Abstract
Thyroid hormones are essential regulators of growth, development and normal bodily function and their release is coordinated by the hypothalamic-pituitary-thyroid (HPT) axis. While the HPT axis has been established as an acutely stress-responsive neuroendocrine system, relatively little is known about the mechanisms of its stress-regulation. The present study examined acute stress-induced changes in peripheral hormone levels [triiodothyronine (T3); thyroxine (T4), thyroid-stimulating hormone (TSH), reverse triiodothyronine (rT3)] and central mRNA levels of regulators of the HPT axis [thyrotropin-releasing hormone (TRH), somatostatin (SST), type II deiodinase (D2)] in response to an inescapable tail-shock, a rodent model of stress. Additionally, we examined whether individual differences in spontaneous exploratory behavior in an open field test predicted basal levels of TH or differential susceptibility to the effects of stress. The stress condition was associated with decreases in peripheral T3, T4 and TSH, but not rT3, when compared with controls. No changes were observed in TRH or SST mRNA levels, but there was a trend suggesting stress-related increases in D2 mRNA. We also found that an animal's exploratory behavior in an unfamiliar open field arena was positively related to peripheral thyroid hormone levels and predicted the magnitude of stress-induced changes. In conclusion, we found suggestive evidence for stress-induced decrease in central drive HPT axis, but the central mechanisms of its stress-regulation remain to be elucidated. Additionally, we found that individual differences in animals' exploratory behavior were correlated with peripheral TH levels.
Thyroid hormones [TH; both 3,5,3′,5′-tetraiodothyronine, thyroxine (T4) and 3,5,3′-triiodothyronine (T3)] are classically considered to play an important role in growth, differentiation, and metabolism. Additionally, thyroid hormones have effects extending beyond development and maturation and are essential for normal bodily function in adults; in adults, thyroid hormones exert profound effects on metabolic regulation, including oxygen consumption and carbohydrate, lipid, and protein metabolism (Wrutniak-Cabello et al., 2001, Zoeller et al., 2007). Comparing endocrine systems across species, TH function as permissive hormones important for transitions and plasticity (Tata, 2006), including puberty (Mann and Plant, 2010), seasonal breeding (Ebling and Barrett, 2008), and metamorphosis in amphibians (Crespi and Denver, 2005). Moreover, the hypothalamic-pituitary-thyroid axis (HPT) is also a stress responsive system (Langer et al., 1983, Armario et al., 1984, Turakulov et al., 1994, Cizza et al., 1996, Kondo et al., 1997, Servatius et al., 2000, Helmreich et al., 2005, Helmreich et al., 2006, Kilburn-Watt et al., 2010).
In mammals, the HPT axis is controlled by neurons located within the parvocellular region of the paraventricular nucleus of the hypothalamus (PVN) that synthesize and release thyrotropin-releasing-hormone (TRH) into the median eminence. TRH stimulates the release of thyroid-stimulating hormone (TSH) from the anterior pituitary, and then TSH travels through the peripheral vasculature to stimulate thyroid hormone (TH) release (both T3 and T4) from the thyroid gland. T3 is considered the biologically active form of thyroid hormone because of its greater affinity for thyroid hormone receptors. T4 is converted to T3 by the activity of deiodinase enzymes located within most target tissues, including the central nervous system. Thyroid hormones exert negative feedback effects at the pituitary and hypothalamus to inhibit the release of TSH (Zoeller et al., 2007). In addition to TRH, other hypothalamic neuropeptides, such as somatostatin (SST), can modulate the release of TSH from the pituitary (Haugen, 2009). Furthermore, deiodinase enzymes, which can alter local or tissue specific levels of T4 and T3, have been shown to play a key role in thyroid-hormone signaling (Gereben et al., 2008). Specifically, deiodinase II (D2) activity in the hypothalamus is important for the set-point of feedback regulation (Fekete and Lechan, 2007) and the decrease in TRH mRNA observed after LPS administration (Sanchez et al., 2008).
We have previously demonstrated that physical stress (foot-shock) causes a decrease in T3 and T4 in adult rats (Helmreich et al., 2005, Helmreich et al., 2006), but overall, stress regulation of the HPT axis has not been completely characterized. Interestingly, the HPT axis is sensitive to stress intensity. Mild stressors may cause a slight increase or no change in peripheral thyroid hormone levels (Armario et al., 1984, Turakulov et al., 1994) while more severe stressors cause a decrease in thyroid hormone levels (Langer et al., 1983, Cizza et al., 1996, Kondo et al., 1997, Kilburn-Watt et al., 2010). Changes in TH caused by fasting and acute stress-induced decreases in thyroid hormone, such as those caused by immobilization and lipopolysaccharide administration appear to be, at least in part, centrally mediated, as evidenced by decreases in TRH mRNA levels within the PVN (Cizza et al., 1996, Kondo et al., 1997, Legradi et al., 1997). In the current studies, we sought to characterize stress-induced changes of the HPT axis at multiple levels within the HPT axis after inescapable tail-shock stress. Functionally, the short-term benefits and advantages of a stress-induced decrease in thyroid hormone levels may reflect a conservation of energy and resources in an unpredictable environment (Engel and Schmale, 1972). However, stress-induced hormonal changes that are initially adaptive can become inappropriate or excessive and may lead to stress-related pathologies and disease (McEwen and Wingfield, 2003, Davis and Tremont, 2007).
In a second set of experiments, we began preliminary studies to ask whether individual differences in animals' behavior may predict the animals' basal TH set-point and any susceptibility to stress-induced TH decrease. Humans differ in TH set-point and maintain this set-point across time (Andersen et al., 2002). Individual differences in TH response to traumatic stimuli have been demonstrated in rats, which may have consequences for successful recovery (Kilburn-Watt et al., 2010). Individual differences in HPA (hypothalamic-pituitary-adrenal) axis function, based on baseline locomotor behavior have been characterized and, interestingly, these differences in baseline behavior not only predict HPA axis responsiveness, but they also predict propensity for drug seeking behavior (Kabbaj et al., 2000). In the current experiments, we sought to determine if an animal's behavior in an open field test correlates with that animal's thyroid hormone set-point and stress-responsivity, employing a widely used out-bred strain of rat (Sprague-Dawley). The identification of individual differences within an experimental population may serve to decrease within experiment variability and also to inform experimental models to explore further links between behavior and endocrine systems (Whishaw and Kolb, 2005).
