Abstract
To test the hypothesis that co-delivery of synergistic drug combinations in the same liposome provides a better anti-tumor effect than the drugs administered in separate liposomes, fluoroorotic acid (FOA) alone and in combination with irinotecan (IRN) were encapsulated in liposomes and evaluated for their anti-tumor activity in the C26 colon carcinoma mouse model. Fluoroorotic acid was dissolved in 7 M urea to increase its solubility so it could be passively loaded into liposomes at a high concentration. IRN was remote loaded into liposomes that contained the ammonium salt of the multi-valent 1,2,3,4-butanetetratcarboxylic acid with a greater than 90% efficiency and at a drug to lipid ratio of 0.2/1. When the two molecules were loaded into the same liposome, FOA was used to remote load IRN. Modulation of the drug/lipid ratio, temperature, and loading time allowed for consistent co-encapsulation of FOA + IRN at various molar ratios. The anti-tumor activity of L-FOA, L-IRN, L-FOA-IRN (5:1), and the L-FOA + L-IRN mixture (5:1) were examined in the C26 mouse model. The maximum tolerated dose of L-FOA was 10 mg/kg given weekly as compared to 100 mg/kg of the non-encapsulated FOA. Delivering two drugs in the same liposome provided a statistically better antitumor effect than delivering the drugs in separate liposomes at the same drug ratio. However, the synergistic activity of the 5:1 ratio of free drugs measured on C26 cells in vitro was not observed in the C26 tumor mouse model. These findings point out the challenges to the design of synergistic treatment protocols based upon results from in vitro cytotoxicity studies. L-FOA at 10 mg/kg as a single agent provided the best anti-tumor efficacy which supports previous suggestions that L-FOA has useful properties as a liposome dependent drug.
Keywords: fluoroorotic acid, C26 colon carcinoma, co-encapsulation, remote loading, synergism
1. Introduction
The combination of a fluoropyrimidine, such as 5-fluorouracil (5-FU), and irinotecan (IRN) is widely used for the treatment of colorectal cancer because of the enhanced tumor killing effect exerted by this drug pair. 5-FU is an antimetabolite that inhibits RNA and DNA synthesis via the enzyme thymidylate synthase. IRN is a camptothecin prodrug that acts by inhibiting the enzyme topoisomerase I, a process which prevents the re-ligation of DNA after replication and causes single strand breaks. A number of studies report that IRN and fluoropyrimidines can be synergistic [1–4], meaning that the combined effect of these drugs is greater than the additive pharmacological effect of the combination. Although the exact mechanism of synergism of this drug combination is not clearly understood, it is believed that IRN recruits cells in S phase that allows increased fluoropyrimidine incorporation into DNA and induces apoptosis [1, 4, 5].
Certain ratios of drug combinations may be synergistic while other ratios may be additive or antagonistic [6]. Therefore, the therapeutic activity of a drug combination such as IRN and 5-FU depends on maintaining the synergistic ratio at the target site. Because drugs have diverse physico-chemical properties, it can be difficult to control the pharmacokinetics of two drugs in a manner that enables the drugs to reach target cells at the optimal ratio and concentration. Phospholipid bilayer vesicles (liposomes) have been used by many groups to enhance the therapeutic activity of anti-cancer drug combinations [5, 7–14]. Bally, Mayer and coworkers have been at the forefront of this new paradigm to improve combination chemotherapy by controlling drug ratios using liposome drug carriers [15, 16]. They have demonstrated that liposomes are able to maintain the encapsulated drug combination at the synergistic ratio for approximately 24 hr after systemic administration in mice [5, 13, 17]. Liposome drug combinations have significantly more therapeutic activity than free drug combinations [5, 13, 14, 18]. Therefore, liposomes are able to synchronize the pharmacokinetics and biodistribution of drug combinations and to control the ratio and dose of the drugs that reach the target site.
The activity of fluoropyrimidines and IRN can be enhanced via liposome delivery [5, 16, 18, 19]. Liposome irinotecan can efficiently encapsulated by remote loading [20–22] and the resulting liposomal IRN (L-IRN) has greater in vivo anti-tumor activity than free IRN. 5-FU, on the other hand, is difficult to retain in liposomes because of its hydrophobicity. FOA is an anionic prodrug of 5-FU that can be encapsulated and retained in liposomes. In in vitro cytotoxicity studies, the liposome encapsulated form is more active than the free form[23–25]. FOA also has significant antitumor activity [26]. However, the in vivo efficacy of liposomal FOA (L-FOA), has not been tested. Nor have FOA and IRN been combined in a liposome formulation.
In this study, we investigated the use of liposomes to deliver FOA and IRN. We describe our efforts to develop effective liposomal formulations encapsulating FOA alone, IRN alone, and FOA + IRN at synergistic molar ratios in order to test the hypothesis that co-encapsulation of two drugs in the same liposome can enhance the efficacy of synergistic agents compared to two drugs delivered in different liposomes. This study illustrates one example of how to co-encapsulate drugs with disparate physico-chemical properties, demonstrates how modifying the drug loading conditions can affect drug co-encapsulation, and provides insights on designing combination chemotherapy studies for assessing drug synergism.
2. Methods and Method
2.1 Materials
5-Fluoroorotic acid (FOA) was purchased from Research Products International (Mt. Prospect, IL). Irinotecan-HCl Trihydrate (IRN) with 98% purity was purchased from Ivy Fine Chemicals (Cherry Hill, NJ). 1,2,3,4-Butanetetracarboxylic acid (BTCA), cholesterol (Chol) and sulforhodamine B (SRB) were purchased from Sigma-Aldrich (St. Louis, MO). Distearoylphosphatidylcholine (DSPC) and methoxy-polyethylene glycol (MW2000)-DSPE (mPEG2000-DSPE) were products from Genzyme (Cambridge, MA). The above chemicals were reagent grade and used as received. C26 and HT29 cells were obtained from the University of California, San Francisco Cell Culture Facility.
2.2 Cell Culture
C26 murine colorectal cancer cells were maintained in RPMI 1640 media supplemented with 10% fetal calf serum. HT29 human colorectal cancer cells were maintained in McCoy’s 5A media supplemented with 10% fetal calf serum. The cells were cultured as a monolayer in 5% CO2 at 37 °C.
2.3 Cytotoxicity Assay
C26 cells were seeded in 96 well plates and incubated for 24 hr at 37 °C to allow for cell attachment. IRN + FOA in a fixed ratio (10:1, 5:1, 1:1, 1:5, and 1:10) were simultaneously added to cells at eight doses that capture the full range of cytotoxicity of the most potent drug. The cells were continuously exposed to the single drugs and pairs of drugs for 72 hr at 37 °C. Each concentration was tested in triplicate per plate. Cytotoxicity was evaluated using the sulforhodamine B assay (SRB) assay [27]. Briefly, the cells were fixed with 50% trichloroacetic acid and stained for 30 min with 0.4% SRB in 1% acetic acid (w/v). The protein bound dye was solubilized with 10 mM unbuffered Tris base, and the absorbance of each well was measured at 564 nm.
