Abstract
Background.
Evidence suggests that gait is influenced by higher order cognitive and cortical control mechanisms. However, less is known about the functional correlates of cortical control of gait.
Methods.
Using functional near-infrared spectroscopy, the current study was designed to evaluate whether increased activations in the prefrontal cortex (PFC) were detected in walking while talking (WWT) compared with normal pace walking (NW) in 11 young and 11 old participants. Specifically, the following two hypotheses were evaluated: (a) Activation in the PFC would be increased in WWT compared with NW. (b) The increase in activation in the PFC during WWT as compared with NW would be greater in young than in old participants.
Results.
Separate linear mixed effects models with age as the two-level between-subject factor, walking condition (NW vs WWT) as the two-level repeated within-subject factor, and HbO2 levels in each of the 16 functional near-infrared spectroscopy channels as the dependent measure revealed significant task effects in 14 channels, indicating a robust bilateral increased activation in the PFC in WWT compared with NW. Furthermore, the group-by-task interaction was significant in 11 channels with young participants showing greater WWT-related increase in HbO2 levels compared with the old participants.
Conclusions.
This study provided the first evidence that oxygenation levels are increased in the PFC during WWT compared with NW in young and old individuals. This effect was modified by age suggesting that older adults may underutilize the PFC in attention-demanding locomotion tasks.
Keywords: Cognition, Gait, fNIRS
DECLINE in gait performance is common in aging and is associated with increased risk of morbidity and mortality, hospitalizations, and poorer quality of life (1,2). The neural substrates underlying gait are not well understood. However, converging evidence from epidemiological, cognitive, and neuroimaging studies suggests that gait is influenced by higher order cognitive and cortical control mechanisms (3–21).
Longitudinal cohort studies have shown that lower walking capacity (3,4), the presence of clinical gait abnormalities (5), as well as performance differences on quantitative parameters of gait (6) are associated with increased risk of incident dementia. Studies examining the relationship between specific cognitive functions and gait have centered on attention and executive functions (7–11). The results typically showed that worse scores on measures of attention and executive functions were associated with poorer gait performance. Moreover, our recent intervention study demonstrated that cognitive remediation of attention and executive functions improved gait velocity in sedentary older adults (12). Age-related decline in gait performance has been documented in divided attention tasks where participants were asked to walk and perform a secondary interference task (13,14). Walking while talking (WWT) paradigms present a twofold advantage in that (a) causal effects of attention resources on gait can be directly assessed and (b) everyday functions often require the individual to walk while encountering interruptions from one or more sensory modalities. Thus, WWT may serve as a more ecologically valid proxy of gait in natural settings.
However, functional or structural neuroimaging correlates of WWT have been lacking. Structural imaging (15) and postmortem (16) studies suggest that frontal and subcortical regions are related to mobility, including gait speed in older adults. These are the same brain areas implicated in attention and executive functions (17). Less is known about the functional brain correlates of normal pace walking (NW) and WWT because active locomotion cannot be assessed in magnetic resonance imaging and positron emission tomography scanners. However, a recent positron emission tomography study used F-fluorodesoxy-glucose (F-FDG) to compare brain activation during human steady-state locomotion to brain activation of imagined walking as assessed with functional magnetic resonance imaging (18). The results revealed both similar distinct brain activation patterns for real and imagined walking (18). Moreover, recent studies have begun to shed light on the functional brain correlates of locomotion using functional near-infrared spectroscopy (fNIRS). Findings from these studies have revealed that the prefrontal, premotor, and the primary sensorimotor cortices were differentially involved in walking, running, and balance control in healthy individuals (19–21) older adults (22) and patients recovering from stroke (23). Moreover, activation levels on fNIRS were shown to be increased in the prefrontal cortex (PFC) when participants were asked to mentally prepare prior to walking (21).
The frontal lobe hypothesis of aging (24) suggests that functions mediated by the PFC including dual tasking (25) decline early with aging. Furthermore, significant reductions in regional cerebral blood flow in anterior relative to posterior cortical regions were observed in older adults (26), suggesting decreased task-specific efficiency of the frontal lobes. The PFC is implicated in dual tasking (27), but the functional involvement of this brain region during WWT in young and old individuals has not been reported to our knowledge. Importantly, current cognitive reserve theories of aging (28) suggest that young compared with older adults may show greater brain activations in response to increased task difficulty and attention demands.
Using fNIRS, the current study was designed to evaluate whether increased activations in the PFC were detected during WWT as compared with NW in young and old participants. Specifically, we aimed to evaluate the following two hypotheses: (a) Activation in the PFC would be increased in WWT compared with NW and (b) the increase in activation in the PFC during WWT as compared with NW would be greater in young than in old participants.