Materials and Methods
Experiment 1
Animals
Seventy-five adult male Sprague-Dawley rats (Charles River Laboratories), body weight 250–310 g at the beginning of the stress sessions were used for this study. All animals were housed two per tub in 40×18×20 cm tubs. Lights were on 0730 to 1930 h and food and water were available ad libitum. All animals were acclimated to the housing facility for at least 5 days before testing. Within each tub, each animal was randomly assigned to either the inescapable tail-shock stress group or control group.
Stress Paradigm
A single session of inescapable tail-shock was used for the experiments. Animals in the stress groups were subjected to 80 trials of 5 sec 1.0 mA tail-shock. Each tail-shock chamber (Med Associates, St. Albans, VT) measured 11.75 × 7.6 × 14.4 cm, with floors composed of 7 grid rods measuring 4.8 mm in diameter spaced 1.6 cm apart. Extending out the back of each chamber was a tail holder; white athletic tape was used to hold the tail in place. Each tail-shock chamber was housed within a sound attenuating chamber (24.13 cm × 41.9 cm × 43.8 cm), with house light and fan. The shock generated by a 24 V regulated power supply (Med Associates) was delivered to the animals' tail through the adhesive electrode tapes. Med-PC IV software was used to control the onset of the tail-shock stimulus. One session consisted of 80 trials; the inter-trial interval was a variable schedule ranging from 5 to 115 sec, with a mean duration of 60 sec. Control animals were placed within a tail-shock chamber, but did not receive tail-shock. The stress session lasted approximately 90 min; animals were returned to their home cage immediately at the end of the session. The animals in both groups were rapidly decapitated at 0, 120, 180 min or 24 hr post-stress initiation (n= 8–9 for each group); animals in the 0 min stress groups were removed directly from their home cage. Stress sessions were staggered so all decapitations occurred at 1300 h to minimize variations caused by diurnal rhythm (Kalsbeek et al., 2000). Brains were quickly removed and frozen; trunk blood was collected in untreated tubes; serum was separated and frozen for later assay.
Experiment 2
Animals were used as described in Experiment 1, except that animals were housed with others from same treatment condition. Additionally, in order to assess individual differences among animals, at 0900 h the day before the stress session, the animals' behavior was quantified in an open field arena. Individual subjects were placed in the center of the arena (81 × 81 cm) under dim lights (20 lux) and behavior was recorded for 10 min using a digital camcorder suspended above. Total distance traveled, time spent in the center of the arena and average velocity in the perimeter were analyzed using TopScan 2.0 Tracking Software (Clever Sys, Reston, VA) and manually inspected for accuracy. The amount of time spent rearing with both forepaws against the wall of the arena was scored manually by a condition-blinded observer using Annostar Software (Clever Sys, Reston, VA). On the same day as the open field test, a tail-nick sample was collected at 1245 h to assess basal T4 levels.
On the following day, the stress session was as described in Experiment 1, except that 100 trials of 1.0 mA tail-shock were utilized in an attempt to elicit less ambiguous between-group differences in peripheral TH levels. Animals were decapitated at 1300 h, either at 0 or 180 min post-stress initiation. The 180 min time point was chosen based on the results of Experiment 1, in order to reduce animal use. Therefore, both groups experienced the open-field test and tail-nicking on the previous day. On the experimental day the 180 min control animals were placed into the stress chamber, but received no tail-shocks; the 0 min animals were not handled before sacrifice, but were housed next to animals that were removed and transported to the experimental room.
The University Committee on Animal Resources of the University of Rochester Medical Center approved all experimental protocols and all animals were treated in accordance with the NIH Guide for Care and Use of Laboratory Animals.
Radioimmunoassays
Peripheral levels of Total T3 and Total T4 were determined using radioimmunoassays kits from MP Biomedicals/Pharmaceuticals (Solon, OH; formerly ICN) using the included protocols (Servatius et al., 2001, Helmreich et al., 2005). Peripheral TSH levels and rT3 were determined via radioimmunoassay kits from ALPCO Diagnostics (Salem, NH), again using included protocols. The rat TSH kit had <0.1 % cross-reactivity for rat GH, rat PRL or rat FSH; the antibody had 5.1 % cross-reactivity with rat LH. The rT3 RIA had <0.01 % cross-reactivity with T3 or T4, and 1.8 % cross-reactivity with 3,3' diiodo-L-thyronine.
In-situ Hybridization
PVN TRH mRNA, periventricular nucleus (PeVN) SST mRNA and medio-basal hypothalamus D2 mRNA levels were determined via in-situ hybridization. Brains were sectioned at 12 μm in the coronal plane throughout the PVN. Four sections per slide were collected onto Super-frost plus slides (Fisher Scientific, Pittsburgh, PA) and stored at −80°C until further processing. In-situ hybridization was conducted using techniques previously described (Schafer et al., 1993). Briefly, sections were removed from the freezer and placed into cold 4% paraformaldehyde for 60 min. Following washes in 2× SSC, sections were placed in 0.1 M triethanolamine for 15 min, to which 0.25 % acetic anhydride had been added. Sections were thoroughly washed with 2× SSC, and then dehydrated through a series of graded alcohol.
The 35S-labeled cRNA probes for TRH, SST and D2 mRNA were generated using standard in-vitro transcription methodology (Riboscribe, Epicenter). The Functional Genomics Center at the University of Rochester Medical Center constructed all DNA templates used for riboprobe synthesis. The rat proTRH cDNA clone, generously provided by Dr. Ron Lechan, Tufts University, yielded a 1241 nt probe. The rat SST cDNA clone, based on (Rogers et al., 1987), yielded a 391 nt probe. The D2 probe was designed based on NCBI NM_031720 (Sanchez et al., 2008), yielding a 355 nt probe. Labeled probe was separated from unincorporated nucleotides using a Micro Bio-Spin 6 column (Bio-Rad, Hercules CA). The probe was diluted in 50 % formamide hybridization buffer (Amresco, Solon, OH) to yield approximately 2.5 × 106 dpm per 70 μl of buffer. Diluted probe (70μl) was applied to each slide and sections were coverslipped. Slides were placed in sealed plastic boxes lined with filter paper moistened with 50% formamide. These boxes were wrapped with plastic wrap and incubated at 55°C for 16 h. Coverslips were removed and slides were washed in 2× SSC. Slides were then incubated in RNase A (200 μg/ml) at 37°C for 60 min, washed successively in decreasing concentrations of SSC (2×, 1×, 0.5×, 0.1×) and then washed in 0.1× SSC at 65°C for 60 min. Slides were subsequently washed in distilled water and dehydrated through graded alcohol. Slides were then exposed to BioMax MR film (Eastman Kodak, Rochester, NY).
