Abstract
Microcystin is a cyanobacterial hepatotoxin that is found worldwide, and poses a serious threat to the ecological communities in which it is found as well as to those who rely on these waters for drinking, sanitation, or as a food source. Microcystin is known to accumulate in fish and other aquatic biota, however the prevalence of microcystin in fish tissue and the human health risks posed by microcystin exposure through fish consumption remain poorly resolved. Here we show that microcystin is pervasive in water and fish from several tropical (Ugandan) and temperate (North American) lakes, including lakes that support some of the largest freshwater fisheries in the world. We establish that fish consumption can be an important and sometimes dominant route of microcystin exposure for humans, and can cause consumers to exceed recommended total daily intake guidelines for microcystin. These results highlight the importance of monitoring microcystin concentrations in fish, and the need to consider potential exposure to microcystin through fish consumption in order to adequately assess human exposure risk.
Introduction
As anthropogenic input of nutrients to freshwater systems continues to alter the total biomass and the composition of algal communities, there are a number of ecological and public health concerns that emerge. Globally, cyanobacterial dominance of freshwater phytoplankton is increasing, as is the occurrence of hazardous blooms of toxic cyanobacteria (1); and it is expected that a warming climate will exacerbate the frequency and duration of such blooms (2). The cyanotoxin microcystin is present in a broad range of aquatic systems (3) and is a potent hepatotoxin as well as a potential tumour promotor (1, 4, 5).
The World Health Organization has set a provisional tolerable daily intake value (TDI) for chronic exposure to microcystin-LR of 0.04 μg/kg body weight (6, 7), and has set a guideline value for microcystin-LR in drinking water of 1 μg/L, based on the assumption that 80 percent of exposure is attributable to water consumption (6, 7). Although accumulation of microcystin in fish and other aquatic organisms is known to occur (1, 8–10), no widely accepted guidelines have been established for microcystin concentrations in fish tissue, and most microcystin exposure scenarios do not consider potential exposure through fish consumption.
We conducted a survey of microcystin in water and fish in two temperate great lakes (Erie and Ontario), three tropical great lakes (Victoria, Albert and Edward) and four other smaller Ugandan lakes (George, Mburo, Nkuruba, Saka). The large lakes sampled all support substantial commercially important fisheries, including the largest temperate (Lake Erie) and tropical (Lake Victoria) lake fisheries in the world. The smaller Ugandan lakes support subsistence fisheries that provide a critically important source of protein and income for riparian communities. These lakes provided a continuum of trophic status, and the fish sampled (491 fish from 33 species) were representative of several trophic levels ranging from planktivores to top predators.
Experimental section
Water samples (integrated euphotic zone) and fish were collected from all Ugandan study sites in April–May of 2007, and then again on a monthly basis between September 2008 and February 2009 for all sites but Lake Albert. The sites sampled included two embayments in northern Lake Victoria (Murchison Bay and Napoleon Gulf), Lake Albert (at a nearshore site in the northeast of the lake), Lake Edward (both nearshore and offshore), and the central portions of Lake George, Lake Mburo, and two crater lakes, Saka and Nkuruba. Water was collected from six stations in both Maumee Bay (Lake Erie) and the Bay of Quinte (Lake Ontario) on four occasions during the summers (May–September) of 2006 and 2007. Fish from these sites were caught during research trawls in July–September of 2006 and 2007.
Water was analyzed for chlorophyll a and nutrient concentrations as in Stainton et al. (11). Microcystin in water was measured using indirect competitive ELISA (Abraxis LLC, Microcystins-ADDA ELISA kits, PN 520011). This is a congener-independent ELISA based on the detection of the Adda side-chain found in microcystins and nodularins (12). Whole water samples were prepared for use in ELISA assays through chemical lysis (using the Abraxis LLC QuikLyse™ method (13)).
Several species of fish from different trophic levels were collected from each lake, with care taken to ensure a representative size range within species. Dorso-lateral fish muscle tissue was dried (at 60 ºC for at least 24 hours) and homogenized using a ball-mill grinder. Where fish were very small and typically consumed whole (including Rastrineobola argentea, Poecelia reticulata, and fish less than approximately 10 cm in total length), whole dried fish were homogenized. Microcystin in fish tissue was analyzed through methanol extraction followed by competitive indirect ELISA (12) based on the method described in Wilson et al. (14) with some modifications. Dry homogenized fish tissue was weighed and then extracted twice, first for 2 hours using 75 % methanol, and then for 24 hours using 75 % methanol in addition to glacial acetic acid (0.002 v/v). After each extraction, samples were centrifuged and supernatant was removed and pooled. The pooled supernatant was filtered to remove particulates, subsampled, and the solvent was evaporated using a Turbovap LV. After evaporation, the remaining solids were resuspended in de-ionized water and analyzed for microcystin using Abraxis anti-ADDA ELISA test kits. Measured dry-weight microcystin concentrations in fish were converted to wet-weight concentrations using a conversion factor of 0.31 (15).