2.4 Drug Interaction Analysis
Dose-effect curves consisting of eight data points were generated for each drug alone and in the combinations. The effect for each concentration was normalized to the untreated controls as a percent of cell survival and then converted to fraction of affected cells. CalcuSyn software (Biosoft, Ferguson, MO) was used to analyze the drug interaction between FOA and IRN. This program uses the median effect principle to determine the combination index (CI), a term which quantitatively describes the degree of synergism or antagonism of a drug interaction [28, 29]. Synergism is indicated for CI < 1, additivity for CI = 1, and antagonism for CI > 1.
2.5 Preparing Liposomal FOA
Liposomes were composed of DSPC:Chol:mPEG-DSPE at a 55:40:5 molar ratio. Lipid mixtures were dissolved in chloroform and dried into a thin film under reduced pressure by rotary evaporation then placed under high vacuum overnight. The films were subsequently hydrated with 500 mM FOA in 7 M urea (adjusted to pH 7 with triethylamine or LiOH) at 65 °C and vortexed to obtain a lipid concentration of 50 mM. The multilamellar vesicles were then sonicated at 65 °C. The preparation was added to a dialysis cassette (10,000 MWCO) (Pierce Chemical Co, Rockford, IL) and dialyzed against 500 mL of 5 mM Hepes, 5% Glucose pH 7.4. For comparison, FOA was passively loaded into liposomes following the method of Heath and coworkers [25]. To assay for the encapsulated FOA concentration, an aliquot of liposomes from all preparations were diluted with phosphate buffered saline (PBS; 2.16 g/L Na2HPO4 7H20, 0.2 g/L KH2PO4, 0.2 g/L KCl, 8.0 g/L NaCl) and mixed with methanol:chloroform (1:1:1 v/v/v), vortexed, and centrifuged at 1,000 rpm for 10 min. Then the upper phase was mixed with 1M HCl. The encapsulated FOA concentration was determined by comparing the absorbance at 284 nm to a standard curve prepared with a solution from a blank lipid extraction. The liposome diameter and particle size distribution were measured by dynamic light scattering (Malvern Instruments, Westborough, MA). The average liposome diameter with encapsulated FOA was ~120 nm.
2.6 Preparing Liposomal IRN
The same lipid mixture was used for IRN encapsulation and was processed as described above. The films were subsequently hydrated with 300 mM BTCA (adjusted to pH 5.0 with NH4OH) at 65 °C and vortexed to obtain a lipid concentration of 100 mM. The liposomes were then sonicated at 65 °C and extruded through 200 nm and 100 nm polycarbonate membranes (Avestin, Ottawa, CA) at 65 °C. The liposomes were exchanged into 5 mM Hepes, 5% Dextrose pH 6.5 by size exclusion chromatography using a Sephadex G25 column. IRN was loaded by incubating the drug with liposomes (0.2/1 drug to lipid molar ratio) at 65°C for 1 hr. The liposome preparation was exchanged into Hepes Buffer (5 mM Hepes, 140 mM NaCl pH 7.4) by size exclusion chromatography using a Sephadex G25 column. To measure the encapsulated IRN concentration, an aliquot of liposomes was mixed with 1% Triton X-100, heated to 100°C until the cloud point was reached, and cooled down room temperature. The encapsulated IRN concentration was determined by comparing the absorbance at 370 nm to an IRN standard curve in the appropriate buffer. The liposome diameter and particle size distribution were measured by dynamic light scattering (Malvern Instruments, Westborough, MA). The average liposome diameter with encapsulated IRN was ~100 nm.
2.7 Liposome Co-encapsulation of FOA and IRN
The same lipid mixture was processed into thin films as outlined above. The lipid films were subsequently hydrated with 500 mM FOA in 7 M urea (adjusted to pH 7 with triethylamine) at 65 °C and vortexed to obtain a lipid concentration of 25 mM. The resulting multilamellar vesicles were then sonicated at 65 °C. The preparations were added to a dialysis cassette (10,000 MWCO) and dialyzed against 500 mL of 5 mM Hepes, 5% glucose pH 6.5. To load IRN into the FOA containing liposomes and achieve an encapsulated FOA:IRN 5:1 molar ratio, IRN was incubated with the liposomes at drug/lipid molar ratios ranging from 0.025/1 to 0.3/1, at loading temperatures of 40, 45, 50, or 60 °C and for incubation periods of 10, 30 or 60 min. The liposome preparations were exchanged into Hepes buffer (5 mM Hepes, 140 mM NaCl pH 7.4) by size exclusion chromatography using a Sephadex G25 column. To assay the drug content of the liposomes, an aliquot was mixed with 1% Triton X-100, heated to 100°C until the cloud point was reached, and then cooled down room temperature. The encapsulated IRN concentration was determined by comparing the absorbance at 370 nm to a standard curve. A second sample was diluted with PBS and mixed with methanol:chloroform (1:1:1 v/v/v), vortexed, and centrifuged at 1000 rpm for 10 min. Then the upper phase was mixed with 1M HCl. The encapsulated FOA concentration was determined by 1) calculating the absorbance due to FOA in the co-formulation at 284 nm according to the equation (A284)FOA = (A284)FOA+IRN − R(A284)IRN where R = [IRN Dilution Factor/FOA Dilution Factor] and 2) comparing (A284)FOA to a standard curve. The liposome diameter and particle size distribution were measured by dynamic light scattering. The average liposome diameter with co-encapsulated drugs was ~120 nm.
2.8 Animals
Eight to ten week old Balb/c mice (for C26 model and maximum tolerated dose studies) and athymic nu/nu mice (for HT29 model) were obtained from Simonsen Laboratories, Inc. (Gilroy, CA). Animal maintenance and experiments adhered to the NIH principles of laboratory animal care under a protocol approved by the Committee on Animal Research at the University of California, San Francisco.
2.9 FOA and L-FOA MTD Studies in Balb/c Mice
A solution of free FOA was made by dissolving the drug in 50 mM MOPS + 50 mM LiCl (pH adjusted to 7.4 with LiOH). L-FOA was prepared by hydrating liposomes with 500 mM FOA in 7 M urea (adjusted to pH 7 with triethylamine) as described above. In the first maximum tolerated dose (MTD) study, Balb/c mice (n=2 mice/group) were administered a single intravenous injection of FOA 100 mg/kg or L-FOA 10 mg/kg on Day 0. In another arm of the study, Balb/c mice (n=2 mice/group) were administered FOA 100 mg/kg or L-FOA 10 mg/kg by intravenous injections on a q4d schedule starting on Day 0. In a second MTD study, Balb/c mice (n=2 mice/group) were administered FOA 100 mg/kg by intravenous injections on a q7d schedule starting on Day 0. Mouse weight and overall health were monitored on alternate days. If a mouse’s body weight decreased by > 15% of the original weight or if a mouse looked unhealthy, treatments were stopped for the group to which the mouse belonged. Mice were sacrificed due to decrease in body weight > 20% of original weight.
