Abstract
The failure of pancreatic β cells to adapt to an increasing demand for insulin is the major mechanism by which patients progress from insulin resistance to type 2 diabetes (T2D) and is thought to be related to dysfunctional lipid homeostasis within those cells. In multiple animal models of diabetes, females demonstrate relative protection from β cell failure. We previously found that the hormone 17β-estradiol (E2) in part mediates this benefit. Here, we show that treating male Zucker diabetic fatty (ZDF) rats with E2 suppressed synthesis and accumulation of fatty acids and glycerolipids in islets and protected against β cell failure. The antilipogenic actions of E2 were recapitulated by pharmacological activation of estrogen receptor α (ERα) or ERβ in a rat β cell line and in cultured ZDF rat, mouse, and human islets. Pancreas-specific null deletion of ERα in mice (PERα–/–) prevented reduction of lipid synthesis by E2 via a direct action in islets, and PERα–/– mice were predisposed to islet lipid accumulation and β cell dysfunction in response to feeding with a high-fat diet. ER activation inhibited β cell lipid synthesis by suppressing the expression (and activity) of fatty acid synthase via a nonclassical pathway dependent on activated Stat3. Accordingly, pancreas-specific deletion of Stat3 in mice curtailed ER-mediated suppression of lipid synthesis. These data suggest that extranuclear ERs may be promising therapeutic targets to prevent β cell failure in T2D.
Introduction
Type 2 diabetes (T2D) occurs when pancreatic β cells fail to compensate for the increased insulin demand in the context of obesity-associated insulin resistance. Thus, developing novel therapeutic strategies to prevent β cell failure in the context of obesity is a major challenge. The likely mechanisms of early β cell demise include fuel overload associated with dysfunctional lipid homeostasis and glucolipotoxicity, which leads to oxidative and endoplasmic reticulum stress, inflammation, and, eventually, β cell apoptosis (1). In diabetic models, females are relatively protected from β cell failure (2). We have shown that the gonadal steroid 17β-estradiol (E2) protects β cells from oxidative stress–induced apoptosis and stimulates insulin biosynthesis via estrogen receptors (ERs) present in β cells, with a predominant ERα effect (3–5). The fact that both human and rodent females are relatively protected from obese forms of T2D with severe β cell failure (2, 6–8) raises the possibility that activation of ERs may also improve lipid homeostasis in β cells. In agreement with this hypothesis, E2 improves metabolic parameters in leptin-resistant mice (9). In addition, in obese Zucker diabetic fatty (ZDF) rats, a model of T2D, males exhibit impaired islet lipid homeostasis and subsequent glucolipotoxic β cell failure, whereas females show reduced accumulation of lipids in islets and are protected from β cell failure (10).
Here we showed that E2 suppressed islet fatty acid (FA) and glycerolipid synthesis and prevented β cell failure in male ZDF rats. Using mice with pancreas-specific null deletion of ERα (referred to herein as PERα–/– mice), we showed that activation of this receptor in vivo suppressed lipid synthesis in islets via a direct action. Accordingly, after exposure to a high-fat (HF) diet, these mice were predisposed to islet lipid accumulation and β cell dysfunction. These E2 actions were dependent on extranuclear ERα, ERβ, and the G protein–coupled ER (GPER) in a pathway involving STAT3 and inhibition of fatty acid synthase (FAS) expression. Our findings provide direct evidence for the involvement of ER signaling in the regulation of islet lipid synthesis in vivo and suggest therapeutic implications for β cell dysfunction in obesity-associated T2D.
Results
E2 prevents β cell failure in ZDF rats.
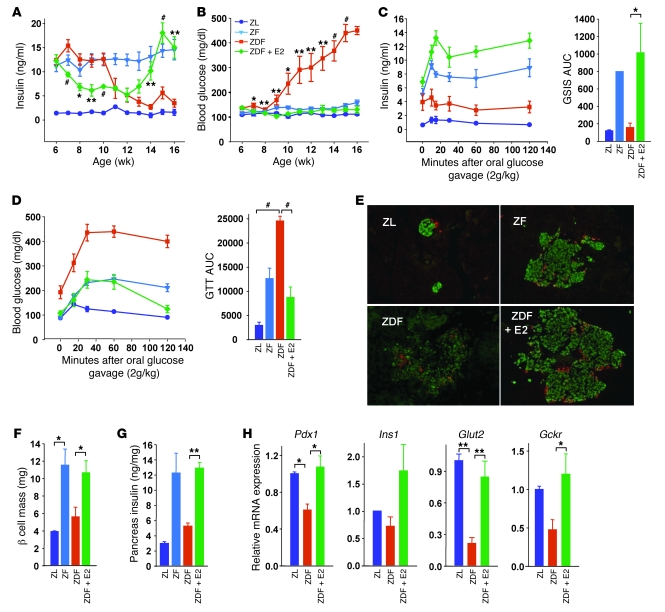
The onset of diabetes in male ZDF rats is characterized by progressive β cell failure in the context of progressive islet triglyceride (TG) accumulation (10). Since female ZDF rats are protected from islet lipid accumulation and β cell failure (10), we explored the hypothesis that E2 prevents islet cell lipogenesis and favors β cell lipid homeostasis. Male ZDF rats were treated s.c. with E2 pellets at 6 weeks of age, and their glucose homeostasis was assessed at 6–16 weeks of age. Age-matched nondiabetic Zucker fatty (ZF) rats were included as controls of leptin resistance without β cell failure. Compared with Zucker lean (ZL) controls, nondiabetic ZF rats were hyperinsulinemic and retained normal blood glucose (Figure 1, A and B). Conversely, ZDF rats showed a progressive decrease in serum insulin concentrations, which was associated with progressive development of hyperglycemia leading to overt diabetes. Maintaining E2 serum concentrations at high physiological levels in ZDF male rats by means of E2 treatment (ZL, 8.5 ± 0.2 pg/ml; ZF, 12.9 ± 1.4 pg/ml; ZDF, 26.0 ± 16.4 pg/ml; E2-treated ZDF, 152.8 ± 45.3 pg/ml) caused an early decrease in serum insulin concentration followed by an increase between 13 and 16 weeks — suggestive of retained β cell ability to compensate for insulin resistance — and prevented hyperglycemia (Figure 1, A and B). The early reduction in insulin levels could reflect an initial improvement in peripheral insulin sensitivity. At 16 weeks, we studied β cell function and the associated glucose tolerance during an oral glucose challenge. Compared with nondiabetic ZF rats, ZDF rats exhibited fasting insulin deficiency associated with severely impaired glucose-stimulated insulin secretion (GSIS) and glucose intolerance, both of which robustly increased in ZDF rats treated with E2 (Figure 1, C and D).
Figure 1. E2 prevents β cell failure in male ZDF rats.
(A–D) Random fed plasma insulin (A) and blood glucose (B) concentrations at the indicated times, as well as plasma insulin (C) and blood glucose (D) concentrations after oral glucose challenge (2.0 g/kg, week 16) and area under the curve (AUC) for GSIS and glucose tolerance test (GTT), in male ZL, ZF, ZDF, and E2-treated ZDF rats. (E) Immunofluorescent labeling of insulin (green) and glucagon (red) in representative islets of male rats at 16 weeks. Images were captured by fluorescence microscopy. Original magnification, ×20. (F) Rat islet β cell mass, calculated from pancreas sections from A. (G) Pancreas insulin concentration in rat islets at 16 weeks. (H) Expression of β cell function genes in rat islets at 9 weeks, normalized to Actb. n = 4–8 (A–D and F); 2 [ZL] and 3–4 [ZF and ZDF] (E, G, and H). *P < 0.05, **P < 0.01, #P < 0.001, ZDF vs. E2-treated ZDF or as indicated by brackets.
