Abstract
During seasonal influenza epidemics, disease burden is shouldered predominantly by the very young and the elderly. Elderly individuals are particularly affected, in part because vaccine efficacy wanes with age. This has been linked to a reduced ability to induce a robust serum antibody response. Here, we show that this is due to reduced quantities of vaccine-specific antibodies, rather than a lack of antibody avidity or affinity. We measured levels of vaccine-specific plasmablasts by ELISPOT 1 week after immunization of young and elderly adults with inactivated seasonal influenza vaccine. Plasmablast-derived polyclonal antibodies (PPAbs) were generated from bulk-cultured B cells, while recombinant monoclonal antibodies (re-mAbs) were produced from single plasmablasts. The frequency of vaccine-specific plasmablasts and the concentration of PPAbs were lower in the elderly than in young adults, whereas the yields of secreted IgG per plasmablast were not different. Differences were not detected in the overall vaccine-specific avidity or affinity of PPAbs and re-mAbs between the 2 age groups. In contrast, reactivity of the antibodies induced by the inactivated seasonal influenza vaccine toward the 2009 pandemic H1N1 virus, which was not present in the vaccine, was higher in the elderly than in the young. These results indicate that the inferior antibody response to influenza vaccination in the elderly is primarily due to reduced quantities of vaccine-specific antibodies. They also suggest that exposure history affects the cross-reactivity of vaccination-induced antibodies.
Introduction
Influenza viruses are respiratory pathogens that cause annual epidemics and intermittent pandemics. Disease burden is especially significant among young children and elderly individuals. Although influenza vaccines effectively protect children and adults against infection, vaccination efficacy wanes with advancing age. When the vaccine and circulating viruses are antigenically similar, the inactivated influenza vaccine protects 70%–90% of younger adults (1), whereas vaccine efficacy ranges 17%–51% in those over 65 years of age (2) and may be even lower in those over 70 (3). Methodological issues in the published cohort studies may have led to overestimation of influenza vaccine efficacy in the elderly; thus, efficacy in the elderly could be even lower than the above estimates (4).
Although reduced vaccine efficacy in the elderly is generally attributed to immunosenescence, the mechanisms leading to this phenomenon are not well understood (reviewed in ref. 5). Numerous studies have indicated that the antibody response to natural influenza infection and vaccination is a critical component of protective immunity (6), and a recent meta-analysis of influenza vaccine studies from the past 20 years concluded that aged individuals (>65 years) had a significantly reduced antibody response to vaccination (2). Thus, 2 basic questions regarding the inferior serum antibody response in the elderly remain largely unanswered. First, is the reduced reactivity observed in the elderly primarily caused by lower quantity (concentration) of the antibodies, or by lower quality (avidity)? Second, if there is a difference in the quantity of influenza-specific antibodies between young and elderly vaccinees, is it caused by different numbers of antibody-secreting cells (ASCs), or by differences in the yield of antibody secreted from each ASC? Resolution of these important questions has been hampered by 2 major limitations of conventional serological techniques: first, the quantity of influenza vaccine–induced antibody in the serum cannot be easily differentiated from background levels of influenza-specific antibody derived from prior exposures to influenza virus or vaccines; and second, the primary source of serum antibody is bone marrow resident plasma cells, a cell population that is generally not accessible in clinical studies.
After administration of a vaccine, naive and memory B cells are activated at the site of immunization and in the draining local lymph nodes. Activated B cells proliferate and differentiate into plasmablasts in germinal centers (GCs) within the local lymph nodes. After 6–8 days, a large number of plasmablasts leave the GC and transiently enter circulation, forming a sharp peak in the peripheral blood that is highly enriched (20%–85%) for vaccine-specific ASCs (7–10). Depending on the trafficking receptors expressed, plasmablasts migrate to the bone marrow and develop into long-lived plasma cells that secrete systemic serum antibody or are targeted to various tissues of the body, including mucosal sites (11, 12).
We and others have used various strategies to characterize the peripheral plasmablast response at day 7 after influenza vaccination. Several groups have used ELISPOT assays to quantify influenza-specific IgG- and IgA-secreting plasmablasts (8, 9, 13, 14). Additionally, vaccine-specific recombinant monoclonal antibodies (re-mAbs) have been generated from individual plasmablasts sorted by flow cytometry based on their surface phenotypes (10). We recently reported that B cells isolated 7 days after vaccination and cultured ex vivo produced plasmablast-derived polyclonal antibodies (PPAbs), including IgG and IgA, that are highly enriched for vaccine specificity, whereas PPAbs generated before vaccination only had negligible reactivity against the vaccine antigens (15). Unlike serum antibodies that reflect the lifelong exposure to a multitude of vaccine immunogens and past influenza strains, PPAbs generated at day 7 after vaccination represent current polyclonal antibody responses to the vaccine without interference from preexisting serum antibodies that cross-react with the new vaccine antigens.
Here we used plasmablast-based assays, including ELISPOT and analysis of PPAbs and plasmablast-derived re-mAbs, to address several basic questions regarding age-related differences in antibody responses after immunization with inactivated seasonal influenza vaccines. We found that the quantitative differences in ASC induction, rather than qualitative differences in antibody avidity/affinity, account for the age-related decline in antibody response to vaccination. Since cross-reactivity is a critical qualitative characteristic of antibody response and the induction of antibodies with cross-reactive protection effects is an important issue for influenza vaccine development, we also compared the cross-reactivity of the antibody response against an influenza strain not present in the vaccines, the 2009 swine-origin pandemic H1N1 (pH1N1) strain. Interestingly, the antibody response induced in the elderly cohort was substantially more cross-reactive to the pH1N1 strain at both polyclonal and monoclonal levels, which suggests that differences between young and elderly vaccinees involve antibody specificity as well as quantity, likely as a result of immunological history.
Results
Reduced serum antibody and PPAb responses in elderly versus young vaccine recipients.
During the 2009–2010 influenza season, 42 volunteers from 2 age groups, 18- to 30-year-olds (n = 21) and 70- to 100-year-olds (n = 21), received 1 dose of the 2009 seasonal trivalent inactivated influenza vaccine (TIV). Blood samples were collected before vaccination (day 0) and on day 7 or 8 and approximately day 28 after vaccination. Sera were prepared from the day 0 and day 28 blood samples. B cells were isolated from the day 7/8 blood samples for ELISPOT analysis and for generating PPAbs.
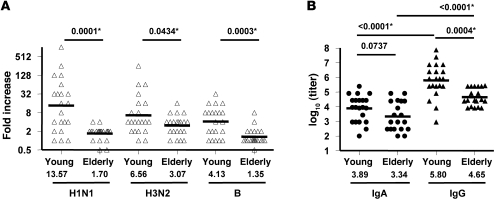
To assess serum antibody responses to vaccination, day 0 and day 28 serum samples were tested for hemagglutination inhibition (HAI) titer against the 3 viral strains used in the vaccine (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI57834DS1). Based on these data, we determined the serum antibody response to vaccination using the fold increase of HAI titer after vaccination. As shown in Figure 1A, the fold increases were significantly higher in young than elderly subjects for each of the 3 component strains. Those whose antibody titers to at least 1 of the 3 strains increased 4-fold or more after vaccination were designated as positive responders. Using this criterion, the frequency of responders was 19 of 21 (90%) in the young volunteers. Only 12 of 21 (57%) of the elderly responded to vaccination, and the difference between groups was significant (P = 0.0033, Fisher exact test). In agreement with previous studies, the serum antibody response was significantly higher in the young than in the elderly.
Figure 1. Antibody responses to 2009 seasonal TIV in young and elderly vaccinees.