2.10 Liposomal FOA+IRN Combination Therapy in C26 Mouse Model
C26 murine colorectal cells (3×105) suspended in 50 μL RPMI 1640 medium were inoculated subcutaneously in the right hind flank of each Balb/c mouse. On Day 8 after tumor implantation, mice were randomly distributed into treatment groups (n = 8). L-FOA-IRN (5:1) was prepared by hydrating liposomes with 500 mM FOA in 7 M urea (adjusted to pH 7 with triethylamine) and incubating IRN with liposomes (0.025/1 drug to lipid molar ratio) at 50 °C for 10 min as described above. Each treatment (~200 μL) was administered by tail vein injection on Day 8 and Day 15. Mouse tumor growth, weight, and overall heath were monitored on alternate days. Tumor volume was determined by measuring the tumor in three dimensions with calipers and calculated using the formula: tumor volume = length × width × height. Mice were sacrificed due to tumor burden (volume ≥ 2000 mm3) or decrease in body weight (> 20% loss). Mouse survival was analyzed by using MedCalc 8.2.1.0 for Windows (MedCalc Software, Mariakerke, Belgium).
2.11 L-IRN Chemotherapy in HT29 Mouse Model
HT29 human colorectal cells (5×106), suspended in 50 μL medium, were inoculated subcutaneously in the right hind flank of each athymic nu/nu mouse. On Day 8 after tumor implantation, mice were randomly distributed into treatment groups (n = 8). Each treatment (~200 μL) was administered by tail vein injection on Days 12, 14, 19, and 21. Mouse tumor growth, weight, and overall health were monitored on alternate days. Tumor volume was determined by measuring the tumor in two dimensions with calipers and calculated using the formula: tumor volume = ½ (length × width2). The percent tumor growth delay (%TGD) was calculated from the equation %TGD = (T-C)/C × 100, where T is the mean time for the tumor volume of a treatment group to reach a designated volume of 300 mm3 and C is the mean time for the control group to reach the designated volume of 300 mm3. Mice were sacrificed due to tumor burden (volume ≥ 2000 mm3) or decrease in body weight (>20% loss). Mouse survival was analyzed by using MedCalc 8.2.1.0 for Windows (MedCalc Software, Mariakerke, Belgium).
3. Results
3.1 Synergism of FOA + IRN
FOA and IRN were screened for synergy in C26 murine colorectal cancer cells at 10:1, 5:1, 1:1, 1:5, and 1:10 molar ratios. To determine whether this combination was synergistic, additive, or antagonistic, we used the median effect method which is the most widely utilized model for analyzing drug interactions[29]. In this method, synergism is indicated for combination index (CI) values < 1, additivity for CI = 1, and antagonism for CI > 1. Table 1 displays the CI values at the EC50, EC75, and EC90 for the five molar ratios tested. This drug combination was very synergistic at the 5:1 molar ratio and slightly synergistic at 10:1 molar ratio over a wide range of concentrations. However, FOA + IRN was mostly antagonistic at the 1:5 and 1:1 molar ratios, and additive at the 1:10 molar ratio.
Table 1.
FOA + IRN Combination Activity in C26 Cells
| Drug Combination | Ratios | Combination Index* | ||
|---|---|---|---|---|
| EC50 | EC75 | EC90 | ||
| FOA + IRN | 1:10 | 1.1 | 0.99 | 0.89 |
| 1:5 | 1.3 | 1.2 | 1.2 | |
| 1:1 | 1.3 | 1.2 | 1.1 | |
| 5:1 | 0.20 | 0.16 | 0.13 | |
| 10:1 | 0.89 | 0.88 | 0.88 | |
Combination Index is a quantity derived from the median effect equation that describes the degree of a drug interaction. Synergism is indicated for CI < 0.9 (Green), additivity for 0.9 < CI < 1.1 (Yellow), and antagonism for CI > 1.1 (Red).
Since delivery of FOA and IRN in liposomes may enhance the efficacy of the combination by maintaining the drugs at their synergistic ratio to tumor cells, we devised liposome formulations for each individual drug and a liposome formulation of the drugs pairs to examine this hypothesis.
3.2 Formulation Development of L-FOA
FOA is a weak acid and is charged in aqueous solution; thus it is difficult to actively load into pre-formed liposomes. Therefore, passive loading methods for encapsulating FOA within liposomes were investigated. Initially, L-FOA was prepared using an approach developed by Heath and coworkers [25]. In this method, the lipid films were hydrated with 50 mM solution of lithium salt of FOA that resulted in the liposomes encapsulating only 1–3 mM of FOA. This drug concentration is low and would require high injection volumes to achieve a therapeutic drug concentration. Therefore, we focused our efforts on ways to increase FOA concentration in liposomes. One method was to increase the solubility of FOA in order to make a more concentrated drug solution for passive loading. FOA was dissolved by using the chaotropic agent 7M urea and adjusted to pH 7 with either LiOH or TEA. By this tactic, we could increase the solubility by greater than 10 fold, and an FOA concentration as high as 650 mM FOA was obtained. This permitted the preparation of L-FOA formulations encapsulating 4–10 mM FOA by passively loading liposomes with 500 mM of TEA-FO or Li-FOA. Remote loading using zinc acetate or calcium acetate were also investigated [30]; however, we were unable to encapsulate FOA to high internal concentrations using these methods.
3.3 Maximum Tolerated Dose (MTD) Analysis of FOA and L-FOA in Balb/c Mice
The toxicity of free FOA in mice and anti-tumor activity of FOA in murine tumors has been described [26, 31]. However, liposomal FOA has only been evaluated in vitro [24, 25, 32]. A MTD study in Balb/c mice is shown in Fig. 1A. The weight of mice did not significantly decrease after one i.v. dose of FOA 100 mg/kg or L-FOA 10 mg/kg during the course of the study. Therefore, one i.v. dose of both formulations was well tolerated. A 2xq4d schedule of FOA 100 mg/kg and L-FOA 10 mg/kg was toxic to the mice.
Fig. 1.