Data Analysis
For in-situ hybridization, relative optical density was determined for the area of interest from the x-ray film; positive signal was considered to be optical density levels above a threshold level that was 3.5 SD above the mean optical density of the background signal. Images from the x-ray film were captured using a Panasonic CCTV camera and Image J software (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/, 1997–2009). Integrated optical density, which incorporates both the intensity and area of positive signal values, was quantified. For TRH measurements, only signal within the PVN was measured, avoiding positive signal within the lateral hypothalamus. SST mRNA was quantified in PeVN (Merchenthaler et al., 1989). For D2, positive signal along the ventral surface of the medial-basal hypothalamus and lining the 3rd ventricle was quantified. For all probes, the 3 sections with the highest levels of expression were averaged together to determine strength of signal in each animal.
Statistical Analysis
All statistical analyses were conducting using Graphpad Prism 5.0 Software. For Experiment 1, separate two-way analyses of variance (ANOVAs) were used to examine the effects of the stress manipulation and time points on levels of plasma TSH, T4, T3, and reverse T3. Subsequent post-hoc tests were conducted using the Bonferroni adjustment for multiple comparisons. Also in Experiment 1, levels of integrated optical density at various time points were compared using a one-way ANOVA, in order to assess the effects of stress on TRH, SST and D2 mRNA. Additionally, the Kruskal-Wallis one-way ANOVA was used to compare groups with significantly different variances in integrated optical densities, which were identified using Bartlett's test for homogeneity of variances.
For Experiment 2, separate one-way ANOVAs were used to compare 0 min, 180 min control and 180 min stress groups in terms of peripheral TSH, T3, T4 and delta T4 (ΔT4; final T4-basal T4). Post-hoc tests were conducted using the Bonferroni adjustment for multiple comparisons. Additionally, separate paired samples T-Tests were used to compare baseline and final T4 within each experimental group.
A correlation matrix was used to examine inter-relationships among behavioral measures, revealing a strong positive relationship (r = .51, p ≤ .02) between total distance traveled and time spent rearing. In contrast, time spent in the center of the arena was not significantly related to other measures. Because locomotion and exploratory rearing appeared to be dominant features of behavior in the open field test, we sought to capture the potentially meaningful covariance between these measures by creating a composite score. For each animal, standardized z-scores were calculated for total distance traveled and time spent rearing and were subsequently averaged, resulting in a standardized exploration score. Bivariate relationships between peripheral hormone levels and standardized exploration were assessed with Pearson correlations using two-tailed tests of significance. For all analyses, p-values ≤ .05 were considered statistically significant.
Results
Experiment 1
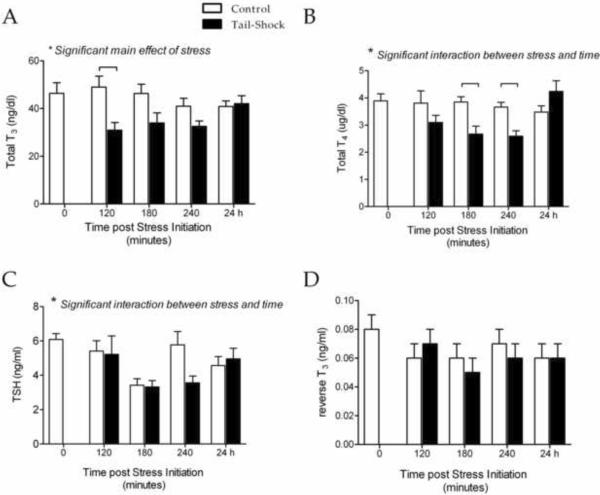
As shown in Figure 1, we found that 80 trials of inescapable tail-shock stress caused a decrease in peripheral TH levels. Specifically for T3, a two-way ANOVA revealed a significant main effect for stress (F(1, 66) = 8.75, p < .01). Bonferroni-corrected post-hoc tests indicated that this decrease was significant at 120 min post-stress initiation (Figure 1A). Stress also caused a significant decrease in circulating levels of T4, indicated by a significant stress × time interaction (F(4, 66) = 3.32, p < .01; Figure 1B). Post-hoc tests indicated significant differences between stress and control animals at 120 and 240 min post-stress initiation. For both T3 and T4, stress-induced decreases in hormone levels were not apparent 24 hr post-stress. Changes in peripheral TSH levels were more complex. A two-way ANOVA revealed a significant stress × time interaction (F(4, 66) = 2.60, p < .05; Figure 1C), but neither visual inspection nor post-hoc tests led to clear-cut conclusions, perhaps due to alterations in control values across time points. We also measured circulating levels of rT3 and found no significant changes related to stress exposure (Figure 1D).
Figure 1.
Total T3 (A), total T4 (B), TSH (C) and rT3 (D) measured in serum samples collected from adult male rats after inescapable tail-shock stress (solid bars), control manipulations (open bars), or without experimental manipulation (0 min). Samples were collected at 1300 h. Significance was determined using a two-way ANOVA and  indicates significant difference via Bonferroni-corrected post-hoc tests; n=8 for all groups. Values are graphed as mean ± s.e.m.
indicates significant difference via Bonferroni-corrected post-hoc tests; n=8 for all groups. Values are graphed as mean ± s.e.m.
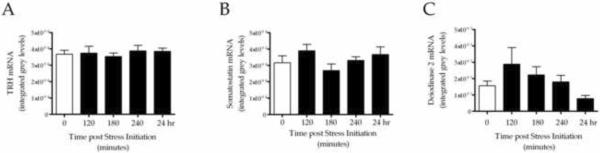
To determine if acute stress-induced changes in TH were, in part, centrally mediated, we used in-situ hybridization to measure changes in mRNA for hypothalamic peptides that are known regulators of the HPT axis. Separate one-way ANOVAs were used to compare levels of mRNA at 0 (pre-stress) 120, 180 and 240 min post-stressor initiation. Firstly, we examined the effects of stress on TRH mRNA within the PVN, but we did not observe a significant change (Figure 2A). Secondly, we examined SST mRNA levels within the periventricular hypothalamus; SST is an inhibitor of TSH release and we hypothesized that stress would stimulate an increase in SST mRNA levels. As shown in Figure 2B, we observed no significant changes in SST mRNA. Additionally, we examined levels of D2 mRNA, hypothesizing that an increase in hypothalamic D2 activity would increase the site-specific conversion of T4 to T3, potentially increasing negative feedback at the level of the hypothalamus and inhibiting TRH activity/release, despite the decrease in feedback resulting from decrease in peripheral levels of T3 and T4. The results of a one-way ANOVA revealed no significant changes in D2 mRNA levels (F(4, 39) = 1.90, p = 0.13; Figure 2C). Based on the observation of significantly different variances between groups, the non-parametric Kruskal-Wallis test was conducted, which revealed a statistical trend for a main effect of stress (H = 8.157; p = .086), such that stressed animals showed increased hypothalamic D2.