METHODS
Participants
Participants were volunteers who had responded to our invitation to participate in clinical research studies. Eleven older adults (7 females), ages 69–88 years, and 11 young adults (7 females), ages 19–29 years, participated in the current study. The old and young samples were comparable in terms of education (mean years of education: old = 13.7 ± 2.7 and young = 14.7 ± 2.6). Mini-Mental State Examination performance (29) was well above the suggested dementia cut score of 24 in the old (mean total score = 27.2 ± 1.8) and young (mean total score = 29.3 ± 0.9) groups. The older adults, also enrolled in a separate pilot study, were sedentary, defined as physically inactive or exercises once weekly or less (30), with gait velocity <1.0 m/s. The older participants did not use any devices to aid their ambulation during the walking trials of this study. Furthermore, based on structured assessment protocols conducted in our medical center, we ascertained that they did not have specific sensory motor deficits that could have interfered with their walking. The following served as the study exclusion criteria: current or recent nonskin neoplastic disease or melanoma, active hepatic disease or primary renal disease requiring dialysis, primary untreated endocrine diseases (eg, Cushing’s disease or primary hypothalamic failure or insulin-dependent diabetes type I or II), HIV infection, any history of psychosis, current or recent (past 5 years) major depressive disorder, bipolar disorder, or anxiety disorder, history of electroconvulsive therapy, current or recent (within past 12 months) alcohol or substance abuse or dependence, recent use (past month) of recreational drugs, brain disorders such as stroke, tumor, infection, epilepsy, multiple sclerosis, neurodegenerative diseases, head injury (loss of consciousness > 5 minutes), diagnosed learning disability, dyslexia, or ADHD, and mental retardation. In addition, any use of medications that target the central nervous system (eg, neuroleptics, anticonvulsants, antidepressants, benzodiazepines) within the last month served as exclusion criteria as well. Written informed consent was obtained at the beginning of each session according to study protocols and approved by the Committee on Clinical Investigation (institutional review board of the Albert Einstein College of Medicine).
Walking Paradigm
The walking paradigm including practice procedures has been validated in several independent studies (11,31).
Normal pace walking.—
Participants were asked to walk at their “normal pace” for six separate trials (trial distance = 15 feet) in a quiet room wearing comfortable footwear with the fNIRS system attached to their forehead. Start and end points for each trial were clearly demarcated. The participants had to turn after each trial was completed, but brain imaging data were not recorded during turns. A tester escorted each participant, ensuring safety and compliance with the start and stop points for each walking trial.
Walking while talking.—
Participants were asked to walk at their normal pace for six additional trials while reciting alternate letters of the alphabet over the same distance. The order of the initial letter on WWT was randomly varied between “A” and “B” to minimize practice effects. Participants were asked to pay equal attention to their walking and talking (31). We have shown that WWT performance was related to increased attention demands (11) and to risk of falls (32).
fNIRS System Used in the Current Experiment
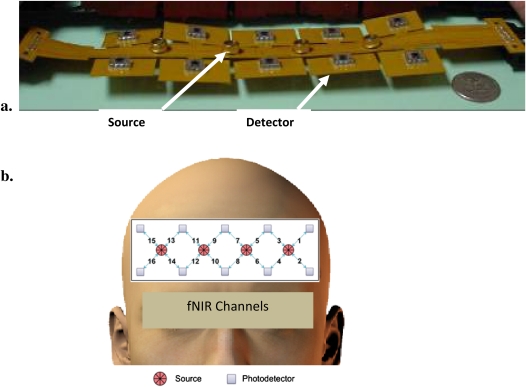
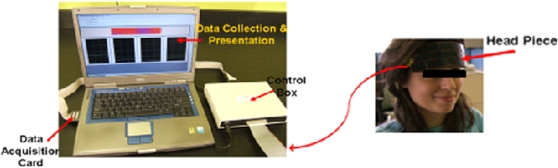
The fNIRS sensor, designed to image the PFC of adult humans, covers the forehead. The fNIRS sensor consists of reusable flexible circuit board that carries the near-infrared light sources and detectors, a replaceable cushioning material, and a disposable single-use medical-grade adhesive tape that serves to attach the sensor to the participant’s forehead. The flexible sensor, developed in the Drexel Biomedical Engineering laboratory, consists of four LED light sources and 10 detectors, which cover the forehead using 16 voxels with a source-detector separation of 2.5 cm. The 16-channel sensor (see Figure 1) has a temporal resolution of 500 ms per scan with 2.5 cm source-detector separation allowing for approximately 1–1.25 cm penetration depth, which has been used to detect hemodynamic changes in response to cognitive challenges and reported in previous studies (34 –41). The light sources (manufactured by Epitex Inc., Kyoto, Japan; type L4X730/4X805/4X850-40Q96-I) contain three built-in LEDs having peak wavelengths at 730, 805, and 850 nm, with an overall outer diameter of 9.2 ± 0.2 mm. The photodetectors (manufactured by Burr-Brown, Tucson, AZ; type OPT101) are monolithic photodiodes with a single-supply transimpedance amplifier. Communication between the data analysis computer and the task presentation computer is established via a serial port connection to time lock fNIR measurement to computer-generated task events (see Figure 2).
Figure 1.
(a). Sixteen-channel functional near-infrared spectroscopy (fNIRS) sensor implemented in this study. (b). Source-detector configuration used for scanning the forehead. This is an adapted version of an image originally published in Behavioural Brain Research (33).
Figure 2.
Overview of the functional near-infrared spectroscopy system with the sensor placed on the forehead of a research volunteer.
Because the circuit board and cushioning materials are flexible, the components move and adapt to the various contours of the participants’ foreheads, allowing the sensor elements to maintain an orthogonal orientation to the skin surface, dramatically improving light-coupling efficiency and signal strength (42). There is a sensor placement procedure followed in all our measurements. The fNIRS is placed on the forehead so that the horizontal symmetry axis central (y axis) coincides with symmetry axis of the head (ie, in between the eyes). On the vertical axis, the sensor is positioned with respect to the international 10–20 system so that FP1 and FP2 marker locations are positioned on the bottom channel row level (43). Validation studies with the fNIRS used by the Drexel group to identify hemodynamic changes in the PFC in response to cognitive challenges have been previously reported (34).
fNIRS localization of brain activation.—
Comparing fNIRS results with the criterion standard functional magnetic resonance imaging measurements poses a challenge because of the inability of the latter to image the brain during motion. However, this challenge has been addressed in recent studies (44). Specifically for the fNIRS system used in this experiment, a coregistration technique was developed using anatomical landmarks and standard structural magnetic resonance imaging templates to visualize the timing and location of brain activations (43). The registration technique utilizes chain-code algorithm and depicts activations over respective locations based on the fixed sensor geometry. The registered data locations can then be used to create spatiotemporal visualization of fNIRS measurements.