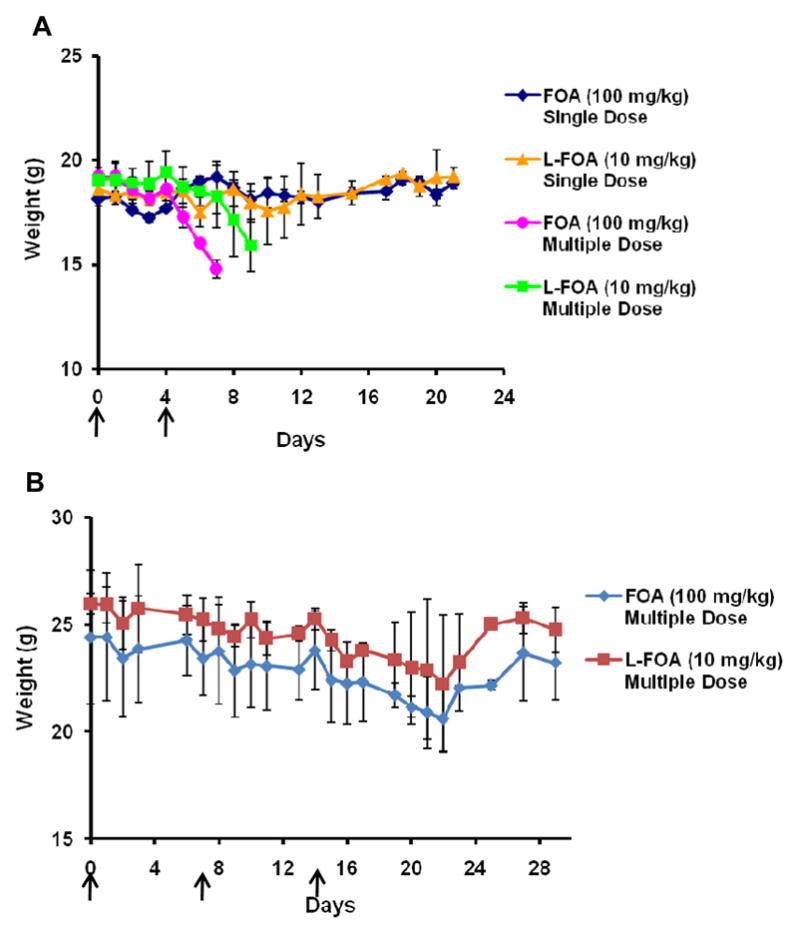
Maximum tolerated dose study of FOA 100 mg/kg and L-FOA 10 mg/kg in Balb/c mice. A. For single dose, FOA and L-FOA administered i.v. on Day 0. For multiple dose, FOA and L-FOA administered i.v. on Day 0 and four days apart (as indicated by arrows). B. FOA 100 mg/kg and L-FOA 10 mg/kg administered i.v. on a 3xq7d schedule starting on Day 0.
We then examined the MTD when FOA 100 mg/kg and L-FOA10 mg/kg were administered i.v. on a 3xq7d schedule, starting on Day 0 to Balb/c mice (Fig. 1B). The weight of the mice showed a non-statistically significant downward trend for twenty-one days after initiating dosing. However the weight of the animals then recovered, indicating that this dose and schedule provided an acceptable maximum tolerated dose in non-tumored mice.
3.4 Development of L-IRN Formulation
There are several published methods for formulating liposomal irinotecan by remote loading, a highly efficient technique used to encapsulate drugs into preformed liposomes via a transmembrane ion or pH gradient [20–22, 33]. Encapsulating IRN in liposomes containing 1,2,3,4-butanetetracarboxylic acid (BTCA) resulted in greater than a 90% encapsulation efficiency of IRN. Therefore, L-IRN was prepared with BTCA as the trapping agent for the single agent studies. Fig. S1 (supplementary information) depicts the proposed mechanism of IRN encapsulation with BTCA as the trapping agent.
3.5 Therapeutic Efficacy of L-IRN in HT29 Tumor Model
To verify that the antitumor efficacy of BTCA loaded L-IRN was similar to that observed in previous studies using sucrose octasulfate as the remote loading gradient generating molecule [20], we determined the anti-tumor activity of L-IRN in HT29 tumor-bearing mice. Free IRN and L-IRN were administered at a dose of 50 mg/kg intravenously to mice twice per week for a total of four doses. This is the MTD of free IRN, and when administered using the dosing regimen is well tolerated [20]. Fig. S2 shows that L-IRN 50 mg/kg had significantly greater tumor growth inhibition than IRN 50 mg/kg (p < 0.05). In fact, the %TGD of L-IRN was 114% whereas the %TGD of IRN was 36%. Also, mice treated with L-IRN 50 mg/kg had a slight increase in survival rate when compared to mice treated with free IRN 50 mg/kg (% increase in life span equaling 50% and 29.3% for L-IRN and IRN, respectively). Thus the BTCA loaded L-IRN had a similar efficacy as other liposomal IRN formulations evaluated in a HT29 tumor xenograft model [20]. L-IRN did not adversely affect the weight of the mice, which indicates that there is an acceptable toxicity profile in the mice at this dose (Fig. S3 in the supplementary information).
3.6 Liposome Co-encapsulation of FOA + IRN
To investigate whether liposomes encapsulating a synergistic ratio had better anti-tumor efficacy in vivo, liposomes co-encapsulating both drugs were formulated. The approach was to first passively load FOA into the liposomes and then to use the weak acid on FOA to remote load IRN. Cholesterol content, drug/lipid ratio, loading temperature, and incubation time can influence co-encapsulation of drugs into liposomes [22]. Therefore, various IRN drug/lipid ratios, loading temperatures, and incubations times were tested in order to load IRN into the FOA encapsulated liposomes and achieve an FOA/IRN 5:1 molar ratio. The approaches examined are summarized in Table 2. The encapsulated FOA concentration is significantly reduced during IRN remote loading. Decreasing the initial IRN drug/lipid ratio, loading temperature, and incubation time increased the retention of FOA in the liposomes. Whereas, increasing the initial IRN drug/lipid ratio and incubation time generally increased the encapsulated IRN concentration. Loading temperature did not significantly affect IRN encapsulation. Thus, the final loading protocol had to balance the competing tendency of the two drugs to be retained in the liposome.
Table 2.
Summary of Conditions and Outcomes of FOA + IRN Co-encapsulation into Liposomes at 5:1 Ratio
| Trial | [FOA]o (mM) | IRN D/L Ratio | Load Temp (°C) | Load Temp (min) | [IRN]f (mM) | [FOA]f (mM) | FOA/IRN Ratio |
|---|---|---|---|---|---|---|---|
| i | 7.2 | 0.1/1 | 50 | 60 | 1.21 | 1.74 | 1.4/1.0 |
| 0.2/1 | 2.18 | 0.94 | 1.0/2.3 | ||||
| 0.1/1 | 65 | 1.05 | 0.51 | 1.0/2.1 | |||
| 0.2/1 | 1.73 | 0.21 | 1.0/8.2 | ||||
| ii | 6.13 | 0.1/1 | 50 | 10 | 1.23 | 3.7 | 3.0/1.0 |
| 30 | 1.14 | 3.11 | 2.7/1.0 | ||||
| 60 | 2.29 | 2.05 | 1.1/1.0 | ||||
| iii | 8.69 | 0.1/1 | 40 | 30 | 1.08 | 2.43 | 2.2/1.0 |
| 0.2/1 | 1.93 | 1.81 | 1.0/1.1 | ||||
| 0.1/1 | 45 | 1.15 | 3.61 | 3.1/1.0 | |||
| 0.2/1 | 2.1 | 1.41 | 1.0/1.5 | ||||
| iv | 4.56 | 0.05/1 | 50 | 30 | 0.56 | 2.03 | 3.6/1.0 |
| 0.075/1 | 0.77 | 1.78 | 2.3/1.0 | ||||
| 0.1/1 | 1.07 | 1.53 | 1.5/1.0 | ||||
| 0.15/1 | 1.51 | 1.21 | 1.0/1.2 | ||||
| v | 4.77 | 0.025/1 | 50 | 30 | 0.39 | 2.13 | 5.4/1.0 |
[FOA]o: initial FOA concentration. D/L: drug to lipid ratio. [IRN]f: final IRN concentration. [FOA]f: final FOA concentration.