Figure 2.
Integrated optical grey level measurements for TRH mRNA levels (A), SST mRNA (B) and D2 mRNA (C) in brains collected from control (0 min) and animals subjected to inescapable tail-shock stress. Data are from the same animals depicted in Figure 1 (Experiment 1). Significance was determined using a one-way ANOVA; n=8 for all groups. Values are graphed as mean ± s.e.m.
Experiment 2
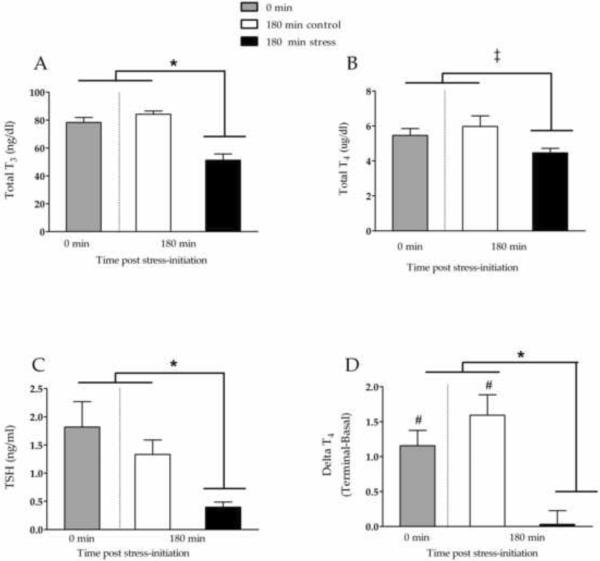
This set of experiments replicated the results from experiment 1, finding that tail-shock stress caused significant decreases in peripheral levels of TH at 180 min post-stressor initiation. Specifically, we observed a stress-related decrease in circulating T3 (F(2, 21) = 24.08, p < .001; Figure 3A) and a trend toward decreased T4 (F(2, 21) = 2.97, p = .07; Figure 3B). We also found a significant stress-induced decrease in circulating TSH levels (F(2, 21) = 6.04, p < .01; Figure 3C), which was ambiguous in Experiment 1. When the change (Δ) in peripheral T4 levels between Day 2 (terminal sample) and Day 1 (basal tail-nick sample) was calculated (terminal-basal) we found a positive change score in the 0 min group and the 180 control animals, indicating that peripheral T4 levels were increased in the terminal sample of animals that experienced mild handling (Figure 3D). Specifically, using paired-samples t-tests, we found significantly higher levels of terminal (Day 2) T4 in the 0 min condition (t(7) = 5.29, p < .001) and 180 min control animals (t(7) = 5.44, p < .001), compared to their Day 1 basal samples. Importantly, this increase was not observed in tail-shock animals; there was no significant change in T4 levels in stressed animals between Day 1 and Day 2 (t(7) < 1). When analyzed across groups, the stress group had a significantly smaller Δ T4 (F(2, 21) = 11.36, p < .001) (Figure 3D), when compared to other groups.
Figure 3.
Total T3 (A), total T4 (B), and TSH (C) measured in terminal serum samples collected from adult male rats 180 min after inescapable tail-shock stress (solid bars) or control manipulations (open bars), or without experimental manipulation (0 min). Change in Total T4 from basal samples (day 1) to terminal samples (day 2) are shown in panel D. Separate paired samples T-Tests indicated that terminal T4 was higher than basal T4 in 0m and 180m controls (denote with #), but not 180m stress animals. All between-group comparisons were made using a one-way ANOVA and Bonferroni-corrected post-hoc tests, with * denoting significant differences with p < .05 and ‡ denoting statistical trends with .1 < p < .05. Values are graphed as mean ± s.e.m.
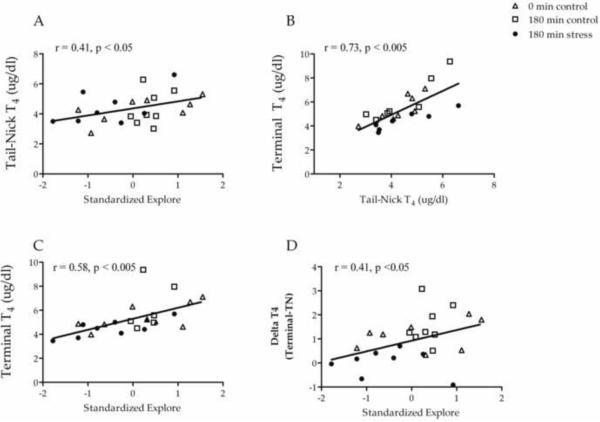
The primary goal of this series of experiments was to determine if variability in the behavior of out-bred rats correlated with peripheral thyroid hormone levels, both basal levels and stress-induced changes. Our data indicate that the animals' behavior indeed predicted TH levels (Figure 4A). In the open field test, we found that the standardized exploration score, which incorporated both total distance and time rearing, was positively correlated with basal T4 (r = .41, p < .05), collected via tail-nick on the day prior to stress. The relationship was such that animals with lower exploration had lower T4 levels and animals with higher T4 levels had higher exploration. As basal T4 values were strongly correlated with terminal T4 values (Figure 4B), this behavior score was positively related to terminal T4 levels (r = .59, p < .01) and Δ T4 (r = .41, p < .05; Figure 4C & 4D). Animals with high exploratory activity had higher terminal T4 levels and also demonstrated more positive Δ T4. In contrast, indices of exploration of the center area of the open field were not significantly related to peripheral T4 levels.
Figure 4.