Results and Discussion
Secchi depth, total phosphorus and chlorophyll a concentrations are often used to indicate lake trophic status (16). Based on the physicochemical observations for the study sites (Table 1) Lake Nkuruba, Lake Albert, and the Bay of Quinte were found to be meso/eutrophic, while Maumee Bay, Napoleon Gulf, and offshore Lake Edward were eutrophic. The remaining sites (Lake George, Lake Mburo, Murchison Bay, Lake Saka, and nearshore Lake Edward) were hypereutrophic.
Table 1.
Summary of select physicochemical properties of study lakes, including microcystin concentrations in water.
| Lake | n | Site Depth (m) | Secchi Depth (m) | Chl (μg/L)a | TP (μg/L) | Trophic Status | MC in Water (μg/L) |
|---|---|---|---|---|---|---|---|
| Albert | 2 | 12.0 | 1.6 (0.3) | 19.2 (3.5) | 32.5 (2.2) | M/E | 0.1 (0.02) |
| Edward | |||||||
| Nearshore | 7 | 3.5 | 0.5 (0.2) | 67.7 (42.3) | 131.5 (50.4) | H | 5.0 (5.7) |
| Offshore | 5 | 7.3 | 1.1 (0.3) | 23.5 (27.5) | 58.9 (9.2) | E | 1.0 (1.1) |
| George | 7 | 2.8 | 0.4 (0.1) | 124.7 (40.7) | 188.7 (24.6) | H | 7.3 (6.6) |
| Mburo | 7 | 3.2 | 0.5 (0.1) | 68.5 (39.0) | 121.3 (39.7) | H | 2.2 (1.1) |
| Victoria | |||||||
| Murchison Bay | 10 | 5.2 | 0.7 (0.1) | 101.8 (48.3) | 106.2 (28.0) | H | 7.3 (5.7) |
| Napoleon Gulf | 12 | 17.5 | 1.4 (0.2) | 24.0 (18.0) | 58.8 (14.6) | E | 1.5 (1.3) |
| Nkuruba | 7 | 33.4 | 1.7 (0.4) | 7.9 (3.5) | 34.4 (8.5) | M | 0.2 (0.1) |
| Saka | 7 | 3.2 | 0.4 (0.1) | 133.8 (84.5) | 182.0 (34.7) | H | 57.1 (67.9) |
| Bay of Quinte | 4 | 3.9 | 2.1 (0.8) | 11.2 (8.4) | 24.1 (6.4) | M/E | 0.9 (1.0) |
| Maumee Bay | 4 | 3.5 | 1.5 (0.7) | 9.5 (7.0) | 46.0 (24.7) | E | 1.3 (2.3) |
Mean values are reported with s.d. in parentheses; n is the sample size. Trophic status was determined based on Vollenweider and Kerekes (11), and reported as: M/E (meso-eutrophic), E (eutrophic), and H (hypereutrophic).
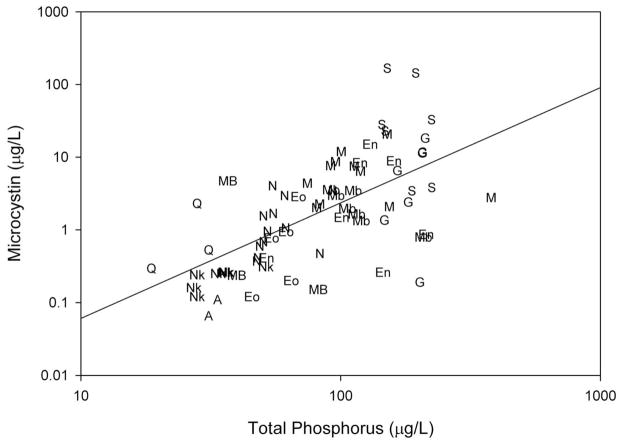
Microcystin concentrations in water consistently exceeded the WHO recommended guideline for microcystin in drinking water at several of the study sites (Table 1), including Lake Victoria, which provides drinking water for more than ten million people (17). This is particularly concerning given that many riparian communities rely on untreated lake water as a primary source of drinking water. While the meso-eutrophic lakes had the lowest observed microcystin concentrations, the highest microcystin concentrations were observed in the hypereutrophic lakes. Across all study sites (both tropical and temperate), significant positive relationships were observed between microcystin concentrations and chlorophyll a concentrations (log transformed data, r2adj = 0.50, n = 72, P<0.001) as well as microcystin concentrations and total phosphorus concentrations (Figure 1, log transformed data, r2adj = 0.40, n = 72, P<0.001). Also, chlorophyll a had a strong positive relationship with total phosphorus (log transformed data, r2adj = 0.69, n = 73, P<0.001). These results confirm that microcystin concentrations in water were higher where algal biomass and total phosphorus concentrations were high, suggesting that eutrophication can increase the prevalence and magnitude of microcystin producing cyanobacterial blooms.
Figure 1.