Compared with control ZL rats, male ZDF rats were obese. Consistent with the E2-induced improvement of obesity in leptin-resistant mouse models (9), treatment with E2 produced an early reduction in body weight after 7 weeks (Supplemental Figure 1A; supplemental material available online with this article; doi: 10.1172/JCI44564DS1). However, because ZDF rats develop insulin deficiency and the maintenance of fat mass is dependent upon insulin action, this difference was minimal after 14 weeks.
E2 protects β cells from lipid accumulation.
We next determined the effect of E2 exposure on β cell mass and islet architecture in male ZDF rats. Compared with ZL controls, and consistent with insulin resistance, nondiabetic ZF rats showed hyperplasic islets with increased insulin content (Figure 1, E–G). Conversely, ZDF rats showed enlarged islets, with destruction of islet architecture, loss of β cell mass, and decreased pancreas insulin content. Treatment of ZDF rats with E2 was associated with preservation of islet architecture and maintenance of β cell mass and pancreatic insulin content (Figure 1, E–G).
In order to understand the mechanism of E2 protection of ZDF islets in vivo, we first assessed mRNA expression of selected key genes involved in β cell function and glucose metabolism in a group of rats during the transition phase, when hyperglycemia is still moderate and prior to the development of overt diabetes (9 weeks; Figure 1B). Expression of the β cell transcription factor pancreatic and duodenal homeobox 1 (Pdx1) and of downstream target genes, such as proinsulin 1 (Ins1), glucose transporter 2 (Glut2), and glucokinase (Gckr), was downregulated in ZDF islets (Figure 1H). This could reflect mild β cell dedifferentiation in these mice, as observed after exposure to chronic hyperglycemia (11). E2 treatment prevented these abnormalities (Figure 1H), in association with normalized blood glucose levels (Figure 1B).
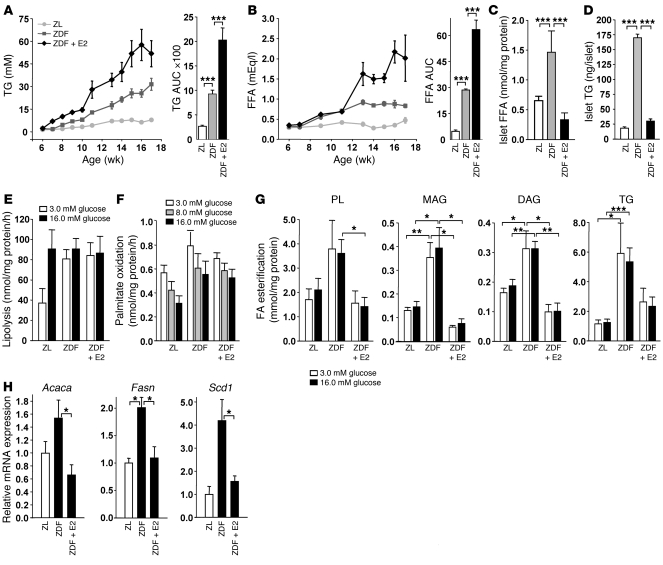
The onset of β cell failure in ZDF rats is preceded by a progressive increase in circulating lipids, leading to a secondary increase in islet TG, which accompanies β cell failure (10). Accordingly, ZDF male rats showed a progressive rise in circulating TG and FFA (Figure 2, A and B). This was associated with increased islet FFA and TG content at 9 weeks of age, corresponding with the development of β cell failure and hyperglycemia (Figure 1, A and B, and Figure 2, C and D). However, whereas E2 treatment caused a rise in serum TG and FFA levels in ZDF rats, it reduced islet TG and FFA accumulation (Figure 2, A–D). Thus, E2 exerts lipopenic effects on islet FFA and TG content that are not mediated by a systemic effect on circulating lipids.
Figure 2. E2 suppresses lipogenesis in ZDF rat islets.
(A and B) Fasting TG (A) and FFA (B) concentrations and area under the curve in male ZL, ZDF, and E2-treated ZDF rats at the indicated time points (n = 4–8). (C) FFA was measured in isolated rat islets at 9 weeks (n = 3–7). (D) TG content in isolated rat islets at 9 weeks (n = 2 [ZL]; 3–4 [ZF and ZDF]). (E) Lipolysis was measured by glycerol release in isolated rat islets at 9 weeks (n = 4–6). (F) Palmitate oxidation was assessed as the release of 3H2O from (9,10[n]-3H) palmitate in rat islets at 9 weeks (n = 4–7). (G) FA esterification was determined as the incorporation of 1-[14C]-palmitate into neutral lipids in isolated islets at 9 weeks (n = 3–7). (H) Gene expression of lipogenesis genes in rat islets at 9 weeks, normalized to Actb (n = 2 [ZL]; 4 [ZF and ZDF]). *P < 0.05, **P < 0.01, ***P < 0.001.
E2 normalizes glycerolipid/FA cycling in ZDF rat islets.
Glycerolipid/FA cycling and gluco-lipo-detoxification in islets involve (a) activation of lipolysis and glycerol release that prevents accumulation of glycerolipids and steatosis (gluco-detoxification), and (b) β-oxidation of FAs that allows for lipo-detoxification (12, 13). Compared with ZL islets, ZDF islets exhibited maximal stimulation of lipolysis at low glucose concentrations without undergoing further stimulation at high glucose levels (Figure 2E). At all tested glucose concentrations, β-oxidation was not significantly different between ZDF and control islets (Figure 2F), indicative of absent β cell lipo-detoxification. However, lipogenesis — measured as FFA accumulation (Figure 1C) — and palmitate esterification into neutral glycerolipids, including phospholipid (PL), monoacylglycerol (MAG), diacylglycerol (DAG), and TG, was markedly increased in ZDF compared with ZL control islets at all glucose concentrations (Figure 2G). Thus, under conditions of high glucose, ZDF islets showed enhanced lipogenesis that was not matched by enhanced lipolysis or β-oxidation, revealing an altered glycerolipid/FA cycle in ZDF islets that leads to accumulation of FFA, TG, and other neutral glycerolipids (DAG and MAG). Importantly, E2 treatment for 3 weeks curtailed FFA and TG accumulation and glycerolipid synthesis in ZDF islets to levels similar to those of ZL control islets without affecting lipolysis or FA oxidation (Figure 3, C–G). Consistently, ZDF islets showed enhanced mRNA levels of enzymes that play a key role in FA synthesis, such as acetyl-CoA carboxylase (Acaca), FAS (Fasn), and stearoyl-CoA desaturase–1 (Scd1), compared with ZL (Figure 2H). Treatment with E2 restored the expression of these lipogenic genes to the level of ZL controls, which suggests that in vivo E2 treatment improves lipid homeostasis in islets by inhibiting all steps of lipogenesis.
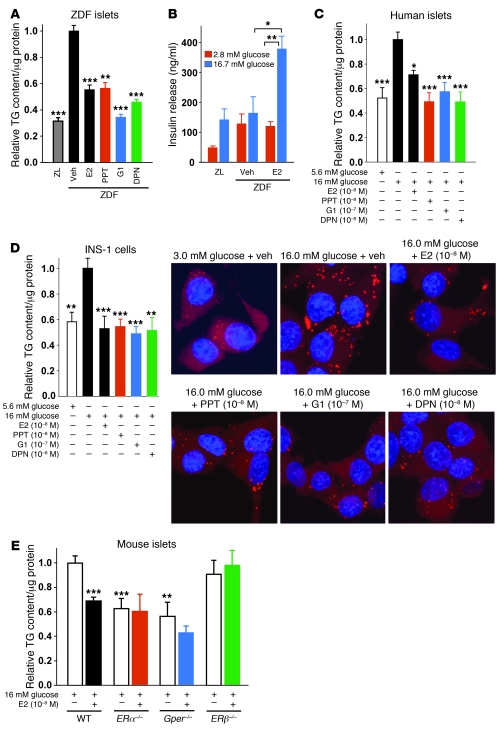
Figure 3. ERα, ERβ, and GPER suppress TG accumulation in β cells.