Scattergrams illustrating correlations between standardized scores for exploration behavior and TH variables from Experiment 2 animals. Experimental condition is indicated using different symbols. Standardized exploration was positively related to basal T4 (A). There was a strong positive relationship between basal (tail-nick) and terminal T4 (B). Standardized exploration was also positively related to terminal T4 (C) and change in T4 from basal to terminal sampling (D).
Discussion
In the current series of experiments, we sought to further characterize acute stress-induced changes in HPT axis function and to also determine if TH levels are correlated with an animals' spontaneous behavior. In Experiment 1 we replicated our previous results, showing that acute stress can cause a decrease in circulating T3 levels within 120 min post-stress initiation (Helmreich et al., 2006). We extended these findings by demonstrating that acute stress also causes a decrease in circulating T4 levels, without a change in peripheral rT3, suggesting that the decrease in peripheral T3 does not result from an alteration in the ratios of conversion pathways of T4 to T3 or rT3. Instead, stress-induced decreases in T3 probably results from a decrease in secretory activity of the thyroid gland. In the second experiment, because we collected a basal sample on the day preceding the stressor and could calculate a change score, we were able to determine that mild handling or arousal that occurred in the 0 min and 180 min control animals stimulated an increase in circulating T4 compared to previous day. Experiencing tail-shock, in addition to the handling experienced by the 0 min and 180 min control animals, blocked this increase in T4. These findings re-iterate the idea that mild stimuli or `arousing' stimuli may lead to an increase in TH, while more potent, aversive stimuli can lead to decrease in TH (Langer et al., 1983, Helmreich et al., 2005).
Additionally, we found TSH levels decreased in animals' subjected to stress. Although the results between Experiment 1 and 2 were not completely consistent, both indicated a stress-induced decrease in TSH levels; the variability in peripheral TSH levels may be due to the pulsatile nature of TSH secretion (Bruhn et al., 1992). The observation that the levels of TSH were inappropriately low or normal in spite of decreasing negative feedback signals from circulating T3 and T4 suggests a decrease in central drive for TSH secretion after acute stress (Braverman and Utiger, 2000). To assess central drive to the HPT axis, we chose to examine molecules that have a direct effect on TRH bioactivity. We therefore measured TRH mRNA levels within the hypophysiotropic PVN neurons, SST mRNA levels, which inhibit TRH activity at the pituitary (Haugen, 2009) and D2 mRNA levels which reflects, in part, site-specific negative feedback tone at the level of the hypothalamus (Tu et al., 1997). Contrary to other homeostatic challenges (stressors) that cause a decrease in HPT that is associated with a decrease in PVN TRH mRNA levels (Cizza et al., 1996, Fekete et al., 2005, Lechan and Fekete, 2006) we did not detect tail-shock-induced changes in TRH mRNA levels or other central regulators of the HPT axis, thus reinforcing the idea that not all stressors impact endocrine systems in the same manner (Herman and Cullinan, 1997). The pattern of changes we measured in D2 mRNA was intriguing but didn't reach statistical significance; it does however warrant future investigation. Also, our list of candidates was certainly not exhaustive; other neuropeptides and neurosystems have been shown to impact the HPT axis (Lechan and Fekete, 2006, Nillni, 2010, Stolakis et al., 2010). TRH activity could also be decreased without accompanying changes in mRNA levels; for example changes in processing of the TRH molecule may alter its bioactivity (Perello et al., 2006) or the TRH specific peptidase PP II (Heuer et al., 1998) located within the tanycytes in the medial-basal hypothalamus (Sanchez et al., 2009) may also alter TRH bioactivity.
Alternatively, stress-induced changes in TSH and subsequent TH may occur at the level of the pituitary (Haugen, 2009). Glucocorticoids (corticosterone), which were certainly elevated by the stress paradigm (Helmreich et al., 1999), have been suggested to inhibit TSH release at the level of the pituitary (Wilber and Utiger, 1969) both in vivo (Hangaard et al., 1996, Roelfsema et al., 2009) and in vitro (Taylor et al., 1995). However, studies also suggest that glucocorticoid effects on the HPT axis are mediated at the level of the hypothalamus (Wilber and Utiger, 1969, Kondo et al., 1997, Haugen, 2009, Roelfsema et al., 2009). Conducting the current experiment in adrenalectomized/corticosterone replaced animals would help clarify the role of corticosterone in stress-induced decreases in TH levels.
Classically, TH hormone effects are thought to take place within the time frame of days to weeks, not within the minutes to hours time-points measured in the current study. However, more recent evidence suggests that changes in TH can have short-term consequences. For example, intraperitoneal administration of T3 to rats leads to an increase in hippocampal BDNF mRNA levels with 2 hrs (Sui et al., 2010) and sub-cutaneous T3 administration can increase food intake within 2 hours in rats (Kong et al., 2004). Furthermore, very rapid direct effects of T3 on hippocampal neuronal excitability have been demonstrated (Caria et al., 2009). Central administration of T3 has been shown to alter sympathetic regulation of brown fat within 1 hr (Lopez et al., 2010), and glucose productions with 2 hr (Klieverik et al., 2009). These effects parallel rapid effects of TH on cardiovascular parameters that have also been reported (Hiroi et al., 2006). These acute effects of TH may be mediated by genomic/membrane bound receptors or non-membrane bound receptors (Davis et al., 2008). Furthermore, recent evidence also suggests that relatively mild hypothyroidism, such as that observed in the current experiments, may have significant effects on an individual (Joffe and Sokolov, 1994, Sait Gonen et al., 2004, Knudsen et al., 2005, Gulseren et al., 2006, Davis and Tremont, 2007).
In the second series of experiments, we determined if the animals' spontaneous behavior in an open field was at all related with TH levels. We found that an animals' behavior was indeed correlated with his thyroid hormone levels; animals with higher exploratory behavior in an open field had higher basal T4 levels and higher T4 levels in the terminal sample, suggesting that the animals with higher exploration scores had a more positive (less inhibited) TH response to the experience/stressor. One way to make sense of these results is to postulate that on an organismal/macro level of analysis, the animals' spontaneous behavior is reflective of the energetic and permissive effects of TH. Low TH hormones, perhaps reflective of conservation-withdrawal, may indicate a less risky exploratory style employed to protect the status quo and hinder change and development. Conversely, higher TH may be indicative of an animal with a more active exploratory style and, on a different level of analysis, one more likely to undergo change and development.
