Abstract
Aging promotes oxidative stress in vascular endothelial and smooth muscle cells, which contribute to the development of cardiovascular diseases. NF-E2–related factor 2 (Nrf2) is a transcription factor, which is activated by reactive oxygen species in the vasculature of young animals, leading to adaptive upregulation of numerous reactive oxygen species detoxifying and antioxidant genes. The present study was designed to elucidate age-associated changes in the homeostatic role of Nrf2-driven free radical detoxification mechanisms in the vasculature of nonhuman primates. We found that carotid arteries of aged rhesus macaques (Macaca mulatta, age: ≥20 years) exhibit significant oxidative stress (as indicated by the increased 8-iso-PGF2α and 4-HNE content and decreased glutathione and ascorbate levels) as compared with vessels of young macaques (age: ∼10 years) that is associated with activation of the redox-sensitive proinflammatory transcription factor, nuclear factor-kappaB. However, age-related oxidative stress does not activate Nrf2 and does not induce Nrf2 target genes (NQO1, GCLC, and HMOX1). In cultured vascular smooth muscle cells (VSMCs) derived from young M mulatta, treatment with H2O2 and high glucose significantly increases transcriptional activity of Nrf2 and upregulates the expression of Nrf2 target genes. In contrast, in cultured vascular smooth muscle cells cells derived from aged macaques, H2O2– and high glucose–induced Nrf2 activity and Nrf2-driven gene expression are blunted. High glucose–induced H2O2 production was significantly increased in aged vascular smooth muscle cells compared with that in vascular smooth muscle cells from young M mulatta. Taken together, aging is associated with Nrf2 dysfunction in M mulatta arteries, which likely exacerbates age-related cellular oxidative stress, promoting nuclear factor-kappaB activation and vascular inflammation in aging.
Keywords: Artery, Inflammation, Oxidative stress, Smooth muscle, Vascular aging
OVER three quarters of deaths from cardiovascular diseases occur among patients over 65 years of age (1). Epidemiological studies show that even in the absence of risk factors related to lifestyle (eg, obesity, hypercholesterolemia, smoking), advanced age, per se, promotes the development of cardiovascular disease [for a recent review, see (2)]. In order to develop novel therapeutic interventions to promote vascular health in older persons, it is essential to understand the mechanisms through which aging impairs homeostatic mechanisms in the vasculature.
The oxidative stress hypothesis of aging postulates that increased production of reactive oxygen species (ROS) induces a variety of macromolecular oxidative modifications and that accumulation of such oxidative damage gradually leads to cellular dysfunction, which is a primary causal factor in the aging process. Although there is currently much debate over the importance of increased cellular ROS levels in regulation of life span (3–5), there is a consensus that oxidative stress contributes to the development of age-associated diseases. Previous studies in laboratory rodents provided ample evidence that oxidative stress develops with age in the arterial system, which impairs endothelial function and promotes vascular inflammation [for a recent review, see (6)]. Vascular oxidative stress and inflammation are thought to promote the development of atherosclerotic vascular diseases (including myocardial infarction, stroke, and vascular dementias), increasing cardiovascular mortality in elderly patients (2).
Recent studies demonstrate that in vascular endothelial and smooth muscle cells of young animals in response to increased production of ROS induced by proatherogenic conditions (ie, diabetes mellitus, cigarette smoke exposure) adaptive mechanisms are invoked that involve induction of NF-E2–related factor 2 (Nrf2)–driven antioxidant defense pathways (7–10). Nrf2 is a key redox-sensitive transcription factor, which regulates the antioxidant response, including induction of numerous genes for proteins that detoxify ROS and regulate synthesis of glutathione (GSH), as well as those with other antioxidant properties. In young organisms, this homeostatic response serves to attenuate vascular oxidative stress and limits the cellular and macromolecular damage caused by the increased free radical production induced by diabetic conditions (8,11,12) and other stressors (13,14). In the arterial system of aged rodents, ROS production both by mitochondria (15) and by plasma membrane–associated NADPH oxidases (16–18) is significantly increased. In cells of young animals, a similar level of ROS would result in an adaptive induction of Nrf2-driven free radical detoxification mechanisms. Despite the current advances in the understanding role of oxidative stress in vascular aging, the role of Nrf2-dependent antioxidant response in the aged vasculature has not been elucidated.
The present study was undertaken to test the hypothesis that aging is associated with dysregulation of Nrf2, and, as a result, in aged organisms, oxidative stress fails to activate Nrf2-regulated ROS detoxification systems in the vasculature. We chose to study aged Macaca mulatta because the nonhuman primate models have the advantage of being phylogenetically closest to humans but exhibiting few of the complicating effects of the cardiovascular diseases (eg, diabetes and hypertension) associated with aging (19,20). To test our hypotheses, we assessed aging-induced changes in markers of oxidative stress in carotid arteries of M mulatta and contrasted them with age-related changes in Nrf2 expression and activity and expression of Nrf2-driven antioxidant enzymes. Using cultured primary smooth muscle cells derived from young and aged M mulatta, we also determined whether aging impairs the ability of vascular smooth muscle cells (VSMCs) to mount an effective antioxidant response in response to oxidative stressors (H2O2 treatment and hyperglycemia) by inducing Nrf2-regulated ROS detoxification systems.
METHODS
Animal Models
All animal use protocols were approved by the Institutional Animal Care and Use Committees of the participating institutions. Four young (10.5 ± 0.9 years, mean relative age: 26% of maximum life span) and four old (22.2 ± 1.7 years; mean relative age: 55% of maximum life span) male rhesus monkeys (M mulatta) were maintained according to National Institutes of Health guidelines and humanely sacrificed according to methods previously described (21). Species longevity record for M mulatta in captivity is 40 years, according to the AnAge database (http://genomics.senescence.info/species/) compiled by de Magalhaes and coworkers (22).
Assessment of Markers of Cellular Oxidative Stress: 8-iso-PGF2α and 4-HNE
In order to assess the level of oxidative stress in the arteries of aged monkeys, we measured tissue levels of the isoprostane 8-iso-PGF2α, a biomarker of lipid peroxidation, using the 8-iso-Prostaglandin F2α Assay (Cell Biolabs, Inc., San Diego, CA) according to the manufacturer’s guideline. Sample protein concentration was used for normalization purposes.