There is a strong correlation between the initial IRN drug/lipid ratio and: 1) the final encapsulated IRN concentration, 2) the final encapsulated FOA concentration, and 3) the co-encapsulated drug ratio (Fig. 2). A FOA/IRN 5:1 co-encapsulated molar ratio could be consistently achieved when remote loading IRN at a 0.025/1 drug/lipid ratio at 50 °C for 30 min. How the aforementioned parameters as well as cholesterol content would affect co-encapsulation of the drugs at a 1:1 molar ratio were also investigated (Table S2 and Fig. S4 in the supplementary information). Thus, the loading parameters were selected to reproducibly achieve a 5:1 or 1:1 molar ratio in the formulations of FOA/IRN in the liposomes.
Fig. 2.
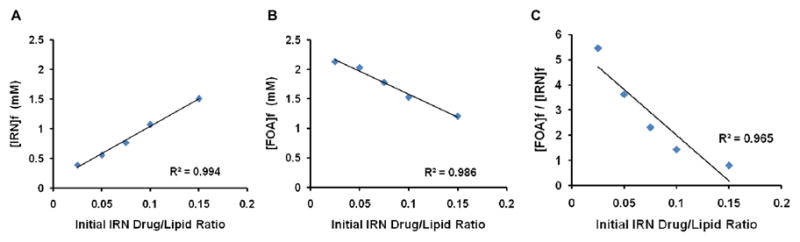
Effect of IRN drug to lipid ratio on FOA and IRN co-encapsulation at 5:1 molar ratio. Loading temperature was 50 °C and loading time was 30 min. A. Effect of initial IRN drug/lipid ratio on the final encapsulated IRN concentration ([IRN]f). B. Effect of initial IRN drug/lipid ratio on the final encapsulated FOA concentration ([FOA]f). C. Effect of initial IRN drug/lipid ratio on the final ratio of FOA and IRN in the liposomes ([FOA]f/[IRN]f).
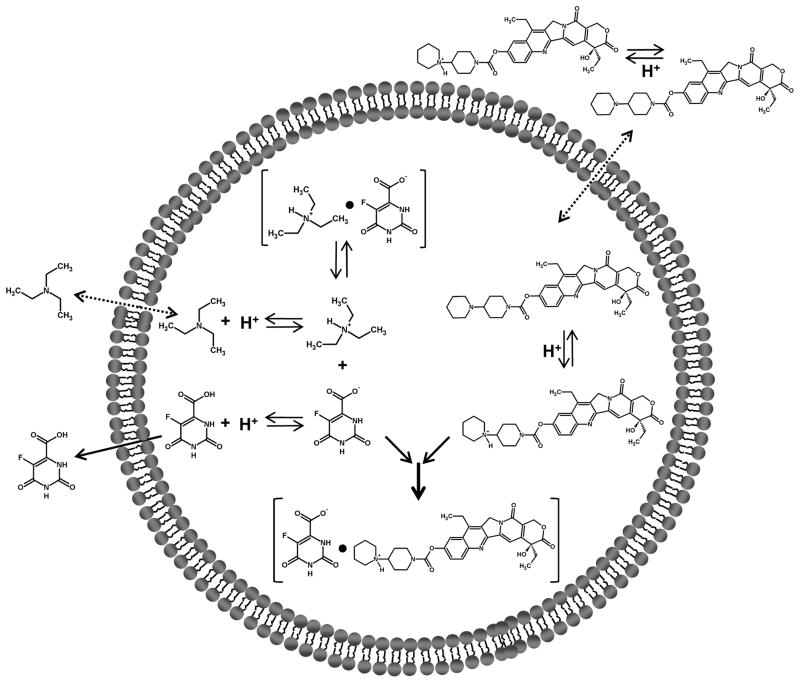
The proposed mechanism of co-encapsulation is shown in Fig. 3. We hypothesize that triethylamine present in the liposome internal buffer partitions out of the liposome interior similar to what occurs in other remote loading process [34, 35]. This causes a transmembrane pH gradient across the liposome bilayer (high [H+] in the interior, low [H+] in the exterior). IRN in the external buffer is then able to cross the liposome bilayer and become protonated. IRN within the liposomes that is positively charged (pKa = 8.1) interacts with FOA that is negatively charged (pKa = 2.4) and probably forms a complex [36].
Fig. 3.
Schematic diagram of proposed mechanism of co-encapsulation of FOA + IRN in liposomes.
3.7 Anti-tumor Effect in and Survival of C26 tumor-bearing mice treated with Liposomal FOA + IRN
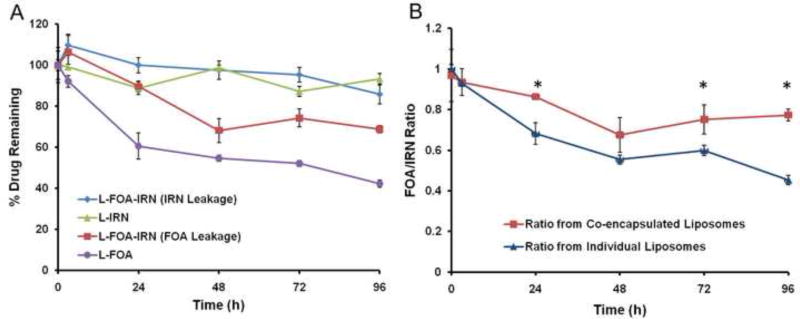
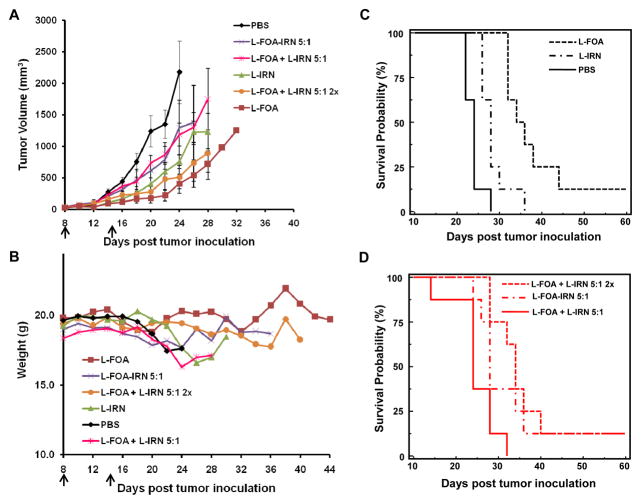
The therapeutic activity of the co-formulation was compared to the single formulations and to mixtures of the single formulations in C26 tumor-bearing mice (Fig. 4). L-FOA 57.4 μmol/kg (10 mg/kg) had a superior tumor growth inhibition and significantly longer survival rate (p=0.0027; log rank test) than L-IRN 73.8 μmol/kg (50 mg/kg). None of the combinations were more effective than L-FOA. Mice treated with drugs in the same liposome, L-FOA-IRN 5:1, had an increased survival compared to mice treated with drugs in separate liposome, L-FOA + L-IRN 5:1 (p=0.0414, log rank test). Furthermore, mice treated with L-FOA + L-IRN 5:1 at twice the dose, which is the same dose given as L-FOA, had a similar survival time as the L-FOA. Mice in both treatment groups lived longer than mice given L-FOA + L-IRN 5:1 at the lower dose (p=0.0015, log rank test). Thus the L-IRN provided no advantage in this combination and perhaps a disadvantage since mice from two groups, L-IRN and L-IRN + L-FOA 5:1, were steadily losing weight after the second i.v. injection (Fig. 4B).