The observation of individual differences within a group of widely used experimental animals replicates previous results (Kabbaj et al., 2000, Korte et al., 2005, Whishaw and Kolb, 2005) and emphasizes the contribution of individual variability to experimental results. Acknowledgement of individual differences within a population may decrease within experiment variability and may allow investigators to explore links between behavior and endocrine system (Whishaw and Kolb, 2005). Specifically related to thyroid hormones, differences in HPT set-point among adult men have been characterized and suggests that detecting changes in TH status is not best accomplished by reference to clinical standards, but to what is “normal” for a particular individual (Andersen et al., 2002). This was also reflected in our T4 results in Experiment 2; when examining experimental group means, there was only a trend for statistically significant stress-induced changes in T4. However, when stress-effects were measured as a change from an animal's own baseline, stress clearly had an inhibitory effect. Individual differences in HPT axis regulation may play a role in behavioral changes induced by a stressor, in both rats and non-human primates (Jarrell et al., 2008). In humans, plasma T3 levels are correlated with the activity of central dopamine and serotonin systems (Strawn et al., 2004) and individual differences in thyroid hormone levels may predict vulnerability to psychiatric disease (Davis and Tremont, 2007).
In summary, we found that acute tail-shock stress leads to a decrease in peripheral levels of T3, T4, and TSH, indicating that a potent stressor can acutely alter the regulation of the HPT axis. We also found that manipulations that may be considered arousing, but not aversive, can lead to an increase in TH, suggesting that changes in TH may `grade' the perceived severity of a stimulus. Beyond that, we found that there are individual differences in regulation of the HPT axis, both basally and in response to stress and that these individual differences can be predicted by the animals' spontaneous exploratory behavior. The results provide insight into the regulation of a vital neuroendocrine system, whose ramifications, especially acutely within the CNS, are beginning to be appreciated.
Highlights
Acute inescapable stress causes a decrease in peripheral thyroid hormones levels in adult male rats within 120 min of stress initiation
Unlike other stressors, these stress-induced change in peripheral hormone levels were not associated with alterations in TRH mRNA levels within the hypothalamus
An animal's exploratory drive is correlated with peripheral thyroid hormone levels
Acknowledgements
We would like to thank the vivarium staff at the University of Rochester for their excellent animal care and James Walton for his excellent technical assistance. Daniel Tylee earned course credit and received an undergraduate research grant from SUNY Geneseo to support his work on these experiments. This work was supported by the Department of Psychiatry at the University of Rochester, and grant MH1R03MH080789 to DLH. The authors appreciate the thoughtful comments from Drs. R. Ader, J. Fudge, J. Moynihan, and D.B Parfitt throughout the experiment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen S, Pedersen KM, Bruun NH, Laurberg P. Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab. 2002;87:1068–1072. doi: 10.1210/jcem.87.3.8165. [DOI] [PubMed] [Google Scholar]
- Armario A, Castellanos J, Balasch J. Effect of acute and chronic psychogenic stress on corticoadrenal and pituitary-thyroid hormones in male rats. Hormone Res. 1984;20:241–245. doi: 10.1159/000180003. [DOI] [PubMed] [Google Scholar]
- Braverman L, Utiger R, editors. Werner's and Ingbar's the thyroid: A fundamental and clinical text. Lippincott Williams and Wilkins; 2000. [Google Scholar]
- Bruhn TO, McFarlane MB, Deckey JE, Jackson IM. Analysis of pulsatile secretion of thyrotropin and growth hormone in the hypothyroid rat. Endocrinology. 1992;131:2615–2621. doi: 10.1210/endo.131.6.1446603. [DOI] [PubMed] [Google Scholar]
- Caria MA, Dratman MB, Kow LM, Mameli O, Pavlides C. Thyroid hormone action: nongenomic modulation of neuronal excitability in the hippocampus. J Neuroendocrinol. 2009;21:98–107. doi: 10.1111/j.1365-2826.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- Cizza G, Brady L, Escpales M, Blackman M, Gold P, Chrousus G. Age and gender influence basal and stress-modulated hypothalamic-pituitary-thyroidal function in Fisher 344/N rats. Neuroendocrinology. 1996;64:440–448. doi: 10.1159/000127150. [DOI] [PubMed] [Google Scholar]
- Crespi EJ, Denver RJ. Ancient origins of human developmental plasticity. Am J Hum Biol. 2005;17:44–54. doi: 10.1002/ajhb.20098. [DOI] [PubMed] [Google Scholar]
- Davis JD, Tremont G. Neuropsychiatric aspects of hypothyroidism and treatment reversibility. Minerva Endocrinol. 2007;32:49–65. [PubMed] [Google Scholar]
- Davis PJ, Leonard JL, Davis FB. Mechanisms of nongenomic actions of thyroid hormone. Front Neuroendocrinol. 2008;29:211–218. doi: 10.1016/j.yfrne.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Ebling FJ, Barrett P. The regulation of seasonal changes in food intake and body weight. J Neuroendocrinol. 2008;20:827–833. doi: 10.1111/j.1365-2826.2008.01721.x. [DOI] [PubMed] [Google Scholar]
- Engel G, Schmale A. Conservation-withdrawal: A primary regulatory process for organismic homeostatsis. CIBA Found Symp. 1972;8:57–75. doi: 10.1002/9780470719916.ch5. [DOI] [PubMed] [Google Scholar]
- Fekete C, Lechan RM. Negative feedback regulation of hypophysiotropic thyrotropin-releasing hormone (TRH) synthesizing neurons: role of neuronal afferents and type 2 deiodinase. Front Neuroendocrinol. 2007;28:97–114. doi: 10.1016/j.yfrne.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete C, Singru PS, Sarkar S, Rand WM, Lechan RM. Ascending brainstem pathways are not involved in lipopolysaccharide-induced suppression of thyrotropin-releasing hormone gene expression in the hypothalamic paraventricular nucleus. Endocrinology. 2005;146:1357–1363. doi: 10.1210/en.2004-1429. [DOI] [PubMed] [Google Scholar]
- Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeold A, Bianco AC. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev. 2008;29:898–938. doi: 10.1210/er.2008-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulseren S, Gulseren L, Hekimsoy Z, Cetinay P, Ozen C, Tokatlioglu B. Depression, anxiety, health-related quality of life, and disability in patients with overt and subclinical thyroid dysfunction. Arch Med Res. 2006;37:133–139. doi: 10.1016/j.arcmed.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Hangaard J, Andersen M, Grodum E, Koldkjaer O, Hagen C. Pulsatile thyrotropin secretion in patients with Addison's disease during variable glucocorticoid therapy. J Clin Endocrinol Metab. 1996;81:2502–2507. doi: 10.1210/jcem.81.7.8675567. [DOI] [PubMed] [Google Scholar]
- Haugen BR. Drugs that suppress TSH or cause central hypothyroidism. Best Pract Res Clin Endocrinol Metab. 2009;23:793–800. doi: 10.1016/j.beem.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmreich D, Crouch M, Dorr N, Parfitt D. Peripheral triiodothyronine (T3) levels during escapable and inescapable shock. Physiology and Behavior. 2006;87:114–119. doi: 10.1016/j.physbeh.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Helmreich D, Parfitt D, Lu X-Y, Akil H, Watson S. Relation between the hypothalamic-pituitary-thyroid (HPT) axis and the hypothalamic-pituitary-adrenal (HPA) axis during repeated stress. Neuroendocrinology. 2005;81:183–192. doi: 10.1159/000087001. [DOI] [PubMed] [Google Scholar]
- Helmreich DL, Watkins LR, Deak T, Maier SF, Akil H, Watson SJ. The effect of stressor controllability on stress-induced neuropeptide mRNA expression within the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 1999;11:121–128. doi: 10.1046/j.1365-2826.1999.00300.x. [DOI] [PubMed] [Google Scholar]
- Herman J, Cullinan W. Neurocircuitry of stress:central control of the hypothalamicadrenocortical axis. Trends Neuroscience. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Heuer H, Schafer MK, Bauer K. The thyrotropin-releasing hormone-degrading ectoenzyme: the third element of the thyrotropin-releasing hormone-signaling system. Thyroid. 1998;8:915–920. doi: 10.1089/thy.1998.8.915. [DOI] [PubMed] [Google Scholar]
- Hiroi Y, Kim HH, Ying H, Furuya F, Huang Z, Simoncini T, Noma K, Ueki K, Nguyen NH, Scanlan TS, Moskowitz MA, Cheng SY, Liao JK. Rapid nongenomic actions of thyroid hormone. Proc Natl Acad Sci U S A. 2006;103:14104–14109. doi: 10.1073/pnas.0601600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiol Behav. 2008;93:807–819. doi: 10.1016/j.physbeh.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe RT, Sokolov ST. Thyroid hormones, the brain, and affective disorders. Crit Rev Neurobiol. 1994;8:45–63. [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20:6983–6988. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Fliers E, Franke A, Wortel J, Buijs R. Functional connections between the suprachiasmatic nucleus and the thyroid gland as revealed by lesioning and viral tracing techniques in the rat. Endocrinology. 2000;141:3832–3841. doi: 10.1210/endo.141.10.7709. [DOI] [PubMed] [Google Scholar]
- Kilburn-Watt E, Banati RB, Keay KA. Altered thyroid hormones and behavioural change in a sub-population of rats following chronic constriction injury. J Neuroendocrinol. 2010;22:960–970. doi: 10.1111/j.1365-2826.2010.02038.x. [DOI] [PubMed] [Google Scholar]
- Klieverik LP, Janssen SF, van Riel A, Foppen E, Bisschop PH, Serlie MJ, Boelen A, Ackermans MT, Sauerwein HP, Fliers E, Kalsbeek A. Thyroid hormone modulates glucose production via a sympathetic pathway from the hypothalamic paraventricular nucleus to the liver. Proc Natl Acad Sci U S A. 2009;106:5966–5971. doi: 10.1073/pnas.0805355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen N, Laurberg P, Rasmussen LB, Bulow I, Perrild H, Ovesen L, Jorgensen T. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. 2005;90:4019–4024. doi: 10.1210/jc.2004-2225. [DOI] [PubMed] [Google Scholar]
- Kondo K, Harbuz M, Levy A, Lightman S. Inhibition of the hypothalamic-pituitary-thyroid axis in response to lipopolysaccharide is independent of changes in circulating corticosteroids. Neuroimmunomodulation. 1997;4:188–194. doi: 10.1159/000097337. [DOI] [PubMed] [Google Scholar]
- Kong WM, Martin NM, Smith KL, Gardiner JV, Connoley IP, Stephens DA, Dhillo WS, Ghatei MA, Small CJ, Bloom SR. Triiodothyronine stimulates food intake via the hypothalamic ventromedial nucleus independent of changes in energy expenditure. Endocrinology. 2004;145:5252–5258. doi: 10.1210/en.2004-0545. [DOI] [PubMed] [Google Scholar]
- Korte S, Koolhaas J, Wingfield J, McEwen B. The darwinian concept of stress: benefits of allostatsis and costs of allostatic load and the trade-offs in health and disease. Neuroscience and Biobehavioral Reviews. 2005;29:3–18. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Langer P, Foldes O, Kvetnansky R, Culman J, Torda T, El Daher F. Pituitary-thyroid function during acute immobilization stress in rats. Exp Clinical Endocrinol. 1983;82:51–60. doi: 10.1055/s-0029-1210255. [DOI] [PubMed] [Google Scholar]
- Lechan RM, Fekete C. The TRH neuron: a hypothalamic integrator of energy metabolism. Prog Brain Res. 2006;153:209–235. doi: 10.1016/S0079-6123(06)53012-2. [DOI] [PubMed] [Google Scholar]
- Legradi G, Emerson C, Ahima R, Flier J, Lechan R. Leptin prevents fasting -induced suppression of prothyrotropin releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinol. 1997;138:2569–2576. doi: 10.1210/endo.138.6.5209. [DOI] [PubMed] [Google Scholar]
