Abstract
Background
Biologic grafts used in ventral hernia repair are derived from various sources and undergo different post-tissue-harvesting processing, handling, and sterilization techniques. It is unclear how these various characteristics impact graft response in the setting of contamination. We evaluated four materials in an infected hernia repair animal model using fluorescence imaging and quantitative culture studies.
Methods
One hundred seven rats underwent creation of a chronic hernia. They were then repaired with one synthetic polyester control material (n = 12) and four different biologic grafts (n = 24 per material). Biologic grafts evaluated included Surgisis (porcine small intestinal submucosa), Permacol (crosslinked porcine dermis), Xenmatrix (noncrosslinked porcine dermis), and Strattice (noncrosslinked porcine dermis). Half of the repairs in each group were inoculated with Staphylococcus aureus at 104 CFU/ml and survived for 30 days without systemic antibiotics. Animals then underwent fluorescence imaging and quantitative bacterial studies.
Results
All clean repairs remained sterile. Rates of bacterial clearance were as follows: polyester synthetic 0%, Surgisis 58%, Permacol 67%, Xenmatrix 75%, and Strattice 92% (P = 0.003). Quantitative bacterial counts had a similar trend in bacterial clearance: polyester synthetic 1 × 106 CFU/g, Surgisis 4.3 × 105 CFU/g, Permacol 1.7 × 103 CFU/g, Xenmatrix 46 CFU/g, and Strattice 31 CFU/g (P = 0.001). Fluorescence imaging was unable to detect low bacterial fluorescence counts observed on bacterial studies.
Conclusion
Biologic grafts, in comparison to synthetic material, are able to clear a Staphylococcus aureus contamination; however, they are able to do so at different rates. Bacterial clearance correlated to the level of residual bacterial burden observed in our study. Post-tissue-harvesting processing, handling, and sterilization techniques may contribute to this observed difference in ability to clear bacteria.
Keywords: Biologic mesh, Infection, Hernia repair
The repair of complex hernias in the setting of infection is a challenging problem that has traditionally been approached with staged repair. The introduction of biologic grafts into surgical practice over the last decade has provided surgeons with a potentially more favorable alternative in the setting of contamination where synthetic materials are otherwise contraindicated.
To date, there are at least 12 biologic grafts approved for the repair of ventral abdominal wall defects. Despite the general term “biologic grafts,” their characteristics are heterogeneous and they are derived from various sources (porcine dermis, bovine dermis, bovine pericardium, porcine small intestinal submucosa, human dermis, and fetal bovine dermis), undergo different tissue processing (no crosslinking, diisocyanate crosslinking, gluteraldehyde crosslinking, or EDAC crosslinking), and different sterilization techniques (ethylene oxide, gamma radiation, liquid alcohol, e-beam radiation) [1].
It is unclear how these various characteristics might impact the graft’s response in the setting of contamination. The availability of clinical studies in the setting of infection for many of these materials is limited, with no single clinical study comparing outcomes associated with two or more materials when exposed to the same infectious challenge. Through the use of fluorescence imaging and quantitative culture studies, we compare four biologic grafts and their ability to clear a Staphylococcus aureus contamination compared to a synthetic mesh in a chronic hernia repair animal model.
Materials and methods
Animals
Female Sprague-Dawley rats (Charles River Inc., Wilmington, MA), weighing between 200 and 220 g, were acclimated and housed under standard conditions. All animal care and operative procedures were performed in accordance with the U.S. Public Health Service Guide for the Care of Laboratory Animals (NIH Publication 85–23, 1985) and were performed with the prior approval of the Case University Institutional Animal Care and Use Committee. Animals were allowed ad libitum intake of standard rat chow and water throughout the study. Prior to both surgeries, animals underwent general anesthesia with inhaled isoflurane, and the abdominal wall was clipped and prepped with 70% chlorhexidine. Following every surgery, pain control consisted of local marcaine (5% diluted 1:10) at the time of incision closure and subcutaneous buprenorphine (0.03 mg/100 g) daily for four days. No systemically administered antibiotics were given during this study.