(A) Fold increase of serum HAI titer of individual vaccinees against the 3 2009 vaccine component strains (H1N1, A/South Dakota/06/2007, an A/Brisbane/59/2007–like strain; H3N2, A/Uruguay/716/2007, an A/Brisbane/10/2007–like strain; B, B/Brisbane/60/2008) approximately 28 days after vaccination. Asterisks denote significant differences. (B) IgA and IgG ELISA binding titer of PPAbs from individual vaccinees collected 1 week after vaccination. The ELISA plates were coated with 2009 TIV. P values were determined by unpaired t test for young vs. elderly or paired t test for IgA vs. IgG within each group. Criteria for statistical significance were adjusted to control type I error rate at 5% across the multiple comparisons; asterisks denote differences that remained statistically significant after the adjustment. Geometric means of fold increase (A) or GMT (B) are shown as bars and numerical values below.
In order to quantify the TIV-specific antibody response directly induced by vaccination, we generated PPAbs by culturing bulk B cells from blood samples collected 1 week after vaccination. The PPAb samples were tested for IgG and IgA binding activity to 2009 TIV by ELISA. As shown in Figure 1B, the IgG geometric mean titer (GMT) of the young group was significantly higher than that of the elderly group. The IgA GMT was also higher in young than in elderly subjects, but this difference was not statistically significant. For both groups, the GMT of the IgG was significantly higher than the GMT of the IgA (approximately 100-fold higher in the young group and 20-fold higher in the elderly group). This indicated that after parenteral immunization with TIV, the total PPAb response was predominantly IgG in both groups. To assess the functional reactivity of PPAbs, HAI titer of each sample was assayed against the vaccinating strains. A subject was classified as PPAb HAI-positive when the HAI titer of the PPAb against at least 1 of the 3 vaccine strains was at least 4-fold higher than that of the negative control. Using this criterion, the frequency of PPAb HAI-positive donors in the young age group was 8 of 20 (40%), whereas none of the 19 elderly subjects was PPAb HAI-positive (P = 0.0003, Fisher exact test). Insufficient sample precluded testing of PPAbs from 3 subjects. The HAI titers of the 8 HAI-positive PPAb samples ranged from 10 to 160, with a GMT of 28.3.
Taken together, these results demonstrate a marked reduction in the potency of antibodies secreted by recently activated plasmablasts in the elderly cohort, which mirrors the reduced serum antibody response seen in the elderly. We believe this provides a new approach to examine age effects on the quantitative and qualitative nature of antibody responses to vaccination.
Reduced quantity of ASCs in elderly versus young vaccine recipients.
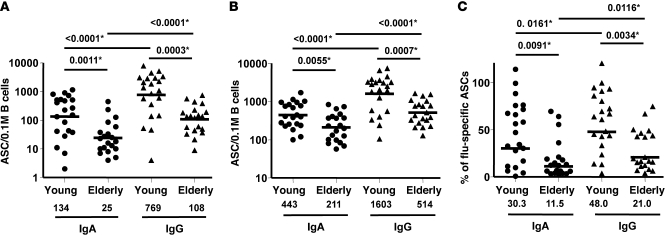
Several theories could explain the lower levels of PPAb and serum antibody responses of elderly individuals to influenza vaccination. Elderly individuals may produce fewer vaccine-specific ASCs in response to vaccination. Alternatively, ASCs of elderly individuals may not secrete the same level of antibodies that younger subjects do. Another possibility is that the quality of the vaccine-induced antibody response may deteriorate with age, yielding a humoral response characterized by low-affinity antibodies. To evaluate each of these possibilities, ELISPOT assays were used to examine the frequencies of vaccine-specific ASCs isolated 1 week after TIV immunization. Tellingly, the frequencies of both TIV-specific IgG ASCs and TIV-specific IgA ASCs were significantly higher in the young subjects than in elderly volunteers (IgA, P = 0.0011; IgG, P = 0.0003; Figure 2A). The ASC response after TIV vaccination was predominantly IgG-mediated rather than IgA-mediated in both groups (on average, 5.7-fold more IgG ASCs in the younger group and 4.3-fold more IgG ASCs in the elderly group; both P < 0.0001). In addition, geometric mean frequency of ASCs with any specificity were significantly higher in samples from the younger cohort than in those of the elderly cohort for both IgA and IgG (IgA, P = 0.0055; IgG, P = 0.0007; Figure 2B). Based on the frequencies of TIV-specific versus total ASCs, we found that the percentage of vaccine-specific ASCs was higher in the younger group than in the elderly group for both IgA and IgG ASCs (IgA, P = 0.0091; IgG, P = 0.0034; Figure 2C). We conclude that frequencies of TIV-specific ASCs circulating after vaccination were significantly lower in the elderly individuals than in younger volunteers.
Figure 2. ASC responses to TIV immunization in the young and elderly.
(A) Frequency of TIV-specific IgA and IgG ASCs. (B) Frequency of total IgA and IgG ASCs. (C) Percent TIV-specific IgA and IgG ASCs, relative to total ASCs. Geometric means of ASC counts per 0.1 million (M) B cells (A and B) or average percentage (C) are shown as bars and numerical values below. P values were determined by unpaired t test for young vs. elderly or paired t test for IgA vs. IgG within each group. Criteria for statistical significance were adjusted to control type I error rate at 5% across the multiple comparisons between age groups and isotypes; asterisks denote differences that remained statistically significant after the adjustment.
Reduced quantity of vaccine-specific PPAbs in elderly versus young vaccine recipients.
To determine the proportion of vaccine-specific antibodies in individual PPAb samples, we first assayed for total concentration of antibodies in each sample using isotype specific ELISA (Figure 3A). Geometric mean concentration of total IgG was significantly higher in younger volunteers than in elderly subjects (P < 0.0001), with levels of total IgG exceeding total IgA in both age groups (P < 0.0001). These differences mirrored those observed in the frequencies of total ASC responses (Figure 2B). In contrast to the difference found in total IgA ASC response, no significant difference between the 2 age groups was detected in geometric mean concentration of total IgA in PPAb samples (P = 0.7135; Figure 3A).
Figure 3. Concentration of total and TIV-specific IgA and IgG in PPAbs generated on day 7 or 8 after TIV immunization.
(A) Concentration of total IgA or IgG in PPAb from each donor. (B) Concentration of TIV-specific IgA or IgG in each individual, determined by multiplying the concentration of total IgA or IgG by the percentage of vaccine-specific IgA ASCs or IgG ASCs (Figure 2C), respectively. Geometric mean concentrations are shown as bars and numerical values below. P values were determined by unpaired t test for young vs. elderly or paired t test for IgA vs. IgG within each age group. Criteria for statistical significance were adjusted to control type I error rate at 5% across the multiple comparisons of Ig concentration-related parameters between age groups and isotypes (Figures 3 and 5); asterisks denote differences that remained statistically significant after the adjustment.
The quantity of vaccine-specific antibodies in PPAb samples was then estimated based on the percentage of vaccine-specific ASCs determined by ELISPOT (Figure 2C) and the concentration of total antibody detected in each donor’s PPAb (Figure 3A). There was significantly higher geometric mean concentration of TIV-specific IgG protein in PPAbs from young than from elderly subjects (P < 0.0001; Figure 3B). Although the vaccine-specific IgA concentration in PPAbs of the young cohort appeared higher than that of the elderly, this difference was not statistically significant after adjustment for multiple comparisons (P = 0.0237). As with the titers (Figure 1B) and ASC frequencies (Figure 2A), the geometric mean concentration of vaccine-specific IgG was statistically significantly higher than the vaccine-specific IgA in both age groups (approximately 10-fold for the young and 4-fold for the elderly; both P < 0.0001).
Similar yield of secreted IgG per ASC in young and elderly vaccine recipients.
