Clinical Features and Epidemiology of Preeclampsia
Preeclampsia is a clinical syndrome, defined as the new onset of hypertension and proteinuria during the second half of pregnancy.1 It afflicts 3–5% of pregnancies and is a leading cause of maternal mortality, especially in developing countries.2–3 Since the only known remedy is delivery of the placenta, in developed countries preeclampsia is an important cause of premature delivery usually medically indicated for the benefit of the mother. This results in infant morbidity and substantial excess health care expenditure.4 Despite the considerable morbidity and mortality, the cause of preeclampsia has remained enigmatic.
Both hypertension and proteinuria implicate the endothelium as the target of the disease. The hypertension of preeclampsia is characterized by peripheral vasoconstriction and decreased arterial compliance.5–6 The proteinuria of preeclampsia is associated with a pathognomonic renal lesion known as glomerular endotheliosis, in which the endothelial cells of the glomerulus swell and endothelial fenestrations are lost.7–8 Podocyturia has been recently associated with preeclampsia during clinical disease 9, however whether this is cause or effect of proteinuria is unknown. The glomerular filtration rate is decreased, compared to normotensive pregnant women; in rare cases, acute renal failure may develop.
Preeclampsia is a systemic vascular disorder which may also affect the liver and the brain in the mothers. When the liver is involved, women may present with abdominal pain, nausea, vomiting, and elevated liver enzymes. Pathologic examination of the liver reveals periportal and sinusoidal fibrin deposition, and in more extreme cases, hemorrhage and necrosis.10 The severe preeclampsia variant, HELLP syndrome (hemolysis, elevated liver enzymes, low platelets), occurs in approximately 20% of women with severe preeclampsia11 and is named not only for the liver involvement but also for the disorder of the coagulation system which develops.12 Approximately 20% of women with HELLP syndrome develop disseminated intravascular coagulation, which carries a poor prognosis for both mother and fetus.11 Placental abruption, ascites, hepatic infarction, hepatic rupture, intra-abdominal bleeding, pulmonary edema, and acute renal failure are all severe clinical manifestations associated with preeclampsia which can result in maternal death.13 Perhaps the most feared complication of preeclampsia is eclampsia itself, defined by the presence of seizures, for which women with severe preeclampsia are often treated with magnesium sulfate prophylaxis.1 The brain injury in eclampsia is associated with cerebral edema and characteristic white matter changes of reversible posterior leukoencephalopathy syndrome (RPLS), that is similar to what is noted in in hypertensive encephalopathy and with cytotoxic immunosuppressive therapies.14 Cerebrovascular complications, including stroke and cerebral hemorrhage, are responsible for the majority of eclampsia related deaths.15 Complications affecting the developing fetus include indicated prematurity16, intrauterine fetal growth restriction (IUGR), oligohydramnios, bronchopulmonary dysplasia17 and increased risk of perinatal death.18
The risk factors for preeclampsia are varied and unique to this condition. Genetic factors are at least partially responsible, as both a maternal and paternal family history of the disease predisposes to preeclampsia.19 There is a 7-fold risk of recurrence for women who have had the disease in a previous pregnancy.20 Multiple gestation is an additional risk factor, and triplet gestation carries a greater risk than twin, suggesting that increased placental mass plays some role.20 Associations between preeclampsia and nulliparity,20 change in paternity from a previous pregnancy,21 increased interpregnancy interval,22 use of barrier contraception,23 and conception by intracytoplasmic sperm injection,24 implicate limited recent exposure to paternal antigen as a predisposing factor. Notably, classic cardiovascular risk factors are associated with preeclampsia: maternal age greater than 40, insulin resistance, obesity, systemic inflammation, and preexisting hypertension, diabetes, or renal disease all increase the risk.20, 25–26 Consistent with this, women with a history of preeclampsia have an elevated risk for cardiovascular disease later in life (see discussion later in this review). Surprisingly, smoking during pregnancy protects against preeclampsia.27
The diagnosis of preeclampsia is clinical. As defined by the American College of Obstetrics and Gynecology, the diagnosis requires blood pressures greater than 140/90 on two occasions, combined with urine protein excretion greater than 300 mg per day. 1 Edema, a classic feature of the disease, is no longer considered a diagnostic feature, given its lack of sensitivity or specificity. 1 Importantly, in 20% of cases, eclampsia may present without preceding hypertension or proteinuria, suggesting that the currently employed diagnostic criteria are imperfect.28 Laboratory tests such as liver function tests, quantification of urine protein, and serum creatinine may be helpful in characterizing the degree of end organ damage, but none is specific for preeclampsia.1 Hyperuricemia, more likely to be present in women with preeclampsia than in normotensive pregnant women, has been used as a diagnostic aid and to predict adverse outcomes in preeclampsia, but its predictive value is generally modest.29–30
Recently work by our group and others has identified an imbalance of pro- and anti-angiogenic proteins as a key factor in the pathogenesis of the preeclampsia.31–38 Here, we review our current understanding of the biology underlying the disease. We first describe the role of the placenta in preeclampsia, then review the mechanisms of angiogenesis and its role in preeclampsia and the role of other contributory pathways in the pathogenesis of preeclampsia. Finally, we comment on the potential mechanisms by which the risk of cardiovascular disease is elevated in women with a history of preeclampsia.
