtremeCT; Scanco Medical AG, Brüttisellen, Switzerland) of the nondominant forearm and leg was performed and analyzed as described previously.14,55,63–66 In brief, all imaging was performed in our laboratory by a dedicated research densitometrist. HR-pQCT of the dominant limb was performed only if there was previous fracture or an arteriovenous fistula or graft in the nondominant limb. The arm or leg was positioned in the scanner and the region of interest was defined on a scout film by manual placement of a reference line at the endplate of the radius or tibia, with the first slice 9.5 and 22.5 mm proximal to the reference line at the radius and tibia, respectively. A stack of 110 parallel computed tomography slices was acquired at the distal end of both sites with a slice thickness of 82 μm, an image matrix size of 1024 × 1024, and a nominal voxel size of 82 μm. Attenuation data were converted to equivalent hydroxyapatite (HA) densities. A phantom was scanned daily for quality control. Image processing and calculation of numerical values were performed using Scanco software. Briefly, the volume of interest is automatically separated into cortical and trabecular regions using a Laplace–Hamming filter and threshold. Mean cortical thickness is defined as the mean cortical volume divided by the outer bone surface. Trabecular bone density is defined as the average bone density within the trabecular volume (TV) of interest; bone volume (BV)/TV (%) is derived from trabecular density (Dtrab) assuming that the density of fully mineralized bone was 1.2 g HA/cm3 (BV/TV = 100 × Dtrab/1200 mg HA/cm3). Because measurements of trabecular microstructure are limited by the resolution of the XtremeCT, which approximates the width of individual trabeculae, trabecular structure is assessed using a semiderived algorithm.63,64 Trabeculae are identified by a mid-axis transformation method and the distance between them is assessed by the distance-transform method.67 Trabecular number is defined as the inverse of the mean spacing of the mid-axes. Trabecular thickness and trabecular separation are derived from BV/TV and trabecular number using formulas from traditional quantitative histomorphometry: trabecular thickness = (BV/TV)/trabecular number and trabecular separation = (1 − BV/TV)/trabecular number. The intraindividual distribution of separation (μm), a parameter that reflects the heterogeneity of the trabecular network, is also measured.
Statistical Analysis
Statistical analyses were conducted using SAS (version 9.2, SAS Institute, Cary, NC). Categorical data were compared using the χ2 test. Continuous data were evaluated for normality before statistical testing and log-transformed when appropriate. Group differences were determined by t test for unequal variances. Generalized linear models were used to adjust for imbalances in covariate structure between fracture and nonfracture groups (Table 1). Preliminary models including gender and race did not demonstrate effects on associations between BTMs and fracture; therefore, these covariates were not included in the final adjusted models. Pearson correlation coefficients were determined after adjustment for kidney function defined by eGFR. Univariate logistic regression was performed to determine univariate relationships between fracture and measures of BTMs after mean standardization. Multiple logistic regression models were used to evaluate independent relationships between BTMs and fracture after adjustment for FN T-score by DXA and unbalanced covariates in this cohort, including age and diabetes status. Because of moderate and significant correlations between BTMs with FN BMD by DXA, BTMs were tertiled before inclusion in multiple logistic models to avoid issues of multicolinearity. Standard ROC curve analysis was performed to determine the ability of DXA, HR-pQCT, and BTMs to discriminate fracture status. In multiple logistic and ROC models, FN T-score was chosen as the referent group because of its common use in clinical practice to evaluate for osteoporosis and its inclusion in the FRAX tool as the skeletal site at which to assess absolute 10-year hip and major osteoporotic fracture risk.15 Diagnostic test characteristics were also determined for a combination of low FN T-score, defined as being −2.0 or less and a BTM level in the upper two tertiles. Data are presented as sensitivity, specificity, and positive and negative predictive values and positive and negative likelihood ratios with their respective 95% confidence intervals.
DISCLOSURES
T.L.N. has a consulting agreement with Roche Diagnostics.