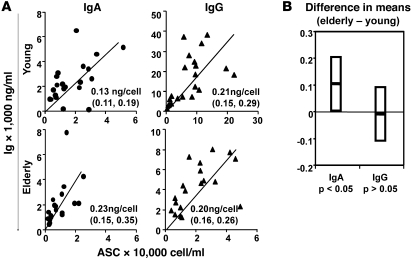
To assess the amount of antibody secreted by individual ASCs, the total IgG and IgA concentrations from each individual (Figure 3A) were plotted against the total numbers of IgG or IgA ASCs per milliliter of B cell culture (based on the ELISPOT data in Figure 2B). A reduced major axis (RMA) regression analysis was used to estimate the mean yield of antibody per cell (see Methods). In both groups, the IgG yield per ASC was approximately 0.2 ng over the culture period (Figure 4A). More IgA was secreted from individual ASCs of the elderly subjects (0.2 ng) compared with the young subjects (0.13 ng); however, the difference was only marginally significant (based on the 95% CIs of estimated mean yield; Figure 4B). Since the IgG response dominated the overall PPAb response in both age groups after immunization with TIV (Figure 1B), it appears that the reduced potency of the antibody response in elderly individuals is not caused by a deficiency in the ability of individual ASCs to secrete antibody, but by the smaller number of plasmablasts induced by vaccination.
Figure 4. Yield of IgG and IgA per ASC in young and aged vaccinees.
(A) Yield of secreted Ig was estimated via RMA regression, using the concentration of total IgA or IgG in PPAb (ng/ml) and the total IgA or IgG ASCs in the B cell culture (cell count/ml) for each donor. Fitted RMA regression line is shown for each Ig isotype and age group, along with estimated mean yield per cell and 95% CI. (B) 95% CI of the difference between the estimated mean yield per cell in the 2 age groups. Because the 95% CI for IgG clearly includes 0, no difference in mean yield per cell between groups is indicated. For IgA, since the 95% CI does not include 0, the results suggest that mean yield per cell was significantly greater (P < 0.05) in the elderly. However, given that the lower confidence bound fell just above 0, this finding should be interpreted with some caution.
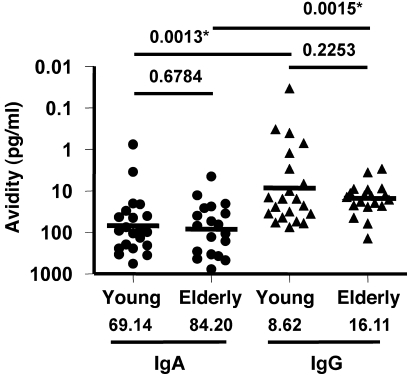
Similar vaccine-specific avidity of PPAbs derived from young and elderly vaccine recipients.
We defined the avidity of vaccine-specific PPAb as the lowest concentration of vaccine-specific PPAb at which binding to the vaccine antigens was detectable with ELISA. A lower concentration indicated a higher avidity. This parameter characterizes the binding quality of vaccine-specific antibodies. To determine the avidity of IgG and IgA for the vaccinating antigens, the concentration of TIV-specific IgG or IgA (Figure 3B) was divided by the highest dilution of PPAb at which binding to the vaccine antigens was detectable by ELISA (Figure 1B). As shown in Figure 5, statistically significant differences were not detected in the avidity of vaccine-specific IgA or IgG between young and elderly groups (IgA, P = 0.6784; IgG, P = 0.2253). The estimated geometric mean avidity of vaccine-specific IgG was higher than that of IgA in both age groups (young, P = 0.0013; elderly, P = 0.0015), which suggests that parenteral immunization with TIV favors the IgG response both quantitatively (Figure 3B) and qualitatively (Figure 5).
Figure 5. Avidity of vaccine-specific PPAbs from young and elderly TIV recipients.
The avidity of vaccine-specific PPAb is defined as the concentration of TIV-specific PPAb (Figure 3B) divided by the TIV-specific ELISA titer of PPAb (Figure 1B) for each donor. Geometric mean avidities are shown as bars and numerical values below. P values were determined by unpaired t test for young vs. elderly or paired t test for IgA vs. IgG within each age group. Criteria for statistical significance were adjusted to control type I error rate at 5% across the multiple comparisons of Ig concentration-related parameters between age groups and isotypes (Figures 3 and 5); asterisks denote differences that remained statistically significant after the adjustment.
Similar vaccine-specific affinity of re-mAbs derived from young and elderly vaccine recipients.
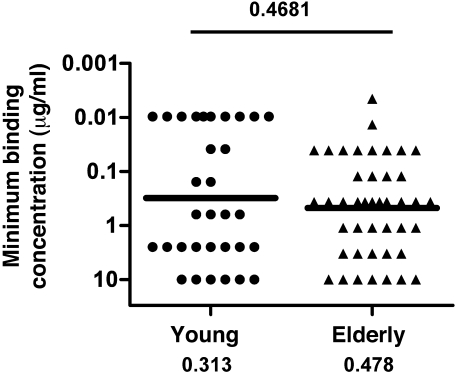
To determine whether age-related differences in antibody quality were evident on the monoclonal level, single plasmablasts taken from volunteers 1 week after vaccination with seasonal TIV were sorted into 96-well plates. The sequences of V regions from both heavy and light chain Ig genes were amplified using single-cell RT-PCR, cloned into expression vectors, and expressed in HEK 293A cells to generate re-mAbs. After purification from culture supernatant, re-mAbs were tested for their ability to bind individual influenza vaccine strains by ELISA. The minimum binding concentration of a re-mAb was defined as its lowest concentration at which binding was detectable at or above a minimum binding threshold; this parameter is inversely related to antibody affinity. The minimum binding concentration of all re-mAbs specific for 1 of the 3 vaccine strains, generated from the young versus elderly age groups, is shown in Figure 6, and re-mAbs derived from each individual vaccinee are shown in Supplemental Figure 2. Similar to the avidity of PPAb derived from the 2 age groups, a statistically significant difference in the minimum binding concentration of influenza-specific re-mAbs was not detected between the 2 age groups (P = 0.4681; Figure 6), which suggests that the overall affinity of vaccine-induced mAbs for the vaccinating antigens is similar in the young and elderly.
Figure 6. Affinity of influenza vaccine virus-specific re-mAbs derived from young and elderly recipients of seasonal TIV.
Binding affinity was measured by ELISA with microtiter plates coated with individual vaccine component viruses. The binding affinity was defined as the minimum concentration of each re-mAb that resulted in an OD405nm greater than 0.607 in the assay. All re-mAbs (32 from the young group; 43 from the elderly group) with a minimum binding concentration up to 10 μg/ml for 1 of the vaccine component viruses were considered vaccine specific and included for this analysis. The OD405nm threshold of 0.607 was set at a level that would exclude 95% of random control re-mAb as vaccine-specific, based on ELISA results of 48 such re-mAbs derived from individual naive B cells. Geometric means of minimum binding concentrations are shown as bars and numerical values below.
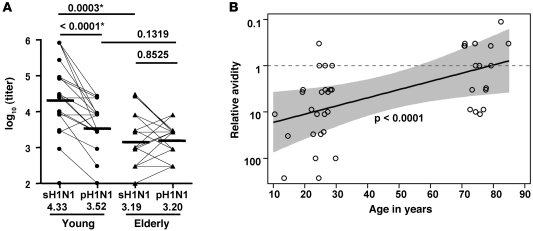
Greater pH1N1-specific heterovariant reactivity of antibodies from elderly versus young vaccine recipients.
To compare the heterovariant reactivity of 2009 seasonal TIV-induced PPAb against the pH1N1 strain between the young and elderly groups, we first measured the IgG binding titer of PPAb samples with ELISA plates coated with either inactivated 2009 seasonal H1N1 (sH1N1) vaccine antigen or inactivated pH1N1 vaccine antigen. Figure 7A shows these 2 titers for each vaccinee in each group. The GMT for the sH1N1 antigen was significantly higher than that for pH1N1 in the young group (P < 0.0001), but elderly individuals had similar GMTs for both sH1N1 and pH1N1 (P = 0.8525). Of note, an increased pH1N1-specific titer compared with the same donor’s sH1N1-specific titer appeared to be rare among young vaccinees (1 of 21), but common among elderly donors (9 of 19). In addition, the titer against sH1N1 antigen was significantly higher in the young than the elderly (P = 0.0003; Figure 7A), mirroring the results in which the ELISA plates were coated with TIV or the mixture of 3 seasonal vaccine antigens (Figure 1B). In contrast, no difference in the titer against pH1N1 was detected between the 2 age groups (P = 0.1319).