(A) Effect of in vitro E2, PPT, G1, and DPN treatment (all 10–8 M except for G1, 10–7 M; 24 hours) on TG content in male rat islets cultured under lipogenic conditions (16 mM glucose without FFA). Results are from 4 wells per group. (B) Effect of in vitro E2 treatment (10–8 M; 48 hours) on glucose-stimulated insulin secretion in cultured islets from male rats (n = 2 [ZL]; 2–4 [ZDF]; 3–4 [ZDF + E2]). (C) Effect of E2, PPT, G1, and DPN treatment (24 hours) on TG content in human islets cultured under lipogenic conditions. (D) Effect of E2, PPT, G1, and DPN treatment (24 hours) on TG content in INS-1 cells cultured under lipogenic conditions, as assessed by TG assay (results are from at least 3 experiments) and Nile red staining (n = 1). Immunofluorescent labeling of lipids (red) and DAPI (blue) is shown. Original magnification, ×100. (E) Effect of in vitro E2 treatment (24 hours) on TG content in ERα–/–, ERβ–/–, and Gper–/– islets. Results are from at least 3 experiments. *P < 0.05, **P < 0.01, ***P < 0.001 vs. respective vehicle (veh) or WT 16 mM glucose control, or as indicated by brackets.
Activation of ERα and ERβ suppresses lipid synthesis in β cells.
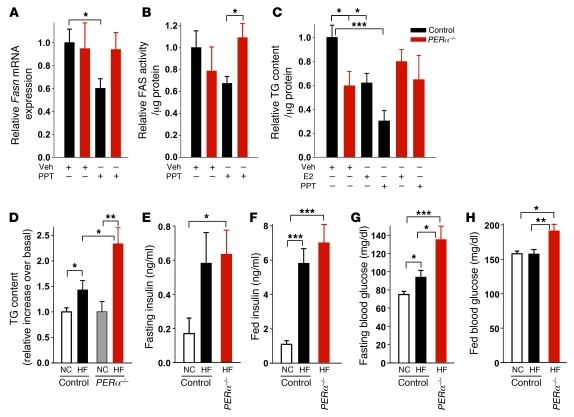
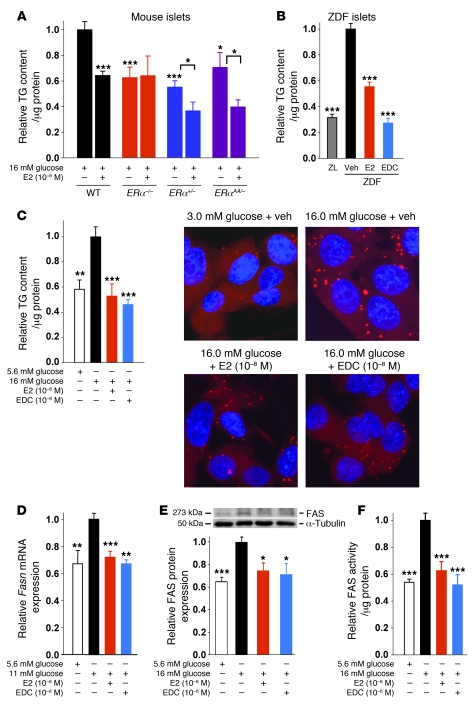
We next sought to determine the extent to which the lipopenic effect of E2 observed in vivo was the result of a direct effect of E2 acting on ERs in β cells. Because E2 inhibits FA synthesis and esterification into TG, we used TG content as a paradigm of lipogenesis. When cultured in lipogenic conditions (16 mM glucose without FFA), ZDF islets showed a rise in TG content compared with ZL control islets (Figure 3A). E2 treatment prevented TG accumulation and improved GSIS in cultured ZDF islets (Figure 3, A and B), confirming a direct antilipogenic effect of E2 on these islets and the regulatory effect of lipid accumulation on GSIS. We have previously described 3 ERs in β cells: the functional ERα and ERβ and the controversial GPER (3, 4). It has been recently proposed that GPER induces expression of ERα36, a short isoform of the classical long isoform of ERα, ERα66 (14). Both ERα66 and ERα36 were expressed in INS-1 cells (Supplemental Figure 2). Treatment with the ERα66-selective agonist propyl-pyrazole-TRIzol (PPT; ref. 15), the GPER/ERα36-agonist GPR30-specific compound–1 (G1; refs. 14, 16), and the ERβ-selective agonist diarylpropionitrile (DPN; ref. 17) prevented TG accumulation to similar extents in ZDF islets (Figure 3A). The antilipogenic actions of E2, PPT, G1, and DPN were recapitulated in cultured human islets and in the glucose-responsive INS-1 rat β cells (Figure 3, C and D), which also showed a glucose-dependent increase in FA synthesis (Supplemental Figure 3, A–D). To further investigate the role of ERs in the suppression of lipid synthesis, we used cultured islets isolated from WT, ERα–/–, ERβ–/–, and Gper–/– mice. ERα–/– and Gper–/– islets showed lower TG content than did control islets (Figure 3E), which could reflect a developmental alteration. Although E2 treatment prevented TG accumulation in WT islets, it had no effect in ERα–/–, ERβ–/–, and Gper–/– islets (Figure 3E), consistent with the relevance of ER activation in suppressing FA and TG synthesis and the nonoverlapping roles of ERα and ERβ.
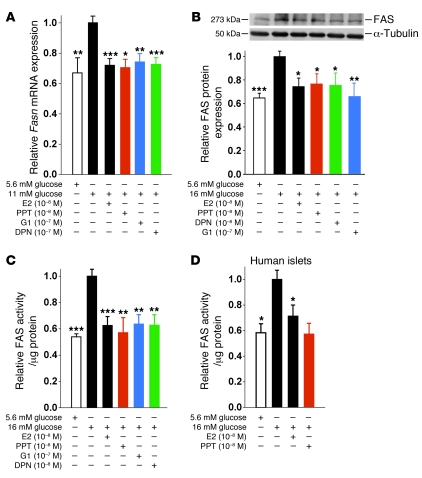
Because INS-1 cells responded to ER agonists to an extent similar to that in rat and human islets, we used them as a model system to study the regulation of lipid synthesis by ERs. We focused on FAS — the master effector of FA synthesis under conditions of glucose surplus — converting malonyl-CoA into saturated long-chain FA (18), which can then undergo β-oxidation or esterification to MAG, DAG, and TG. Exposure of INS-1 cells to high glucose increased Fasn mRNA and FAS protein expression as well as FAS enzymatic activity (Figure 4, A–C). Consistent with ER suppression of TG accumulation (Figure 3D), treatment with E2, PPT, G1, and DPN decreased Fasn mRNA and FAS protein levels to similar extents and suppressed FAS enzymatic activity to basal levels (Figure 4, A–C). E2 suppression of FAS activity was also observed in human islets (Figure 4D). Thus, activation of ERs in islets in a hyperglycemic/diabetic environment prevents the synthesis and accumulation of saturated long-chain FA and, consequently, glycerolipids.
Figure 4. ERα, ERβ, and GPER suppress lipid synthesis in β cells.
(A) Effect of E2, PPT, G1, and DPN treatment (4 hours) on Fasn gene expression, normalized to Actb, in INS-1 cells cultured under lipogenic conditions. (B) Effect of E2, PPT, G1, and DPN treatment (24 hours) on FAS protein expression in INS-1 cells cultured under lipogenic conditions. (C) Effect of E2, PPT, G1, and DPN treatment (24 hours) on FAS enzymatic activity, as measured by incorporation of [14C] malonyl-CoA, in INS-1 cells cultured under lipogenic conditions. (D) Effect of E2 treatment (24 hours) on FAS enzymatic activity in human islets cultured under lipogenic conditions. Results are from at least 3 experiments. *P < 0.05, **P < 0.01, ***P < 0.001 vs. 11 mM (A) or 16 mM (B–D) glucose alone.
Islet ERα is necessary for E2 suppression of lipid synthesis in vivo.