Acknowledgments
This research was supported by grants from the National Institutes of Health (K23 DK080139 [T.L.N.], K24 DK076808 [M.B.L.], and K24 AR052665 [E.S.]), Amgen (Young Investigator Award [T.L.N.]), and the International Society for Clinical Densitometry (Special Projects Award [T.L.N.]). Part of this material was presented in abstract form at the annual meeting of the American Society of Nephrology; November 18 through 21, 2010; Denver, CO.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Nickolas TL, McMahon DJ, Shane E: Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol 17: 3223–3232, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Dooley AC, Weiss NS, Kestenbaum B: Increased risk of hip fracture among men with CKD. Am J Kidney Dis 51: 38–44, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Fried LF, Biggs ML, Shlipak MG, Seliger S, Kestenbaum B, Stehman-Breen C, Sarnak M, Siscovick D, Harris T, Cauley J, Newman AB, Robbins J: Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol 18: 282–286, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Nitsch D, Mylne A, Roderick PJ, Smeeth L, Hubbard R, Fletcher A: Chronic kidney disease and hip fracture-related mortality in older people in the UK. Nephrol Dial Transplant 24: 1539–1544, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Klawansky S, Komaroff E, Cavanaugh PF, Jr, Mitchell DY, Gordon MJ, Connelly JE, Ross SD: Relationship between age, renal function and bone mineral density in the US population. Osteoporos Int 14: 570–576, 2003 [DOI] [PubMed] [Google Scholar]
- 6. United States Renal Data System: Annual data report: Incidence and prevalence of ESRD (2003). Am J Kidney Dis Suppl S37–S173, 2003 [Google Scholar]
- 7. Rix M, Andreassen H, Eskildsen P, Langdahl B, Olgaard K: Bone mineral density and biochemical markers of bone turnover in patients with predialysis chronic renal failure. Kidney Int 56: 1084–1093, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Chaitou A, Boutroy S, Vilayphiou N, Munoz F, Delmas PD, Chapurlat R, Szulc P: Association between bone turnover rate and bone microarchitecture in men: The STRAMBO study. J Bone Miner Res 25: 2313–2323, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Schaffler MB: Role of bone turnover in microdamage. Osteoporos Int 14[Suppl 5]: S73–S77, discussion S77–S80, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Schaffler MB, Choi K, Milgrom C: Aging and matrix microdamage accumulation in human compact bone. Bone 17: 521–525, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Parfitt AM: Bone age, mineral density, and fatigue damage. Calcif Tissue Int 53[Suppl 1]: S82–S85, discussion S85–S86, 1993 [DOI] [PubMed] [Google Scholar]
- 12. Gerdhem P, Ivaska KK, Alatalo SL, Halleen JM, Hellman J, Isaksson A, Pettersson K, Vaananen HK, Akesson K, Obrant KJ: Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res 19: 386–393, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Kaji H, Yamauchi M, Yamaguchi T, Sugimoto T: Urinary deoxypyridinoline is a BMD-independent marker for prevalent vertebral fractures in postmenopausal women treated with glucocorticoid. Osteoporos Int 21: 1585–1590, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Nickolas TL, Stein E, Cohen A, Thomas V, Staron RB, McMahon DJ, Leonard MB, Shane E: Bone mass and microarchitecture in CKD patients with fracture. J Am Soc Nephrol 21: 1371–1380, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Watts NB, Lewiecki EM, Miller PD, Baim S: National Osteoporosis Foundation 2008 Clinician's Guide to Prevention and Treatment of Osteoporosis and the World Health Organization Fracture Risk Assessment Tool (FRAX): What they mean to the bone densitometrist and bone technologist. J Clin Densitom 11: 473–477, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Glover SJ, Gall M, Schoenborn-Kellenberger O, Wagener M, Garnero P, Boonen S, Cauley