Figure 7. Heterovariant versus homotypic reactivity of vaccine-induced PPAbs in young and elderly recipients.
(A) Binding titer of 2009 seasonal TIV-induced PPAbs (IgG) against sH1N1 and pH1N1 vaccine antigens. Binding was measured with ELISA plates coated with monovalent sH1N1 or pH1N1 vaccines. GMTs are shown as horizontal bars and numerical values below. Criteria for statistical significance were adjusted to control type I error rate at 5% across the multiple comparisons; asterisks denote differences that remained statistically significant after the adjustment. (B) Effects of age on the relative avidity of vaccine-induced PPAbs (IgG) against the pH1N1 antigen. Relative avidity was defined as the ratio of titer against sH1N1 to titer against pH1N1 (see Methods). The dashed line demarcates relative avidity of 1. The P value is from a test of the null hypothesis that slope is 0 for weighted linear regression fit on the logarithmic scale. The shaded area defines the 95% Working-Hotelling confidence band of geometric mean relative avidity as a function of age. Data for 1 donor aged >89 years (relative avidity, 0.124) was not plotted because HIPAA rules prohibit publication of exact age information in this age group; if included, the value remained P < 0.0001.
Since the titer of PPAbs for binding the vaccinating sH1N1 antigen was different between young and elderly individuals immunized with the same seasonal vaccine, we compared the PPAb binding reactivity against the pH1N1 antigen after normalization to their titer against the vaccinating sH1N1 antigen. The ratio of sH1N1- to pH1N1-specific titer was determined for each PPAb sample, and this ratio was defined as the relative avidity for pH1N1 (see Methods). Relative avidity was regressed on age for all young and elderly vaccinees to estimate the effects of age on this parameter (Figure 7B). To extend the age range for this analysis, we also included data from 3 children aged 10–15 years, who were studied with the same protocol. After immunization with 2009 seasonal TIV containing sH1N1 strain, the relative PPAb avidity for pH1N1 increased significantly with age (P < 0.0001; Figure 7B). In particular, the PPAbs in all 5 donors older than 78 years had equal or higher titers for the pH1N1 antigen than for the sH1N1 vaccine antigen (relative avidity, ≤1).
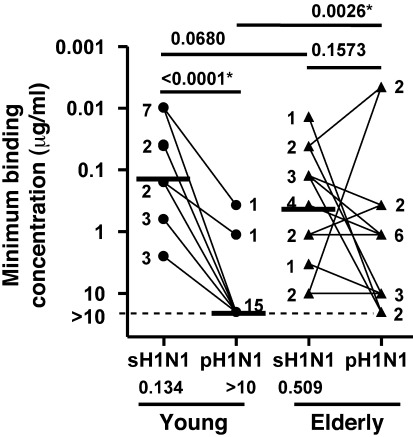
We then investigated age-related differences in the cross-reactivity of antibody response at the monoclonal level after immunization with the seasonal TIV. Re-mAbs that tested positive for binding to the sH1N1 strain were examined using ELISA for their ability to bind the pH1N1 strain. As shown in Figure 8, there was a significant decrease in the binding of younger vaccinee–derived re-mAbs to the pH1N1 relative to the vaccinating sH1N1 antigen (P < 0.0001): all 17 mAbs in the young group had lower affinity (i.e., higher minimum binding concentration) for pH1N1 than for sH1N1, with 15 of them falling beyond the limit of detection (>10 μg/ml). In contrast, a statistically significant difference was not detected in the ability of elderly vaccinee–derived re-mAbs to bind pH1N1 versus sH1N1 (P = 0.1573): 5 of 15 mAbs in this group had higher or equal affinity for pH1N1 compared with sH1N1, and 13 mAbs had a measurable affinity for pH1N1 (≤10 μg/ml). The elderly vaccinee–derived re-mAbs had higher affinity for pH1N1 than did re-mAbs from younger subjects (P = 0.0026). Taken together, Figures 7 and 8 showed that at day 7 after immunization with seasonal TIV, plasmablast-derived polyclonal and monoclonal antibodies from the older individuals had greater heterovariant cross-reactivity against the pH1N1 strain. Of note, although a statistically significant difference in the ability of binding sH1N1 was not detected between the re-mAbs derived from elderly and younger vaccinees based on the current dataset (P = 0.0680; Figure 8), the estimated geometric mean of minimum binding concentrations for sH1N1 was smaller in the young than the elderly group (0.134 μg/ml vs. 0.509 μg/ml). Potential strain-specific effects on the affinity of vaccine-specific mAbs in the young and the elderly should be explored with larger sets of re-mAbs generated from the 2 age groups in future studies.
Figure 8. Heterovariant versus homotypic affinity of re-mAbs derived from young and elderly recipients of seasonal TIV.
Re-mAbs with minimum binding concentration up to 10 μg/ml for sH1N1 (17 from the young group; 15 from the elderly group) were included for this analysis. Affinity of re-mAb for binding sH1N1 or pH1N1 viruses was defined as the minimum binding concentration for the corresponding influenza strains, determined by ELISA (see Figure 6 legend). The minimum binding concentrations greater than 10 μg/ml were right-censored. Data were analyzed using a parametric model for interval-censored data with terms for young vs. elderly, sH1N1 vs. pH1N1, and their interaction plus a γ frailty term. The numeral beside each symbol indicates the number of mAbs with the same minimum binding concentration. Estimated geometric means of minimum binding concentration based on the parametric model are shown as horizontal bars and numerical values below. (This parameter could not be determined for pH1N1 in the elderly group because of the distribution of right-censored observations and the other observations.) Criteria for statistical significance were adjusted to control type I error rate at 5% across the multiple comparisons; asterisks denote differences that remained statistically significant after the adjustment.
Discussion
A major goal of this study was to evaluate antibody responses to seasonal influenza vaccines in young and elderly individuals by isolating and characterizing plasmablast-derived polyclonal and monoclonal antibodies 7–8 days after vaccination. Of particular interest were the quantity and quality of influenza-specific antibodies. Antibody titer reveals the overall reactivity of an antibody sample and is a function of both quantity and quality of an antibody. Distinguishing the role of these parameters in antibody activity is important, as each one is modulated by distinct biological pathways. The quantity of antibody is determined by the number of ASCs and the yield of antibody from each ASC, whereas the quality, or avidity, depends on the affinity of individual Ig components. This characteristic is a function of the structure of individual antibody molecules and is ultimately determined by the Ig gene sequences.
We demonstrated that the quantity of vaccine-specific IgG was significantly greater in young than in elderly individuals at day 7–8 after vaccination. Mechanistically, this was associated with higher numbers of IgG ASCs in younger subjects. The average yield of secreted IgG per ASC was comparable between the 2 age groups. For IgA, the yield per ASC was higher in the elderly; however, this finding must be verified, given its marginal statistical significance (Figure 4B). Since the IgG response is the predominant component of the plasmablast and PPAb responses after TIV immunization in both age groups, our results clearly indicate a quantitative difference in the vaccine-specific antibody response between young and elderly subjects. At least for IgG responses, the reduced antibody quantity in elderly individuals was caused by fewer ASCs, and not by a lower yield of antibody per ASC. Therefore, when evaluating new strategies to enhance influenza vaccine efficacy in the elderly population, such as use of vaccine adjuvants and increased vaccine doses, the quantity of ASCs induced should be considered.