Using ERα as a paradigm of ER actions in β cells, we investigated its role in the control of islet lipid synthesis in vivo using a mouse with pancreas-specific deletion of ERα (PERα–/– mice). The characterization of female PERα–/– mice has previously been described (5). Characterization of ERαlox/lox control and PERα–/– male mice revealed no difference between fasting and fed blood glucose, serum insulin, and glucose tolerance or in body weight or serum leptin (Supplemental Figure 4). This demonstrated that ERα elimination in the pancreas did not substantially alter glucose homeostasis and body weight in male mice fed normal chow (NC). However, PERα–/– mice showed lower islet TG content than did control mice (Figure 5C), consistent with findings in cultured ERα–/– islets (Figure 3E). We next studied the in vivo contribution of ERα to the suppression of islet lipid synthesis after in vivo treatment with E2 or the ERα-selective agonist PPT. Selectively activating ERα decreased Fasn gene expression, FAS enzymatic activity, and islet TG accumulation in control islets, but not PERα–/– islets (Figure 5, A–C). The suppression of TG content in control but not PERα–/– islets was recapitulated by E2 (Figure 5C). These data demonstrate that in vivo ERα activation decreases islet FA synthesis and accumulation into TG. Conversely, failure of ERα activation in other tissues to suppress islet FAS and TG in PERα–/– mice further support the essential role of islet ERα in this function.
Figure 5. E2 acts directly on islet ERα to suppress lipid synthesis.
(A) Gene expression of Fasn, normalized to Actb, in islets of male ERαlox/lox control and PERα–/– mice treated with 200 μg/d PPT for 2 days (n = 8–24). (B) FAS enzymatic activity in islets of PPT-treated control and PERα–/– mice (n = 6–18). (C) TG content in islets of control and PERα–/– mice treated with 4 μg/d E2 or 200 μg/d PPT for 2 days (n = 9–25). (D) Relative fold increase in TG content in mouse islets after sacrifice at 11 weeks. PERα–/– NC data are duplicated from C (n = 9–36). (E–H) Fasting (E) and fed (F) plasma insulin concentrations, and fasting (G) and fed (H) blood glucose, in control and PERα–/– mice at 9 weeks (n = 10–21). *P < 0.05, **P < 0.01, ***P < 0.001.
We next explored the function of ERα in protecting islet lipid homeostasis and function in the T2D model of HF feeding. Compared with NC, HF feeding produced the same increase in body weight and fat pad mass in control and PERα–/– mice (Supplemental Figure 5, A and B). HF feeding provoked a greater rise in islet TG accumulation in PERα–/– compared with control mice (Figure 5D), demonstrating a predisposition to islet lipid accumulation. HF diet–fed control and PERα–/– mice showed similar hyperinsulinemia compared with NC-fed control mice (Figure 5, E and F), consistent with the development of insulin resistance after HF feeding. However, in both fasting and fed states, PERα–/– mice displayed an increase in blood glucose without difference in insulin concentrations compared with control mice (Figure 5, G and H), which suggests a failure of β cell compensation for insulin resistance in PERα–/– mice. Thus, HF feeding causes more lipid accumulation in PERα–/– islets in association with mild β cell dysfunction and hyperglycemia.
Extranuclear ERs suppresses lipid synthesis via STAT3.
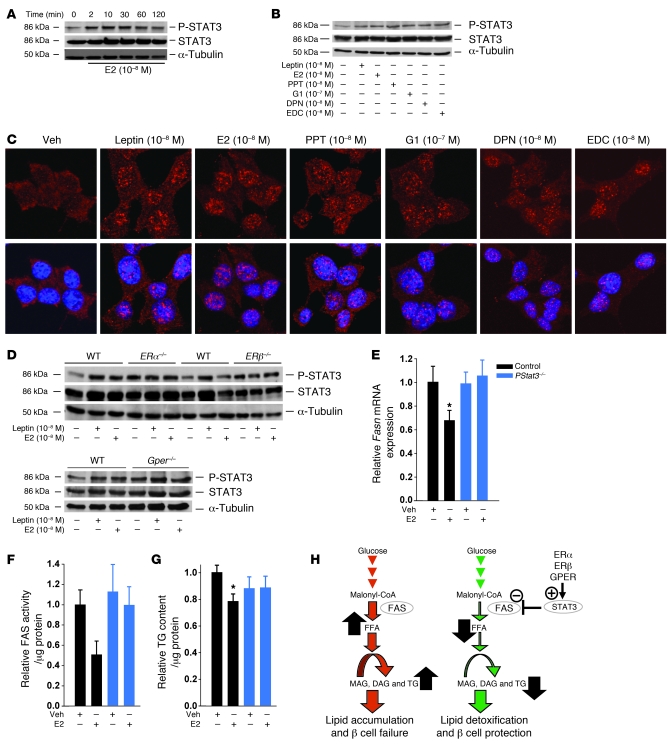
We recently showed that ERα signals outside the nucleus in β cells to enhance cell survival and amplify insulin biosynthesis (4, 5). In the classical ER signaling pathway, E2-activated ERs binds as homodimers to an estrogen response element (ERE) in target promoters or indirectly to an AP-1 or Sp-1 response element through association with other transcription factors that tether the activated ERs to DNA (19). This classical, genomic mechanism leads to up- or downregulation of gene transcription. To investigate whether ERE is involved in ERα suppression of lipid synthesis, we used an ERα knockin mouse with a mutation of the DNA-binding domain of ERα that eliminates ERα binding to ERE (ERαAA/– mice; ref. 20). Treatment with E2 reduced TG content in cultured WT islets (Figure 6A). Consistent with our in vitro and in vivo findings in ERα–/– and PERα–/– islets (Figure 3E and Figure 5C), cultured ERα+/– islets showed a constitutive decrease in TG content (Figure 6A). E2 treatment lowered TG content in ERα+/– and ERαAA/– islets, but not ERα–/– islets (Figure 6A). This demonstrated that E2 suppression of TG required only 1 ERα allele in an ERE-independent manner. Since ERα suppressed TG accumulation through an ERE-independent pathway, we explored the possibility that this is mediated via an extranuclear ERα. We used INS-1 β cells treated with an estrogen dendrimer conjugate (EDC) that remains outside the nucleus and is used to study extranuclear ER actions (21, 22). EDC was as efficient as E2 or PPT in preventing TG accumulation in ZDF rat islets and INS-1 cells (compare Figure 6, B and C, and Figure 3, A and D), as well as in inhibiting Fasn mRNA expression, FAS protein levels, and FAS enzymatic activity (compare Figure 6, D–F, and Figure 4, A–C). This is suggestive of an extranuclear ER mechanism suppressing islet FA synthesis and esterification in TG. Because ERs suppress lipogenesis in leptin-resistant ZDF rats, we investigated whether the downstream target of leptin involved in regulating glucose and lipid metabolism (23–25), namely STAT3 (26), mediates extranuclear ERs suppression of lipid synthesis. E2 rapidly (within 2 minutes) stimulated STAT3 phosphorylation — and, hence, activation — in INS-1 cells (Figure 7A). Accordingly, E2, PPT, G1, DPN, and EDC activated STAT3 and its nuclear translocation to an extent comparable to that of leptin (Figure 7, B and C). Moreover, E2 induced an increase in STAT3 phosphorylation in islets derived from WT mice, but not ERα–/–, ERβ–/–, or Gper–/– mice (Figure 7D), consistent with the importance of ERs in STAT3 activation in islets. To further explore the role of STAT3 in the inhibition of lipid synthesis in islets by ERs in vivo, we used a mouse with pancreas-specific deletion of STAT3 (PStat3–/–) that does not develop glucose intolerance or obesity (27). In vivo treatment with E2 decreased islet Fasn mRNA levels, FAS enzymatic activity, and TG content in control islets, but not PStat3–/– islets (Figure 7, E–G). Together, the data demonstrate that suppression of FA synthesis and esterification into TG in islets by ERs requires direct activation of STAT3.