JA, Black DM, Delmas PD, Eastell R: Establishing a reference interval for bone turnover markers in 637 healthy, young, premenopausal women from the United Kingdom, France, Belgium, and the United States. J Bone Miner Res 24: 389–397, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Glover SJ, Garnero P, Naylor K, Rogers A, Eastell R: Establishing a reference range for bone turnover markers in young, healthy women. Bone 42: 623–630, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Catalona WJ, Smith DS, Ratliff TL, Dodds KM, Coplen DE, Yuan JJ, Petros JA, Andriole GL: Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med 324: 1156–1161, 1991 [DOI] [PubMed] [Google Scholar]
- 19. Stein EM, Liu XS, Nickolas TL, Cohen A, Thomas V, McMahon DJ, Zhang C, Yin PT, Cosman F, Nieves J, Guo XE, Shane E: Abnormal microarchitecture and reduced stiffness at the radius and tibia in postmenopausal women with fractures. J Bone Miner Res 25: 2296–2305, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boutroy S, Bouxsein ML, Munoz F, Delmas PD: In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 90: 6508–6515, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD: Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res 23: 392–399, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Sornay-Rendu E, Cabrera-Bravo JL, Boutroy S, Munoz F, Delmas PD: Severity of vertebral fractures is associated with alterations of cortical architecture in postmenopausal women. J Bone Miner Res 24: 737–743, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Melton LJ, III, Christen D, Riggs BL, Achenbach SJ, Muller R, van Lenthe GH, Amin S, Atkinson EJ, Khosla S: Assessing forearm fracture risk in postmenopausal women. Osteoporos Int 21: 1161–1169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsuchida T, Ishimura E, Miki T, Matsumoto N, Naka H, Jono S, Inaba M, Nishizawa Y: The clinical significance of serum osteocalcin and N-terminal propeptide of type I collagen in predialysis patients with chronic renal failure. Osteoporos Int 16: 172–179, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Okuno S, Inaba M, Kitatani K, Ishimura E, Yamakawa T, Nishizawa Y: Serum levels of C-terminal telopeptide of type I collagen: A useful new marker of cortical bone loss in hemodialysis patients. Osteoporos Int 16: 501–509, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Maeno Y, Inaba M, Okuno S, Yamakawa T, Ishimura E, Nishizawa Y: Serum concentrations of cross-linked N-telopeptides of type I collagen: New marker for bone resorption in hemodialysis patients. Clin Chem 51: 2312–2317, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Ueda M, Inaba M, Okuno S, Maeno Y, Ishimura E, Yamakawa T, Nishizawa Y: Serum BAP as the clinically useful marker for predicting BMD reduction in diabetic hemodialysis patients with low PTH. Life Sci 77: 1130–1139, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Urena P, De Vernejoul MC: Circulating biochemical markers of bone remodeling in uremic patients. Kidney Int 55: 2141–2156, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Coen G, Ballanti P, Bonucci E, Calabria S, Centorrino M, Fassino V, Manni M, Mantella D, Mazzaferro S, Napoletano I, Sardella D, Taggi F: Bone markers in the diagnosis of low turnover osteodystrophy in haemodialysis patients. Nephrol Dial Transplant 13: 2294–2302, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Sherrard DJ, Baylink DJ, Wergedal JE, Maloney NA: Quantitative histological studies on the pathogenesis of uremic bone disease. J Clin Endocrinol Metab 39: 119–135, 1974 [DOI] [PubMed] [Google Scholar]