We also compared vaccine-specific antibody avidity between the 2 age groups, taking advantage of the PPAb assay combined with ELISPOT to assess concentrations of vaccine-specific antibodies. Furthermore, we generated and analyzed re-mAbs from individual plasmablasts to assess antibody affinity at the monoclonal level. A statistically significant difference was not detected between the 2 age groups in avidity at the polyclonal level (Figure 5), or in overall affinity at the monoclonal level for the vaccinating antigens (Figure 6). Taken together, our findings suggest that the inferior antibody responses raised after TIV immunization in the elderly are primarily caused by reduced quantity rather than by reduced avidity of vaccine-specific antibodies.
We next examined the heterovariant reactivity of seasonal influenza vaccine–induced antibodies to the pH1N1 strain, an antigen not included in the seasonal TIV. Although the heterovariant titers of vaccine-induced PPAb were significantly lower than the homotypic vaccine-specific titer in young vaccinees, there was no significant difference in the titers of PPAbs from elderly individuals for sH1N1 and pH1N1 (Figure 7A). Furthermore, we showed that the relative heterovariant avidity of PPAbs for pH1N1 increased significantly with age (Figure 7B), which indicates that when adjusted to the same concentration, the polyclonal antibodies induced by the sH1N1 vaccine antigen in elderly vaccinees had greater heterovariant reactivity against pH1N1 than did those in young adults. We confirmed these findings at the monoclonal level by demonstrating that the sH1N1-specific re-mAbs had similar affinity for pH1N1 in the elderly group, but significantly lower affinity for pH1N1 in the younger group, compared with their homotypic affinity for the vaccinating sH1N1 antigens (Figure 8).
Age-related changes in the humoral response to antigenic exposure have been studied in mouse models and, to a lesser extent, in humans, focusing on the T cell–dependent B cell response that is critical for effective long-term humoral immunity. Age-related decline of functional antibody responses has been attributed to intrinsic defects in B cells as well as their accessory cells (reviewed in refs. 16–18). B cells from older mice showed decreased in vitro proliferation in the absence of exogenous IL-4 compared with their counterparts from younger animals (19), whereas in a study with human samples, difference in in vitro proliferation was not detected between young and elderly subjects in purified B cells cultured with various stimuli (20). It has been well established that GC formation and kinetics are impaired in aged mice during both primary and secondary responses (21). Of special interest is the marked reduction in the expression level of CD154 (the CD40 ligand) in aged, activated T helper cells (22). Because interaction between CD40 and CD154 on antigen-specific T and B cells is required for GC formation and antibody class switching, reduced CD154 level on aged CD4+ helper T cells could result in poor antibody responses in the elderly (23, 24). In the current study, we demonstrated a significantly reduced quantity of vaccine-specific ASCs in elderly individuals after TIV immunization compared with young vaccinees. Although other possibilities can be envisioned, 2 potential mechanisms could directly contribute to the difference in ASC quantity: (a) reduced CD154 expression on CD4+ helper T cells, resulting in reduced formation of GCs and reduced proliferation of vaccine-activated B cells; and (b) defects in the intrinsic proliferation capability of aged B cells. Future studies should be directed at assessing the extent to which these 2 mechanisms affect the number of influenza-specific ASCs in humans after influenza vaccination.
A functional B cell response to antigenic stimulus relies on the GC-dependent processes of affinity maturation, during which somatic hypermutation (SHM) diversifies the Ig variable regions of proliferating B cells, and class switch recombination (CSR). These processes are mediated by activation-induced cytidine deaminase (AID) (25). The activity of AID declines with aging in both mice and humans (26, 27) and is associated with the reduced serum antibody response to influenza vaccine in the elderly (28). This is in agreement with studies showing that, compared with young counterparts, elderly individuals undergo less de novo SHM of their Ig heavy chain genes, possess reduced functional antibody activity, and have reduced protective efficacy in response to pneumococcal polysaccharide vaccine (29). In the case of the polysaccharide vaccine, the reduced antibody response in the elderly is related to low antibody avidity rather than low antibody concentration (30–33). In this study of the response to influenza vaccine, we did not observe statistically significant differences in overall polyclonal or monoclonal antibody affinity between young and elderly recipients of influenza vaccine. This discrepancy might be due to the facts that the antibody response to the bacterial polysaccharide antigens does not involve immunological memory, but memory is key in influenza vaccination.
Most influenza vaccine recipients, except very young children, have been previously exposed to influenza antigens by natural infection, prior vaccination, or both. Hence, each adult vaccinee will have certain levels of preexisting immunity, including memory B cells that cross-react with the new vaccine antigen to various degrees. Influenza virus–specific memory B cells have been shown to survive 90 years in human beings (34). Because of a lower activation threshold of memory B cells, they are able to enter division more easily and produce a greater antibody response than naive B cells (35, 36). By analyzing the Ig gene sequences of activated B cells, we showed that the B cell response in adult recipients of influenza vaccine was predominantly recall of memory B cells rather than activation of naive cells (10). We propose that the existence of closely related cross-reactive memory B cell clones — resulting from prior exposure in the elderly vaccinees — compensates, at least in part, for the defects in AID reactivity and antibody affinity maturation found in the elderly vaccinees and results in production of vaccine-specific antibodies with similar avidity, although in quantities smaller than those in younger vaccinees. This hypothesis might be addressed in future studies by comparing the Ig gene repertoire and sequences in prevaccination memory B cells and vaccine-induced plasmablasts in the young and elderly recipients of influenza vaccines. Based on the current findings, we propose that the inferior protection efficacy of influenza vaccines in the elderly population is primarily caused by lower quantity, rather than quality, of influenza-specific antibodies induced by vaccination.
The potential effects of age-related changes in the influenza memory B cell repertoire on antibody responses to a new seasonal vaccination were further examined by analysis of the heterovariant reactivity of antibodies induced by the seasonal influenza vaccines. We found that after seasonal influenza vaccination, heterovariant reactivity for the pH1N1 antigen increased with age: in young vaccinees, heterovariant avidity and affinity were significantly lower than homotypic avidity and affinity for the seasonal vaccine antigens, whereas in the elderly, statistically significant differences were not detected (Figures 7 and 8). In fact, in all 5 elderly vaccinees older than 78 years, the titer of PPAbs against the pH1N1 antigen was higher than that against the sH1N1 antigen (Figure 7B). In a previous 2009 influenza vaccine study, prevaccination serum antibody titers against pH1N1 were detected in 34% of individuals born before 1950, but only in 4% of those born after 1980, which suggests that a substantial fraction of the population 60 years or older had been exposed to influenza strains that were circulating before 1950 and were antigenically related to pH1N1 (37). A few such influenza A/H1N1 strains were recently identified in a study using a mouse model (38). These findings are supported by the crystal structure of the HA protein from the pH1N1 virus, which shows an antigenic structure extremely similar to those of human H1N1 viruses circulating in the early 1900s (39). Conceivably, elderly individuals carry a larger population of memory B cells primed by pH1N1-related viruses. We propose that these memory B cells can be activated by seasonal vaccine antigens, proliferate, and develop into plasmablasts with limited SHM, resulting in production of antibodies that recognize the new vaccinating antigen while retaining high affinity for the pH1N1-related antigen. Interestingly, some PPAbs and re-mAbs from the elderly individuals in our study even had higher reactivity against pH1N1 than sH1N1 (Figures 7 and 8). However, it remains to be determined whether the greater cross-reactivity of vaccine-induced antibodies in the elderly has any beneficial effect with respect to vaccine-induced cross-protection.