Figure 6. Extranuclear ERs suppress lipid synthesis.
(A) Effect of in vitro E2 treatment (24 hours) on TG content in WT, ERα–/–, ERα+/–, and ERαAA/– islets cultured under lipogenic conditions. ERα–/– islet data were pooled with data from experiments using PERα–/– islets. (B) Effect of in vitro E2 and EDC treatment (both 10–8 M; 24 hours) on TG content in male rat islets cultured under lipogenic conditions. Data are from 4 wells (ZL, ZDF, ZDF + E2) or 2 wells (ZDF + EDC). (C) Effect of E2 and EDC treatment (24 hours) on TG content in INS-1 cells cultured under lipogenic conditions, assessed by TG assay and Nile red staining (n = 1). Original magnification, ×100. (D) Effect of E2 and EDC treatment (4 hours) on Fasn gene expression, normalized to Actb, in INS-1 cells cultured under lipogenic conditions. (E) Effect of E2 and EDC treatment (24 hours) on FAS protein expression in INS-1 cells cultured under lipogenic conditions. (F) Effect of E2 and EDC treatment (24 hours) on FAS enzymatic activity in INS-1 cells cultured under lipogenic conditions. Results are from at least 3 experiments. *P < 0.05, **P < 0.01, ***P < 0.001 vs. 11 mM (D) or 16 mM (A–C, E, and F) glucose alone.
Figure 7. ERs suppress lipid synthesis via STAT3.
(A and B) INS-1 cells were treated with E2 for up to 2 hours (A) or with leptin, E2, PPT, G1, DPN, or EDC for 10 minutes (B), and phosphorylation of STAT3 was determined by Western blotting. Blots are representative of at least 3 experiments. (C) Immunofluorescent labeling of phosphorylated STAT3 (red) and DAPI (blue) in INS-1 cells treated with leptin, E2, PPT, G1, DPN, or EDC for 10 minutes. Images are representative of 2 experiments. Original magnification, ×100. (D) Cultured islets from the indicated mice were treated with E2 for 10 minutes, and STAT3 phosphorylation was determined by Western blotting. (E) Gene expression of Fasn, normalized to Actb, in control and PStat3–/– mouse islets treated with 6 μg/d E2 for 2 days (n = 11–20). (F) FAS enzymatic activity in islets of E2-treated control and PStat3–/– mice (n = 8–20). (G) TG content in islets of E2-treated control and PStat3–/– mice (n = 13–22). Data in E–G are from at least 3 experiments. *P < 0.05. (H) Proposed mechanism for ER-mediated inhibition of islet FA synthesis and lipid accumulation. Left: Lipid accumulation in islets leads to β cell failure. Right: ER activation suppresses FAS expression and activity, thus preventing lipid accumulation and protecting against β cell failure in a STAT3-dependent manner.
Discussion
We have shown that the steroid E2 reduced islet FA and glycerolipid synthesis in vivo via ERα in β cells and prevented β cell failure in ZDF rats, a model of T2D. We have also shown that pharmacological activation of ERα66, GPER/ERα36, and ERβ suppressed lipogenesis in cultured rodent and human β cells.
Several nonexclusive mechanisms could underline the protective effect of ER activation against β cell failure in ZDF rats. First, enhanced insulin gene expression may be involved. The ZDF rat is also a model of β cell exhaustion of insulin stores in the face of fuel surfeit and massive obesity. Unlike its nondiabetic ZF counterpart, the ZDF rat harbors a mutation in the insulin promoter, decreasing insulin transcription (28) and potentially participating in failure of ZDF islets to meet the insulin biosynthesis demand of insulin resistance. We have previously shown that ERα potentiates insulin gene transcription and increases islet insulin stores, which probably contribute to the compensatory increase in insulin secretion from β cells in insulin resistance (5). Consistent with this view, E2 enhances PDX-1 and proinsulin gene expression in ZDF islets and causes a rise in islet insulin content. Second, E2 could enhance β cell survival in the face of hyperglycemia and hyperlipidemia (glucolipotoxicity). With ER activation preventing β cell apoptosis in streptozotocin-treated mice (3, 4), it is likely that E2 preserves β cell mass of ZDF rats via a similar antiapoptotic mechanism. Third, E2 improves obesity and insulin sensitivity in ZDF rats, potentially protecting β cells in an indirect manner (2, 29). However, our current findings support an instrumental role for E2 in preventing β cell failure via direct autocrine regulation of islet lipid homeostasis. Unlike insulin sensitizers like metformin or thiazolidinediones that improve β cell function and prevent islet lipotoxicity by suppressing circulating FFA (30, 31), E2 prevented β cell failure despite an elevation in circulating lipids in ZDF rats. Moreover, E2 reduced TG content and restored GSIS in cultured ZDF islets, demonstrating the importance of lipid homeostasis to β cell function in this model and suggesting a direct protective effect for E2 against lipotoxicity in these cells. Finally, the disruption of lipid homeostasis in PERα–/– mice fed HF diet was associated with mild β cell failure to compensate for insulin resistance and hyperglycemia, a classical sign of early T2D. Taken together, these data argue that E2 improvement of lipid homeostasis is instrumental in protecting β cell function in obesity-associated models of T2D.
E2 protection likely involves the lipogenic arm of the glycerolipid/FA cycle in ZDF islets. Islets from the nondiabetic ZF rats are characterized by balanced increased lipolysis and β-oxidation in the face of increased lipogenesis, allowing islet gluco-lipo-detoxification and favoring compensation for insulin resistance (12). In contrast, islets from diabetic ZDF rats show an imbalance between enhanced lipid esterification processes (lipogenesis) and lipolysis/β-oxidation, leading to the accumulation of MAG, DAG, and TG. By reducing FA synthesis and esterification into glycerolipid in ZDF islets, E2 prevents the sustained accumulation of potentially toxic lipid intermediates in the lipogenic arm, thus preventing tissue glucolipotoxicity (1, 12, 13).
Previous studies have reported that E2 suppresses lipogenesis in white adipose tissue and liver in vivo (32, 33). However, it is unknown whether the antilipogenic effects of E2 are mediated via activation of adipocyte or hepatocyte ERs or via the central nervous system, similar to leptin signals from the mediobasal hypothalamus that trigger sympathetic outflow to white adipose tissue (34). Several arguments support the view that E2 directly suppresses islet lipid synthesis, at least in part, via ER activation in β cells. First, E2 and selective agonists for all ERs reduced FA synthesis and TG accumulation in cultured rodent and human β cells. Second, in vivo activation of ERα with a selective agonist also suppressed islet FA synthesis and TG accumulation in mice. Conversely, in vivo activation of ERα in nonislet tissues failed to suppress islet lipid synthesis in PERα–/– mice, which have islet-specific null deletion of ERα, and islets accumulated more lipids when these mice were fed a HF diet, in association with β cell dysfunction and mild hyperglycemia. Thus, we conclude that activation of ERα in islets is critical to prevent islet lipid synthesis and accumulation and β cell dysfunction, independent of an effect on the central nervous system or other tissues. Additionally, ERα, ERβ, and GPER/ERα36 reduced lipid synthesis to the same extent in cultured β cells when activated by synthetic agonists, despite E2 having no effect in cultured islets individually deficient in ERα, ERβ, or GPER. This suggests that ERα and ERβ exhibit nonredundant antilipogenic functions in islets.
We observed that ERs suppressed islet TG synthesis independently of an ERE. Moreover, the extranuclear ER ligand EDC and the agonist for the recently characterized extranuclear ERα36, lacking transcriptional activity (14), both suppressed Fasn mRNA levels and FAS activity. This demonstrated that E2 suppresses FA synthesis via input from extranuclear ERs, rather than from ERs acting as classical ligand-activated transcription factors. Furthermore, we present in vivo evidence that the extranuclear ERs signal via STAT3 activation to suppress islet FA synthesis and lipid accumulation. Further studies are needed to identify the pathways inhibiting FAS level and activity downstream of STAT3. A proposed mechanism for ER-mediated inhibition of islet lipid synthesis and accumulation is shown in Figure 7H.