The Preeclamptic Placenta
The placenta is the central organ in the pathogenesis of preeclampsia. Removal of the placenta abolishes the disease;39 moreover, only the placenta, and not the fetus, is required for its development. This is best demonstrated by the case of molar pregnancy, which carries an elevated risk for preeclampsia.40 Pathologic examination of placentas from women with severe preeclampsia reveals several abnormalities including infarcts, atherosis, thrombosis, and chronic inflammation.41 Likely, some of the abnormalities seen in the preeclamptic placenta are consequences of the hypertension and endothelial injury induced by the disease. However, there are abnormalities of placental development which precede the maternal derangements.
During normal placentation, the embryo-derived cytotrophoblast cells invade the maternal uterine wall. Post-invasion, cytotrophoblasts are found in the smooth muscle and endothelial layers of the maternal decidual arteries. This interaction acts to induce the remodeling of these maternal vessels into high capacitance, low resistance vessels which provide access to maternal oxygen and nutrients for the placenta and developing fetus.42 As part of this process, the cytotrophoblasts adopt an endothelial phenotype, expressing adhesion molecules classically found on the surface of endothelial cells.43 In preeclampsia, this process is aberrant. The invasion of the cytotrophoblasts is incomplete, with cytotrophoblast cells present only in the superficial layers of the decidua.43 The spiral arteries fail to be invaded or remodeled, resulting in constricted, high resistance vessels, visible upon pathologic examination of preeclamptic placentas. This shallow invasion has been shown to be related to a failure of the cytotrophoblasts to adopt an endothelial adhesion phenotype.44 (see Figure 1)
Figure 1. Abnormal Placentation in Preeclampsia.
In normal placental development, invasive cytotrophoblasts of fetal origin invade the maternal spiral arteries, transforming them from small-caliber resistance vessels to high-caliber capacitance vessels capable of providing placental perfusion adequate to sustain the growing fetus. During the process of vascular invasion, the cytotrophoblasts differentiate from an epithelial phenotype to an endothelial phenotype, a process referred to as “pseudovasculogenesis” or “vascular mimicry” (Upper Panel). In preeclampsia, cytotrophoblasts fail to adopt an invasive endothelial phenotype. Instead, invasion of the spiral arteries is shallow and they remain small caliber, resistance vessels (Lower Panel). Figure reproduced with permission from Lam et al. 45
Hypoxia may contribute to the abnormal placental development described above, as the failure of cytotrophoblasts to fully invade and to switch adhesion molecules can also be reproduced in vitro when cytotrophoblasts are cultured under hypoxic conditions.46 Consistent with this, the risk for preeclampsia is higher in women living at high altitude.47 However, hypoxia resulting from abnormal placentation also contributes to the fetal and maternal complications of the disease. Clinically, abnormal uterine artery Doppler wave forms herald the development of preeclampsia, suggesting decreased placental perfusion.48 Decreased placental perfusion in its more extreme cases results in fetal growth restriction, oligohydramnios, or intrauterine fetal demise. Interestingly, pregnant rats and baboons develop hypertension and proteinuria in response to surgically induced uteroplacental ischemia,49–50 implicating placental hypoxia in the development of the maternal disease.