Taken together, our findings suggest that under certain circumstances, immunization with a current influenza vaccine induces an antibody response to previously circulated influenza strains. Of note, although heterovariant binding activity to pH1N1 antigen was clearly detected in vaccine-induced PPAbs and re-mAbs generated during the current work, Hancock et al. did not observe an increase in serum neutralization antibody titers against the pH1N1 virus in the vast majority of adult recipients aged 60 years or older (37). To address this discrepancy, it will be critical to carry out properly powered analyses of vaccine-specific and heterovariant functional reactivity of PPAbs and re-mAbs. Such studies will help determine whether this discrepancy reflects the difference between total binding activity (measured by ELISA) and functional activity (measured by HAI and virus neutralization assays) or, more intriguingly, intrinsic differences between the antibodies derived from peripheral plasmablasts and those from bone marrow resident plasma cells (15). If the latter is the case, analysis of the peripheral plasmablast responses could result in more accurate predictors of vaccine efficacy.
Methods
Human participants, vaccination protocol, and blood sample collection.
Volunteers in 3 age groups (elderly adults, 70–100 years of age; younger adults, 18–51 years of age; children, 10–15 years of age) were enrolled during the 2007–2008 and 2009–2010 influenza seasons. The study protocols were approved by the institutional review boards at Stanford University, University of Chicago, and Oklahoma Medical Research Foundation. Informed consent was obtained from participants and the parents of pediatric participants. In addition, assent was obtained from the child participants. Participants were immunized with 1 dose of either the 2007 or the 2009 seasonal TIV (Fluzone; Sanofi Pasteur). The 2007 vaccine contained an A/Solomon Islands/3/2006–like H1N1 virus, an A/Wisconsin/67/2005–like H3N2 virus, and a B/Malaysia/2506/2004–like virus. The 2009 vaccine contained an A/Brisbane/59/2007–like H1N1 virus, an A/Brisbane/10/2007–like H3N2 virus, and a B/Brisbane/60/2008–like virus. Blood samples were collected from each participant at 3 time points: day 0, before vaccination; day 7 or 8 after vaccination; and day 28 (±4) after vaccination. Sera were isolated from the day 0 and day 28 blood samples. From the day 7–8 whole blood samples, B cells were isolated by negative selection with the RosetteSep Human B cell Enrichment Cocktail (Stemcell Technologies) following the manufacturer’s instructions. The average purity of CD19+ B cells was 70%, with no significant difference between the young and elderly age groups (71% and 69%, respectively; P = 0.782, unpaired t test).
Enumeration of total and influenza-specific ASCs.
Total and influenza vaccine–specific IgA or IgG ASCs in the isolated B cells were enumerated using ELISPOT as previously described (14), with minor modifications. This assay was performed for all study participants enrolled at Stanford University during the 2009 influenza season (elderly adults, aged 70–100 years, n = 21; young adults, aged 18–30 years, n = 21; children, aged 10–15 years, n = 3), except for 1 elderly donor, from whom an insufficient amount of blood was collected. In brief, 96-well MultiScreen HTS plates (Millipore) were coated with affinity-purified goat anti-human IgA+IgG+IgM (H+L) (KPL Inc.) at a concentration of 4 μg/ml in PBS to detect total ASCs or coated with 2009 TIV (Fluzone) at a concentration of 9 μg/ml in PBS to detect vaccine-specific ASCs. Wells coated with PBS served as negative controls. Plates were incubated overnight at 4°C and then blocked for 2 hours at 37°C with complete medium (RPMI 1640 supplemented with 10% heat inactivated FCS, 100 U/ml penicillin G, and 100 μg/ml streptomycin). Freshly isolated B cells were resuspended in complete medium containing phosphatase-conjugated goat anti-human IgA (KPL Inc.) at 0.2 μg/ml or phosphatase-conjugated goat anti-human IgG (H+L) (KPL Inc.) at 0.2 μg/ml, dispensed into wells of the coated and blocked plates at 2-fold serial dilutions, and incubated for 4 hours or longer at 37°C in an atmosphere of 5% CO2. Plates were washed with PBS and developed with a Blue alkaline phosphatase substrate kit (Vector Laboratories). The number of IgG and IgA ASCs in each well was determined with an ELISPOT plate reader and ImmunoSpot software (Cellular Technologies). Nonspecific spots detected in the negative control (PBS) wells were subtracted from the counts of influenza-specific and total ASCs.
Generation of PPAbs.
PPAbs were collected as previously described (15) from all study participants enrolled at Stanford University during the 2009 influenza season, except for 2 elderly donors from whom recovered B cell counts were not sufficient for this purpose. In brief, freshly isolated B cells were resuspended in complete medium at 3 × 106 cells per ml and cultured in 5% CO2 at 37°C for 7 days. The conditioned media with secreted PPAbs were collected and stored at –20°C. Concentrations of IgA and IgG in the PPAb samples were determined with the IMMUNO-TEK Quantitative Human IgA or IgG ELISA kits (Zeptometrix Corp.), respectively, following the manufacturer’s instructions.
Generation of re-mAbs from individual plasmablasts.
Generation of re-mAbs was preformed as previously described (10, 40, 41) using blood samples collected from elderly and younger participants. In 2007, 4 elderly individuals (aged 71–79 years) were enrolled at Stanford, and 6 younger individuals (aged 29–51 years) were enrolled at University of Chicago and Oklahoma Medical Research Foundation, for generating re-mAbs. In 2009, 4 elderly individuals (aged 74–92 years) were randomly selected from those enrolled at Stanford University for generating re-mAbs. In brief, single plasmablasts were sorted into 96-well PCR plates containing RNase inhibitor (Promega) on a FACSAria II Flow Cytometer equipped with ACDU (BD Biosciences), based on the phenotype of CD3–CD19+CD20–CD27+CD38+. VH and Vk genes from each cell were amplified by RT-PCR and nested PCR reactions using previously published cocktails of primers (41) and then sequenced. Antibody sequences were deposited in GenBank (accession nos. HQ689701–HQ689792). For cloning, restriction sites were incorporated by PCR with primers to the particular variable and junctional genes. VH or Vk genes amplified from each single cell were cloned into IgG1 or Igk expression vectors as previously described (10, 40, 41). Heavy and light chain plasmids were cotransfected into the HEK 293A cell line for expression of re-mAbs. Secreted re-mAbs were purified with Pierce protein A sepharose (Thermo Fisher Scientific).
ELISA for influenza vaccine antigen–specific binding activity of PPAbs.
96-well Vinyl Microtiter Microplates were coated with 2009 TIV (Fluzone) at 2.7 μg/ml in PBS, monovalent-inactivated sH1N1 (A/Brisbane/59/2007) vaccine antigen, or inactivated pH1N1 (A/California/7/2009) vaccine antigen (provided by P. Dormitzer, Novartis Co., Cambridge, Massachusetts, USA), both at 0.9 μg/ml in PBS. Plates were incubated overnight at 4°C, washed with wash buffer (0.1% Tween 20 in PBS), and blocked with 3% BSA in PBS for 1 hour at 37°C. PPAb samples were serially diluted with complete medium, added to the wells of coated/blocked plates, and incubated for 1 hour at 37°C. Wells incubated with complete medium served as negative controls. The plates were then washed and incubated for 1 hour at 37°C with peroxidase-conjugated goat anti-IgG(γ) (KPL Inc.) diluted 1:4,000 with 3% BSA in PBS, or with peroxidase-conjugated goat anti IgA(α) (KPL Inc.) diluted 1:2,000. After washing, the plates were developed with TMB substrate (KPL Inc.), and the OD450nm of each well was determined with an ELISA plate reader. The cutoff OD450nm value was set at mean plus 2 SD of average OD450nm values of all negative control wells. Titer of each sample was determined as the highest dilution at which the mean OD450nm value of duplicate wells was greater than the cutoff.
ELISA for influenza virus–specific binding activity of re-mAbs.