We have also assessed tissue content of 4-hydroxy-2-nonenal (4-HNE; an oxidized secondary product that forms a relatively stable adduct with proteins), a useful biomarker of oxidative stress, by Western blotting using a primary antibody directed against 4-HNE (Abcam, ab46544).
Determination of Endogenous GSH and Ascorbate Using HPLC Electrochemical Detection
In aged rodents, vascular oxidative stress is associated with a significant decline in cellular redox-active GSH and ascorbate content (23). Thus, as an additional measure of vascular oxidative stress in the present study, we assessed concentrations of GSH and ascorbate in homogenates of M mulatta arteries using a Perkin-Elmer HPLC equipped with an eight-channel amperometric array detector as described (24,25). In brief, 10-mg aliquots of tissue samples were washed with ice-cold phosphate-buffered saline (PBS) and homogenized in 5% (w/v) metaphosphoric acid. Samples were centrifuged at 10,000g for 10 minutes to sediment protein, and the supernatant fraction was stored for analysis of redox-sensitive compounds. Precipitated proteins were dissolved in 0.1 N NaOH and stored for protein determination by a spectrophotometric quantitation method using BCA reagent (Pierce Chemical Co., Rockford, IL). Concentrations of GSH and ascorbic acid in supernatant fractions were determined by injecting 5-μL aliquots onto an ultrasphere 5 μ, 4.6 × 250 mm, C18 column and eluting with mobile phase of 50 mM NaH2PO4, 0.05 mM octane sulfonic acid, and 1.5% acetonitrile (pH 2.62) at a flow rate of 1 mL/min. The detectors were set at 200, 350, 400, 450, 500, 550, 600, and 700 mV, respectively. Peak areas were analyzed using ESA, Inc. software and concentrations of GSH and ascorbate are reported as nanomole per milligrams of protein.
Nuclear NF-κB–Binding Activity Assay
Nuclei were isolated from carotid arteries of young and aged M mulatta using the Nuclear Extraction kit from Active Motif (Carlsbad, CA) as reported (15,26). Using the nuclear extract obtained, nuclear factor-kappaB (NF-κB)–binding activity was assayed using the TransAM NF-κB ELISA Kit (Active Motif), as reported (15).
Immunofluorescent Labeling for Nrf2
To assess nuclear translocation of Nrf2, immunolabeling for Nrf2 was performed using paraffin-embedded sections of the carotid arteries isolated from young and aged monkeys. In brief, after deparaffinization, arterial sections were incubated in ice-cold acetone (10 minutes) followed by washes in Tween-PBS (10 minutes) and then in PBS (3 × 5 minutes). Triton (0.5%, 10 minutes) was used for permeabilization. The sections were blocked with 10% fetal calf serum in PBS for 1 hour and then immunolabeling was performed using a rabbit polyclonal antibody directed against Nrf2 (Abcam, ab31163; 1:50, overnight, at 4°C, in PBS containing 1% bovine serum albumin). Thereafter, slides were washed with PBS (3 × 10 minutes) before adding the secondary antibody (Alexa Fluor 688 goat anti-rabbit IgG; for 1 hour, at room temperature). After washing with PBS (3 × 5 minutes), Hoechst 33342 (5 μg/mL) was added for 5 minutes. The sections were visualized by confocal laser scanning microscopy (Leica SP2 MP). Cells treated with the canonical Nrf2 activator sulforaphane (2 μmol/L) were also immunolabelled using the aforementioned protocol and were used as positive controls (data not shown).
Nuclear Nrf2–Binding Activity Assay
Using nuclear extracts obtained from carotid arteries of young and aged M mulatta, Nrf2-binding activity was assayed using the TransAM Nrf2 ELISA Kit (Active Motif) according to the manufacturer's guidelines.
Quantitative Real-Time Reverse Transcription–Polymerase Chain Reaction
A quantitative real-time reverse transcription–polymerase chain reaction technique was used to analyze messenger RNA (mRNA) expression of the NF-κB target genes; inducible nitric oxide synthase (iNOS), intercellular adhesion molecule 1 (ICAM-1), and interleukin-6; and the Nrf2/ARE target genes, NAD(P)H:quinone oxidoreductase 1 (Nqo1), heme oxygenase-1 (Hmox1), and gamma-glutamylcysteine synthetase (Gclc) in carotid arteries of M mulatta as well as in vascular smooth muscle samples, using a Strategen MX3000, as previously reported (15,16,18,27). In brief, total RNA was isolated with a Mini RNA Isolation Kit (Zymo Research, Orange, CA) and was reverse transcribed using Superscript III RT (Invitrogen, Carlsbad, CA) as described previously (16,28). Amplification efficiencies were determined using a dilution series of a standard vascular sample. Quantification was performed using the efficiency-corrected ΔΔCq method. The relative quantities of the reference genes Hprt and Actb were determined, and a normalization factor was calculated based on the geometric mean for internal normalization. Fidelity of the polymerase chain reactions was determined by melting temperature analysis and visualization of the product on a 2% agarose gel.
Western Blotting
To analyze protein expression of Nrf2 and the Nrf2 targets NQO1 and GCLC, Western blotting was performed as described (29), using the following primary antibodies: rabbit anti-Nrf2 (Abcam, ab31163; 1:1,000 5% milk), rabbit anti-GCLC (Abcam, ab41463; 1 μg/mL in 5% milk), and rabbit anti-NQO1 (Abcam, ab34173; 1:2,000 in 5% milk). All PVDF membranes were incubated in primary antibodies overnight at 4°C. A donkey anti-rabbit secondary antibody was used (Abcam, ab16284; 1:2,000 in 5% milk). Mouse anti-β-actin (Abcam, ab6276; 1:10,000 in 5% milk) and Coomassie staining were used for normalization purposes.
Cell Cultures, Keap-1 Overexpression
Early passage (Passages 4–5) arterial VSMCs; derived from young and aged M mulatta were used. VSMCs were isolated, as previously described (30). VSMCs were cultured in Smooth Muscle Cell Growth Medium (Cell Applications Inc.). In some experiments, VSMCs were treated with high glucose (30 mmol/L) or H2O2 (from 10−6 to 10−4 mol/L). Mannitol was used as osmotic control for the high-glucose experiments. To disrupt Nrf2 signaling, Keap-1 overexpression was achieved in VSMCs by transfection with a Keap-1 full-length cDNA-encoding plasmid (Origen) as described (12,31).