Hernia induction
One hundred seven rats underwent creation of a chronic hernia. A 1-cm transverse incision was made at the level of the xiphoid. A full-thickness skin flap (5 cm) was created at the avascular prefascial plane above the linea alba. The linea alba was then sharply cut under direct visualization for 4 cm to create a full-thickness laparotomy. The skin was then closed 4-0 Vicryl. After surviving 30 days, rats developed an abdominal wall hernia.
Chronic hernia repair
Animals were randomly assigned to undergo biologic mesh bridge repair (0.5-cm mesh-tissue overlap) with Permacol (Covidien, Mansfield, MA) (crosslinked porcine dermis; n = 24), Surgisis (Cook, Bloomington, IN) (noncrosslinked porcine small intestinal submucosa; n = 24), Xenmatrix (Bard Davol, Warwick, RI) (noncrosslinked porcine dermis; n = 24), or Strattice (LifeCell, Branchburg, NJ) (noncrosslinked porcine dermis; n = 24). Prior to implantation, all materials were properly rehydrated or soaked as directed by the manufacturer. Another group of animals were randomized to the synthetic control arm in which Parietex (Covidien) (three-dimensional polyester woven mesh; n = 12) was used. A full-thickness skin flap was created in the prefascial avascular plane beginning at the prior xiphoid incision and extended caudally to create a flap beginning 1 cm lateral to the midline. Following hernia sac excision, the hernia defect was measured and the mesh was then secured in place with interrupted 4-0 Prolene sutures approximately 0.5 cm apart. Following skin closure, half the animals in each group were inoculated as described below and survived for 30 days. The remaining animals underwent sterile closure to serve as the uninfected clean controls.
Bacterial preparation and infecting hernia repairs
A clinical strain (Seattle 1945) of Staphylococcus aureus (SA) transformed with a green fluorescent protein (GFP)-labeled plasmid to produce SA 1945GFPuvr was used in this study [2]. The strain was recovered from storage at −70°C by subculture onto a tryptic soy agar (TSA) plate with 5% sheep blood, which was then incubated at 37°C for 24 h. An individual colony was then inoculated in 5 ml of brain heart infusion (BHI) broth and incubated in a 37°C shaker at 200 rpm for 24 h. A 1:50 dilution of the overnight bacterial broth was then created and placed in a 37°C shaker at 200 rpm for 2.5 h, allowing the bacteria to reach log phase growth at a concentration of 108 CFU/ml based on optical density. This growth was then serially diluted in sterile 0.9% normal saline (NS) to a concentration of 105 CFU/ml and a 100-µl volume was used as the inoculum for the infected animals. Half the animals in each group were inoculated with GFP SA (contaminated cases, n = 12 per biologic group; n = 6 per synthetic mesh). The inoculum was introduced onto each mesh following skin closure via a skin puncture with a 25-gauge needle. The viability and inoculum concentration of the culture were verified on the day of the study. For our in vitro Maestro imaging studies, the same methodology was used and serial dilutions (103–108 CFU/ml) of GFP SA broth were created.
Necropsy and culture studies
Animals were euthanized 30 days after hernia repair and the abdominal wall was clipped and prepped with chlorhexidine. After the skin was sharply removed from the abdominal wall, a full-thickness abdominal wall explant was procured. Abdominal wall explants underwent immediate fluorescence imaging using Maestro (methods described below). Following imaging, a 6-mm sterile punch biopsy of both tissue and mesh was obtained, weighed, placed in 1 cc of sterile 0.9% saline, and homogenized. Serial tenfold dilutions of the mesh and tissue homogenate were made in 0.9% saline and 100-µl volumes of the original suspension, then the dilutions were plated on TSA plates with 5% sheep blood and incubated for 48 h at 37°C. Bacterial growth was quantified from plates showing 30–300 CFU per plate and expressed as CFU/g of specimen based on the weight of each specimen. Bacterial clearance, as a percentage, was defined as the number of animals with sterile cultures divided by the total number of infected animals. GFP SA was identified on plates by green fluorescence emitted from colonies after UV light exposure. Colonies that did not fluoresce were identified by conventional microbiological methods.