Figure 4.
FOA and IRN release profile from L-FOA-IRN, L-IRN, and L-FOA. A. In vitro leakage from liposomes during 96 hr incubation in 33% serum at 37°C. B. Ratio of IRN and FOA released from the co-encapsulated and the individual liposomes over time. *Statistical significance (P < 0.05) between the ratio from co-encapsulated and separate liposomes as measured by Student’s t-test.
4. Discussion
The objective of this study was to investigate the hypothesis that co-encapsulation of two drugs in the same liposome can enhance the efficacy of synergistic agents compared to two drugs delivered in different liposomes. This required us to devise liposome formulations of FOA, IRN, and FOA + IRN combination.
The combination activity of FOA + IRN was tested in C26 cells. FOA + IRN at a 5:1 molar ratio was synergistic in C26 cells in vitro (Table 1). It was surprising that IRN showed good activity since it is a prodrug that has to be activated by a carboxylesterase to the active compound SN38. The results from the screen indicated that the synergism exhibited by FOA and IRN is ratio-dependent. This makes it important to control the ratios in vivo in order to achieve maximum therapy.
To prepare L-FOA, a method developed by Heath and coworkers [25] was initially used which allowed encapsulation of 1–3 mM of FOA. Dissolving FOA in 7M urea, which can disrupt hydrogen bonding between molecules, allowed us to make a more concentrated FOA solution. As a result, liposome that encapsulated higher concentrations (~10mM) of FOA could be prepared. With the L-FOA formulation, one could deliver a 10 mg/kg dose to mice in a 200 μL volume.
The MTD of L-FOA was established because: 1) L-FOA has never been tested in mice and 2) we needed to determine whether L-FOA could be administered safely at a schedule similar to an IRN dosing schedule. The maximum tolerated dose of L-FOA is 10 mg/kg, which is a 10 fold less dose than the MTD of free FOA. This enhanced toxicity is probably due to the longer circulation, sustained release, and enhanced accumulation of FOA to sites of toxicity due to the liposome formulation. This increased toxicity for a water soluble anti-metabolite has been observed in liposomal cytosine arabinoside formulations [37]. Balb/c mice were able to tolerate a single i.v. dose of both treatments, but could not endure multiple i.v. doses of FOA 100 mg/kg given four days apart (Fig. 1A). The mice also could not tolerate multiple i.v. doses of 10 mg/kg L-FOA given four days apart. Therefore, L-FOA could not be administered on the schedule followed in the L-IRN therapy study in HT29 mice. The MTD of the two formulations were also evaluated in the Balb/c mice on a 3×q7d dosing schedule (Fig. 1B). This is the schedule that Mayer and coworkers used to evaluate the therapeutic activity of their liposome IRN + floxuridine co-formulations [5]. The formulations were not toxic to the mice at this schedule; therefore, the weekly schedule was selected for use in animal studies with the FOA and IRN liposome formulations.
The therapeutic efficacy of BTCA loaded L-IRN was tested in a HT29 human xenograft mouse model at a similar dose and schedule used by Drummond and coworkers [20]. The data in Fig. S2 and Fig. S3 (supplementary information) demonstrate that L-IRN was efficacious and safe at the dose and schedule administered.
Lastly, a liposome formulation that encapsulated both FOA and IRN was developed. Co-encapsulating these two drugs in one liposome formulation was challenging because of the disparate physico-chemical properties of these two drugs. Weakly acidic drugs like FOA are traditionally passively loaded into liposomes; while amphipathic drugs like IRN can be actively loaded into preformed liposomes. Passive loading of drugs occurs through hydrating a lipid film with an aqueous solution of drug. This method is inefficient, and the resulting encapsulated drug concentration relies on the maximum solubility of the drug in solution [34]. It is difficult to remote load FOA because it is deprotonated in aqueous solution; therefore, FOA cannot readily cross a lipid bilayer. Whereas, IRN can partition into and diffuse across a bilayer when deprotonated. To co-encapsulate FOA and IRN in liposomes, FOA was passively loaded into vesicles and then was used to remote load IRN (Fig. 3). In this protocol, some of the FOA leaked out during IRN remote loading (Fig. 2, Table 2, as well as Table S1 and Fig. S4 in the supplementary information). Reducing the loading temperature, loading time and IRN drug/lipid ratio enhanced FOA retention but minimized IRN loading. The IRN drug/lipid ratio had the biggest impact on FOA retention and IRN loading, and adjusting the loading conditions allowed us to reproducibly encapsulate FOA + IRN at ratios between 5:1 to 1:1.
We investigated the combination therapy of FOA + IRN when delivered in the same liposome or when delivered together in separate liposomes in C26 tumor-bearing mice. L-FOA (57.4 μmol/kg) was more effective than L-IRN (73.8 μmol/kg). Although IRN + floxuridine, another fluoropyrimidine, co-encapsulated in liposomes had greater anti-tumor activity than the single liposome agents [5], we did not observe the same results. Although delivering the two drugs at the 5:1 ratio in the same liposome was statistically superior than delivering the two drugs at the 5:1 ratio in separate liposome, none of the combinations were more effective than L-FOA alone at 10 mg/kg (Fig. 4). This might have been due to: 1) lower dose of FOA in the co-encapsulated formulations than in the single liposome formulation or 2) co-delivery of the drugs in separate liposomes. The dose of FOA in the co-encapsulated formulation, L-FOA-IRN 5:1, was 28.7 μmol/kg while that in L-FOA was 57.4 μmol/kg. It was challenging to devise a co-encapsulated formulation with this concentration of FOA because of the leakage of FOA due to IRN remote loading in the liposome. To deliver a co-formulation with FOA at the dose of the single agent formulation, we had to deliver the drugs in separate liposomes. The L-FOA + L-IRN 5:1 (double strength) combination was administered at the same dose of FOA as the L-FOA formulation and had slightly but not significantly less, tumor growth inhibition as L-FOA.