Measurement of Mitochondrial O2· − and H2O2 Production in Cultured VSMCs
Mitochondrial O2· − production in VSMCs from young and aged monkeys was measure by flow cytometry (Guava, Hayward, CA) using MitoSOX Red (Invitrogen), a mitochondrion-specific hydroethidine-derivative fluorescent dye, as previously reported (32). Cell debris (low forward and side scatter) and dead cells (Sytox Green) were gated out for analysis (32). As a positive control, mitochondrial ROS production was increased to maximum levels in VSMCs by coadministration of antimycin A (AA; 10−6 mol/L, which inhibits Complex III by binding to the UQI site, blocking electron transfer from haem bH to ubiquinone) (33,34) plus succinate (10 mmol/L, a substrate of Complex II) (15).
In other experiments, the cell-permeant oxidative fluorescent indicator dye 5,6-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate-acetyl ester (CM-H2DCFDA; Invitrogen) was used to assess H2O2 production in VSMCs from young and aged monkeys. To assess the contribution of mitochondrial sources to cellular peroxide production, CM-H2DCFDA fluorescence was measured in the presence and absence of the mitochondrial uncoupler carbonylcyanide-p-trifluoromethoxyphenylhydrazone (FCCP; 1 μmol/L), which effectively inhibits mitochondrial ROS generation (15).
Furthermore, cellular H2O2 production was also assessed in VSMCs from young and aged monkeys cultured in the presence and absence of high glucose (30 mmol/L, for 24 hours). For these experiments, mannitol was used as osmolarity control.
Transient Transfection, NF-κB, and Nrf2 Reporter Gene Assays
Transcriptional activity of NF-κB was tested in VSMCs derived from young and old M mulatta by a reporter gene assay as described (28). We used a NF-κB reporter comprised an NF-κB response element upstream of firefly luciferase (NF-κB-Luc; Stratagene) and a renilla luciferase plasmid under the control of the CMV promoter. The role of oxidative stress in NF-κB activation was tested by treating VSMCs with PEG-catalase (200 U/mL, for 24 hours).
The effect of treatment with H2O2 (from 10−6 to 10−4 mol/L) on transcriptional activity of Nrf2 was tested in VSMCs derived from young and old M mulatta by a reporter gene assay, as described (10,12). We used an antioxidant response element (ARE) reporter composed of tandem repeats of the ARE transcriptional response element upstream of firefly luciferase (SA Biosciences, Frederick, MD) and a renilla luciferase plasmid under the control of the CMV promoter (as an internal control). All transfections in VSMCs were performed using the Amaxa Nucleofector technology (Amaxa, Gaithersburg, MD), as we have previously reported (26,35,36). Firefly and renilla luciferase activities were assessed after 24 hours using the Dual Luciferase Reporter Assay Kit (Promega) and a Tecan Infinite M200 plate reader.
Data Analysis
Data were normalized to the respective control mean values and are expressed as means ± SEM. Statistical analyses of data were performed by Student’s t test or by two-way analysis of variance followed by the Tukey’s post hoc test, as appropriate. The p < .05 was considered statistically significant.
RESULTS
Age-Associated Vascular Oxidative Stress and Inflammation in Macaca mulatta
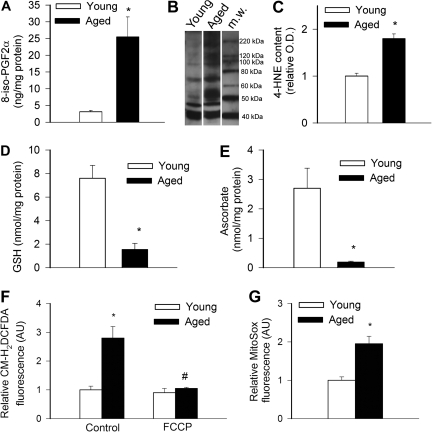
8-Isoprostane content in the arterial wall was increased in aged M mulatta as compared with young vessels (Figure 1A). 4-HNE content was significantly increased compared with that of young animals (Figure 1B and C). Consistent with the presence of aging-induced oxidative stress, vascular GSH content, and ascorbate concentrations were significantly reduced in aged M mulatta (Figure 1D and E).
Figure 1.
(A) 8-iso-PGF2α content in carotid arteries of young and aged Macaca mulatta. Data are mean ± SEM (n = 4 for each group), *p < .05. (B) Representative Western blot showing immunolabeling for 4-HNE in samples of carotid arteries of young and aged M mulatta (m.w., molecular weight markers). Equal protein loading was confirmed using Coomassie staining. Bar graphs (C) are summary densitometric data, *p <.05. (D–E) GSH (D) and ascorbate (E) content, determined using HPLC electrochemical detection, in carotid arteries of young and aged M mulatta, *p < .05. Data are mean ± SEM (n = 4 for each group). (E) H2DCFDA (F) and MitoSox (G) fluorescence intensities, representing cellular peroxide and mitochondrial O2· − production, in cultured vascular smooth muscle cells derived from young and aged M mulatta (flow cytometry data). The effect of treatment with the mitochondrial uncoupler FCCP on H2DCFDA fluorescence is also shown, *p < .05 versus young, #p < .05 versus untreated. Data are mean ± SEM (n = 4–5).
In VSMCs derived from aged M mulatta, H2DCFDA (Figure 1F) and MitoSox (Figure 1G) fluorescence, indicating cellular peroxide production and mitochondrial O2· − generation, respectively, were significantly increased compared with those in cells from young animals. Treatment with FCCP significantly decreased H2DCFDA fluorescence in aged VSMCs (Figure 1F), suggesting that mitochondrial sources contribute significantly to age-related oxidative stress in these cells.