Preprocessing for artifact removal.—
Raw intensity measurements at 730, 805, and 850 nm were first low-pass filtered with a finite impulse response filter of cutoff frequency at 0.14 Hz to eliminate possible respiration and heart rate signals and unwanted high-frequency noise (45). Then using a combined independent component analysis/principal component analysis, environmental and equipment noise and signal drifts are removed from the raw intensity measurements (34).
Hemodynamic signal extraction.—
Using modified Beer–Lambert law, artifact-removed raw intensity measurements at 730, 805, and 850 nm are converted to oxygenated hemoglobin (HbO2), deoxygenated hemoglobin (Hb), and oxygenation or oxygen index (Oxy = HbO2 − Hb) signals for each of the 16 channels that covers the entire forehead. In the current experiment, HbO2 values that are more reliable and sensitive to locomotion-related changes in cerebral blood flow (20,22) were used to characterize changes in the PFC in walking and in WWT. Also, using one index for task-related hemodynamic changes in the PFC reduced the number comparisons and thus the probability of increased Type I error. Relative changes in the concentrations of HbO2 were obtained using a 5-second baseline, which was collected immediately before the start of the NW and WWT gait protocols.
Trial epoch extraction for NW and WWT.—
There were six trial epochs for each walking condition. The time course of each trial was divided into 0.5-second intervals starting at 0 seconds. fNIRS parameters fluctuate and return to baseline preactivation levels after reaching maximal values. Thus, consistent with previous studies (46,47), maximal HbO2 values of each separate trial epoch obtained for each task condition (NW or WWT), channel and participant, were used for statistical analysis. We have used the maximal HbO2 values of the averaged time course of six trial epochs assessed in NW and WWT to eliminate possible intertrial differences and to obtain a more robust signal. In order to extract the maximum HbO2 values, common ground for the timing of the data epochs had to be determined. We selected the minimum time elapsed from start to end of each walking protocol for the old (4 seconds) and young (3.5 seconds) participants. HbO2 values for NW and WWT were calculated according to a 5-second baseline assessed immediately prior to the start of each walking condition. Proximal baseline conditions ranging from 1 to 5 seconds have been previously reported in fNIRS studies (48,49). Participants were standing during this time period. For each walking condition, the starting baseline levels for the 5-second baseline period were adjusted to a zero mean value in HbO2 values. Hence, the changes in HbO2 values for NW and WWT were normalized to the same level of starting baseline condition.
Statistical Analysis
Descriptive statistics for gait velocity (centimeters per second) per age group in NW and WWT were provided. Linear mixed effects model with age as the two-level between-subject factor, walking condition (NW vs. WWT) as the two-level repeated within-subject factor, and gait velocity as the dependent measures was used to ascertain the increased attention demands attributed to the dual-task interference. The interaction term of age-by-walking condition was included to explicitly evaluate whether age influenced the effect of dual-task interference on gait velocity. The advantage of the linear mixed model is that the heterogeneity and correlation of repeated measures under different conditions are taken into account. A random intercept was included in the model to allow the entry point to vary across individuals.
Descriptive statistics for HbO2 levels in NW and WWT were depicted for each of the 16 fNIRS channels per age group. Separate linear mixed effects models with age as the two-level between-subject factor, walking condition (NW vs WWT) as the two-level repeated within-subject factor, and HbO2 levels in each of the 16 channels as the dependent measure were run to examine whether oxygenation levels increased as a function of dual-task interference. The interaction term of age-by-walking condition was included in each model to evaluate whether age influenced the effect of dual-task interference on HbO2 levels. For all statistical analyses in this study, the level of significance was set at p = .05.
RESULTS
As expected, gait velocity was higher in young compared with old individuals in NW (old = 71.6 cm/s ± 17.7 and young = 122.2 cm/s ±17.5) and in WWT (old = 36.2 cm/s ± 13.1 and young = 81.0 cm/s ± 12.9]). Consistent with the descriptive data, the linear mixed effects model revealed significant task effect demonstrating that gait velocity was much slower in WWT compared with NW (β = 41.10, 95% confidence interval = 22.92−59.29, p < .001). Gait velocity was higher in the young compared with the old participants as evidenced by the significant group effect (β = −44, 95% confidence interval = −66.20 to 23.53, p < .001). However, the group-by-task interaction was not significant (β = −5.70, 95% confidence interval = −31.41 to 20.01, p = .656), indicating that the dual-task interference reduced gait velocity in both age groups.
HbO2 levels per age group and walking condition for each of the 16 fNIRS channels are summarized in Table 1.
Table 1.