It is possible that this co-formulation did not show enhanced efficacy compared to L-FOA because the drugs were in separate liposomes. Delivering drugs in the same liposome formulation may help to coordinate the release of the drugs in the cell such that the encapsulated drugs leak at similar rates [15, 16]. Perhaps the L-FOA + L-IRN 5:1 (double dose) mixture did not deliver the 5:1 synergistic ratio into the cell.
In conclusion, we describe the development and evaluation of liposomal formulations for FOA and IRN alone and in combination. An optimized method for encapsulating FOA into liposomes was developed which allowed us to encapsulate up to 10mM of FOA. This method enabled us to co-encapsulate FOA and IRN, which have disparate physico-chemical properties, at different molar ratios. L-FOA as a single agent has anti-tumor efficacy in the C26 tumor mouse model that was superior in tumor growth inhibition and in the increase in survival time compared to L-IRN 73.8 μmol/kg (50 mg/kg). However, the co-delivery of IRN with FOA in either the same or different liposomes failed to improve further the anti-tumor activity in the C26 model.
Supplementary Material
Fig. 5.
L-FOA-IRN combination therapy in C26 tumor-bearing mice. Balb/c mice (n=8) were treated with i.v. injections on Days 8 and 15 (as indicated by arrows). A. Anti-tumor activity. Error bars represent SEM. B. Effect of combination therapy on weight of C26 tumor-bearing mice. C and D. Survival curves. The treatment group s are PBS, L-FOA (10 mg/kg; 57.4 μmol/kg), L-IRN (50 mg/kg; 73.8 μmol/kg), L-FOA-IRN 5:1 (5 mg/kg or 28.7 μmol/kg FOA; 3.9 mg/kg or 5.7 μmol/kg IRN), L-FOA + L-IRN 5:1 (5 mg/kg or 28.7 μmol/kg FOA; 3.9 mg/kg or 5.7 μmol/kg IRN), and L-FOA + L-IRN 5:1 double strength (10 mg/kg or 57.4 μmol/kg FOA; 7.8 mg/kg or 11.5 μmol/kg IRN).
Acknowledgments
We are grateful for the financial support from the NIH (RO1 GM061851), and a PhRMA Foundation Pre-Doctoral Fellowship as well as an UNCF-Merck Dissertation Fellowship to KR. We thank Nichole Macaraeg and Megan E. Fox for their technical assistance, and Dr. B. Mark Evers at the University of Texas for providing us with the HT29 tumor cell line.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Azrak RG, Cao S, Slocum HK, Toth K, Durrani FA, Yin MB, Pendyala L, Zhang W, McLeod HL, Rustum YM. Therapeutic synergy between irinotecan and 5-fluorouracil against human tumor xenografts. Clin Cancer Res. 2004;10(3):1121–1129. doi: 10.1158/1078-0432.ccr-0913-3. [DOI] [PubMed] [Google Scholar]
- 2.Fischel JL, Rostagno P, Formento P, Dubreuil A, Etienne MC, Milano G. Ternary combination of irinotecan, fluorouracil-folinic acid and oxaliplatin: results on human colon cancer cell lines. Br J Cancer. 2001;84(4):579–585. doi: 10.1054/bjoc.2000.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grivicich I, Regner A, da Rocha AB, Kayser GB, Schunemann DP, Grass LB, Alves PA, Henriques JA, Schwartsmann G. The irinotecan/5-fluorouracil combination induces apoptosis and enhances manganese superoxide dismutase activity in HT-29 human colon carcinoma cells. Chemotherapy. 2005;51(2–3):93–102. doi: 10.1159/000085617. [DOI] [PubMed] [Google Scholar]
- 4.Peters GJ, van der Wilt CL, van Moorsel CJ, Kroep JR, Bergman AM, Ackland SP. Basis for effective combination cancer chemotherapy with antimetabolites. Pharmacol Ther. 2000;87(2–3):227–253. doi: 10.1016/s0163-7258(00)00086-3. [DOI] [PubMed] [Google Scholar]
- 5.Mayer LD, Harasym TO, Tardi PG, Harasym NL, Shew CR, Johnstone SA, Ramsay EC, Bally MB, Janoff AS. Ratiometric dosing of anticancer drug combinations: controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther. 2006;5(7):1854–1863. doi: 10.1158/1535-7163.MCT-06-0118. [DOI] [PubMed] [Google Scholar]
- 6.Tallarida RJ. Drug synergism: its detection and applications. J Pharmacol Exp Ther. 2001;298(3):865–872. [PubMed] [Google Scholar]
- 7.Vaage J, Donovan D, Mayhew E, Uster P, Woodle M. Therapy of mouse mammary carcinomas with vincristine and doxorubicin encapsulated in sterically stabilized liposomes. Int J Cancer. 1993;54(6):959–964. doi: 10.1002/ijc.2910540616. [DOI] [PubMed] [Google Scholar]
- 8.Abraham SA, McKenzie C, Masin D, Ng R, Harasym TO, Mayer LD, Bally MB. In vitro and in vivo characterization of doxorubicin and vincristine coencapsulated within liposomes through use of transition metal ion complexation and pH gradient loading. Clin Cancer Res. 2004;10(2):728–738. doi: 10.1158/1078-0432.ccr-1131-03. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Goh B, Lu W, Zhang Q, Chang A, Liu XY, Tan TM, Lee H. In vitro cytotoxicity of Stealth liposomes co-encapsulating doxorubicin and verapamil on doxorubicin-resistant tumor cells. Biol Pharm Bull. 2005;28(5):822–828. doi: 10.1248/bpb.28.822. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita Y, Krauze MT, Kawaguchi T, Noble CO, Drummond DC, Park JW, Bankiewicz KS. Convection-enhanced delivery of a topoisomerase I inhibitor (nanoliposomal topotecan) and a topoisomerase II inhibitor (pegylated liposomal doxorubicin) in intracranial brain tumor xenografts. Neuro Oncol. 2007;9(1):20–28. doi: 10.1215/15228517-2006-016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krauze MT, Noble CO, Kawaguchi T, Drummond D, Kirpotin DB, Yamashita Y, Kullberg E, Forsayeth J, Park JW, Bankiewicz KS. Convection-enhanced delivery of nanoliposomal CPT-11 (irinotecan) and PEGylated liposomal doxorubicin (Doxil) in rodent intracranial brain tumor xenografts. Neuro Oncol. 2007;9(4):393–403. doi: 10.1215/15228517-2007-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J, Lu Y, Lee A, Pan X, Yang X, Zhao X, Lee RJ. Reversal of multidrug resistance by transferrin-conjugated liposomes co-encapsulating doxorubicin and verapamil. J Pharm Pharm Sci. 2007;10(3):350–357. [PubMed] [Google Scholar]