Costar 3369 96-well Easy-Wash plates were coated using carbonate binding buffer and 8 HA units per well of live virus. The 3 virus strains for the 2007 seasonal TIV and 3 virus strains for the 2009 seasonal TIV were used for re-mAb generated from 2007 and 2009 vaccine recipients, respectively. In addition, the pH1N1 strain (A/California/7/2009) was used for testing the heterovariant binding activity. Plates were sealed and incubated overnight at 4°C. Plates were then washed 4 times with 0.05% Tween-20 in PBS and blocked for 1 hour at 37°C with 20% FCS in PBS. The re-mAb samples were serially diluted in PBS, starting from a concentration of 10 μg/ml. Blocked plates were washed 4 times with 0.05% Tween-20 in PBS, and the serially diluted re-mAbs were applied to the plates in duplicate and incubated for 1 hour at 37°C. After washing, a 1:1,000 dilution of peroxidase-conjugated affinity-purified goat anti-human IgG (Jackson ImmunoResearch Laboratories) was added to the plate and allowed to incubate for 1 hour at 37°C. Super AquaBlue ELISA substrate (eBiosciences) was applied after washing, and the OD405nm value was recorded when positive controls (10, 42) reached an OD value of 3.000.
Comparison of vaccine-specific avidity of PPAbs derived from young and elderly vaccine recipients.
The specific avidity of vaccine-specific PPAb, defined as the minimum concentration or the highest dilution at which a specific binding was detected by ELISA, was determined for individuals in each age group — as (specific Ab concentration)/titer, calculated as (fraction of specific ASC) × (total Ab concentration)/titer — and then compared between the 2 groups (Figure 5). Alternately, comparison of IgG avidity can be achieved by using another parameter. Specific avidity is (fraction of specific ASC) × (total Ab concentration)/titer, as described above; this can therefore be expressed as [(specific ASC number)/(total ASC number)] × (total Ab concentration)/titer, which further becomes (specific ASC number) × [(total Ab concentration)/(total ASC number)]/titer, and thus specific avidity can also be determined by (specific ASC number) × (yield of Ab per cell)/titer. Since the yield of IgG per ASC was comparable between the young and elderly groups (Figure 4), it can be considered as a constant. Thus, the value of IgG avidity is in proportion to the ratio (specific ASC number)/titer, which can be used as a surrogate for avidity. In agreement with the results shown in Figure 5, statistically significant difference was not detected in this ratio for IgG avidity between the young and elderly groups (data not shown).
Determination of heterovariant relative avidity of PPAb for pH1N1.
We defined relative avidity of sH1N1-induced PPAb for the pH1N1 antigen as the ratio ApH1N1/AsH1N1, in which ApH1N1 is the avidity for pH1N1 and AsH1N1 is the avidity for sH1N1 antigen. Based on the following reasoning, this relative avidity is equal to the ratio TsH1N1 /TpH1N1, in which TsH1N1 and TpH1N1 represent the ELISA binding titers of PPAb against sH1N1 and pH1N1 antigens, respectively.
The total concentration of PPAb secreted from cultured bulk B cells isolated on day 7 after vaccination (Figure 3A), or Ctotal, can be considered as the sum of 2 components: Cvaccine, the quantity of antibodies secreted from plasmablasts activated by the vaccine, and Cbackground, the quantity of antibodies derived from the plasmablasts activated by irrelevant environmental antigens or polyclonal B cell stimulants (43). Since the contribution of these background antibodies to the overall reactivity (or titer) of PPAb against the influenza vaccine antigens is negligible (15), we defined AsH1N1 as Cvaccine/TsH1N1 and ApH1N1 as Cvaccine/TpH1N1. Therefore, if relative avidity is ApH1N1/AsH1N1, this would be calculated as (Cvaccine/TpH1N1)/(Cvaccine/TsH1N1), which is equal to TsH1N1/TpH1N1. A smaller value of this ratio indicates higher relative avidity to pH1N1.
HAI assay.
The HAI assay was performed using a standard technique (44). In brief, 25-μl aliquots of serially diluted serum or PPAb samples in PBS were mixed with 25-μl aliquots of the vaccine component viruses (provided by G. Kemble, MedImmune LLC, Mountain View, California, USA; G. Air, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, USA; and BEI Resources), corresponding to 4 HA units, in V-bottom 96-well plates (Nunc; Thermo Fisher Scientific) and incubated for 30 minutes at room temperature. At the end of the incubation, 50 μl of 0.5% chicken red blood cells was added and incubated for a minimum of 45 minutes before determination of HAI activity. The HAI titer of a given serum sample was defined as the reciprocal of the last dilution with no HAI activity. A titer of 2 was assigned to all serum samples in which the first dilution (1:4) was negative. Complete medium was used as the negative control for PPAb samples. A titer of 2.5 was assigned to all PPAb samples in which the first dilution (1:5) was negative, and a sample with a titer of 10 or higher was considered HAI positive.
Statistics.
Unless otherwise indicated, unpaired 2-tailed t test adjusted for unequal variances or paired 2-tailed t test was used to compare means between age groups (young vs. elderly), testing antigens (sH1N1 vs. pH1N1), or antibody isotypes (IgA vs. IgG). All data were logarithm transformed for comparison of GMTs. Association on 2 × 2 contingency tables was assessed by Fisher exact test.
To estimate yield of secreted IgG or IgA per ASC, the assumptions were made that average yield per cell did not vary with the antigen specificity of ASCs within each donor or the number of ASCs within an age group and that no antibody was produced in the absence of ASCs. This allowed fitting of the simple no-intercept regression model A = βQ, where A is the amount of IgA (or IgG) secreted and Q is the quantity of ASCs. The regression coefficient β is the yield per ASC. Because both A and Q may have contained measurement error, β was estimated using RMA regression (45). A variance-stabilized bootstrap t (46) was used to construct confidence intervals and compare RMA regression coefficients between age groups. To enhance coverage accuracy, confidence intervals were obtained as the quantiles from a fit of a skew-normal distribution (47) to the bootstrap distribution.
Hypothesis tests were declared statistically significant for P values less than 0.05. The type I error rate was controlled at 5% across multiple comparisons within each set of analyses using sequential Bonferroni adjustment (48).
Supplementary Material
Acknowledgments
This study was supported by NIH grants AI057229, DK56339, AI057158, AI057266, AI090023, and HHSN266200500026C. We thank our study subjects for their participation; R. Ahmed for constructive discussions; P. Dormitzer, G. Kemble, and G. Air for providing critical reagents; C. Zhang, T. Tse, C. Helms, L. Garman, M. Huang, M. Morrissey, and J. Hwei-Lee for technical assistance; S. Mackey for coordinating the clinical study; and T. Quan, K. Spann, S. Batra, B. Tse, S. Swope, S. Cathey, C. Walsh, S. French, and M. Ugur for enrolling subjects and collecting blood samples. We also thank V.C. Maino (BD Biosciences) for allowing us access to the FACSAria II flow cytometer in his laboratory and M.C. Jaimes for single-cell sorting some of the blood samples.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(8):3109–3119. doi:10.1172/JCI57834.
Carlos F. Narvaez’s present address is: Facultad de Salud, Programa de Medicina, Universidad Surcolombiana, Neiva, Colombia.
See the related Commentary beginning on page 2981.