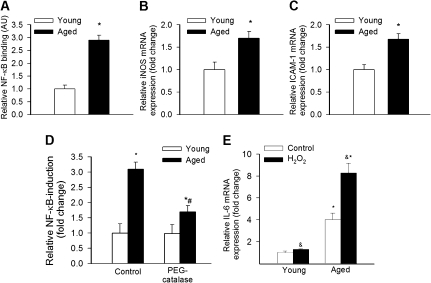
Previous studies demonstrated that age-associated oxidative stress in laboratory rodents promotes vascular inflammation by activating the redox-sensitive transcription factor NF-κB (15). We found that in carotid arteries of M mulatta, aging-induced oxidative stress is also associated with an increased nuclear NF-κB–binding activity (Figure 2A) and upregulation of the NF-κB target genes iNOS (Figure 2B) and iCAM-1 (Figure 2C).
Figure 2.
NF-κB–binding activity in nuclear extracts from carotid arteries of young and aged Macaca mulatta. Data are mean ± SEM (n = 4 in each group), *p < .05 vs young. (B–C) Expression of iNOS (B) and ICAM-1 (C) mRNA in carotid arteries of young and aged M mulatta. Data are mean ± SEM (n = 4 in each group; *p < .05). (D) Reporter gene assay showing that in VSMCs derived from aged M mulatta transcriptional activity of NF-κB is significantly increased (*p < .05 vs young VSMCs). Data are mean ± SEM (n = 4–5). (E) Expression of interleukin-6 mRNA in cultured vascular smooth muscle cells derived from young and aged M mulatta. The effects of treatment with H2O2 (10 μmol/L, for 24 hours) are also shown (*p < .05 vs young VSMCs and &p < .05 vs untreated).
In VSMCs derived from aged M mulatta, transcriptional activity of NF-κB was also significantly increased (Figure 2D), and this was partially inhibited by treatment with PEG-catalase. In VSMCs from young M mulatta, treatment with H2O2 upregulated expression of interleukin-6 (Figure 2E). Interestingly, in VSMCs of aged M mulatta, H2O2 elicited significantly greater increases in interleukin-6 expression than in those of young M mulatta (Figure 2E).
Age-Associated Oxidative Stress Is Not Associated With Adaptive Increases in Nrf2 Activation in Arteries of Macaca mulatta
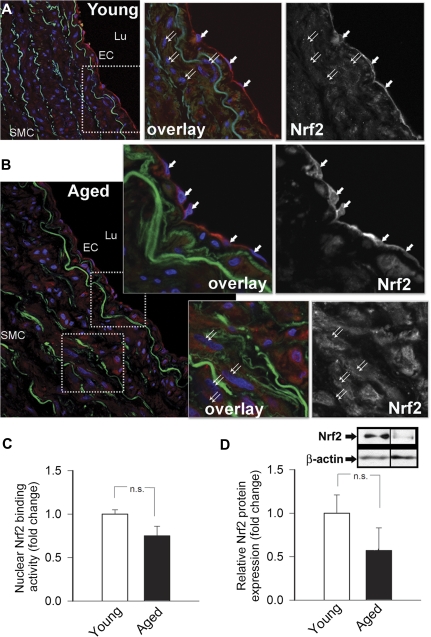
Upon ROS-induced activation in young animals, Nrf2 translocates to the nucleus, where it binds to the ARE to activate transcription of Phase II and antioxidant defense enzymes. Thus, to determine whether age-related oxidative stress is associated with Nrf2 activation, we tested nuclear translocation of Nrf2 and expression of known Nrf2 target genes. As shown in Figure 3A and B, predominantly cytoplasmic labeling of Nrf2 with no significant nuclear staining was observed in arteries of aged M mulatta, and this staining pattern did not differ from Nrf2 staining in arteries of young macaques. Nuclear Nrf2–binding activity (Figure 3C) and protein expression of Nrf2 (Figure 3D) tended to decrease in arteries of aged M mulatta; however, the differences did not reach statistical significance.
Figure 3.
Representative confocal images showing immunofluorescent labeling for Nrf2 (red) in sections of carotid arteries of young (A, left) and aged (B, left) Macaca mulatta. Green autofluorescence of elastic laminae is shown for orientation purposes, Hoechst 33342 (blue) was used for nuclear staining (original magnification: 20×, SMC, smooth muscle cells; EC, endothelial cells; Lu, lumen). Insets: arrows and double arrows point to the nuclei of endothelial cells and smooth muscle cells, respectively (middle panels: overlay images and right panels: respective monochrome images showing immunolabeling for Nrf2. Note cytoplasmic localization of Nrf2 in each image.). (C) Nuclear Nrf2–binding activity in carotid arteries of young and aged M mulatta. Data are mean ± SEM (n = 4 in each group). (D) Protein expression of Nrf2 in carotid arteries of young and aged M mulatta (Western blotting, β-actin was used for normalization purposes). Bar graphs are summary densitometric values. Data are mean ± SEM (n = 4 in each group).
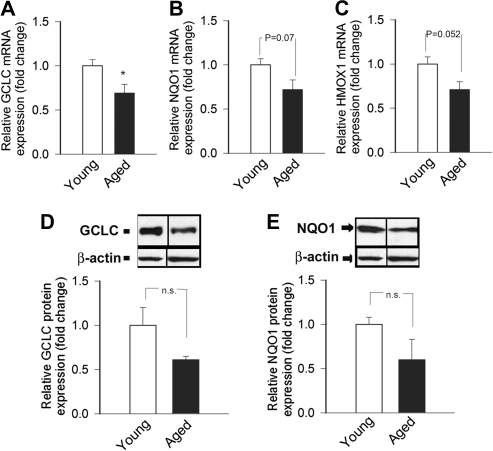
A quantitative real-time reverse transcription–polymerase chain reaction technique and Western blotting was used to analyze mRNA and protein expression of known Nrf2 targets in M mulatta arteries. We found that expression of GCLC (Figure 4A and D), NQO1 (Figure 4B and E), and heme oxygenase-1 (Figure 4C) are not upregulated in arteries of aged M mulatta, despite the presence of significant oxidative stress in these vessels (Figure 1).
Figure 4.
Expression of GCLC (A), NQO1 (B), and HMOX1 (C) mRNA in carotid arteries of young and aged Macaca mulatta. Data are mean ± SEM (n = 4 in each group; *p < .05). (D and E) Protein expression of GCLC (D) and NQO1 (E) in carotid arteries of young and aged M mulatta (Western blotting, β-actin was used for normalization purposes). Bar graphs are summary densitometric values. Data are mean ± SEM (n = 4 in each group).