- 13.Tardi P, Johnstone S, Harasym N, Xie S, Harasym T, Zisman N, Harvie P, Bermudes D, Mayer L. In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res. 2009;33(1):129–139. doi: 10.1016/j.leukres.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 14.Tardi PG, Dos Santos N, Harasym TO, Johnstone SA, Zisman N, Tsang AW, Bermudes DG, Mayer LD. Drug ratio-dependent antitumor activity of irinotecan and cisplatin combinations in vitro and in vivo. Mol Cancer Ther. 2009;8(8):2266–2275. doi: 10.1158/1535-7163.MCT-09-0243. [DOI] [PubMed] [Google Scholar]
- 15.Ramsay EC, Dos Santos N, Dragowska WH, Laskin JJ, Bally MB. The formulation of lipid-based nanotechnologies for the delivery of fixed dose anticancer drug combinations. Curr Drug Deliv. 2005;2(4):341–351. doi: 10.2174/156720105774370294. [DOI] [PubMed] [Google Scholar]
- 16.Mayer LD, Janoff AS. Optimizing combination chemotherapy by controlling drug ratios. Mol Interv. 2007;7(4):216–223. doi: 10.1124/mi.7.4.8. [DOI] [PubMed] [Google Scholar]
- 17.Bayne WF, Mayer LD, Swenson CE. Pharmacokinetics of CPX-351 (cytarabine/daunorubicin HCl) liposome injection in the mouse. J Pharm Sci. 2009;98(7):2540–2548. doi: 10.1002/jps.21620. [DOI] [PubMed] [Google Scholar]
- 18.Harasym TO, Tardi PG, Harasym NL, Harvie P, Johnstone SA, Mayer LD. Increased preclinical efficacy of irinotecan and floxuridine coencapsulated inside liposomes is associated with tumor delivery of synergistic drug ratios. Oncol Res. 2007;16(8):361–374. doi: 10.3727/000000006783980937. [DOI] [PubMed] [Google Scholar]
- 19.Batist G, Gelmon KA, Chi KN, Miller WH, Jr, Chia SK, Mayer LD, Swenson CE, Janoff AS, Louie AC. Safety, pharmacokinetics, and efficacy of CPX-1 liposome injection in patients with advanced solid tumors. Clin Cancer Res. 2009;15(2):692–700. doi: 10.1158/1078-0432.CCR-08-0515. [DOI] [PubMed] [Google Scholar]
- 20.Drummond DC, Noble CO, Guo Z, Hong K, Park JW, Kirpotin DB. Development of a highly active nanoliposomal irinotecan using a novel intraliposomal stabilization strategy. Cancer Res. 2006;66(6):3271–3277. doi: 10.1158/0008-5472.CAN-05-4007. [DOI] [PubMed] [Google Scholar]
- 21.Dicko A, Tardi P, Xie X, Mayer L. Role of copper gluconate/triethanolamine in irinotecan encapsulation inside the liposomes. Int J Pharm. 2007;337(1–2):219–228. doi: 10.1016/j.ijpharm.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Tardi PG, Gallagher RC, Johnstone S, Harasym N, Webb M, Bally MB, Mayer LD. Coencapsulation of irinotecan and floxuridine into low cholesterol-containing liposomes that coordinate drug release in vivo. Biochim Biophys Acta. 2007;1768(3):678–687. doi: 10.1016/j.bbamem.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Heath TD. Methodology and experimental design for the study of liposome-dependent drugs. Methods Enzymol. 2005;391:186–199. doi: 10.1016/S0076-6879(05)91011-2. [DOI] [PubMed] [Google Scholar]
- 24.Heath TD, Lopez NG, Lewis GP, Stern WH. Antiproliferative and anticontractile effects of liposome encapsulated fluoroorotate. Invest Ophthalmol Vis Sci. 1987;28(8):1365–1372. [PubMed] [Google Scholar]
- 25.Heath TD, Lopez NG, Stern WH, Papahadjopoulos D. 5-Fluoroorotate: a new liposome-dependent cytotoxic agent. FEBS Lett. 1985;187(1):73–75. doi: 10.1016/0014-5793(85)81217-5. [DOI] [PubMed] [Google Scholar]
- 26.Heidelberger C, Griesbach L, Montag BJ, Mooren D, Cruz O, Schnitzer RJ, Grunberg E. Studies on fluorinated pyrimidines. II. Effects on transplanted tumors. Cancer Res. 1958;18(3):305–317. [PubMed] [Google Scholar]
- 27.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82(13):1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 28.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 29.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 30.Clerc S, Barenholz Y. Loading of amphipathic weak acids into liposomes in response to transmembrane calcium acetate gradients. Biochim Biophys Acta. 1995;1240(2):257–265. doi: 10.1016/0005-2736(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 31.Bono VH, Jr, Cheng CC, Frei E, 3rd, Kelly MG. Mehtyl-5-Fluoroorotate: Synthesis and Comparison with 5-Fluoroorotic Acid with Respect to Biological Activity and Cell Entry. Cancer Res. 1964;24:513–517. [PubMed] [Google Scholar]
- 32.Straubinger RM, Lopez NG, Debs RJ, Hong K, Papahadjopoulos D. Liposome-based therapy of human ovarian cancer: parameters determining potency of negatively charged and antibody-targeted liposomes. Cancer Res. 1988;48(18):5237–5245. [PubMed] [Google Scholar]
- 33.Drummond DC, Noble CO, Hayes ME, Park JW, Kirpotin DB. Pharmacokinetics and in vivo drug release rates in liposomal nanocarrier development. J Pharm Sci. 2008;97(11):4696–4740. doi: 10.1002/jps.21358. [DOI] [PubMed] [Google Scholar]
- 34.Abraham SA, Waterhouse DN, Mayer LD, Cullis PR, Madden TD, Bally MB. The liposomal formulation of doxorubicin. Methods Enzymol. 2005;391:71–97. doi: 10.1016/S0076-6879(05)91004-5. [DOI] [PubMed] [Google Scholar]
- 35.Zucker D, Marcus D, Barenholz Y, Goldblum A. Liposome drugs’ loading efficiency: a working model based on loading conditions and drug’s physicochemical properties. J Control Release. 2009;139(1):73–80. doi: 10.1016/j.jconrel.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 36.Dicko A, Frazier AA, Liboiron BD, Hinderliter A, Ellena JF, Xie X, Cho C, Weber T, Tardi PG, Cabral-Lilly D, Cafiso DS, Mayer LD. Intra and inter-molecular interactions dictate the aggregation state of irinotecan co-encapsulated with floxuridine inside liposomes. Pharm Res. 2008;25(7):1702–1713. doi: 10.1007/s11095-008-9561-z. [DOI] [PubMed] [Google Scholar]
- 37.Mayhew E, Rustum YM, Szoka F, Papahadjopoulos D. Role of cholesterol in enhancing the antitumor activity of cytosine arabinoside entrapped in liposomes. Cancer Treat Rep. 1979;63(11–12):1923–1928. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.