Regression of microcystin concentrations against total phosphorus concentrations from all study sites (r2adj: 0.40, n=72, P<0.001). Site labels in Figure 1 are indicated as follows: A (Lake Albert), En (Lake Edward nearshore), Eo (Lake Edward offshore), G (Lake George), Mb (Lake Mburo), M (Murchison Bay, Lake Victoria), N (Napoleon Gulf, Lake Victoria), Nk (Lake Nkuruba), S (Lake Saka), MB (Maumee Bay, Lake Erie), Q (Bay of Quinte, Lake Ontario).
Microcystin was found to have accumulated in fish at all of the study sites, and observed concentrations ranged from 0.5–1917 μg/kg and 4.5–215.2 μg/kg of wet weight (w.w.) in muscle tissue and whole fish respectively (Table 2, Figure 2). Microcystin concentrations in fish tended to be higher in lakes where microcystin concentrations in water were also high. Within lakes, microcystin concentrations in fish exhibited a great deal of variability. This variability is likely attributable to the seasonal variability in microcystin in water, the differences in diet between the species sampled, and the wide range in size within species.
Table 2.
Microcystin concentrations in fish from all study sites.
| Name | Microcystin (μg/kg wet weight)
|
||
|---|---|---|---|
| n | mean ± s.d. | range | |
| Lake Albert | |||
| Lates niloticus (> 25 cm) | 5 | 6.7 ± 3.5 | 3.9–11.6 |
| Tilapia zilli | 5 | 4.0 ± 1.3 | 2.7–6.2 |
| Lake Edward | |||
| Bagrus docmac | 5 | 6.2 ± 3.0 | 2.1–9.3 |
| Barbus bynni | 5 | 5.5 ± 2.7 | 1.3–8.6 |
| Clarias gariepinus | 5 | 8.6 ± 7.5 | 2.0–21.3 |
| Haplochromis spp. | 5 | 10.0 ± 3.2 | 5.2–13.6 |
| Haplochromis squamipinnis | 5 | 8.6 ± 3.4 | 3.7–12.6 |
| Oreochromis leucostictus | 4 | 21.9 ± 30.7 | 2.9–67.7 |
| Oreochromis niloticus | 20 | 6.7 ± 4.5 | 1.1–16.7 |
| Protopterus aethiopicus | 5 | 5.3 ± 4.9 | 1.4–13.4 |
| Lake George | |||
| Bagrus docmac | 5 | 9.4 ± 6.9 | 4.4–21.2 |
| Clarias gariepinus | 5 | 6.9 ± 2.0 | 4.4–9.9 |
| Haplochromis spp. | 5 | 5.6 ± 4.7 | 2.6–13.7 |
| Haplochromis squamipinnis | 4 | 6.7 ± 3.5 | 2.9–9.9 |
| Haplochromis squamipinnis (whole) | 1 | 11.8 | ~ |
| Oreochromis esculentus | 4 | 13.8 ± 6.5 | 6.3–21.5 |
| Oreochromis leucostictus | 5 | 21.2 ± 32.3 | 0.9–78.4 |
| Oreochromis niloticus | 18 | 10.2 ± 8.6 | 1.7–33.9 |
| Protopterus aethiopicus | 5 | 2.4 ± 1.2 | 1.5–4.6 |
| Tilapia zilli | 1 | 2.0 | ~ |
| Lake Mburo | |||
| Bagrus docmac | 1 | 13.4 | ~ |
| Clarias gariepinus | 5 | 20.6 ± 19.5 | 3.0–51.3 |
| Haplochromis spp. | 4 | 6.4 ± 3.9 | 2.5–11.8 |
| Haplochromis spp. (whole) | 1 | 12.1 | ~ |
| Oreochromis esculentus | 6 | 23.9 ± 18.4 | 1.3–54.2 |
| Oreochromis leucostictus | 5 | 8.4 ± 6.7 | 2.2–16.2 |
| Oreochromis niloticus | 15 | 7.4 ± 7.6 | 1.3–23.6 |
| Protopterus aethiopicus | 5 | 2.3 ± 2.1 | 0.8–6.1 |
| Lake Nkuruba | |||
| Oreochromis leucostictus | 10 | 7.2 ± 5.1 | 1.6–17.2 |
| Poecelia reticulata (whole) | 2 | 38.9 ± 48.6 | 4.5–73.3 |
| Tilapia zilli | 9 | 10.0 ± 5.5 | 2.1–19.3 |
| Tilapia zilli (whole) | 3 | 49.2 ± 11.4 | 42.5–62.3 |
| Lake Saka | |||