Oxidative Stress Elicits a Blunted Nrf2-Driven Antioxidant Response in VSMCs From Aged Macaca mulatta
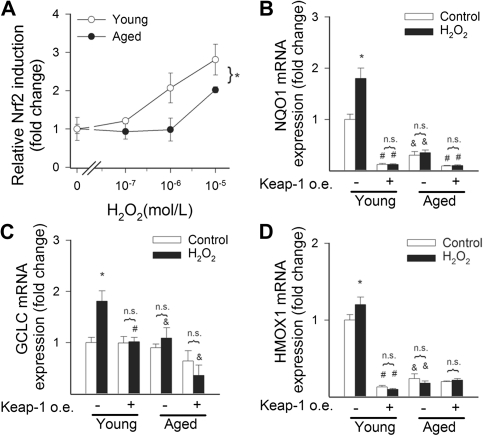
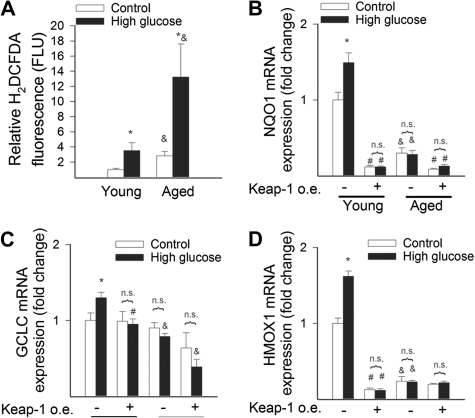
To determine whether age-associated Nrf2 dysregulation impairs the ability of vascular cells to mount an effective antioxidant response to oxidative stressors, we treated VSMCs from young and aged M mulatta with H2O2 (10−7 to 10−5 mol/L) and high glucose. We found that H2O2 significantly increased transcriptional activity of Nrf2 in VSMCs of young M mulatta, whereas H2O2–induced Nrf2 activation was significantly attenuated in VSMCs derived from aged monkeys (Figure 5A). H2O2 and high glucose elicited substantial upregulation of the Nrf2 target genes NQO1, GCLC, and HMOX1 in young VSMCs (Figures 5B–D and 6B–D, respectively). In contrast, H2O2– and hyperglycemia-induced changes in mRNA expression of GCLC, NQO1, and HMOX1 were blunted in VSMCs derived from aged monkeys (Figures 4B–D and 5B–D, respectively). In young VSMCs, overexpression of Keap-1 significantly downregulated basal expression levels of NQO1, GCLC, and HMOX1 and prevented H2O2– and hyperglycemia-induced changes in Nrf2 target genes, mimicking the aging phenotype (Figures 5B–D and 6B–D, respectively). In aged VSMCs, overexpression of Keap-1 did not elicit marked changes in the expression of Nrf2-driven genes. Lack of induction of Nrf2 target genes in response to model hyperglycemia was associated with significantly greater increases in cellular H2DCFDA fluorescence in VSMCs-derived aged M mulatta as compared with young VSMCs (Figure 6A).
Figure 5.
(A) Reporter gene assay showing that in VSMCs derived from aged Macaca mulatta H2O2–induced transcriptional activity of Nrf2 is significantly attenuated as compared with VSMCs derived from young monkeys. Data are mean ± SEM (n = 4–5 for each data point), *p < .05 versus young VSMCs. (B) Expression of NQO1, GCLC (C), and heme oxygenase-1 (D) mRNA in VSMCs derived from young and aged M mulatta. The effects of H2O2 (10 μmol/L, for 24 hours) and overexpression of Keap-1 are also shown. Data are mean ± SEM (n = 4–5 in each group; *p < .05).
Figure 6.
(A) In VSMCs from aged Macaca mulatta, high-glucose treatment (30 mmol/L, for 24 hours) results in significantly higher peroxide levels as compared with VSMCs derived from young monkeys. Cellular peroxide levels were measured by flow cytometry using the redox-sensitive fluorescent dye CM-H2DCFDA. Data are mean ± SEM (n = 4–5 in each group; *p < .05 vs untreated and &p < .05 vs young VSMCs). (B) Expression of NQO1, GCLC (C), and heme oxygenase-1 (D) mRNA in VSMCs derived from young and aged M mulatta. The effects of high glucose (30 mmol/L, for 24 hours) and overexpression of Keap-1 are also shown. Data are mean ± SEM (n = 4–5 in each group; *p < .05 vs untreated, #p < .05 vs no Keap-1 overexpression, and & p < .05 vs young VSMCs).
DISCUSSION
Results from the present study demonstrate for the first time an age-dependent increase in carotid arteries of M mulatta in oxidative stress, as indicated by the increased levels of several independent markers of cellular oxidative stress (Figure 1A–E). These results extend those of previous studies in rodents (16,18,37). Our findings that increased peroxide production in VSMCs derived from aged M mulatta was attenuated by a mitochondrial uncoupler (Figure 1F), and the demonstration of increased mitochondrial O2· − in aged VSMCs (Figure 1G) suggest that mitochondria importantly contribute to the age-related increase in oxidative stress in carotid arteries of M mulatta. This conclusion is in accord with the results of previous studies showing increased mitochondrial oxidative stress in arteries of aged rodents (15,27). Additional mechanisms of increased ROS production with advancing age also include activation of NADPH oxidases (16,18,38). From a pathophysiological standpoint, an increasing vascular oxidative stress with increasing age is expected to promote vascular inflammation and endothelial dysfunction contributing to the development of vascular diseases. Accordingly, age-associated oxidative stress is linked to increased activity of the redox-sensitive transcription factor NF-κB and upregulation of NF-κB–driven proinflammatory gene expression both in arteries and in VSMCs derived from aged M mulatta (Figure 2), extending previous findings in vessels of aged rats (15) and mice (17,39). Aging is also associated with generalized endothelial dysfunction in primate models (19) and elderly humans (38), similar to the findings in laboratory rodents (16,18,40).