Maestro fluorescence imaging
Spectral fluorescence images of serial bacterial broth concentrations and explanted abdominal wall were obtained using the Maestro™ In-Vivo Imaging System (CRi, Inc., Woburn, MA). A bandpass filter appropriate for the fluorochrome of interest (GFP; Ex = 445–490 nm, Em = 515 long pass filter; acquisition settings = 500–720) was used for excitation and emission light, respectively. The tunable filter was automatically stepped in 10-nm increments while the camera captured images at an autoexposure. To evaluate signal intensities, regions of interest (ROI) were selected over the subject of interest and the total fluorescence signal was determined. Total signal in the ROI (in photons) measured at the surface of the subject of interest is divided by its area (in pixels) as well as exposure time (in ms). The spectral fluorescent images consisting of autofluorescence spectra and GFP SA were captured and unmixed based on their spectral patterns using Maestro™ software (CRi, Inc.). Spectral libraries were generated by assigning spectral peaks to background autofluorescence (biologic mesh and tissue), background from the imaging stage and in vitro plate, and fluorescence from GFP bacteria. The spectral libraries were manually computed using the Maestro software. All analysis was based on the spectral library determined from the spectral shift. Maestro signal intensities are represented as relative fluorescence units (RFUs).
In vitro imaging
Using a 24-black-well plate, Maestro imaging was performed on serial concentrations (103–108 CFU/ml) of GFP SA in 1-cc volumes as described above. This method allowed us to determine the minimal level of GFP SA signal detection using Maestro imaging.
Abdominal wall explants imaging
The same Maestro settings were then used when imaging the explanted abdominal walls. In order to subtract any background autofluorescence derived from the biologic material, abdominal wall explants of control (clean) repairs were used as the imaging control. Half the animals in the biologic graft group were imaged (n = 12 per material; total n = 48), while all the synthetic abdominal wall explants were imaged (n = 12).
Data analysis
Data were analyzed using Stata 10 (StataCorp LP, College Station, TX). A correlation curve was determined for the in vitro study. Percent bacterial clearance and average bacterial counts (in CFU/g) were obtained following culture studies. For statistical purposes, when no GFP SA was detected on TSA plates, the minimum number of colonies detectable was used (300 CFU/ml) in place of a 0-CFU/ml count. Culture results were otherwise reported based on obtained bacterial growth numbers. Mann-Whitney, post-hoc, and Fisher’s exact tests were performed where appropriate, and P < 0.05 was considered significant.
Results
One hundred seven animals underwent hernia repair and 106 survived 30 days following repair. One early technical related death occurred in a sterile repair group (Strattice). Low numbers of coagulase negative and positive staphylococci (not the GFP SA inoculated) were isolated from a few samples, likely derived from skin contamination at the time of surgical necropsy and were excluded from analysis.
Microbiology results
Clean cases
All clean repair cultures (Permacol, Surgisis, Xenmatrix, Strattice, and Parietex) remained sterile after a 30-day survival.