| Astatoreochromis alluaudi | 4 | 56.1 ± 94.4 | 7.1–197.7 |
| Astatoreochromis alluaudi (whole) | 1 | 32.5 | ~ |
| Barbus neumayerii | 1 | 9.5 | ~ |
| Haplochromis spp. | 8 | 719.4 ± 800.3 | 9.5–1917 |
| Haplochromis spp. (whole) | 2 | 21.3–215.2 | ~ |
| Lates niloticus (> 25 cm) | 4 | 16.4 ± 18.3 | 4.1–43.7 |
| Oreochromis niloticus | 19 | 19.3 ± 19.4 | 0.8–63.4 |
| Lake Victoria (Murchison Bay) | |||
| Clarias gariepinus | 1 | 23.9 | ~ |
| Haplochromis spp. | 4 | 35.6 ± 36.3 | 9.0–88.9 |
| Haplochromis spp. (whole) | 1 | 19.9 | ~ |
| Lates niloticus (< 25 cm) | 7 | 21.2 ± 14.8 | 3.1–49.5 |
| Lates niloticus (> 25 cm) | 17 | 8.0 ± 6.8 | 1.3–25.0 |
| Oreochromis leucostictus | 5 | 30.3 ± 18.1 | 14.9–59.8 |
| Oreochromis niloticus | 28 | 12.8 ± 11.9 | 1.4–57.7 |
| Protopterus aethiopicus | 5 | 4.1 ± 2.4 | 1.7–7.7 |
| Rastrineobola argentea (whole) | 2 | 36.2–41.2 | ~ |
| Synodontis spp. | 10 | 22.8 ± 17.1 | 3.8–64.4 |
| Tilapia zilli | 5 | 15.4 ± 10.8 | 7.1–33.8 |
| Lake Victoria (Napoleon Gulf) | |||
| Astatoreochromis alluaudi | 1 | 6.2 | ~ |
| Bagrus docmac | 1 | 15.1 | ~ |
| Brycinus sadleri | 1 | 24.6 | ~ |
| Haplochromis spp. | 5 | 15.2 ± 8.0 | 2.8–24.2 |
| Lates niloticus (< 25 cm) | 6 | 12.4 ± 7.0 | 3.9–23.5 |
| Lates niloticus (> 25 cm) | 17 | 5.9 ± 4.9 | 0.5–16.7 |
| Mormyrus kannume | 5 | 21.1 ± 7.1 | 12.5–29.8 |
| Oreochromis leucostictus | 2 | 3.2–4.3 | ~ |
| Oreochromis niloticus | 24 | 9.7 ± 7.6 | 1.2–29.1 |
| Oreochromis variabilis | 5 | 30.1 ± 34.1 | 3.5–87.6 |
| Protopterus aethiopicus | 5 | 2.8 ± 1.3 | 1.1–4.5 |
| Rastrineobola argentea (whole) | 8 | 80.9 ± 36.0 | 39.0–128.5 |
| Synodontis spp. | 7 | 22.8 ± 15.0 | 8.2–44.9 |
| Tilapia zilli | 5 | 7.4 ± 4.0 | 3.4–14.1 |
| Lake Ontario (Bay of Quinte) | |||
| Alosa pseudoharengus (alewife) | 3 | 25.9 ± 10.1 | 20.0–37.5 |
| Ameiurus nebulosus (brown bullhead) | 6 | 4.4 ± 0.6 | 3.3–5.0 |
| Aplodinotus grunniens (freshwater drum) | 3 | 0.8 ± 0.3 | 0.5–1.1 |
| Esox lucius (northern pike) | 8 | 10.2 ± 7.6 | 1.6–25.8 |
| Lepomis gibbosus (pumpkinseed) | 4 | 1.9 ± 1.0 | 0.7–2.9 |
| Lepomis macrochirus (bluegill) | 1 | 4.8 | ~ |
| Morone americana (white perch) | 9 | 4.5 ± 4.1 | 0.7–14.8 |
| Perca flavescens (yellow perch) | 7 | 3.1 ± 2.0 | 0.5–5.6 |
| Pomoxis nigromaculatus (black crappie) | 2 | 1.7 ± 0.3 | 1.5–1.9 |
| Stizostedion vitreum (walleye) | 14 | 2.1 ± 1.6 | 0.5–6.1 |
| Lake Erie (Western Basin) | |||
| Aplodinotus grunniens (freshwater drum) | 2 | 2.4 ± 6.0 | 1.7–10.1 |
| Coregonus clupeaformis (whitefish) | 5 | 4.1 ± 1.0 | 2.9–5.4 |
| Micropterus dolomieu (smallmouth bass) | 5 | 13.4 ± 17.8 | 1.5–43.6 |
| Morone americana (white perch) | 6 | 5.6 ± 4.9 | 1.9–15.0 |
| Morone chrysops (white bass) | 5 | 18.3 ± 8.7 | 4.2–27.1 |
| Perca flavescens (yellow perch) | 4 | 5.0 ± 1.4 | 3.6–7.0 |
| Stizostedion vitreum (walleye) | 5 | 23.9 ± 17.2 | 5.3–41.2 |
Where samples represent fish that were analyzed whole, this has been indicated in parentheses after the species name. All other values in this table are for fish muscle tissue.