There is increasing evidence to suggests that stress-activated “cap’n’collar” transcription factors, including Nrf2, play an important role in regulating the aging process by orchestrating the transcriptional response of cells to oxidative stress (41–44). Regulation of the expression of antioxidant enzymes by homologues of Nrf2 is evolutionarily highly conserved, and studies on Caenorhabditis elegans demonstrate that knockdown of SKN-1, the worm homolog of Nrf2, shortens life span (45). Previous studies also suggest that Nrf2 mediates the antiaging effects of caloric restriction (42). Recent studies have demonstrated that activation of Nrf2 and upregulation of its downstream target enzymes provide vascular protection in oxidative stress by conferring important antioxidative and anti-inflammatory effects (8,12,46–49). Here, we demonstrate, for the first time, that development of vascular oxidative stress in aged nonhuman primates is associated with a homeostatic failure due to dysregulation of Nrf2-mediated antioxidant responses (Figures 3 and 4). Our findings extend the results of recent studies showing that in the liver (50,51) and in the vasculature (52) of rodents, Nrf2-depedent cytoprotection against oxidative stress diminishes with aging. The available data also suggest an age-related decline in antioxidant enzymes in humans (53), although a detailed analysis of age-related changes in Nrf2 target gene expression in human vessels is yet to be conducted. We postulate that aging-induced Nrf2 dysfunction contributes to the age-related dysregulation of GSH synthesis in various tissues (54–56), which likely contributes to the observed age-related decline in vascular GSH content as well. The mechanisms underlying dysregulation of Nrf2-mediated antioxidant response in aging are likely multifaceted. Although our recent findings in F344×BN rats demonstrate that aging is associated with a downregulation of basal vascular Nrf2 expression both at the mRNA and protein levels (Ungvari and Csiszar, unpublished data, 2010), age-associated changes in Nrf2 expression in arteries of M mulatta are less pronounced (Figure 3D). In addition, aging may also impair the pathways that regulate Nrf2 activation and nuclear translocation. Accordingly, results from the present study demonstrate that exogenous administration of H2O2 elicits significant Nrf2 activation (Figure 5A) and upregulates Nrf2-depedenent genes (Figure 5B–D) in VSMCs of young M mulatta. In contrast, in cells of aged monkeys, H2O2 fails to increase transcriptional activity of Nrf2 and upregulates Nrf2-dependent free radical detoxification pathways.
There are multiple mechanisms through which dysregulation of Nrf2 may promote the development of cardiovascular diseases in aging. Adaptive activation of the Nrf2/ARE pathway in young animals plays a key role in vasoprotection in a diabetic context (10,12). Importantly, genetic lack of a functional Nrf2/ARE pathway results in significant increases in vascular ROS levels and a more severe endothelial functional impairment in arteries of young type 2 diabetic Nrf2−/− mice compared with vessels of young wild-type controls (10). Also, in isolated blood vessels and endothelial cells from young animals, high glucose elicits significant mitochondrial ROS production (57,58), which significantly increases the transcriptional activity of Nrf2 (8,11,12). Our present studies provide evidence that aging impairs the ability of primate VSMCs to mount an effective Nrf2-dependent antioxidant defense in response to hyperglycemia (Figure 6B–D), which results in more robust oxidative stress in VSMCs derived from aged monkeys than in those of young animals (Figure 6A). We posit that impaired ability of aged cells to mount an effective Nrf2/ARE-mediated antioxidant response (50,52) would render the vascular system vulnerable to the deleterious effects of metabolic disease. Because type 2 diabetes is a disease of aging (affecting almost one in five of people aged 65 years or more), future studies should investigate the interaction of aging and type 2 diabetes, with special emphasis on the role of Nrf2 dysfunction in exaggerated vascular complications observed in aged diabetics (59). Both aging (16) and diabetes mellitus (60) exacerbates the production of reactive nitrogen species in the cardiovascular system, thus subsequent studies should also elucidate whether Nrf2 dysregulation renders the aged vasculature more sensitive to the adverse effects of diabetes-related nitrosative stress as well (61).
In conclusion, our studies provide evidence that aging in a nonhuman primate impairs the ability of vascular cells to mount an effective Nrf2-dependent antioxidant defense in response to age-related increases in ROS production. Nrf2 may provide a therapeutic target for countering oxidative stress associated with aging and pathological conditions characterized by accelerated vascular aging. In that regard, it is significant that in vascular cells, Nrf2 can be activated pharmacologically by resveratrol (10), a polyphenolic compound with diverse antiaging properties (17,62–65). Importantly, resveratrol was shown to confer vasoprotection in rodent models of aging, upregulating antioxidant systems, decreasing oxidative stress, improving endothelial function, and attenuating vascular inflammation (17). Further studies are warranted to determine whether resveratrol and/or other activators of Nrf2 can confer similar vasoprotective effects in aged primates as well.
FUNDING
This work was supported by grants from the American Diabetes Association (to Z. Ungvari), American Federation for Aging Research (to A. Csiszar), the University of Oklahoma College of Medicine Alumni Association (to A. Csiszar), the National Institutes of Health (AG031085 to A. Csiszar; AT006526 and HL077256 to Z. Ungvari; P01 AG11370 to W. E. Sonntag), and the Intramural Research Program of the National Institute on Aging (M. Wang, E. Lakatta, R. de Cabo).