- 31. Bervoets AR, Spasovski GB, Behets GJ, Dams G, Polenakovic MH, Zafirovska K, Van Hoof VO, De Broe ME, D'Haese PC: Useful biochemical markers for diagnosing renal osteodystrophy in predialysis end-stage renal failure patients. Am J Kidney Dis 41: 997–1007, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Garnero P, Hausherr E, Chapuy MC, Marcelli C, Grandjean H, Muller C, Cormier C, Breart G, Meunier PJ, Delmas PD: Markers of bone resorption predict hip fracture in elderly women: The EPIDOS Prospective Study. J Bone Miner Res 11: 1531–1538, 1996 [DOI] [PubMed] [Google Scholar]
- 33. Garnero P, Sornay-Rendu E, Duboeuf F, Delmas PD: Markers of bone turnover predict postmenopausal forearm bone loss over 4 years: The OFELY study. J Bone Miner Res 14: 1614–1621, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Garnero P, Dargent-Molina P, Hans D, Schott AM, Breart G, Meunier PJ, Delmas PD: Do markers of bone resorption add to bone mineral density and ultrasonographic heel measurement for the prediction of hip fracture in elderly women? The EPIDOS prospective study. Osteoporos Int 8: 563–569, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Johnell O, Oden A, De Laet C, Garnero P, Delmas PD, Kanis JA: Biochemical indices of bone turnover and the assessment of fracture probability. Osteoporos Int 13: 523–526, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Wesseling-Perry K, Pereira RC, Wang H, Elashoff RM, Sahney S, Gales B, Juppner H, Salusky IB: Relationship between plasma fibroblast growth factor-23 concentration and bone mineralization in children with renal failure on peritoneal dialysis. J Clin Endocrinol Metab 94: 511–517, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B: Fracture prevention with vitamin D supplementation: A meta-analysis of randomized controlled trials. JAMA 293: 2257–2264, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Ambrus C, Almasi C, Berta K, Deak G, Marton A, Molnar MZ, Nemeth Z, Horvath C, Lakatos P, Szathmari M, Mucsi I: Vitamin D insufficiency and bone fractures in patients on maintenance hemodialysis. Int Urol Nephrol 43, 475–482, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL: Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int 71: 31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Stehman-Breen CO, Sherrard DJ, Alem AM, Gillen DL, Heckbert SR, Wong CS, Ball A, Weiss NS: Risk factors for hip fracture among patients with end-stage renal disease. Kidney Int 58: 2200–2205, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Piraino B, Chen T, Cooperstein L, Segre G, Puschett J: Fractures and vertebral bone mineral density in patients with renal osteodystrophy. Clin Nephrol 30: 57–62, 1988 [PubMed] [Google Scholar]
- 42. Coco M, Rush H: Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis 36: 1115–1121, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Atsumi K, Kushida K, Yamazaki K, Shimizu S, Ohmura A, Inoue T: Risk factors for vertebral fractures in renal osteodystrophy. Am J Kidney Dis 33: 287–293, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Danese MD, Kim J, Doan QV, Dylan M, Griffiths R, Chertow GM: PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am J Kidney Dis 47: 149–156, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Jadoul M, Albert JM, Akiba T, Akizawa T, Arab L, Bragg-Gresham JL, Mason N, Prutz KG, Young EW, Pisoni RL: Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 70: 1358–1366, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Ha SK, Park CH, Seo JK, Park SH, Kang SW, Choi KH, Lee HY, Han DS: Studies on bone markers and bone mineral density in patients with chronic renal failure. Yonsei Med J 37: 350–356, 1996 [DOI] [PubMed] [Google Scholar]
- 47. Bacchetta J, Boutroy S, Guebre-Egziabher F, Juillard L, Drai J, Pelletier S, Richard M, Charrie A, Carlier MC, Chapurlat R, Laville M, Fouque D: The relationship between adipokines, osteocalcin and bone quality in chronic kidney disease. Nephrol Dial Transplant 24: 3120–3125, 2009 [DOI] [PubMed] [Google Scholar]