HbO2 Levels During Normal Walking and WWT in Young and Old Participants
| Channel | Entire Sample (n = 22) |
Young (n = 11) |
Old (n = 11) |
|||
| NW | WWT | NW | WWT | NW | WWT | |
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| 1 | **0.38 (0.39) | 0.84 (0.99) | 0.32 (0.46) | 0.86 (1.33) | 0.43 (0.33) | 0.82 (0.55) |
| 2 | **0.37 (0.55) | 1.4 (1.1) | 0.38 (0.68) | 2.24 (0.95) | 0.36 (0.42) | 0.56 (0.46) |
| 3 | *0.51 (0.74) | 1.4 (1.6) | 0.65 (1.00) | 2.17 (1.97) | 0.37 (0.34) | 0.63 (0.49) |
| 4 | *0.62 (1.1) | 1.9 (1.7) | 0.84 (1.62) | 3.31 (1.48) | 0.40 (0.40) | 0.59 (0.44) |
| 5 | **0.35 (0.73) | 1.2 (1.3) | 0.35 (0.97) | 1.84 (1.55) | 0.35 (0.42) | 0.47 (0.33) |
| 6 | **0.38 (0.66) | 1.4 (1.3) | 0.42 (0.82) | 2.35 (1.28) | 0.34 (0.48) | 0.49 (0.25) |
| 7 | **0.38 (0.66) | 1.2 (1.2) | 0.16 (0.54) | 1.82 (1.34) | 0.59 (0.72) | 0.69 (0.96) |
| 8 | **0.37 (0.73) | 1.2 (1.0) | 0.17 (0.52) | 1.78 (1.13) | 0.56 (0.87) | 0.65 (0.67) |
| 9 | **0.47 (0.90) | 0.94 (0.90) | 0.57 (1.18) | 1.20 (1.08) | 0.37 (0.52) | 0.69 (0.64) |
| 10 | **0.43 (0.61) | 1.2 (1.2) | 0.25 (0.55) | 1.81 (1.35) | 0.60 (0.64) | 0.56 (0.49) |
| 11 | **0.45 (0.70) | 1.1 (1.0) | 0.52 (0.85) | 1.62 (1.14) | 0.37 (0.53) | 0.64 (0.80) |
| 12 | **0.48 (0.88) | 1.5 (1.3) | 0.67 (1.23) | 2.48 (1.24) | 0.28 (0.18) | 0.60 (0.66) |
| 13 | 0.21 (0.32) | 1.1 (0.99) | 0.16 (0.37) | 1.71 (1.06) | 0.27 (0.26) | 0.56 (0.46) |
| 14 | *0.45 (0.67) | 1.6 (1.4) | 0.49 (0.82) | 2.48 (1.26) | 0.42 (0.51) | 0.69 (0.83) |
| 15 | *0.29 (0.38) | 1.1 (1.0) | 0.27 (0.48) | 1.38 (1.20) | 0.30 (0.27) | 0.73 (0.74) |
| 16 | *0.67 (1.0) | 1.5 (1.1) | 0.67 (1.19) | 2.23 (1.02) | 0.68 (1.02) | 0.84 (0.78) |
Notes: Linear mixed effects models examined whether maximal HbO2 levels during the time course were significantly greater than maximal HbO2 levels at Time 0 for NW. Significant increases were observed in 15 of the 16 channels. NW = normal pace walking; WWT = walking while talking.
*p < .05. **p < .01.
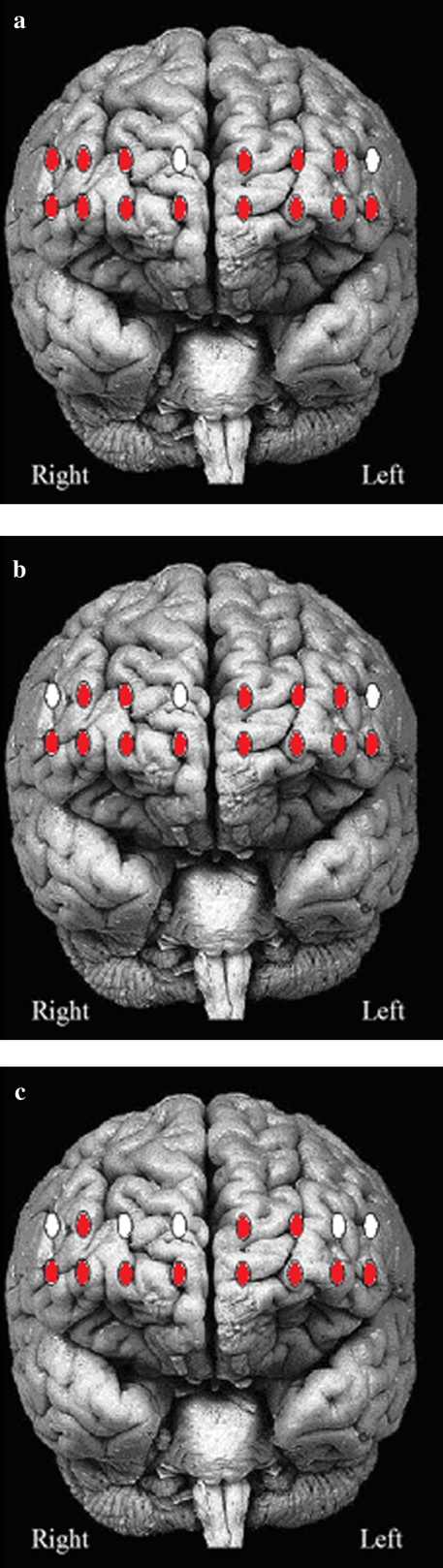
Descriptively, the results suggested that activation levels increased in WWT compared with NW but that this effect was modified by age with the young participants showing greater dual-task–related increase in HbO2 levels than the old participants. Because NW served as the control condition for WWT, we also sought to determine whether PFC activations increased during the time course of NW relative to Time 0. The results revealed significant and comparable increases in HbO2 levels in 15 of the 16 channels in the young and old participants (see Table 1). Separate linear mixed effects models with age as the two-level between-subject factor, walking condition (NW vs WWT) as the two-level repeated within-subject factor, and HbO2 levels in each of the 16 channels as the dependent measure revealed significant task effect in 14 channels indicating a robust bilateral increased activation in the PFC in WWT compared with NW. The group effect was significant in 13 of the 16 channels revealing that overall HbO2 levels were higher in the young compared with the old participants. However, this was primarily attributed to higher activation levels in WWT in the young compared with the old participants. Furthermore, the group-by-task interaction was significant in 11 channels with young participants showing, bilaterally, greater WWT-related increase in HbO2 levels compared with the old participants. The results of the mixed linear effect models were summarized in Table 2 and depicted visually in Figure 3a–c.