References
- 1.Gurwitz JH, Goldberg RJ, Gore JM. Coronary thrombolysis for the elderly? JAMA. 1991;265:1720–1723. [PubMed] [Google Scholar]
- 2.Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010;65:1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jang YC, Perez VI, Song W, et al. Overexpression of Mn superoxide dismutase does not increase life span in mice. 2009;64(11):1114–1125. doi: 10.1093/gerona/glp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Ikeno Y, Qi W, et al. Mice deficient in both Mn superoxide dismutase and glutathione peroxidase-1 have increased oxidative damage and a greater incidence of pathology but no reduction in longevity. J Gerontol A Biol Sci Med Sci. 2009;64:1212–1220. doi: 10.1093/gerona/glp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ran Q, Liang H, Ikeno Y, et al. Reduction in glutathione peroxidase 4 increases life span through increased sensitivity to apoptosis. J Gerontol A Biol Sci Med Sci. 2007;62:932–942. doi: 10.1093/gerona/62.9.932. [DOI] [PubMed] [Google Scholar]
- 6.Csiszar A, Wang M, Lakatta EG, Ungvari ZI. Inflammation and endothelial dysfunction during aging: role of NF-{kappa}B. J Appl Physiol. 2008;105:1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoh K, Hirayama A, Ishizaki K, et al. Hyperglycemia induces oxidative and nitrosative stress and increases renal functional impairment in Nrf2-deficient mice. Genes Cells. 2008;13:1159–1170. doi: 10.1111/j.1365-2443.2008.01234.x. [DOI] [PubMed] [Google Scholar]
- 8.Xue M, Qian Q, Antonysunil A, Rabbani N, Babaei-Jadidi R, Thornalley PJ. Activation of NF-E2-related factor-2 reverses biochemical dysfunction of endothelial cells induced by hyperglycemia linked to vascular disease. Diabetes. 2008;57(10):2809–2817. doi: 10.2337/db06-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res. 2008;102:519–528. doi: 10.1161/CIRCRESAHA.107.168369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ungvari Z, Bagi Z, Feher A, et al. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–H24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He X, Kan H, Cai L, Ma Q. Nrf2 is critical in defense against high glucose-induced oxidative damage in cardiomyocytes. J Mol Cell Cardiol. 2009;46:47–58. doi: 10.1016/j.yjmcc.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Ungvari Z, Bailey-Downs L, Gautam T, et al. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol. 2011;300(4):H1133–H1140. doi: 10.1152/ajpheart.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warabi E, Takabe W, Minami T, et al. Shear stress stabilizes NF-E2-related factor 2 and induces antioxidant genes in endothelial cells: role of reactive oxygen/nitrogen species. Free Radic Biol Med. 2007;42:260–269. doi: 10.1016/j.freeradbiomed.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 14.Afonyushkin T, Oskolkova OV, Philippova M, et al. Oxidized phospholipids regulate expression of ATF4 and VEGF in endothelial cells via NRF2-dependent mechanism: novel point of convergence between electrophilic and unfolded protein stress pathways. Arterioscler Thromb Vasc Biol. 2010;30:1007–1013. doi: 10.1161/ATVBAHA.110.204354. [DOI] [PubMed] [Google Scholar]
- 15.Ungvari Z, Orosz Z, Labinskyy N, et al. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 16.Csiszar A, Ungvari Z, Edwards JG, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 17.Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-TNFalfa treatment in aging. Am J Pathol. 2007;170:388–698. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asai K, Kudej RK, Shen YT, et al. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler Thromb Vasc Biol. 2000;20:1493–1499. doi: 10.1161/01.atv.20.6.1493. [DOI] [PubMed] [Google Scholar]
- 20.Shi Q, Aida K, Vandeberg JL, Wang XL. Passage-dependent changes in baboon endothelial cells—relevance to in vitro aging. DNA Cell Biol. 2004;23:502–509. doi: 10.1089/1044549041562294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaitkevicius PV, Lane M, Spurgeon H, et al. A cross-link breaker has sustained effects on arterial and ventricular properties in older rhesus monkeys. Proc Natl Acad Sci U S A. 2001;98:1171–1175. doi: 10.1073/pnas.98.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Magalhaes JP, Costa J, Church GM. An analysis of the relationship between metabolism, developmental schedules, and longevity using phylogenetic independent contrasts. J Gerontol A Biol Sci Med Sci. 2007;62:149–160. doi: 10.1093/gerona/62.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Addabbo F, Ratliff B, Park HC, et al. The Krebs cycle and mitochondrial mass are early victims of endothelial dysfunction: proteomic approach. Am J Pathol. 2009;174:34–43. doi: 10.2353/ajpath.2009.080650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Csiszar A, Labinskyy N, Jimenez R, et al. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ungvari Z, Gautam T, Koncz P, et al. Vasoprotective effects of life span-extending peripubertal GH replacement in Lewis dwarf rats. J Gerontol A Biol Sci Med Sci. 2010;65:1145–1156. doi: 10.1093/gerona/glq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Csiszar A, Smith KE, Koller A, Kaley G, Edwards JG, Ungvari Z. Regulation of bone morphogenetic protein-2 expression in endothelial cells: role of nuclear factor-kappaB activation by tumor necrosis factor-alpha, H2O2, and high intravascular pressure. Circulation. 2005;111:2364–2372. doi: 10.1161/01.CIR.0000164201.40634.1D. [DOI] [PubMed] [Google Scholar]
- 27.Ungvari ZI, Labinskyy N, Gupte SA, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol. 2008;294:H2121–H2128. doi: 10.1152/ajpheart.00012.2008. [DOI] [PubMed] [Google Scholar]
- 28.Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-{alpha}-induced activation of coronary arterial endothelial cells: role of NF-{kappa}B inhibition. Am J Physiol. 2006;291:H1694–H1699. doi: 10.1152/ajpheart.00340.2006. [DOI] [PubMed] [Google Scholar]
- 29.Csiszar A, Labinskyy N, Zhao X, et al. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell. 2007;6:783–797. doi: 10.1111/j.1474-9726.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- 30.Krug AW, Allenhofer L, Monticone R, et al. Elevated mineralocorticoid receptor activity in aged rat vascular smooth muscle cells promotes a proinflammatory phenotype via extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase and epidermal growth factor receptor-dependent pathways. Hypertension. 2010;55:1476–1483. doi: 10.1161/HYPERTENSIONAHA.109.148783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Csiszar A, Labinskyy N, Podlutsky A, et al. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008;294:H2721–H2735. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Csiszar A, Labinskyy N, Perez V, et al. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol. 2008;295:H1882–H1894. doi: 10.1152/ajpheart.412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 34.Lambert AJ, Brand MD. Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH:ubiquinone oxidoreductase (complex I) J Biol Chem. 2004;279:39414–39420. doi: 10.1074/jbc.M406576200. [DOI] [PubMed] [Google Scholar]