Our findings have therapeutic implications for T2D. Previously, 2 randomized trials have shown a 30% reduction in the incidence of T2D in postmenopausal women on estrogen replacement therapy and provided some evidence for the protective effect of this therapy on β cells (35, 36). In ZDF rats, E2 treatment produces a massive increase in circulating lipids, as can be observed with oral estrogen (37), which indicates that global activation of ER could be deleterious in T2D. Although ER ligands with selective extranuclear ER actions (21) or ER pathway–selective activity (38) have been developed, their effect is global. The results of the current study, combined with our previous findings that ERs improve islet survival (3, 4) and insulin biosynthesis (5), suggest that selective enhancement or targeting of ER action in β cells may prove a novel therapeutic avenue to prevent β cell failure in T2D.
Methods
Rodents.
Male ZDF, nondiabetic ZF, and ZL rats were obtained at 6 weeks of age (Charles Rivers Laboratory). After anesthetization with ketamine (100 mg/kg) and xylazine (10 mg/kg) i.p., rats were implanted with a slow-release E2 pellet (0.72 mg/pellet; Innovative Research of America) s.c. behind the neck using a trocar. Rats were studied between 6–16 weeks of age. The generation of PERα–/– mice has been described previously (5). Stat3lox/lox mice were provided by D.E. Levy (New York University School of Medicine, New York, New York, USA; ref. 39). PStat3–/– mice were generated as previously described (27). The generation of ERα–/–, ERβ–/–, and Gper–/– mice has previously been described (3, 40, 41). ERαAA/– mice were provided by J.L. Jameson (Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA; ref. 20). The generation of ERαAA/– mice has previously been described (4). Mice were studied between 9 and 20 weeks of age. For in vivo drug stimulation, E2 (4 or 6 μg/d), PPT (200 μg/d), or vehicle (10% ethanol, 90% sesame oil) were injected s.c. twice daily for 2 days. All animal experiments were approved by the Northwestern University Animal Care and Use Committees and were conducted in accordance with the NIH Guide for the Care and Use of Animals.
Metabolic studies.
ZDF rat body weight was assessed at the indicated time points, and fat pads were weighed after sacrifice at 16 weeks. Blood glucose and serum insulin were measured as indicated using a glucose monitor (OneTouch Ultra) and insulin ELISA (Linco Research), respectively. For rats, at 16 weeks, in vivo GSIS and in vivo glucose tolerance test were performed simultaneously after an oral glucose (2.0 g/kg) gavage. Blood glucose and serum insulin were measured as above for the indicated times. Serum TG (Sigma-Aldrich) and serum FFA (Wako Chemicals) were measured weekly for the duration of the study using an enzymatic colorimetric assay (see below). Mouse serum leptin was assessed by ELISA (LINCO Research).
HF diet.
Mice were weaned onto a HF diet (30% AMF Diet; 14.9% kcal protein, 33.2% kcal carbohydrates, 51.9% kcal fat; Harlan) at 3 weeks of age. Body weight, blood glucose, and serum insulin were assessed at the indicated time points. After sacrifice at 11 weeks, fat pads were weighed, and islets were isolated for assessment of TG content. Islet TG content data was represented as fold increase from basal; values for control and PERα–/– mice were both assigned as 1.
Immunohistochemistry and Nile red.
Pancreases were fixed (10% neutral-buffered formalin), embedded in paraffin, and sectioned at 5 μm. Heat-induced antigen retrieval was performed in deparaffinized pancreatic sections in citrate buffer (0.01 M citric acid, 0.05% Tween-20, pH 6.0). Sections were permeabilized (0.2% Triton X-100), blocked (5% goat serum, 1% BSA, and 0.02% Triton X-100 for 30 minutes at room temperature), and incubated (overnight at 4°C) with primary antibodies: guinea pig anti-insulin (all 1:1,000 except for ZDF, 1:500; Linco) and rabbit anti-glucagon (all 1:1,000 except for ZDF, 1:500; Linco). Slides were washed and incubated (1 hour at room temperature) with secondary antibodies: FITC-conjugated goat anti–guinea pig (1:400; Jackson ImmunoResearch) and Cy3-conjugated goat anti-rabbit (1:400; Jackson ImmunoResearch). After treatment, cells were washed in assay buffer, fixed with fixative, washed in assay buffer, and stained with Nile red (Cayman Chemical). After treatment, cells were fixed in acetone, permeabilized (0.1% Triton X-100), blocked (5% goat serum, 1% BSA, and 0.02% Triton X-100 for 60 minutes at room temperature), and incubated (overnight at 4°C) with primary antibody: polyclonal rabbit anti–phospho-Stat3 (Tyr705) (1:100; Cell Signaling Technology). Cells were washed and incubated (4 hours at room temperature) with secondary antibody, Cy3-conjugated goat anti-rabbit (1:200; Jackson ImmunoResearch), and counterstained with DAPI (1:50,000; 5 minutes). Images were captured using a Zeiss LSM 510 META laser scanning confocal microscope.
Pancreas insulin concentration.
After treatment, pancreases were collected, weighed, and homogenized in ice-cold acid ethanol as described previously (4). After overnight incubation at 4°C, homogenates were centrifuged (16,100 g at 4°C), and supernatant were stored (–20°C) until insulin quantitation by RIA (Linco Research).
Calculation of β cell mass.
Deparaffinized pancreas sections (4 μm) were stained for insulin as described above, and insulin-positive cells were imaged on an epifluorescent microscope (Nikon Eclipse E400) at ×20 magnification. Insulin-positive cell area per section and per entire-pancreas section (original magnification, ×2.5) was quantified with MetaMorph (Molecular Devices). β Cell mass was determined by dividing total insulin-positive area for each section by section area (percentage area of β cells), multiplied by pancreatic weight. All islets were imaged from 1 section per animal (n = 1–4) per group.
Islet isolation and insulin secretion in static incubation.
Islet isolation was performed as described previously (3). Briefly, pancreases were excised, incubated (37°C, 12–15 minutes) in HBSS with collagenase (2 mg/ml; Sigma-Aldrich), filtered, rinsed with ice-cold HBSS, and separated by density gradient in Histopaque (Sigma-Aldrich). After an ice-cold HBSS wash, islets were hand-picked under a dissection microscope or transferred en masse to culture dish. Insulin secretion from islets was measured by static incubation as described previously (3). Briefly, after overnight recovery, islets were transferred to a 24-well plate (10 islets/well) containing phenol red–free RPMI media and treated for 48 hours at 37°C. After treatment, islets were preincubated in KRB buffer, washed in PBS, and incubated for 30 minutes in the presence of glucose (2.8 or 16.7 mM), after which media were collected and stored at –20°C until insulin quantification by ELISA (Linco Research).
Islet lipolysis.
After a 1-hour recovery in RPMI 1640 medium, isolated islets were transferred into 12-well plates (60 islets/well) and incubated for 2 hours in 1 ml RPMI medium (3 mmol/l glucose). Islets were washed in Krebs-Ringer bicarbonate HEPES buffer (KRBH) with 0.07% BSA and 3 mmol/l glucose, then transferred into 0.2 ml KRBH with 0.07% BSA in a 96-well plate with final glucose concentration of 3 or 16 mmol/l. Islets were incubated (3 hours at 37°C) in a humidified 5% CO2 atmosphere. For background measurements, 100 μl incubation solution was removed after 5 minutes’ incubation. After incubation, 150 μl incubation solution was removed for glycerol release determinations. The remaining islets were recollected, and, after successive washes in cold PBS, were frozen at –20°C in 50 μl lysis buffer for further protein measurements.
FA oxidation.