Contaminated cases
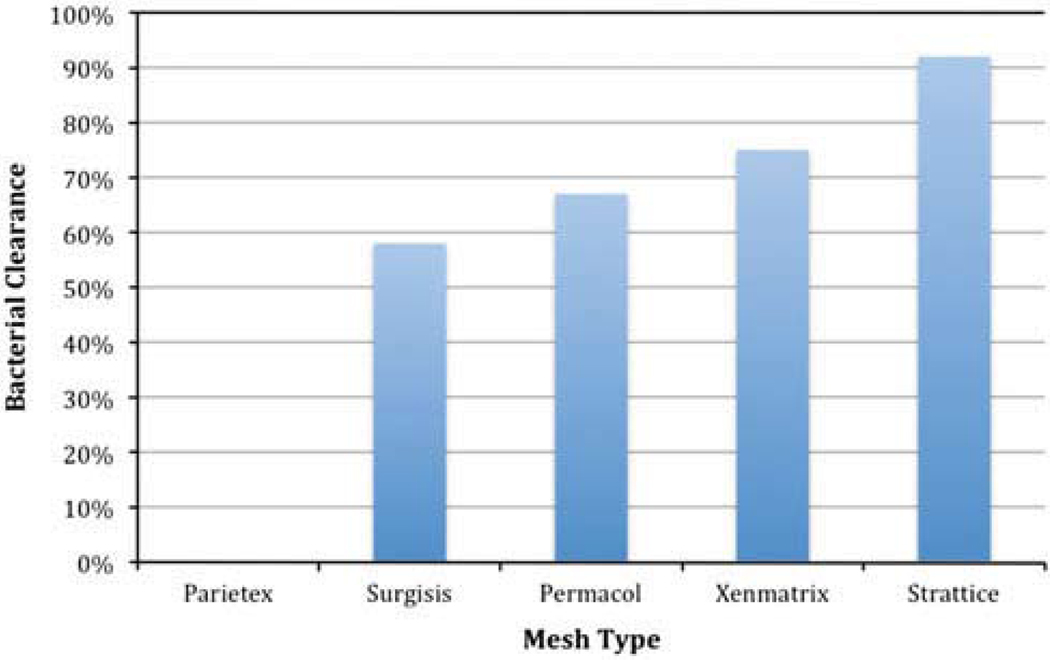
None of the infected synthetic Parietex repairs cleared the GFP SA infection (0%; n = 0/6). A decreased and varied rate of bacterial clearance was observed across the four biologic grafts: Surgisis 58% (n = 7/12), Permacol 67% (n = 8/12), Xenmatrix 75% (n = 9/12), and Strattice 92% (n = 11/12) (Fig. 1). There was a statistically significant difference in rates of GFP SA bacterial clearance across all five infected groups (P = 0.003). Quantitative bacterial counts for GFP SA showed a similar trend for bacterial clearance: Parietex 1 × 106 CFU/g (SD = 6.7 × 105 CFU/g), Surgisis 4.3 × 105 CFU/g (SD = 1.4 × 106 CFU/g), Permacol 1.7 × 103 CFU/g (SD = 4.3 × 103 CFU/g), Xenmatrix 46 CFU/g (SD = 88 CFU/g), and Strattice 31 CFU/g (SD = 1 × 102 CFU/g) (P = 0.001). When the bacterial counts of the four biologic grafts were compared alone, there was a significant difference among them as well (P = 0.01).
Fig. 1.
Rates of Staphylococcus aureus bacterial clearance achieved among various hernia repair materials after a 30-day survival in an infected hernia repair rat model (P = 0.003)
In vitro Maestro imaging
There was a positive linear correlation (R2 = 0.9121; Y = 715X + 186) between the Maestro fluorescence imaging signal and bacterial counts in GFP SA broth serial dilutions above a concentration of 105 CFU/ml. Below this concentration, Maestro fluorescence imaging was unable to detect an adequate GFP signal. This was determined to be the lower limit of GFP SA detection with Maestro fluorescence imaging.
Explant Maestro imaging
At baseline, all biologic materials had a high inherent green autofluorescence, which fell into a range similar to that of the GFP fluorescence spectra. Imaging of abdominal wall explants was unable to detect the presence of GFP SA in the contaminated animals or a difference in fluorescence signal between clean and contaminated cases (0 RFU signal/group). While quantitative microbiology studies more accurately represented GFP SA growth in some specimens, the persistent bacterial burden appeared to be below the detectable limit of Maestro fluorescence imaging. The discussion that follows is based primarily on our quantitative microbiology results.
Discussion
Use of the widely available biologic grafts in the setting of contaminated abdominal wall reconstruction is increasing despite a lack of extensive evaluation of their ability to clear bacteria under contaminated conditions. To our knowledge, this is the first animal study that compares four biologic grafts with the same bacterial contamination methodology. Using quantitative bacterial culture studies, we showed that biologic grafts, compared to a synthetic material, can decrease or clear a Staphylococcus aureus (SA) contamination in the setting of a chronic hernia repair model, with varied ability noted across the different materials.