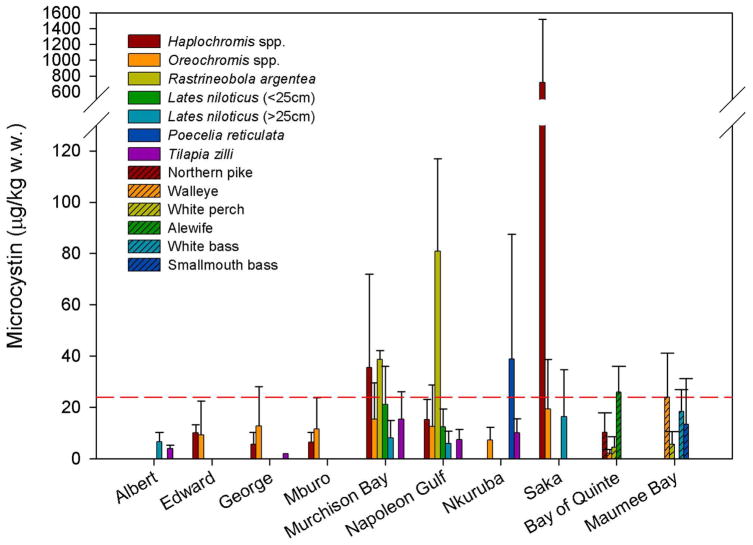
Figure 2.
Microcystin concentrations in several species of fish. The red line represents the fish microcystin concentration that would cause a consumer to exceed tolerable daily intake (TDI) values recommended by the WHO for chronic exposure (0.04 μg/kg body weight/day; which yields a threshold concentration of microcystin in fish of 24 μg/kg wet weight for an individual weighing 60 kg and consuming 100 g of fish/day).
The fish collected from the Ugandan study sites included a broad range of species, many of which form the basis of large commercial fisheries (e.g. Lates niloticus, Oreochromis spp., Rastrineobola argentea) and all of which are important local food sources. In the Ugandan lakes, the highest microcystin concentrations in fish were observed in Haplochromis spp. from hypereutrophic Lake Saka, where microcystin concentrations in water were also extremely high. This suggests that regular consumption of fish or untreated water from this lake may be associated with health risks. We also observed high microcystin concentrations in Rastrineobola argentea, a small zooplanktivorous cyprinid that now dominates landings in Lake Victoria and is of considerable importance to both commercial and subsistence fisheries (18). Microcystin concentrations in these fish ranged from 36.2–41.2 μg/kg w.w. in Murchison Bay, and from 39.0–129 μg/kg w.w. in Napoleon Gulf (Table 2, Figure 2). The WHO TDI for microcystin yields a threshold concentration of microcystin in fish of 24 μg/kg w.w. for a consumer weighing 60 kg and consuming 100 g of fish daily. The concentrations observed in R. argentea would be cause a consumer to exceed the WHO TDI for microcystin by a factor of 1.5–5.4. R. argentea is typically consumed whole, and as such, we analyzed whole fish for microcystin because this most accurately represents the exposure risk to consumers. Microcystin concentrations were likely high due to the inclusion of the viscera in the analyzed sample as well as cyanobacteria present in the gut of these small fish. High microcystin concentrations were also observed in the muscle tissue of small (<25 cm) Lates niloticus (Nile perch), Haplochromis spp., and some tilapiine cichlids (Oreochromis spp. and Tilapia zilli) (Table 2, Figure 2). These trends are of importance because the fish with the highest observed microcystin concentrations represent the less commercially marketable and less profitable fish that tend to be consumed by low-income local residents and those living in fishing communities. Large Lates niloticus from Lakes Victoria and Albert, which are economically important fish for export (18), pose no risk to consumers given the low microcystin concentrations observed in fish exceeding a total length of 25 cm (mean concentrations of 8.0, 5.9, and 6.7 μg/kg w.w. in Murchison Bay, Napoleon Gulf and Lake Albert respectively).
Microcystin concentrations in fish collected in summer from embayments on the North American great lakes experiencing seasonal cyanobacterial blooms (19) can reach levels similar to those observed at the Ugandan study sites (Table 2, Figure 2). In the western basin of Lake Erie, the highest microcystin concentrations were observed in walleye (5.3–41.2 μg/kg w.w.), white bass (4.2–27.1 μg/kg w.w.) and smallmouth bass (1.5–43.6 μg/kg w.w.). In the Bay of Quinte (Lake Ontario), the zooplanktivorous alewives had the highest microcystin concentrations (20.0–37.5 μg/kg w.w.), followed by northern pike (1.6–25.8 μg/kg w.w.). Many of these fish are important species for both sport and commercial fisheries, and, if eaten, several of the fish sampled would cause a consumer to exceed the WHO TDI for microcystin.
It is important to note that the WHO drinking water guidelines and TDI values have been developed based on the toxicity of the microcystin-LR congener. However, the anti-ADDA ELISA used in the current study is “congener independent” and as such the measured microcystin concentrations are not directly comparable with microcystin-LR based guideline values, and may in fact include high concentrations of congeners with different toxicity than microcystin-LR. However, the total microcystin concentrations in both fish and water often greatly exceeded WHO TDI values, confirming that even if the microcystin congeners present in these lakes are not the most toxic congeners, they are still likely to pose a considerable risk to consumers.