- 35.Csiszar A, Ahmad M, Smith KE, et al. Bone morphogenetic protein-2 induces proinflammatory endothelial phenotype. Am J Pathol. 2006;168:629–638. doi: 10.2353/ajpath.2006.050284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;17:21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- 37.Csiszar A, Labinskyy N, Orosz Z, Xiangmin Z, Buffenstein R, Ungvari Z. Vascular aging in the longest-living rodent, the naked mole rat. Am J Physiol. 2007;293:H919–H927. doi: 10.1152/ajpheart.01287.2006. [DOI] [PubMed] [Google Scholar]
- 38.Donato AJ, Eskurza I, Silver AE, et al. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 39.Lesniewski LA, Durrant JR, Connell ML, Folian BJ, Donato AJ, Seals DR. Salicylate treatment improves age-associated vascular endothelial dysfunction: potential role of nuclear factor {kappa}B and forkhead box O phosphorylation. J Gerontol A Biol Sci Med Sci. 2011;66:409–418. doi: 10.1093/gerona/glq233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lesniewski LA, Connell ML, Durrant JR, et al. B6D2F1 mice are a suitable model of oxidative stress-mediated impaired endothelium-dependent dilation with aging. J Gerontol A Biol Sci Med Sci. 2009;64:9–20. doi: 10.1093/gerona/gln049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jodar L, Mercken EM, Ariza J, et al. Genetic deletion of nrf2 promotes immortalization and decreases life span of murine embryonic fibroblasts. J Gerontol A Biol Sci Med Sci. 2011;66:247–256. doi: 10.1093/gerona/glq181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pearson KJ, Lewis KN, Price NL, et al. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci U S A. 2008;105:2325–2330. doi: 10.1073/pnas.0712162105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minor RK, Allard JS, Younts CM, Ward TM, de Cabo R. Dietary interventions to extend life span and health span based on calorie restriction. J Gerontol A Biol Sci Med Sci. 2010;65:695–703. doi: 10.1093/gerona/glq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin-Montalvo A, Villalba JM, Navas P, de Cabo R. NRF2, cancer and calorie restriction. Oncogene. 2011;30:505–520. doi: 10.1038/onc.2010.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jasper H. SKNy worms and long life. Cell. 2008;132:915–916. doi: 10.1016/j.cell.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Chen XL, Varner SE, Rao AS, et al. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel anti-inflammatory mechanism. J Biol Chem. 2003;278:703–711. doi: 10.1074/jbc.M203161200. [DOI] [PubMed] [Google Scholar]
- 47.Jyrkkanen HK, Kansanen E, Inkala M, et al. Nrf2 regulates antioxidant gene expression evoked by oxidized phospholipids in endothelial cells and murine arteries in vivo. Circ Res. 2008;103:e1–e9. doi: 10.1161/CIRCRESAHA.108.176883. [DOI] [PubMed] [Google Scholar]
- 48.Zakkar M, Van der Heiden K, Luong LA, et al. Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler Thromb Vasc Biol. 2009;29(11):1851–1857. doi: 10.1161/ATVBAHA.109.193375. [DOI] [PubMed] [Google Scholar]
- 49.Fledderus JO, Boon RA, Volger OL, et al. KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:1339–1346. doi: 10.1161/ATVBAHA.108.165811. [DOI] [PubMed] [Google Scholar]
- 50.Suh JH, Shenvi SV, Dixon BM, et al. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bloomer SA, Zhang HJ, Brown KE, Kregel KC. Differential regulation of hepatic heme oxygenase-1 protein with aging and heat stress. J Gerontol A Biol Sci Med Sci. 2009;64:419–425. doi: 10.1093/gerona/gln056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collins AR, Lyon CJ, Xia X, et al. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res. 2009;104:e42–e54. doi: 10.1161/CIRCRESAHA.108.188771. [DOI] [PubMed] [Google Scholar]
- 53.Espinoza SE, Guo H, Fedarko N, et al. Glutathione peroxidase enzyme activity in aging. J Gerontol A Biol Sci Med Sci. 2008;63:505–509. doi: 10.1093/gerona/63.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu J, Rong S, Xie B, et al. Procyanidins extracted from the lotus seedpod ameliorate age-related antioxidant deficit in aged rats. J Gerontol A Biol Sci Med Sci. 2010;65:236–241. doi: 10.1093/gerona/glp211. [DOI] [PubMed] [Google Scholar]
- 55.Chen CN, Brown-Borg HM, Rakoczy SG, Ferrington DA, Thompson LV. Aging impairs the expression of the catalytic subunit of glutamate cysteine ligase in soleus muscle under stress. J Gerontol A Biol Sci Med Sci. 2010;65:129–137. doi: 10.1093/gerona/glp194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen CN, Brown-Borg HM, Rakoczy SG, Thompson LV. Muscle disuse: adaptation of antioxidant systems is age dependent. J Gerontol A Biol Sci Med Sci. 2008;63:461–466. doi: 10.1093/gerona/63.5.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ungvari Z, Labinskyy N, Mukhopadhyay P, et al. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–H1881. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mukhopadhyay P, Rajesh M, Hasko G, Hawkins BJ, Madesh M, Pacher P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat Protoc. 2007;2:2295–2301. doi: 10.1038/nprot.2007.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bruce DG, Davis WA, Casey GP, et al. Predictors of cognitive decline in older individuals with diabetes. Diabetes Care. 2008;31:2103–2107. doi: 10.2337/dc08-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szabo C, Zanchi A, Komjati K, et al. Poly(ADP-Ribose) polymerase is activated in subjects at risk of developing type 2 diabetes and is associated with impaired vascular reactivity. Circulation. 2002;106:2680–2686. doi: 10.1161/01.cir.0000038365.78031.9c. [DOI] [PubMed] [Google Scholar]
- 62.Ryan MJ, Jackson JR, Hao Y, et al. Suppression of oxidative stress by resveratrol after isometric contractions in gastrocnemius muscles of aged mice. J Gerontol A Biol Sci Med Sci. 2010;65:815–831. doi: 10.1093/gerona/glq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Labbe A, Garand C, Cogger VC, et al. Resveratrol improves insulin resistance hyperglycemia and hepatosteatosis but not hypertriglyceridemia, inflammation, and life span in a mouse model for Werner syndrome. J Gerontol A Biol Sci Med Sci. 2011;66:264–278. doi: 10.1093/gerona/glq184. [DOI] [PubMed] [Google Scholar]
- 64.Miller RA, Harrison DE, Astle CM, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giovannelli L, Pitozzi V, Jacomelli M, et al. Protective effects of resveratrol against senescence-associated changes in cultured human fibroblasts. J Gerontol A Biol Sci Med Sci. 2011;66:9–18. doi: 10.1093/gerona/glq161. [DOI] [PubMed] [Google Scholar]