Considering the quantity and variety of biologic grafts available to the reconstructive surgeon, there is a lack of studies evaluating these materials in a preclinical setting. Of the available studies in the literature, most are limited to three materials: Permacol, Surgisis, and Alloderm [3–19]. While these studies evaluate important characteristics associated with biologic grafts, they do so in a sterile setting. Such studies serve as measures of safety and possible outcomes under the most ideal circumstances. These studies are often difficult to translate into current clinical practice because most biologic grafts will be placed in a contaminated setting, where synthetic meshes are otherwise contraindicated. While there are a few studies in which biologic materials are exposed to a bacterial challenge, a short survival time and varied bacterial techniques limit interpretation [20–22].
In our study we exposed four biologic grafts to a moderate level of bacterial inoculum with SA at the time of repair (104 CFU/ml GFP SA) and evaluated them at 30 days with quantitative cultures (CFU/g) and fluorescence imaging. When compared to our synthetic infection control group, all biologic materials appeared to clear the contamination more effectively, with 50% or less of them actually growing any SA on quantitative bacterial cultures. In addition, the biologic grafts had decreased bacterial burden (CFU/g) compared to the synthetic control mesh. Maestro fluorescence imaging was unable to detect the presence of GFP SA across our materials primarily because of the overall low bacterial burden present on the biologic grafts. The ability to clear contamination, however, was quite varied among the different materials as evidenced by our quantitative microbiology results.
The biologic grafts compared in our study are widely available materials that are inherently different based on source and post-tissue-harvesting processing techniques. Their inherent differences might play an important role in how well they can clear SA contamination. While all materials in our study are porcine-derived, three are from dermal sources (Permacol, Xenmatrix, and Strattice), one has small intestinal submucosa as its source (Surgisis), and one undergoes the chemical processing of crosslinking (Permacol). Surgisis had the highest overall bacterial recovery on quantitative cultures, an outcome that might have been related to the delamination process and the subsequent central region of poor tissue infiltration found in our animals on gross inspection. In our study we had available the 8-ply design of Surgisis. It would be interesting to evaluate and compare our current findings to those of the more recent Surgisis designs as their modification in processing techniques might change our results.
In comparison to Surgisis, the dermal-based products (Permacol, Xenmatrix, and Strattice) all appeared intact with no gross material breakdown noted on necropsy. This lack of breakdown might have provided a more favorable scenario for the host to address the infection. Despite appearing grossly unchanged, Permacol had the second highest bacterial burden on quantitative cultures. This may be a characteristic that could be related to its crosslinking processing. While the process of crosslinking is intended to prevent host and bacterial collagenase-induced degradation, there is also concern that crosslinking may prevent fibroblast infiltration and adequate native tissue ingrowth [1, 23, 24]. This may have impeded the ability of the host to decrease the bacterial burden. Indeed, prior histopathology-based animal studies have noted decreased tissue ingrowth with this material [6, 8]. The last two materials, Xenmatrix and Strattice, two noncrosslinked dermal-based biologics, had the least persistent bacterial burden on 30-day quantitative cultures. In addition to these characteristics, these two materials are both e-beam sterilized which is a different processing technique compared to Permacol (γ irradiation sterilized) and Surgisis (EO sterilized). There may certainly be other processing techniques that influence how these materials are able to clear bacteria, but this information is largely proprietary, thus limiting their evaluation.
In our study we chose to evaluate persistent bacterial burden by using both quantitative bacterial cultures and quantitative fluorescence Maestro imaging. Our quantitative approach was important as it provided “tissue-level” bacterial burden. The importance of showing bacterial burden as a measure of the ability to close a wound successfully was shown in the classic work by Krizek et al. [25]. Their work in quantitative bacteriology attempted to predict successful closure of a wound in the face of bacterial contamination. Through their work, a bacterial burden of 105 CFU/g or less predicted the successful closure of a contaminated wound. Similar studies in allograft burn and wound coverage have shown successful “take” of the grafts when placed in the setting of 105 CFU/g or less [25, 26]. Such a reference (105 CFU/g) may have potential relevance and importance in how we evaluate currently used biologic grafts in the setting of contaminated abdominal wall reconstruction.