Table 2.
Linear Mixed Effects Models With Age as the two-Level Between-Subject Factor, Walking Condition (NW vs WWT) as the Two-Level Repeated Within-Subject Factor, and HbO2 Levels for Each of the 16 fNIRS Channels as the Dependent Measure
| Channel | Task |
Task × Group |
Group |
||||||
| β | 95% CI | p | β | 95% CI | p | β | 95% CI | p | |
| 1 | .86 | −1.21 to 0.14 | .11 | .14 | −0.81 to 1.11 | .75 | −.03 | −0.94 to 0.87 | .93 |
| 2 | −1.85 | −2.43 to −1.2 | <.001 | 1.65 | 0.83–2.46 | <.001 | −1.6 | −2.34 to −1.00 | <.001 |
| 3 | −1.53 | −2.52 to −0.52 | .004 | 1.2 | −0.15 to 2.6 | .079 | −1.52 | −2.81 to −0.25 | .021 |
| 4 | −2.46 | −3.44 to −1.48 | <.001 | 2.27 | 0.88–3.66 | .002 | −2.71 | −3.68 to −1.74 | <.001 |
| 5 | −1.48 | −2.31 to −0.655 | .001 | 1.36 | 0.19–2.54 | .024 | −1.37 | −2.37 to −0.36 | .01 |
| 6 | −1.92 | −2.62 to −1.22 | <.001 | 1.77 | 0.78–2.76 | .001 | −1.86 | −2.68 to −1.03 | <.001 |
| 7 | −1.66 | −2.48 to −0.83 | <.001 | 1.56 | 0.39–2.72 | .01 | −1.13 | −2.17 to −0.09 | .034 |
| 8 | −1.60 | −2.32 to −0.88 | <.001 | 1.52 | 0.50–2.54 | .004 | −1.13 | −1.96 to −0.30 | .01 |
| 9 | −.63 | −1.41 to 0.146 | .108 | .31 | −0.78 to 1.41 | .565 | −.51 | −1.3 to 0.27 | .192 |
| 10 | −1.55 | −2.28 to −0.83 | <.001 | 1.60 | 0.57–2.63 | .003 | −1.2 | −2.15 to −0.34 | .009 |
| 11 | −1.10 | −1.84 to −0.35 | .005 | .83 | −0.21 to 1.89 | .116 | −.98 | −1.86 to −0.10 | .030 |
| 12 | −1.80 | −2.61 to −0.98 | <.001 | 1.48 | 0.33–2.63 | .013 | −1.87 | −2.76 to −0.98 | <.001 |
| 13 | −1.55 | −2.10 to −1.00 | <.001 | 1.26 | 0.48–2.03 | .002 | −1.15 | −1.88 to −0.42 | .004 |
| 14 | −1.98 | −2.77 to −1.20 | <.001 | 1.71 | 0.60–2.81 | .003 | −1.78 | −2.73 to −0.83 | .001 |
| 15 | −1.10 | −1.77 to 1.43 | .002 | .67 | −0.26 to 1.62 | .151 | −.65 | −1.54 to 0.239 | .142 |
| 16 | −1.55 | −2.43 to −0.67 | .001 | 1.39 | 0.15–2.64 | .029 | −1.39 | −2.02 to −0.57 | .002 |
Note: CI = confidence interval.
Figure 3.
(a) Red (dark grey in print) channels indicate significant increases in HbO2 levels during WWT as compared with NW. (b) Red (dark grey in print) channels indicate significant increases in HbO2 levels in young as compared with older participants across walking conditions. (c) Red (dark grey in print) channels indicate significant group-by-task interactions revealing greater increases in HbO2 levels in young than in older adults during WWT as compared with NW. Brain image templates copyrighted, 1998, University of Washington, Sundsten J.W, Mulligan K.
DISCUSSION
The current study used fNIRS to investigate the PFC hemodynamic correlates of NW and WWT in young and old individuals. Consistent with recent research in healthy (19–21), old (22), and pathological (23) samples, we found increased PFC activation during NW (see Table 1). However, this study is the first to report on increased PFC activation levels in walking when paired with a simultaneous secondary interference task compared with NW. This effect was robust and uniformly bilateral as evidenced by the increased activation levels in 14 of the 16 fNIRS channels (7 on each side). Furthermore, applying a more stringent significance threshold would not have changed the results as significance levels in 11 of the 14 channels were equal to or smaller than 0.001. The increased oxygenation levels in the PFC during WWT as compared with NW are consistent with the documented role of this brain region in monitoring and coordinating attention resources to competing task demands (27). Furthermore, NW serves as an excellent control condition for WWT as the physiological demands of walking in both conditions are equivalent. The left hemisphere including the PFC plays a pivotal role in cortical control of speech as evidenced by positron emission tomography and magnetic resonance imaging (50) methods. However, it is noteworthy that the WWT-related increases in oxygenation levels, observed in this study, were uniformly bilateral. Hence, it appears that this effect cannot be attributed exclusively to the speech demands that are inherent in WWT. Although the fNIRS sensor used in the current study is not designed to assess activations in the primary speech areas, we assessed the PFC correlates of reciting the alphabet in a few selected participants (data not shown) and found no evidence that reciting the alphabet could account for the significant increases in activation in this brain region during WWT. Nonetheless, in the context of WWT, the PFC correlates of reciting the alphabet should be further evaluated in future studies.