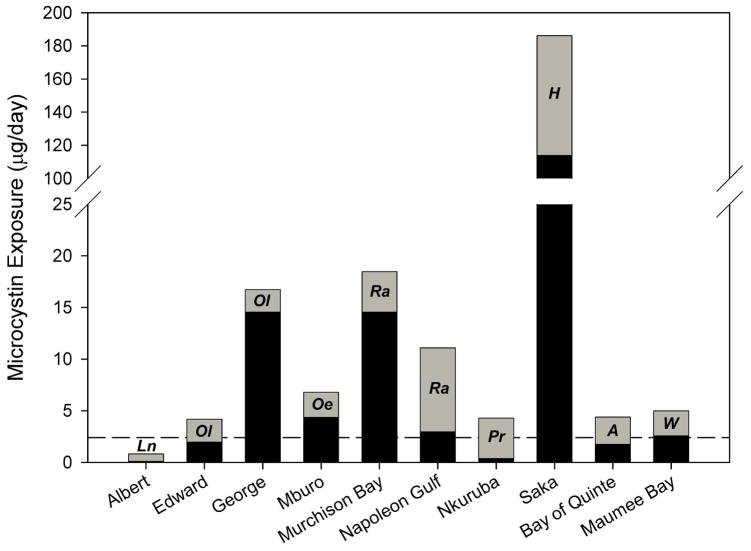
Figure 3 displays estimates of potential daily microcystin exposure based on an individual (weighing 60 kg) consuming water (2 L) and fish (100 g) from each of the study lakes. Potential daily exposure was calculated for each lake based on mean microcystin concentrations in water and mean microcystin concentrations in the fish taxa with the highest mean microcystin concentrations, as this provides a worst-case scenario of chronic daily exposure. At all sites but Lake Albert, such daily exposure estimates exceeded the WHO TDI for chronic exposure.
Figure 3.
Potential daily microcystin exposure for individuals consuming water and fish from the study lakes. The reference line indicates the threshold value at which a 60 kg consumer would exceed the WHO TDI (2.4 μg/day). Exposure from water is indicated in black, and is based on daily consumption of 2 L using mean microcystin concentrations in water. Exposure from fish is indicated in grey, and is based on daily consumption of 100 g using mean microcystin concentration in the fish taxa with the highest mean concentrations. The species used in calculating exposure from fish are indicated as follows: Ln (Lates niloticus), Ol (Oreochromis leucostictus), Oe (Oreochromis esculentus) Ra (Rastrineobola argentea), Pr (Poecelia reticulata), H (Haplochromis spp.), A (alewife), and W (walleye).
At the majority of study sites, potential exposure from water exceeded potential exposure from fish, so where people are getting drinking water and fish from the same lake, water tends to be the main source of exposure. However, fish can represent a significant and sometimes dominant source of microcystin to consumers, particularly where people are consuming fish species that have elevated microcystin concentrations. Based on the scenario outlined in Figure 3, the high relative importance of fish as a source of microcystin exposure in Lake Albert, Lake Edward, Napoleon Gulf (Lake Victoria), Lake Nkuruba, and the Bay of Quinte (Lake Ontario) indicates that even where chlorophyll and microcystin concentrations in the water are relatively low, microcystin exposure through fish consumption may increase total daily exposure to potentially detrimental levels. Given the high potential for microcystin exposure through fish consumption at several of the study sites, avoiding or treating drinking water would not eliminate the risk of exposure to microcystin. For example, in Napoleon Gulf, an individual eating 100 g of R. argentea would be exposed to 8.1 μg of microcystin, which is high enough to greatly exceed the recommended WHO TDI before even considering potential exposure from drinking water. Avoiding the consumption of some fish species (such as R. argentea and Haplochromis spp.) may be necessary to reduce significant exposure risk; however, in many households this may not be an option. Also, it is of importance to note that neither boiling water nor cooking fish prior to consumption is expected to appreciably reduce the risk of microcystin exposure (20, 21).
From a chronic exposure standpoint, year round presence of microcystin in the water and food webs of the Ugandan lakes is a likely scenario, given that cyanobacterial blooms can occur year-round in tropical lakes (22, 23), indicating the potential for persistent exposure of fish (and their human consumers) to possibly harmful levels of microcystin. However, in temperate lakes, where phytoplankton biomass is much lower during winter periods (24), year-round chronic exposure of aquatic food webs and human consumers to microcystin would not be expected. Although observed microcystin concentrations and potential daily microcystin exposure values for Maumee Bay and the Bay of Quinte sometimes exceeded the WHO TDI for chronic exposure to microcystin, these values were based on samples collected in the summer and early fall, a time period which is likely to capture the highest microcystin concentrations experienced throughout the year. Because fish are able to depurate microcystin when no longer exposed (25, 26), microcystin concentrations in fish are likely to decline in concert with microcystin in water as the winter season approaches. Also, for the Ugandan lakes, the likelihood that an individual is consuming both water and fish daily from the same lake is much higher than for the North American study sites, where individuals often have alternative sources of drinking water and food. These differences between the tropical and temperate study sites suggest that while the daily exposure scenario in Figure 3 may be realistic for Ugandan consumers, North American consumers are unlikely to experience chronic exposure to microcystin that exceeds WHO TDI guidelines.