Maestro imaging in our model was certainly limited by a detection threshold and possibly the location and depth of the fluorescence. The chosen inoculum for this study (104 CFU/ml) started off below the detectable limit for Maestro fluorescence imaging. Given the overall low GFP SA bacterial counts among our biologics, it is apparent that the organism did not proliferate to its potential. One of the drawbacks of Maestro imaging is that it is unable to accurately detect fluorescence that is deeply embedded in tissue. For the field of biologic grafts, Maestro fluorescence imaging using green fluorescence certainly has it limitations. Several innovative alternatives can be considered in order to overcome the challenges associated with abdominal wall explant or in vivo imaging. The use of red fluorescent-tagged bacteria or luciferase-tagged bacteria could allow for in vivo bacterial detection [27]. Despite this imaging limitation, we were still able to detect the bacteria’s green fluorescence under UV light during our quantitative bacterial cultures, thus allowing us to accurately compare the varied responses across materials to this setting of contamination.
Longer serial evaluations of these biomaterials are important in understanding the materials’ ability to provide a durable hernia repair in the setting of contamination. This is a critical question in light of their apparent differences in their ability to clear infection. It would be important to translate these studies to include other bacterial agents involved in abdominal wall repair infections as well. MRSA, for example, is an important pathogen in mesh infections containing different virulence factors compared to MSSA, which may change the ability of these materials to clear infection. Furthermore, histology and biomechanics would be important in understanding their true potential for allowing neovascularization and fibroblast ingrowth with eventual native tissue replacement leading to a strong repair.
Biologic grafts can better clear a SA contamination than a synthetic material. However, the ability to do so varied across the different materials evaluated in our study. This may have been secondary to their differences in tissue source, processing, and sterilization techniques. Further studies evaluating the potential of these materials to serve as a long-term durable hernia repair in the setting of infection are needed.
Acknowledgments
The authors acknowledge Joeseph Furlan, BA, and Ki Hoon Jung, MD, for their invaluable contribution to this work. This research was supported by a 2009 SAGES Educational Foundation grant awarded to Dr. Rosen and a Case Western Reserve University CTSA Grant awarded to Dr. Broome (UL1 RR024989).
Footnotes
Disclosures Dr. Rosen has served as consultant and received honoraria from Covidien, LifeCell, and Gore. Drs. K. C. Harth, A.-M. Broome, M. R. Jacobs, J. A. Blatnik, F. Zeinali, and Mrs. S. Bajaksouzian have no conflicts of interest or financial ties to disclose.
Presented at the 12th WCES, April 14–17, 2010, National Harbor, MD
Contributor Information
K. C. Harth, Department of Surgery, University Hospitals Case Medical Center, 11100 Euclid Avenue, Cleveland, OH 44106-5047, USA
A.-M. Broome, Departments of Biomedical Engineering and Radiology, Case Western Reserve School of Medicine, Cleveland, OH 44106, USA
M. R. Jacobs, Department of Pathology, Case Western Reserve University and University Hospitals Case Medical Center, Cleveland, OH 44106, USA
J. A. Blatnik, Department of Surgery, University Hospitals Case Medical Center, 11100 Euclid Avenue, Cleveland, OH 44106-5047, USA