- Lopez M, Varela L, Vazquez MJ, Rodriguez-Cuenca S, Gonzalez CR, Velagapudi VR, Morgan DA, Schoenmakers E, Agassandian K, Lage R, Martinez de Morentin PB, Tovar S, Nogueiras R, Carling D, Lelliott C, Gallego R, Oresic M, Chatterjee K, Saha AK, Rahmouni K, Dieguez C, Vidal-Puig A. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med. 2010;16:1001–1008. doi: 10.1038/nm.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DR, Plant TM. The role and potential sites of action of thyroid hormone in timing the onset of puberty in male primates. Brain Res. 2010;1364:175–185. doi: 10.1016/j.brainres.2010.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B, Wingfield J. The concept of allostatis in biology and biomedicine. Hormones and Behavior. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Setalo G, Csontos C, Petrusz P, Flerko B, Negro-Vilar A. Combined retrograde tracing and immunocytochemical identification of luteinizing hormone-releasing hormone- and somatostatin-containing neurons projecting to the median eminence of the rat. Endocrinology. 1989;125:2812–2821. doi: 10.1210/endo-125-6-2812. [DOI] [PubMed] [Google Scholar]
- Nillni EA. Regulation of the hypothalamic thyrotropin releasing hormone (TRH) neuron by neuronal and peripheral inputs. Frontiers in neuroendocrinology. 2010;31:134–156. doi: 10.1016/j.yfrne.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perello M, Friedman T, Paez-Espinosa V, Shen X, Stuart RC, Nillni EA. Thyroid hormones selectively regulate the posttranslational processing of prothyrotropin-releasing hormone in the paraventricular nucleus of the hypothalamus. Endocrinology. 2006;147:2705–2716. doi: 10.1210/en.2005-1609. [DOI] [PubMed] [Google Scholar]
- Roelfsema F, Pereira AM, Biermasz NR, Frolich M, Keenan DM, Veldhuis JD, Romijn JA. Diminished and irregular TSH secretion with delayed acrophase in patients with Cushing's syndrome. Eur J Endocrinol. 2009;161:695–703. doi: 10.1530/EJE-09-0580. [DOI] [PubMed] [Google Scholar]
- Rogers KV, Vician L, Steiner RA, Clifton DK. Reduced preprosomatostatin messenger ribonucleic acid in the periventricular nucleus of hypophysectomized rats determined by quantitative in situ hybridization. Endocrinology. 1987;121:90–93. doi: 10.1210/endo-121-1-90. [DOI] [PubMed] [Google Scholar]
- Sait Gonen M, Kisakol G, Savas Cilli A, Dikbas O, Gungor K, Inal A, Kaya A. Assessment of anxiety in subclinical thyroid disorders. Endocr J. 2004;51:311–315. doi: 10.1507/endocrj.51.311. [DOI] [PubMed] [Google Scholar]
- Sanchez E, Singru PS, Fekete C, Lechan RM. Induction of type 2 iodothyronine deiodinase in the mediobasal hypothalamus by bacterial lipopolysaccharide: role of corticosterone. Endocrinology. 2008;149:2484–2493. doi: 10.1210/en.2007-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez E, Vargas MA, Singru PS, Pascual I, Romero F, Fekete C, Charli JL, Lechan RM. Tanycyte pyroglutamyl peptidase II contributes to regulation of the hypothalamic-pituitary-thyroid axis through glial-axonal associations in the median eminence. Endocrinology. 2009;150:2283–2291. doi: 10.1210/en.2008-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer K-H, Herman J, Watson S. In Situ hybridization histochemistry. In: London E, editor. Imaging Drug Action in the Brain. CRC Press; Boca Raton: 1993. pp. 337–377. [Google Scholar]
- Servatius R, Brennan F, Moldow R, Pogach L, Natelson B, Ottenweller J. Persistent hormonal effects of stress are not due to reduced food intake or exposure to stressed rats. Endocrine. 2001;14:181–187. doi: 10.1385/ENDO:14:2:181. [DOI] [PubMed] [Google Scholar]
- Servatius R, Natelson B, Moldow R, Pogach L, Brennan F, Ottenweller J. Persistent neuroendocrine changes in multiple hormonal axes after a single or repeated stressor exposures. Stress. 2000;3:263–274. doi: 10.3109/10253890009001132. [DOI] [PubMed] [Google Scholar]
- Stolakis V, Kalafatakis K, Botis J, Zarros A, Liapi C. The regulatory role of neurotensin on the hypothalamic-anterior pituitary axons: emphasis on the control of thyroid-related functions. Neuropeptides. 2010;44:1–7. doi: 10.1016/j.npep.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Strawn JR, Ekhator NN, D'Souza BB, Geracioti TD., Jr. Pituitary-thyroid state correlates with central dopaminergic and serotonergic activity in healthy humans. Neuropsychobiology. 2004;49:84–87. doi: 10.1159/000076415. [DOI] [PubMed] [Google Scholar]
- Sui L, Ren WW, Li BM. Administration of thyroid hormone increases reelin and brain-derived neurotrophic factor expression in rat hippocampus in vivo. Brain Res. 2010;1313:9–24. doi: 10.1016/j.brainres.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Tata JR. Amphibian metamorphosis as a model for the developmental actions of thyroid hormone. Mol Cell Endocrinol. 2006;246:10–20. doi: 10.1016/j.mce.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Taylor AD, Flower RJ, Buckingham JC. Dexamethasone inhibits the release of TSH from the rat anterior pituitary gland in vitro by mechanisms dependent on de novo protein synthesis and lipocortin 1. J Endocrinol. 1995;147:533–544. doi: 10.1677/joe.0.1470533. [DOI] [PubMed] [Google Scholar]
- Tu HM, Kim SW, Salvatore D, Bartha T, Legradi G, Larsen PR, Lechan RM. Regional distribution of type 2 thyroxine deiodinase messenger ribonucleic acid in rat hypothalamus and pituitary and its regulation by thyroid hormone. Endocrinology. 1997;138:3359–3368. doi: 10.1210/endo.138.8.5318. [DOI] [PubMed] [Google Scholar]
- Turakulov Y, Burikhanov R, Pakitdinov P, Myslitskaya A. Influence of immobilization stress on the levels of thyroid hormones. Neuroscience Behav Physio. 1994;24:462–464. doi: 10.1007/BF02360166. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Kolb B, editors. The behavior of the laboratory rat: A handbook with tests. Oxford University Press; 2005. [Google Scholar]
- Wilber JF, Utiger RD. The effect of glucocorticoids on thyrotropin secretion. J Clin Invest. 1969;48:2096–2103. doi: 10.1172/JCI106176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrutniak-Cabello C, Casas F, Cabello G. Thyroid hormone action in mitochondria. Journal of Molecular Endocrinology. 2001;26:67–77. doi: 10.1677/jme.0.0260067. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Tan SW, Tyl RW. General background on the hypothalamic-pituitary-thyroid (HPT) axis. Crit Rev Toxicol. 2007;37:11–53. doi: 10.1080/10408440601123446. [DOI] [PubMed] [Google Scholar]