These results demonstrate the broad prevalence of microcystin in water and fish from temperate and tropical lakes that support important commercial, sport, and subsistence fisheries and are critical sources of drinking water. Our observed microcystin concentrations in fish and water fall within the range of concentrations reported in other studies from around the world, including previously reported data from East Africa, North America, Egypt, Brazil, Argentina, and China (10, 27–34), confirming that accumulation of microcystin in fish is of global concern. As such, current guidelines for quantifying risk may not adequately reflect the potential for fish to make up a considerable proportion of microcystin exposure. Of particular concern are riparian fishing communities consuming water and small fish from the tropical study sites, where there is risk of chronic year-round exposure to microcystin, and potential detrimental health effects.
Acknowledgments
We thank the personnel of the National Fisheries Resources Research Institute (Uganda) and the Lake Erie Centre (Ohio) for support during fieldwork. We also thank the Ontario Ministry of Natural Resources and the Ministry of the Environment for providing fish samples from the Laurentian great lakes. This research was supported by NIH and GLFC grants to S.J.G, and an International Development Research Council Doctoral Research Award to A.E.P.
References
- 1.de Figueiredo DR, Azeiteiro UM, Esteves SM, Goncalves FJM, Pereira MJ. Microcystin-producing blooms––a serious global public health issue. Ecotox Environ Safe. 2004;59:151–163. doi: 10.1016/j.ecoenv.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Paerl HW, Huisman J. Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environ Microbiol Reports. 2009;1:27–37. doi: 10.1111/j.1758-2229.2008.00004.x. [DOI] [PubMed] [Google Scholar]
- 3.Sivonen L, Jones G. In: Toxic Cyanobacteria in Water: A Guide to Public Health Significance, Monitoring and Management. Chorus I, Bartram J, editors. E&FN Spon; London: 1999. pp. 41–111. [Google Scholar]
- 4.Falconer I, Humpage A. Tumour promotion by cyanobacterial toxins. Phycologia. 1996;35:6–11. [Google Scholar]
- 5.Chen J, Xie P, Li L, Xu J. First identification of the hepatotoxic microcystins in the serum of a chronically exposed human population together with indication of hepatocellular damage. Toxicol Sci. 2009;108:81–89. doi: 10.1093/toxsci/kfp009. [DOI] [PubMed] [Google Scholar]
- 6.WHO. Addendum to Volume 2: Health Criteria and Other Supporting Information. 2. World Health Organisation; Geneva: 1998. Guidelines for Drinking-water Quatlity. [Google Scholar]
- 7.Falconer I, Bartram J, Chorus I, Duiper-Goodman T, Utkilen H, Burch M, Codd GA. In: Toxic Cyanobacteria in Water: A Guide to Public Health Significance, Monitoring and Management. Chorus I, Bartram J, editors. E&FN Spon; London: 1999. pp. 156–178. [Google Scholar]
- 8.Ibelings BW, Chorus AR. Accumulation of cyanobacterial toxins in freshwater “seafood” and its consequences for public health: A review. Environ Pollut. 2007;150:177–192. doi: 10.1016/j.envpol.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Kotak BG, Zurawell RW, Prepas EE, Holmes FB. Microcystin-LR concentration in aquatic food web compartments from lakes of varying trophic status. Can J Fish Aquat Sci. 1996;53:1974–1985. [Google Scholar]
- 10.Magalhães VF, Marinho MM, Domingos P, Oliveira AC, Costa SM, Azevedo LO, Azevedo SM. Microcystins (cyanobacterial hepatotoxins) bioaccumulation in fish and crustaceans from Septiba Bay (Brasil, RJ) Toxicon. 2003;42:289–295. doi: 10.1016/s0041-0101(03)00144-2. [DOI] [PubMed] [Google Scholar]
- 11.Stainton MP, Capel MJ, Armstrong FAJ. The Chemical Analysis of Fresh Water. Canadian Fisheries and Marine Service; 1977. [Google Scholar]
- 12.Fischer WJ, Garthwaite I, Miles CO, Ross KM, Aggen JB, Chamberlin AR, Towers NR, Dietrich DR. Congener-independent immunoassay for microcystins and nodularins. Environ Sci Technol. 2001;35:4849–4856. doi: 10.1021/es011182f. [DOI] [PubMed] [Google Scholar]
- 13.Loftin KA, Meyer MT, Rubio F, Kamp L, Humphries E, Whereat E. Comparison of two cell lysis procedures for recovery of microcystins in water samples from Silver Lake in Dover, Delaware with microcystin producing cyanobacterial accumulations. USGS Open-File Report 2008–1341. 2008 [Google Scholar]