F. Zeinali, Department of Surgery, University Hospitals Case Medical Center, 11100 Euclid Avenue, Cleveland, OH 44106-5047, USA
S. Bajaksouzian, Department of Pathology, Case Western Reserve University and University Hospitals Case Medical Center, Cleveland, OH 44106, USA
M. J. Rosen, Department of Surgery, University Hospitals Case Medical Center, 11100 Euclid Avenue, Cleveland, OH 44106-5047, USA, Michael.rosen@UHhospitals.org
References
- 1.Bellows CF, Alder A, Helton WS. Abdominal wall reconstruction using biological tissue grafts: present status and future opportunities. Expert Rev Med Device. 2006;3(5):657–675. doi: 10.1586/17434440.3.5.657. [DOI] [PubMed] [Google Scholar]
- 2.Leid JG, Shirtliff ME, Costerton JW, Stoodley AP. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect Immun. 2002;70(11):6339–6345. doi: 10.1128/IAI.70.11.6339-6345.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelley P, Gordley K, Higuera S, Hicks J, Hollier LH. Assessing the long-term retention and permanency of acellular cross-linked porcine dermal collagen as a soft-tissue substitute. Plast Reconstr Surg. 2005;116(6):1780–1784. doi: 10.1097/01.prs.0000186529.60982.0a. [DOI] [PubMed] [Google Scholar]
- 4.Richter G, Smith J, Spencer H, Fan C, Vural E. Histological comparison of implanted cadaveric and porcine dermal matrix grafts. Otolaryngol Head Neck Surg. 2007;137(2):239–242. doi: 10.1016/j.otohns.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Konstantinovic ML, Lagae P, Zheng F, Verbeken EK, De Ridder D, Deprest JA. Comparison of host response to polypropylene and non-cross-linked porcine small intestine serosal-derived collagen implants in a rat model. BJOG. 2005;112(11):1554–1560. doi: 10.1111/j.1471-0528.2005.00688.x. [DOI] [PubMed] [Google Scholar]
- 6.Macleod TM, Williams G, Sanders R, Green CJ. Histological evaluation of Permacol as a subcutaneous implant over a 20-week period in the rat model. Br J Plast Surg. 2005;58(4):518–532. doi: 10.1016/j.bjps.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Petter-Puchner AH, Fortelny RH, Mittermayr R, Walder N, Ohlinger W, Redl H. Adverse effects of porcine small intestine submucosa implants in experimental ventral hernia repair. Surg Endosc. 2006;20(6):942–946. doi: 10.1007/s00464-005-0568-9. [DOI] [PubMed] [Google Scholar]
- 8.Petterpuchner A, Fortelny R, Walder N, Mittermayr R, Ohlinger W, Vangriensven M, Redl H. Adverse effects associated with the use of porcine cross-linked collagen implants in an experimental model of incisional hernia repair. J Surg Res. 2008;145(1):105–110. doi: 10.1016/j.jss.2007.03.090. [DOI] [PubMed] [Google Scholar]
- 9.Silverman RP, Li EN, Holton LH, Sawan KT, Goldberg NH. Ventral hernia repair using allogenic acellular dermal matrix in a swine model. Hernia. 2004;8(4):336–342. doi: 10.1007/s10029-004-0241-6. [DOI] [PubMed] [Google Scholar]
- 10.Eppley BL. Experimental assessment of the revascularization of acellular human dermis for soft-tissue augmentation. Plast Reconstr Surg. 2001;107(3):757–762. doi: 10.1097/00006534-200103000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Menon NG, Rodriguez ED, Byrnes CK, Girotto JA, Goldberg NH, Silverman RP. Revascularization of human acellular dermis in full-thickness abdominal wall reconstruction in the rabbit model. Ann Plast Surg. 2003;50(5):523–527. doi: 10.1097/01.SAP.0000044252.76804.6B. [DOI] [PubMed] [Google Scholar]
- 12.Poulose BK, Scholz S, Moore DE, Schmidt CR, Grogan EL, Lao OB, Nanney L, Davidson J, Holzman MD. Physiologic properties of small intestine submucosa. J Surg Res. 2005;123(2):262–267. doi: 10.1016/j.jss.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Rauth TP, Poulose BK, Nanney LB, Holzman MD. A comparative analysis of expanded polytetrafluoroethylene and small intestinal submucosa—implications for patch repair in ventral herniorrhaphy. J Surg Res. 2007;143(1):43–49. doi: 10.1016/j.jss.2007.03.079. [DOI] [PubMed] [Google Scholar]