- 48. MacNeil JA, Boyd SK: Load distribution and the predictive power of morphological indices in the distal radius and tibia by high resolution peripheral quantitative computed tomography. Bone 41: 129–137, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Melton LJ, III, Riggs BL, van Lenthe GH, Achenbach SJ, Muller R, Bouxsein ML, Amin S, Atkinson EJ, Khosla S: Contribution of in vivo structural measurements and load/strength ratios to the determination of forearm fracture risk in postmenopausal women. J Bone Miner Res 22: 1442–1448, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Burghardt AJ, Kazakia GJ, Ramachandran S, Link TM, Majumdar S: Age and gender related differences in the geometric properties and biomechanical significance of intra-cortical porosity in the distal radius and tibia. J Bone Miner Res 25: 983–993, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yeni YN, Brown CU, Wang Z, Norman TL: The influence of bone morphology on fracture toughness of the human femur and tibia. Bone 21: 453–459, 1997 [DOI] [PubMed] [Google Scholar]
- 52. van der Linden JC, Homminga J, Verhaar JA, Weinans H: Mechanical consequences of bone loss in cancellous bone. J Bone Miner Res 16: 457–465, 2001 [DOI] [PubMed] [Google Scholar]
- 53. Silva MJ, Gibson LJ: Modeling the mechanical behavior of vertebral trabecular bone: Effects of age-related changes in microstructure. Bone 21: 191–199, 1997 [DOI] [PubMed] [Google Scholar]
- 54. Chapter 3.1: Diagnosis of CKD-MBD: biochemical abnormalities. Kidney Int 76[Suppl 113]: S22–S49, 2009 [DOI] [PubMed] [Google Scholar]
- 55. Laib A, Hauselmann HJ, Ruegsegger P: In vivo high resolution 3D-QCT of the human forearm. Technol Health Care 6: 329–337, 1998 [PubMed] [Google Scholar]
- 56. Black DM, Cummings SR, Genant HK, Nevitt MC, Palermo L, Browner W: Axial and appendicular bone density predict fractures in older women. J Bone Miner Res 7: 633–638, 1992 [DOI] [PubMed] [Google Scholar]
- 57. Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Cummings SR: BMD at multiple sites and risk of fracture of multiple types: Long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res 18: 1947–1954, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 59. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 60. Black DM, Palermo L, Nevitt MC, Genant HK, Epstein R, San VR, Cummings SR: Comparison of methods for defining prevalent vertebral deformities: The Study of Osteoporotic Fractures. J Bone Miner Res 10: 890–902, 1995 [DOI] [PubMed] [Google Scholar]
- 61. KDOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease 2003. Available at http://www.kidney.org/professionals/kdoqi/guidelines_bone/index.htm Accessed December 1, 2010
- 62. Chapter 4.1: Treatment of CKD-MBD targeted at lowering high serum phosphorus and maintaining serum calcium. Kidney Int 76: S50–S99, 2009 [DOI] [PubMed] [Google Scholar]
- 63. Laib A, Ruegsegger P: Calibration of trabecular bone structure measurements of in vivo three-dimensional peripheral quantitative computed tomography with 28-microm-resolution microcomputed tomography. Bone 24: 35–39, 1999 [DOI] [PubMed] [Google Scholar]
- 64. Hildebrand T, Laib A, Muller R, Dequeker J, Ruegsegger P: Direct three-dimensional morphometric analysis of human cancellous bone: Microstructural data from spine, femur, iliac crest, and calcaneus. J Bone Miner Res 14: 1167–1174, 1999 [DOI] [PubMed] [Google Scholar]
- 65. MacNeil JA, Boyd SK: Accuracy of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys 29: 1096–1105, 2007 [DOI] [PubMed] [Google Scholar]
- 66. Liu XS, ZX, Sekhon KK, Adam MF, McMahon DJ, Bilezikian JP, Shane E, Guo XE: High-resolution peripheral quantitative computed tomography can assess microstructural and mechanical properties of human distal tibial bone. J Bone Miner Res 25: 746–756, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hildebrand T, Ruegsegger P: Quantification of bone microarchitecture with the structure model index. Comput Methods Biomech Biomed Engin 1: 15–23, 1997 [DOI] [PubMed] [Google Scholar]