Total FA oxidation (from exogenous and endogenous FFA sources) was measured after a 1-hour recovery in RPMI 1640 medium. Islets were transferred to 12-well plates (50 islets/well) containing 1 ml RPMI medium with 5.5 mmol/l glucose (0.25% w/v), defatted BSA, and 0.1 mmol/l (9,10[n]-3H) palmitate (2 μCi/ml) and were cultured for 16 hours at 37°C. Islets were then washed in KRBH with 0.25% BSA, 3 mmol/l glucose, and 0.1 mmol/l palmitate and incubated for 2 hours in KRBH with 0.25% BSA, 0.1 mmol/l (9,10[n]-3H) palmitate (2 μCi/ml), 1 mmol/l carnitine, and 3 or 16 mmol/l glucose. Incubation media was collected at the end of incubations after centrifugation, and supernatants were frozen for further separation of 3H2O from labeled FAs. Radioactivity of 3H2O was counted in a liquid scintillation counter.
FA esterification.
After a 1-hour recovery in RPMI media, islets were transferred to 12-well plates (50 islets/well) containing 1 ml RPMI complete medium with 3 or 16 mM glucose, 0.25% defatted BSA, 0.1 mM [1-14C] palmitate (1 μCi/ml), and cultured for 16 hours at 37°C. Islets were collected, washed in cold PBS, and resuspended in 3 ml Folch reagent. Total lipids were extracted and separated by thin-layer chromatography using petroleum ether/ether/acetic acid at a 70:30:1 ratio. The spots corresponding to PL, MAG, DAG, and TG visualized by autoradiography were individually scraped and mixed with scintillation fluid, and radioactivity was measured using a scintillation counter.
Islet and INS-1 TG content.
After treatment or islet isolation, cells or islets were washed twice in PBS, and lipids were extracted overnight (4°C) in 3 ml chloroform/methanol at a 2:1 ratio. The following day, lipids were purified by addition of 1.5 ml dH2O, centrifugation at 1,800 g for 10 minutes, and transferring the bottom layer to clean tube for drying under N2 gas. Lipids were resuspended in 50% isopropanol and 50% dH2O, and TG amount was determined using an enzymatic colorimetric assay (Sigma-Aldrich).
FAS enzyme activity.
FAS activity was measured by the incorporation of radiolabeled malonyl-CoA into palmitate as described previously (42). Briefly, after treatment and 2× PBS wash, cells were lysed in buffer (20 mM Tris, pH 7.5; 1.0 mM EDTA; 1.0 mM DTT; and phosphatase and protease inhibitors) and centrifuged at 12,000 g for 30 minutes at 4°C. Lysates were incubated for 20 minutes at 37°C with 166.6 μM acetyl-CoA, 100 mM potassium phosphate (pH 6.6), 0.1 μCi [14C] malonyl-CoA, and 25 nM malonyl-CoA in the absence or presence of 500 μM NADPH. The reaction was stopped with 1:1 chloroform/methanol solution, mixed for 30 minutes at 20°C, and centrifuged at 12,500 g for 30 minutes. The supernatant was vacuum-dried, and the pellet was resuspended in 200 μl water-saturated butanol. After addition of 200 μl ddH2O, vortexing, and spinning for 1 minute, the upper layer was removed for reextraction. The butanol layer was dried and counted. Protein was quantified by Bio-Rad protein assay, and results were expressed as relative cpm of [14C] incorporated per microgram cell lysate.
Cell and islet culture and drug stimulation.
Glucose-responsive INS-1 cells (clone 832/13) were provided by C. Newgard (Duke University Medical Center, Durham, North Carolina, USA; ref. 43). Cells or islets were cultured in phenol red–free RPMI 1640 or DMEM media with 2.8 mM, 5.6 mM, 11 mM, or 16.0 mM glucose and 10% charcoal-stripped FBS for the indicated time periods prior to experimentation. Human islets (provided by the Integrated Islet Distribution Program) were cultured in phenol red–free CMRL 1066 with 10% charcoal-stripped FBS. Cells were seeded to approximately 80%, and 200–250 islets were used for experimentation. Cells or islets were incubated with either leptin (10–8 M; R&D Systems), E2 (10–8 M; Steraloids Inc.), PPT (10–8 M), DPN (10–8 M), G1 (10–7 M), EDC (10–8 M), or vehicle (ethanol or DMSO) for the indicated times. EDC was synthesized by J.A. Katzenellenbogen (University of Illinois, Urbana, Illinois, USA).
Western blot analysis.
After treatment in media with or without serum, cells were homogenized in lysis buffer as described previously (44). Immunoblotting primary antibodies were as follows: polyclonal rabbit anti–phospho-Stat3 (Tyr705) (1:1,000; Cell Signaling Technology), rabbit anti-Stat3 (1:1,000; Cell Signaling Technology), monoclonal rabbit anti-FAS (C20G5) (1:1,000; Cell Signaling Technology), monoclonal rat anti-ERα (H222) (1:100; Fitzgerald), polyclonal rabbit anti-ERα (HC-20) (1:400; Santa Cruz Biotechnology), polyclonal rabbit-anti-ERα (MC-20) (1:400; Santa Cruz Biotechnology), and monoclonal mouse anti–tubulin-α Ab-2 (1:10,000; Lab Vision Corp.). Secondary antibodies were horseradish peroxidase–conjugated goat anti-mouse, goat anti-rabbit, or goat anti-rat, and detection of immunoreactive bands was performed using the ECL Western Blotting Substrate (Pierce).
Real-time quantitative RT-PCR.
After treatment or islet isolation, total RNA was extracted in TRIzol Reagent (Invitrogen). 1 μg RNA was reverse transcribed using iScript cDNA Synthesis Kit (Bio-Rad) with random hexamers (iCycler; Bio-Rad). mRNA expression of target genes was normalized to Actb expression, as described previously (45). Primer sequences (Sigma-Aldrich) are shown in Supplemental Table 1.
Statistics.
Results are presented as mean ± SEM unless otherwise indicated. Data were analyzed using unpaired 2-tailed Student’s t test or 1-way ANOVA as appropriate. A P value less than 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We are grateful to Winifred P.S. Wong for technical assistance in generating and characterizing the ERαlox/lox and PERα–/– male mice. Imaging work was performed at the Northwestern University Cell Imaging Facility, supported by National Cancer Institute Cancer Center Support Grant P30 CA060553. We are thankful to the Integrated Islet Distribution Program, funded by the National Institutes of Diabetes and Digestive and Kidney Disease with support from the Juvenile Diabetes Research Foundation International, for providing human islets. This research was supported by grants from the NIH (R01DK074970-01 and P50HD044405), the Juvenile Diabetes Research Foundation (1-2006-837), and the March of Dimes (6-FY07-678) to F. Mauvais-Jarvis. J.P. Tiano was supported in part by NIH Training Grant T32 DK007169, and C. Le May was the recipient of a Juvenile Diabetes Research Foundation Post-Doctoral Fellowship. ERαlox/lox mouse generation was funded by NIH/NIEHS division of intramural research grant Z01ES70065 to K.S. Korach. Experiments of lipid cycling in Figure 3 were partially funded by grants from the Canadian Diabetes Association and the Canadian Institute of Health Research to M. Prentki. FAS activity assays were funded by grants from the NIH (R01DK054254 and R01DK083850) and the United States Department of Agriculture (USDA 38903-19826) to S.M. Najjar.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(8):3331–3342. doi:10.1172/JCI44564.