Our second hypothesis was confirmed as well with old age attenuating the increase in oxygenation levels in the PFC during WWT as compared with NW. This effect was bilateral and robust as evidenced by the significant task-by-group interactions in 11 fNIRS channels (see Table 2 and Figure 3c). The age-related attenuation of the increase in PFC activation levels during WWT cannot be attributed to group differences in compliance with or performance on the verbal interference task because the decline in gait velocity in WWT relative to NW was comparable in both age groups. This finding is consistent with the frontal lobe hypothesis of aging (24) and with cognitive reserve theory proposing that young adults demonstrate greater increase in brain activation in response to increased cognitive task demands (28). Worse attention has been linked to poor WWT performance in older adults (11,13,14), which in turn was predictive of the risk of falls in nondemented older adults (32). Furthermore, lower scores on measures of attention and executive functions have also been associated with increased risk of falls (51–53). The effects reported herein provide compelling support to these findings in that underutilization of the PFC to monitor and coordinate attention resources may account for the relationship between impaired attention, walking performance, and the risk of falls in this population. To our knowledge this study is the first to demonstrate this evidence. It remains to be evaluated whether including direct measures of mobility task-related PFC activation will improve current risk assessment of mobility limitations in older adults.
The limitations of this study should be considered. Locomotion requires complex visuo-sensorimotor coordination and is therefore dependent on multiple brain regions and networks that clearly extend beyond the PFC and include the spinal cord, brainstem, cerebellum, basal ganglia, and motor cortex (54). Furthermore, the PFC itself is intricate both structurally and functionally and has extensive connection to subcortical and posterior regions (55). Hence, the task-related changes in oxygenation levels observed here, although robust, provide a limited window as to how the PFC in concert with other brain regions and networks functionally controls locomotion under normal and dual-task conditions. Nonetheless, the focus on the PFC is justified in light of previous cognitive (cf. (56) for review) and structural magnetic resonance imaging (15) studies implicating this region in higher order control of gait in humans. Although the older adults were sedentary, it is noteworthy that limited physical activity is common among the elderly participants. Although, it is impossible to separate the effects of disease from normal aging in this small sample, participants were screened for a number of diseases and conditions that affect the central nervous system. Nonetheless, it will be critical for future research to replicate and validate these findings in larger and more representative cohorts of older adults. The lack of structural imaging data is a limitation as the higher atrophy and greater structural variability in the PFC in older adults may have influenced the findings reported herein. However, it should be emphasized that the study hypotheses evaluated the increase in PFC activation during WWT compared with NW and the influence of aging on the increased involvement of this brain region during WWT. Hence, structural differences alone cannot account for our reported findings. Assessing higher order control of gait in conditions other than NW and WWT may be of interest. For instance, fast walking was uniquely related to cognitive decline in a nondemented cohort (57), suggesting differential brain involvement that can potentially be captured by advanced neuroimaging methods such as fNIRS. Herein, we identified robust and bilateral age-related increases in HbO2 levels in the PFC during WWT. However, future research should examine the possible differential involvement of the PFC, as measured by the different fNIRS channels, in a range of walking conditions that vary in terms of their cognitive demands and relations to clinical outcomes.
It is noteworthy that methodologically, this investigation differs from previous fNIRS studies of gait (19–22) in several important aspects. Gait was not assessed on a treadmill. Instead, the gait protocols, which require discrete and relatively short trials, were consistent with our previous studies linking quantitative gait performance in older adults to cognitive processes (11), dementia (6), and the catechol-O-methyltransferase genotype that is involved in dopamine degradation in the PFC and striatum (58). This was critical in terms of the applicability and relevance of these findings to our previous research and to common clinical assessment of gait. Previous fNIRS studies assessed gait over a relatively longer time frame that afforded depiction of hemodynamic changes during the course of the task as well. Here the time course was much shorter, suggesting that fNIRS can reliably identify changes in brain activation attributed to walking and WWT even in this relatively short time interval. However, future studies should investigate whether and how oxygenation levels vary over time during NW and WWT.
In summary, this study provided the first evidence that oxygenation levels are increased in the PFC in WWT compared with NW in young and old individuals. Furthermore, this effect was modified by age with young individuals showing greater increases in PFC oxygenation levels compared with the old participants. This finding suggests that older adults may underutilize the PFC in attention-demanding locomotion tasks.
FUNDING
Dr. R.H. is supported by the National Institute on Aging Paul B. Beeson Award K23 AG030857 and by grant R01AG036921. This study was supported, in part, by an intramural grant from the Albert Einstein College of Medicine, Bronx, NY.
Acknowledgments
This paper was orally presented in part at the Third International Congress on Gait and Mental Function, Washington DC, USA, in February 2010.