References
- 1.Fiore AE, et al. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep. 2007;56(RR-6):1–54. [PubMed] [Google Scholar]
- 2.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24(8):1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 3.Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. JAMA. 1994;272(21):1661–1665. doi: 10.1001/jama.272.21.1661. [DOI] [PubMed] [Google Scholar]
- 4.Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis. 2007;7(10):658–666. doi: 10.1016/S1473-3099(07)70236-0. [DOI] [PubMed] [Google Scholar]
- 5.Chen WH, Kozlovsky BF, Effros RB, Grubeck-Loebenstein B, Edelman R, Sztein MB. Vaccination in the elderly: an immunological perspective. Trends Immunol. 2009;30(7):351–359. doi: 10.1016/j.it.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerhard W. The role of the antibody response in influenza virus infection. Curr Top Microbiol Immunol. 2001;260:171–190. doi: 10.1007/978-3-662-05783-4_9. [DOI] [PubMed] [Google Scholar]
- 7.Cox RJ, Brokstad KA, Zuckerman MA, Wood JM, Haaheim LR, Oxford JS. An early humoral immune response in peripheral blood following parenteral inactivated influenza vaccination. Vaccine. 1994;12(11):993–999. doi: 10.1016/0264-410X(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 8.el-Madhun AS, Cox RJ, Soreide A, Olofsson J, Haaheim LR. Systemic and mucosal immune responses in young children and adults after parenteral influenza vaccination. J Infect Dis. 1998;178(4):933–939. doi: 10.1086/515656. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki S, et al. Influence of prior influenza vaccination on antibody and B-cell responses. PLoS One. 2008;3(8):e2975. doi: 10.1371/journal.pone.0002975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wrammert J, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453(7195):667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16(1):1–4. doi: 10.1016/S1074-7613(01)00261-8. [DOI] [PubMed] [Google Scholar]
- 12.Kunkel EJ, Butcher EC. Plasma-cell homing. Nat Rev Immunol. 2003;3(10):822–829. doi: 10.1038/nri1203. [DOI] [PubMed] [Google Scholar]
- 13.Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol. 2004;59(1):1–15. doi: 10.1111/j.0300-9475.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki S, et al. Comparison of the influenza virus-specific effector and memory B-cell responses to immunization of children and adults with live attenuated or inactivated influenza virus vaccines. J Virol. 2007;81(1):215–228. doi: 10.1128/JVI.01957-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He XS, et al. Plasmablast–derived polyclonal antibody response after influenza vaccination. J Immunol Methods. 2011;365(1–2):67–75. doi: 10.1016/j.jim.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cancro MP, et al. B cells and aging: molecules and mechanisms. Trends Immunol. ul 2009. 30 7 313 318 10.1016/j.it.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9(3):185–194. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 18. Frasca D, Blomberg BB. Aging affects human B cell responses [published online ahead of print February 12, 2011].J Clin Immunol . doi:10.1007/s10875-010-9501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frasca D, Nguyen D, Riley RL, Blomberg BB. Effects of aging on proliferation and E47 transcription factor activity induced by different stimuli in murine splenic B cells. Mech Ageing Dev. 2003;124(4):361–369. doi: 10.1016/S0047-6374(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 20.Bancos S, Phipps RP. Memory B cells from older people express normal levels of cyclooxygenase-2 and produce higher levels of IL-6 and IL-10 upon in vitro activation. Cell Immunol. 2010;266(1):90–97. doi: 10.1016/j.cellimm.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng B, Han S, Takahashi Y, Kelsoe G. Immunosenescence and germinal center reaction. Immunol Rev. 1997;160:63–77. doi: 10.1111/j.1600-065X.1997.tb01028.x. [DOI] [PubMed] [Google Scholar]
- 22.Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200(12):1613–1622. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han S, Marinova E, Zheng B. Rectification of age-related impairment in Ig gene hypermutation during a memory response. Int Immunol. 2004;16(4):525–532. doi: 10.1093/intimm/dxh054. [DOI] [PubMed] [Google Scholar]
- 24.Zheng B, Switzer K, Marinova E, Wansley D, Han S. Correction of age-associated deficiency in germinal center response by immunization with immune complexes. Clin Immunol. 2007;124(2):131–137. doi: 10.1016/j.clim.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Nussenzweig MC, Alt FW. Antibody diversity: one enzyme to rule them all. Nat Med. 2004;10(12):1304–1305. doi: 10.1038/nm1204-1304. [DOI] [PubMed] [Google Scholar]
- 26.Frasca D, et al. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. . J Immunol. 2008;180(8):5283–5290. doi: 10.4049/jimmunol.180.8.5283. [DOI] [PubMed] [Google Scholar]
- 27.Frasca D, Landin AM, Riley RL, Blomberg BB. Mechanisms for decreased function of B cells in aged mice and humans. J Immunol. 2008;180(5):2741–2746. doi: 10.4049/jimmunol.180.5.2741. [DOI] [PubMed] [Google Scholar]
- 28.Frasca D, et al. Intrinsic defects in B cell response to seasonal influenza vaccination in elderly humans. Vaccine. 2010;28(51):8077–8084. doi: 10.1016/j.vaccine.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolibab K, Smithson SL, Rabquer B, Khuder S, Westerink MA. Immune response to pneumococcal polysaccharides 4 and 14 in elderly and young adults: analysis of the variable heavy chain repertoire. Infect Immun. 2005;73(11):7465–7476. doi: 10.1128/IAI.73.11.7465-7476.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero-Steiner S, et al. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin Infect Dis. 1999;29(2):281–288. doi: 10.1086/520200. [DOI] [PubMed] [Google Scholar]
- 31.Usinger WR, Lucas AH. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect Immun. 1999;67(5):2366–2370. doi: 10.1128/iai.67.5.2366-2370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carson PJ, Nichol KL, O’Brien J, Hilo P, Janoff EN. Immune function and vaccine responses in healthy advanced elderly patients. Arch Intern Med. 2000;160(13):2017–2024. doi: 10.1001/archinte.160.13.2017. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, Hwang Y, Nahm MH. Avidity, potency, and cross-reactivity of monoclonal antibodies to pneumococcal capsular polysaccharide serotype 6B. Infect Immun. 2001;69(1):336–344. doi: 10.1128/IAI.69.1.336-344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu X, et al. Neutralizing antibodies derived from the B cells of 1918 influenza pandemic survivors. Nature. 2008;455(7212):532–536. doi: 10.1038/nature07231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tangye SG, Avery DT, Deenick EK, Hodgkin PD. Intrinsic differences in the proliferation of naive and memory human B cells as a mechanism for enhanced secondary immune responses. J Immunol. 2003;170(2):686–694. doi: 10.4049/jimmunol.170.2.686. [DOI] [PubMed] [Google Scholar]
- 36.Good KL, Tangye SG. Decreased expression of Kruppel-like factors in memory B cells induces the rapid response typical of secondary antibody responses. Proc Natl Acad Sci U S A. 2007;104(33):13420–13425. doi: 10.1073/pnas.0703872104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hancock K, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361(20):1945–1952. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 38.Skountzou I, et al. Immunity to pre-1950 H1N1 influenza viruses confers cross-protection against the pandemic swine-origin 2009 A (H1N1) influenza virus. J Immunol. 2010;185(3):1642–1649. doi: 10.4049/jimmunol.1000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu R, Ekiert DC, Krause JC, Hai R, Crowe JE, Jr, Wilson IA. Structural basis of preexisting immunity to the 2009 H1N1 pandemic influenza virus. Science. 2010;328(5976):357–360. doi: 10.1126/science.1186430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 41.Smith K, et al. Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat Protoc. 2009;4(3):372–384. doi: 10.1038/nprot.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wrammert J, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011;208(1):181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298(5601):2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 44. Prevention CfDCa.The 1998-99 Who Influenza Reagent Kit For The Identification Of Influenza Isolates . Atlanta, Georgia, USA: Centers for Disease Control and Prevention; 1998. [Google Scholar]
- 45.McArdle B. The structural relationship: regression in biology. Can J Zool. 1988;66(11):2329–2339. [Google Scholar]
- 46. Efron B, Tibshirani R.An Introduction To The Bootstrap . New York, New York, USA: Chapman and Hall; 1993. [Google Scholar]
- 47.Azzalini A. A class of distributions which includes the normal ones. Scand J Statist. 1985;12(2):171–178. [Google Scholar]
- 48.Holm S. A simple sequentially rejective multiple test procedure. Scand J Statist. 1979;6(2):65–70. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.