- 14.Wilson AE, Gossiaux DC, Höök TO, Berry JP, Landrum PF, Dyble J, Guildford SJ. Evaluation of the human health threat associated with the hepatotoxin microcystin in the muscle and liver tissues of yellow perch (Perca flavescens) Can J Fish Aquat Sci. 2008;65:1487–1497. [Google Scholar]
- 15.Ramlal P, Bugenyi F, Kling G, Nriagu J, Rudd J, Campbell L. Mercury concentrations in water, sediment, and biota from Lake Victoria, East Africa. J Great Lakes Res. 2003;29:283–291. [Google Scholar]
- 16.Vollenweider R, Kerekes J. Eutrophication of Waters: Monitoring, Assessment and Control. OECD; Paris: 1982. [Google Scholar]
- 17.Mugidde R, Hecky RE, Hendzel LL, Talyor WD. Pelagic nitrogen fixation in Lake Victoria (East Africa) J Great Lakes Res. 2003;29:76–88. [Google Scholar]
- 18.Kolding J, van Zwieten P, Mkumbo O, Silsbe G, Hecky R. In: The Ecosystem Approach to Fisheries. Bianchi G, Skjodal HR, editors. CABI; Wallingford: 2008. pp. 309–354. [Google Scholar]
- 19.Watson SB, Ridal J, Boyer GL. Microcystin-LR concentration in aquatic food web compartments from lakes of varying trophic status. Can J Fish Aquat Sci. 2008;65:1779–1796. [Google Scholar]
- 20.Harada K, Tsuji K, Watanabe M, Kondo F. Stability of microcystins from cyanobacteria. Effect of pH and temperature. Phycologia. 1996;35:83–88. [Google Scholar]
- 21.Zhang D, Xie P, Chen J. Effects of temperature on the stability of microcystins in muscle of fish and its consequences for food safety. Bulletin of Environmental Contamination and Toxicology. 2010;84:202–207. doi: 10.1007/s00128-009-9910-6. [DOI] [PubMed] [Google Scholar]
- 22.Kling HJ, Mugidde R, Hecky RE. In: Great Lakes of the World: Food webs, health and integrity. Munawar M, Hecky RE, editors. Backhuys; Leiden: 2001. pp. 47–66. [Google Scholar]
- 23.Oliver RL, Ganf GG. In: The Ecology of Cyanobacteria. Whitton BA, Potts M, editors. Kluwer Academic Publishers; Netherlands: 2000. pp. 149–194. [Google Scholar]
- 24.Munawar M, Munawar IF. The seasonality of phytoplankton in the North American Great Lakes, a comparative synthesis. Hydrobiologia. 1986;138:85–115. [Google Scholar]
- 25.Tencalla F, Dietrich D. Biochemical characterization of microcystin toxicity in rainbow trout (Oncorhynchus mykiss) Toxicon. 1997;35:583–595. doi: 10.1016/s0041-0101(96)00153-5. [DOI] [PubMed] [Google Scholar]
- 26.Xie L, Xie P, Ozawa K, Honma T, Yokoyama A, Park H. Dynamics of microcystins-LR and –RR in the phytoplanktivorous silver carp in a sub-chronic toxicity experiment. Env Pollution. 2004;127:431–439. doi: 10.1016/j.envpol.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Mohamed ZA, Carmichael WW, Hussein AA. Estimation of microcystins in the freshwater fish Oreochromis niloticus in an Egyptian fish farm containing a Microcystis bloom. Environ Toxicol. 2003;18:137–141. doi: 10.1002/tox.10111. [DOI] [PubMed] [Google Scholar]
- 28.Sekadende BC, Lylmo TJ, Kurmayer R. Microcystin production by cyanobacteria in the Mwanza Gulf (Lake Victoria, Tanzania) Hydrobiologia. 2005;543:299–304. [Google Scholar]
- 29.Xie L, Xie P, Guo L, Li L, Miyabara Y, Park H. Organ distribution and bioaccumulation of microcystins in freshwater fish at different trophic levels from the eutrophic Lake Chaohu, China. Environ Toxicol. 2005;20:293–300. doi: 10.1002/tox.20120. [DOI] [PubMed] [Google Scholar]
- 30.Deblois CP, Aranda-Rodriguez R, Giani A, Bird DF. Microcystin accumulation in liver and muscle of tilapia in two large Brazilian hydroelectric reservoirs. Toxicon. 2008;51:435–448. doi: 10.1016/j.toxicon.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Okello W, Portmann C, Erhard M, Gademann K, Kurmayer R. Occurrence of microcystin-producing cyanobacteria in Ugandan freshwater habitats. Environ Toxicol. 2009 doi: 10.1002/tox.20522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang D, Xie P, Liu Y, Qiu T. Transfer, distribution and bioaccumulation of microcystins in the aquatic food web in Lake Taihu, China, with potential risks to human health. Sci Total Environ. 2009;407:2191–2199. doi: 10.1016/j.scitotenv.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 33.Amé MV, Galanti LN, Menone ML, Gerpe MS, Moreno VJ, Wunderlin DA. Microcystin–LR, –RR, –YR and –LA in water samples and fishes from a shallow lake in Argentina. Harmful Algae. 2010;9:66–73. [Google Scholar]
- 34.Semyalo R, Rohrlack T, Naggawa C, Nyakairu GW. Microcystin concentrations in Nile tilapia (Oreochromis niloticus) caught from Murchison Bay, Lake Victoria and Lake Mburo, Uganda. Hydrobiologia. 2010;638:235–244. [Google Scholar]