References
- 1.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 2.Verghese J, LeValley A, Hall CB, Katz MJ, Ambrose AF, Lipton RB. Epidemiology of gait disorders in community-residing older adults. J Am Geriatr Soc. 2006;54:255–261. doi: 10.1111/j.1532-5415.2005.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and dementia in physically capable elderly men. JAMA. 2004;292:1447–1453. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- 4.Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 5.Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-Alzheimer's dementia. N Engl J Med. 2002;347:1761–1768. doi: 10.1056/NEJMoa020441. [DOI] [PubMed] [Google Scholar]
- 6.Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007;78:929–935. doi: 10.1136/jnnp.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atkinson HH, Rapp SR, Williamson JD, et al. The relationship between cognitive function and physical performance in older women: results from the women's health initiative memory study. J Gerontol A Biol Sci Med Sci. 2010;65:300–306. doi: 10.1093/gerona/glp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watson NL, Rosano C, Boudreau RM, et al. Executive function, memory, and gait speed decline in well-functioning older adults. J Gerontol A Biol Sci Med Sci. 2010;65(10):1093–1100. doi: 10.1093/gerona/glq111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atkinson HH, Rosano C, Simonsick EM, et al. Cognitive function, gait speed decline, and comorbidities: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2007;62:844–850. doi: 10.1093/gerona/62.8.844. [DOI] [PubMed] [Google Scholar]
- 10.Ble A, Volpato S, Zuliani G, et al. Executive function correlates with walking speed in older persons: the InCHIANTI study. J Am Geriatr Soc. 2005;53:410–415. doi: 10.1111/j.1532-5415.2005.53157.x. [DOI] [PubMed] [Google Scholar]
- 11.Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: results from the Einstein Aging Study. Neuropsychology. 2006;20:215–223. doi: 10.1037/0894-4105.20.2.215. [DOI] [PubMed] [Google Scholar]
- 12.Verghese J, Mahoney J, Ambrose AF, Wang C, Holtzer R. Effect of cognitive remediation on gait in sedentary seniors. J Gerontol A Biol Sci Med Sci. 2010;65(12):1338–1343. doi: 10.1093/gerona/glq127. [DOI] [PubMed] [Google Scholar]
- 13.Li KZH, Lindenberger U, Freund AM, Baltes PB. Walking while memorizing: age-related differences in compensatory behavior. Psychol Sci. 2001;12:230–237. doi: 10.1111/1467-9280.00341. [DOI] [PubMed] [Google Scholar]
- 14.Lindenberger U, Marsiske M, Baltes PB. Memorizing while walking: increase in dual-task costs from young adulthood to old age. Psychol Aging. 2000;15:417–436. doi: 10.1037//0882-7974.15.3.417. [DOI] [PubMed] [Google Scholar]
- 15.Rosano C, Aizenstein HJ, Studenski S, Newman AB. A regions-of-interest volumetric analysis of mobility limitations in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007;62:1048–1055. doi: 10.1093/gerona/62.9.1048. [DOI] [PubMed] [Google Scholar]
- 16.Whitman GT, DiPatre PL, Lopez IA, et al. Neuropathology in older people with disequilibrium of unknown cause. Neurology. 1999;53:375–382. doi: 10.1212/wnl.53.2.375. [DOI] [PubMed] [Google Scholar]
- 17.Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- 18.la Fougere C, Zwergal A, Rominger A, et al. Real versus imagined locomotion: a [18F]-FDG PET-fMRI comparison. Neuroimage. 2010;50(4):1589–1598. doi: 10.1016/j.neuroimage.2009.12.060. [DOI] [PubMed] [Google Scholar]
- 19.Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S. Role of the prefrontal cortex in human balance control. Neuroimage. 2008;43(2):329–336. doi: 10.1016/j.neuroimage.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 20.Miyai I, Tanabe HC, Sase I, et al. Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. Neuroimage. 2001;14:1186–1192. doi: 10.1006/nimg.2001.0905. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki M, Miyai I, Ono T, Kubota K. Activities in the frontal cortex and gait performance are modulated by preparation. An fNIRS study. Neuroimage. 2008;39:600–607. doi: 10.1016/j.neuroimage.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 22.Harada T, Miyai I, Suzuki M, Kubota K. Gait capacity affects cortical activation patterns related to speed control in the elderly. Exp Brain Res. 2009;193:445–454. doi: 10.1007/s00221-008-1643-y. [DOI] [PubMed] [Google Scholar]
- 23.Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S. Sustained prefrontal activation during ataxic gait: a compensatory mechanism for ataxic stroke? Neuroimage. 2007;37:1338–1345. doi: 10.1016/j.neuroimage.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 24.West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- 25.Holtzer R, Stern Y, Rakitin BC. Age-related differences in executive control of working memory. Mem Cognit. 2004;32:1333–1345. doi: 10.3758/bf03206324. [DOI] [PubMed] [Google Scholar]
- 26.Gur RC, Gur RE, Obrist WD, Skolnick BE, Reivich M. Age and regional cerebral blood flow at rest and during cognitive activity. Arch Gen Psychiatry. 1987;44:617–621. doi: 10.1001/archpsyc.1987.01800190037006. [DOI] [PubMed] [Google Scholar]
- 27.Stelzel C, Brandt SA, Schubert T. Neural mechanisms of concurrent stimulus processing in dual tasks. Neuroimage. 2009;48:237–248. doi: 10.1016/j.neuroimage.2009.06.064. [DOI] [PubMed] [Google Scholar]
- 28.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 30.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 31.Verghese J, Kuslansky G, Holtzer R, et al. Walking while talking: effect of task prioritization in the elderly. Arch Phys Med Rehabil. 2007;88:50–53. doi: 10.1016/j.apmr.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verghese J, Buschke H, Viola L, et al. Validity of divided attention tasks in predicting falls in older individuals: a preliminary study. J Am Geriatr Soc. 2002;50:1572–1576. doi: 10.1046/j.1532-5415.2002.50415.x. [DOI] [PubMed] [Google Scholar]