- 14.Sandor M, Xu H, Connor J, Lombardi J, Harper JR, Silverman RP, McQuillan DJ. Host response to implanted porcine-derived biologic materials in a primate model of abdominal wall repair. Tissue Eng Part A. 2008;14(12):2021–2031. doi: 10.1089/ten.tea.2007.0317. [DOI] [PubMed] [Google Scholar]
- 15.Kaleya RN. Evaluation of implant/host tissue interactions following intraperitoneal implantation of porcine dermal collagen prosthesis in the rat. Hernia. 2005;9(3):269–276. doi: 10.1007/s10029-005-0003-0. [DOI] [PubMed] [Google Scholar]
- 16.Gaertner WB, Bonsack ME, Delaney JP. Experimental evaluation of four biologic prostheses for ventral hernia repair. J Gastrointest Surg. 2007;11(10):1275–1285. doi: 10.1007/s11605-007-0242-8. [DOI] [PubMed] [Google Scholar]
- 17.Soiderer EE, Lantz GC, Kazacos EA, Hodde JP, Wiegand RE. Morphologic study of three collagen materials for body wall repair. J Surg Res. 2004;118(2):161–175. doi: 10.1016/S0022-4804(03)00352-4. [DOI] [PubMed] [Google Scholar]
- 18.Ayubi FS, Armstrong PJ, Mattia MS, Parker DM. Abdominal wall hernia repair: a comparison of Permacol and Surgisis grafts in a rat hernia model. Hernia. 2008;12(4):373–378. doi: 10.1007/s10029-008-0359-z. [DOI] [PubMed] [Google Scholar]
- 19.Badylak S, Kokini K, Tullius B, Simmons-Byrd A, Morff R. Morphologic study of small intestinal submucosa as a body wall repair device. J Surg Res. 2002;103(2):190–202. doi: 10.1006/jsre.2001.6349. [DOI] [PubMed] [Google Scholar]
- 20.Carbonell AM, Matthews BD, Dreau D, Foster M, Austin CE, Kercher KW, Sing RF, Heniford BT. The susceptibility of prosthetic biomaterials to infection. Surg Endosc. 2005;19(3):430–435. doi: 10.1007/s00464-004-8810-4. [DOI] [PubMed] [Google Scholar]
- 21.Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28(25):3587–3593. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 22.Milburn ML, Holton LH, Chung TL, Li EN, Bochicchio GV, Goldberg NH, Silverman RP. Acellular dermal matrix compared with synthetic implant material for repair of ventral hernia in the setting of peri-operative Staphylococcus aureus implant contamination: a rabbit model. Surg Infect. 2008;9(4):433–442. doi: 10.1089/sur.2007.044. [DOI] [PubMed] [Google Scholar]
- 23.Liang H-C, Chang Y, Hsu C-K, Lee M-H, Sung H-W. Effects of crosslinking degree of an acellular biological tissue on its tissue regeneration pattern. Biomaterials. 2004;25(17):3541–3552. doi: 10.1016/j.biomaterials.2003.09.109. [DOI] [PubMed] [Google Scholar]
- 24.Jarman-Smith ML, Bodamyali T, Stevens C, Howell JA, Horrocks M, Chaudhuri JB. Porcine collagen crosslinking, degradation and its capability for fibroblast adhesion and proliferation. J Mater Sci Mater Med. 2004;15(8):925–932. doi: 10.1023/B:JMSM.0000036281.47596.cc. [DOI] [PubMed] [Google Scholar]
- 25.Krizek TJ, Robson MC. Evolution of quantitative bacteriology in wound management. Am J Surg. 1975;130(5):579–584. doi: 10.1016/0002-9610(75)90516-4. [DOI] [PubMed] [Google Scholar]
- 26.Liedberg NCF, Reiss E, Artz CP. The effect of bacteria on the take of split-thickness skin grafts in rabbits. Ann Surg. 1955;142(1):92–96. doi: 10.1097/00000658-195507000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engelsman A, van Dam G, van der Mei H, Busscher H, Ploeg R. In vivo evaluation of bacterial infection involving morphologically different surgical meshes. Ann Surg. 2010;251(1):133–137. doi: 10.1097/SLA.0b013e3181b61d9a. [DOI] [PubMed] [Google Scholar]