References
- 1.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116(7):1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu S, Mauvais-Jarvis F. Minireview: Estrogenic protection of beta-cell failure in metabolic diseases. Endocrinology. 2010;151(3):859–864. doi: 10.1210/en.2009-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le May C, et al. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci U S A. 2006;103(24):9232–9237. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu S, et al. Importance of extranuclear estrogen receptor-alpha and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes. 2009;58(10):2292–2302. doi: 10.2337/db09-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong WP, et al. Extranuclear estrogen receptor-alpha stimulates NeuroD1 binding to the insulin promoter and favors insulin synthesis. Proc Natl Acad Sci U S A. 2010;107(29):13057–13062. doi: 10.1073/pnas.0914501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pick A, et al. Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes. 1998;47(3):358–364. doi: 10.2337/diabetes.47.3.358. [DOI] [PubMed] [Google Scholar]
- 7.Mauvais-Jarvis F, et al. Ketosis-prone type 2 diabetes in patients of sub-Saharan African origin: clinical pathophysiology and natural history of beta-cell dysfunction and insulin resistance. Diabetes. 2004;53(3):645–653. doi: 10.2337/diabetes.53.3.645. [DOI] [PubMed] [Google Scholar]
- 8.Louet JF, et al. Gender and neurogenin3 influence the pathogenesis of ketosis-prone diabetes. Diabetes Obes Metab. 2008;10(10):912–920. doi: 10.1111/j.1463-1326.2007.00830.x. [DOI] [PubMed] [Google Scholar]
- 9.Gao Q, et al. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nature Medicine. 2007;13(1):89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- 10.Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, Unger RH. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc Natl Acad Sci U S A. 1994;91(23):10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jonas JC, et al. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. J Biol Chem. 1999;274(20):14112–14121. doi: 10.1074/jbc.274.20.14112. [DOI] [PubMed] [Google Scholar]
- 12.Nolan CJ, et al. Beta cell compensation for insulin resistance in Zucker fatty rats: increased lipolysis and fatty acid signalling. Diabetologia. 2006;49(9):2120–2130. doi: 10.1007/s00125-006-0305-5. [DOI] [PubMed] [Google Scholar]
- 13.Delghingaro-Augusto V, et al. Islet beta cell failure in the 60% pancreatectomised obese hyperlipidaemic Zucker fatty rat: severe dysfunction with altered glycerolipid metabolism without steatosis or a falling beta cell mass. Diabetologia. 2009;52(6):1122–1132. doi: 10.1007/s00125-009-1317-8. [DOI] [PubMed] [Google Scholar]
- 14.Kang L, et al. Involvement of estrogen receptor variant ER-alpha36, not GPR30, in nongenomic estrogen signaling. Mol Endocrinol. 2010;24(4):709–721. doi: 10.1210/me.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stauffer SR, et al. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43(26):4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- 16.Bologa CG, et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2(4):207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 17.Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44(24):4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- 18.Wakil SJ. Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry. 1989;28(11):4523–4530. doi: 10.1021/bi00437a001. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson S, et al. Mechanisms of estrogen action. Physiol Rev. 2001;81(4):1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 20.Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, Jameson JL. An estrogen receptor (ER)alpha deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol Endocrinol. 2002;16(10):2188–2201. doi: 10.1210/me.2001-0174. [DOI] [PubMed] [Google Scholar]
- 21.Harrington WR, et al. Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol. 2006;20(3):491–502. doi: 10.1210/me.2005-0186. [DOI] [PubMed] [Google Scholar]
- 22.Chambliss KL, et al. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest. 2010;120(7):2319–2330. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue H, et al. Role of STAT-3 in regulation of hepatic gluconeogenic genes and carbohydrate metabolism in vivo. Nat Med. 2004;10(2):168–174. doi: 10.1038/nm980. [DOI] [PubMed] [Google Scholar]
- 24.Ueki K, Kondo T, Tseng YH, Kahn CR. Central role of suppressors of cytokine signaling proteins in hepatic steatosis, insulin resistance, and the metabolic syndrome in the mouse. Proc Natl Acad Sci U S A. 2004;101(28):10422–10427. doi: 10.1073/pnas.0402511101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinoshita S, et al. Role of hepatic STAT3 in the regulation of lipid metabolism. Kobe J Med Sci. 2008;54(4):E200–E208. [PubMed] [Google Scholar]
- 26.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet. 1996;14(1):95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 27.Lee JY, Hennighausen L. The transcription factor Stat3 is dispensable for pancreatic beta-cell development and function. Biochem Biophys Res Commun. 2005;334(3):764–768. doi: 10.1016/j.bbrc.2005.06.162. [DOI] [PubMed] [Google Scholar]
- 28.Griffen SC, Wang J, German MS. A genetic defect in beta-cell gene expression segregates independently from the fa locus in the ZDF rat. Diabetes. 2001;50(1):63–68. doi: 10.2337/diabetes.50.1.63. [DOI] [PubMed] [Google Scholar]
- 29.Mauvais-Jarvis F. Estrogen and androgen receptors: regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol Metab. 2011;22(1):24–33. doi: 10.1016/j.tem.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sreenan S, Sturis J, Pugh W, Burant CF, Polonsky KS. Prevention of hyperglycemia in the Zucker diabetic fatty rat by treatment with metformin or troglitazone. Am J Physiol. 1996;271(4 pt 1):E742–E747. doi: 10.1152/ajpendo.1996.271.4.E742. [DOI] [PubMed] [Google Scholar]
- 31.Pickavance LC, Brand CL, Wassermann K, Wilding JP. The dual PPARalpha/gamma agonist, ragaglitazar, improves insulin sensitivity and metabolic profile equally with pioglitazone in diabetic and dietary obese ZDF rats. Br J Pharmacol. 2005;144(3):308–316. doi: 10.1038/sj.bjp.0706041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem. 2005;280(43):35983–35991. doi: 10.1074/jbc.M507339200. [DOI] [PubMed] [Google Scholar]
- 33.Gao H, et al. Long-term administration of estradiol decreases expression of hepatic lipogenic genes and improves insulin sensitivity in ob/ob mice: a possible mechanism is through direct regulation of signal transducer and activator of transcription 3. Mol Endocrinol. 2006;20(6):1287–1299. doi: 10.1210/me.2006-0012. [DOI] [PubMed] [Google Scholar]
- 34.Buettner C, et al. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med. 2008;14(6):667–675. doi: 10.1038/nm1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Margolis KL, et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia. 2004;47(7):1175–1187. doi: 10.1007/s00125-004-1448-x. [DOI] [PubMed] [Google Scholar]
- 36.Kanaya AM, et al. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138(1):1–9. doi: 10.7326/0003-4819-138-1-200301070-00005. [DOI] [PubMed] [Google Scholar]
- 37.Carr MC, et al. Effect of raloxifene on serum triglycerides in women with a history of hypertriglyceridemia while on oral estrogen therapy. Diabetes Care. 2005;28(7):1555–1561. doi: 10.2337/diacare.28.7.1555. [DOI] [PubMed] [Google Scholar]
- 38.Chadwick CC, et al. Identification of pathway-selective estrogen receptor ligands that inhibit NF-kappaB transcriptional activity. Proc Natl Acad Sci U S A. 2005;102(7):2543–2548. doi: 10.1073/pnas.0405841102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raz R, Lee CK, Cannizzaro LA, d’Eustachio P, Levy DE. Essential role of STAT3 for embryonic stem cell pluripotency. Proc Natl Acad Sci U S A. 1999;96(6):2846–2851. doi: 10.1073/pnas.96.6.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127(19):4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 41.Wang C, et al. GPR30 contributes to estrogen-induced thymic atrophy. Mol Endocrinol. 2008;22(3):636–648. doi: 10.1210/me.2007-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Najjar SM, et al. Insulin acutely decreases hepatic fatty acid synthase activity. Cell Metab. 2005;2(1):43–53. doi: 10.1016/j.cmet.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 2000;49(3):424–430. doi: 10.2337/diabetes.49.3.424. [DOI] [PubMed] [Google Scholar]
- 44.Mauvais-Jarvis F, et al. Reduced expression of the murine p85alpha subunit of phosphoinositide 3-kinase improves insulin signaling and ameliorates diabetes. J Clin Invest. 2002;109(1):141–149. doi: 10.1172/JCI13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cariou B, et al. Cellular and molecular mechanisms of adipose tissue plasticity in muscle insulin receptor knockout mice. Endocrinology. 2004;145(4):1926–1932. doi: 10.1210/en.2003-0882. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.