- 33.León-Carrión J, Izzetoglu M, Izzetoglu K, et al. Efficient learning produces spontaneous neural repetition suppression in prefrontal cortex. Behav Brain Res. 2010;208(2):502–508. doi: 10.1016/j.bbr.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 34.Izzetoglu M, Bunce SC, Izzetoglu K, Onaral B, Pourrezaei K. Functional brain imaging using near-infrared technology. IEEE Eng Med Biol Mag. 2007;26:38–46. doi: 10.1109/memb.2007.384094. [DOI] [PubMed] [Google Scholar]
- 35.Kato T, Kamei A, Takashima S, Ozaki T. Human visual cortical function during photic stimulation monitoring by means of near-infrared spectroscopy. J Cereb Blood Flow Metab. 1993;13:516–520. doi: 10.1038/jcbfm.1993.66. [DOI] [PubMed] [Google Scholar]
- 36.Villringer A, Chance B. Non-invasive optical spectroscopy and imaging of human brain function. Trends Neurosci. 1997;20:435–442. doi: 10.1016/s0166-2236(97)01132-6. [DOI] [PubMed] [Google Scholar]
- 37.Kim MN, Durduran T, Frangos S, et al. Noninvasive measurement of cerebral blood flow and blood oxygenation using near-infrared and diffuse correlation spectroscopies in critically brain-injured adults. Neurocrit Care. 2010;12(2):173–180. doi: 10.1007/s12028-009-9305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okada E, Firbank M, Schweiger M, Arridge SR, Cope M, Delpy DT. Theoretical and experimental investigation of near-infrared light propagation in a model of the adult head. Appl Opt. 1997;36(1):21–31. doi: 10.1364/ao.36.000021. [DOI] [PubMed] [Google Scholar]
- 39.Leenders KL, Perani D, Lammertsma AA, et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain. 1990;113(Pt 1):27–47. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- 40.Leon-Carrion J, Martin-Rodriguez JF, Damas-Lopez J, et al. A lasting post-stimulus activation on dorsolateral prefrontal cortex is produced when processing valence and arousal in visual affective stimuli. Neurosci Lett. 2007;422(3):147–152. doi: 10.1016/j.neulet.2007.04.087. [DOI] [PubMed] [Google Scholar]
- 41.Leon-Carrion J, Damas J, Izzetoglu K, et al. Differential time course and intensity of PFC activation for men and women in response to emotional stimuli: a functional near-infrared spectroscopy (fNIRS) study. Neurosci Lett. 2006;403(1–2):90–95. doi: 10.1016/j.neulet.2006.04.050. [DOI] [PubMed] [Google Scholar]
- 42.Bunce SC, Izzetoglu M, Izzetoglu K, Onaral B, Pourrezaei K. Functional near-infrared spectroscopy. IEEE Eng Med Biol Mag. 2006;25:54–62. doi: 10.1109/memb.2006.1657788. [DOI] [PubMed] [Google Scholar]
- 43.Ayaz H, Izzetoglu M, Platek SM, et al. Registering fNIR data to brain surface image using MRI templates. Conf Proc IEEE Eng Med Biol Soc. 2006;1:2671–2674. doi: 10.1109/IEMBS.2006.260835. [DOI] [PubMed] [Google Scholar]
- 44.Okamoto M, Dan I. Automated cortical projection of head-surface locations for transcranial functional brain mapping. Neuroimage. 2005;26:18–28. doi: 10.1016/j.neuroimage.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 45.Huppert TJ, Diamond SG, Franceschini MA, Boas DA. HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl Opt. 2009;48:D280–D298. doi: 10.1364/ao.48.00d280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quaresima V, Ferrari M, van der Sluijs MC, Menssen J, Colier WN. Lateral frontal cortex oxygenation changes during translation and language switching revealed by non-invasive near-infrared multi-point measurements. Brain Res Bull. 2002;59:235–243. doi: 10.1016/s0361-9230(02)00871-7. [DOI] [PubMed] [Google Scholar]
- 47.Sakatani K, Xie Y, Lichty W, Li S, Zuo H. Language-activated cerebral blood oxygenation and hemodynamic changes of the left prefrontal cortex in poststroke aphasic patients: a near-infrared spectroscopy study. Stroke. 1998;29:1299–1304. doi: 10.1161/01.str.29.7.1299. [DOI] [PubMed] [Google Scholar]
- 48.Hull R, Bortfeld H, Koons S. Near-infrared spectroscopy and cortical responses to speech production. Open Neuroimag J. 2009;3:26–30. doi: 10.2174/1874440000903010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hyde DC, Boas DA, Blair C, Carey S. Near-infrared spectroscopy shows right parietal specialization for number in pre-verbal infants. Neuroimage. 2010;53:647–652. doi: 10.1016/j.neuroimage.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herman T, Mirelman A, Giladi N, Schweiger A, Hausdorff JM. Executive control deficits as a prodrome to falls in healthy older adults: a prospective study linking thinking, walking, and falling. J Gerontol A Biol Sci Med Sci. 2010;65(10):1086–1092. doi: 10.1093/gerona/glq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hausdorff JM, Doniger GM, Springer S, Yogev G, Simon ES, Giladi N. A common cognitive profile in elderly fallers and in patients with Parkinson's disease: the prominence of impaired executive function and attention. Exp Aging Res. 2006;32:411–429. doi: 10.1080/03610730600875817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holtzer R, Friedman R, Lipton RB, Katz M, Xue X, Verghese J. The relationship between specific cognitive functions and falls in aging. Neuropsychology. 2007;21:540–548. doi: 10.1037/0894-4105.21.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takakusaki K. Forebrain control of locomotor behaviors. Brain Res Rev. 2008;57:192–198. doi: 10.1016/j.brainresrev.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 55.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 56.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23:329–342. doi: 10.1002/mds.21720. quiz 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deshpande N, Metter EJ, Bandinelli S, Guralnik J, Ferrucci L. Gait speed under varied challenges and cognitive decline in older persons: a prospective study. Age Ageing. 2009;38(5):509–514. doi: 10.1093/ageing/afp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holtzer R, Ozelius L, Xue X, Wang T, Lipton RB, Verghese J. Differential effects of COMT on gait and executive control in aging. Neurobiol Aging. 2010;31(3):523–531. doi: 10.1016/j.neurobiolaging.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]