Pro-Angiogenic factors and Vascular Homeostasis
Abnormalities in the placenta and resulting consequences to the fetus are a hallmark of preeclampsia, but the maternal features of the disease have been its most mysterious feature. Recently, circulating anti-angiogenic proteins have been implicated in the pathogenesis of many of the maternal features of the disease (see Figure 2). Prior to describing how release of these factors into the circulation may lead to the maternal syndrome, we review the evidence for the role of pro-angiogenic growth factors and their receptors in vascular homeostasis.
Figure 2. sFlt1 and sEng Causes Endothelial Dysfunction by Antagonizing VEGF and TGF-β1 signaling.
There is mounting evidence that VEGF and TGF-β1 are required to maintain endothelial health in several tissues including the kidney and perhaps the placenta. During normal pregnancy, vascular homeostasis is maintained by physiological levels of VEGF and TGF-β1 signaling in the vasculature. In preeclampsia, excess placental secretion of sFlt1 and sEng (two endogenous circulating anti-angiogenic proteins) inhibits VEGF and TGF-β1 signaling respectively in the vasculature. This results in endothelial cell dysfunction, including decreased prostacyclin, nitric oxide production and release of procoagulant proteins.
Vascular Endothelial Growth Factors
The vascular endothelial growth factors are secreted dimeric glycoproteins involved in vasculogenesis (the process by which new blood vessels are formed in embryonic life) and angiogenesis (the process by which blood vessels branch to form new blood vessels). In humans and other mammals this family of growth factors includes VEGF-A (VEGF) and placental growth factor (PlGF), among others. VEGF-A (hereafter VEGF), the first discovered and prototypical protein in this family, is a pro-angiogenic factor which promotes the proliferation and survival of endothelial cells and induces vascular permeability.51–52 PlGF is a VEGF homolog released by the placenta which also has pro-angiogenic activity.53
VEGF family receptors present on vascular endothelial cells include Flt-1 (VEGFR-1) and KDR (VEGFR-2, murine Flk-1). While VEGF binds to both Flt-1 and KDR receptors, PlGF homodimers bind exclusively to Flt-1.54 KDR is thought to be primarily responsible for VEGF's action on endothelial cells.55–57 KDR null-mice die at embryonic day 8.5–9.5 with an absence of organized blood vessels and widespread necrosis, suggesting lack of perfusion to vital structures.58 Flt-1 null mice die in embryonic life, with death due to the overgrowth of endothelial cells and resultant blood vessel disarray.58 Mice with Flt-1 lacking the tyrosine kinase domain but with intact ligand binding domain have normal blood vessels and survive,59 implying that Flt-1 acts as a negative regulator of angiogenesis through sequestration of extracellular VEGF rather than through intracellular action. More recently work by Chappell et al suggest that the role of Flt1 gene may be to express sFlt-1 (a soluble VEGF signaling inhibitor) which acts by regulating guidance of emerging vessel sprouts by modulating local VEGF availability60
VEGF is essential for embryonic vasculogenesis and angiogenesis. The ablation of a single VEGF allele results in markedly abnormal vasculature including the placental vasculature with death at embryonic day 10–12.61 Besides its essential role in placental and embryonic vasculogenesis and angiogenesis, VEGF is involved in the survival of endothelial cells and vascular homeostasis in mature vessels and tissues. In adult mice VEGF is expressed by cell types located adjacent to fenestrated endothelia including the epithelial cells of the choroid plexus, renal podocytes, and hepatocytes.62 In vitro, VEGF induces endothelial fenestrations,63 while inhibition of VEGF in adult mice reduces the density of so-called VEGF dependent fenestrated capillaries.64 Accordingly, targeted inhibition of VEGF in vivo leads to pathology in many of the organs with fenestrated endothelia, also affected in preeclampsia. Specifically, in the mouse kidney, podocyte selective knockout of VEGF in early post-natal life results in proteinuria, nephrotic syndrome, endotheliosis and eventual disappearance of endothelial cells from the glomerular tuft, recapitulating the classic renal lesion seen in preeclampsia.65 In the liver, inhibition of VEGF signaling in early post-natal life leads to abnormal liver development with small hepatocytes and immature sinusoidal vasculature.66 In adult mice, activation of the Flt-1 receptor on liver sinusoidal endothelial cells by VEGF or PlGF leads to elaboration of hepatocyte growth factor and liver enlargement.67 Additionally, in the brain, inhibition of VEGF signaling results in decreased perfusion of choroid plexus vasculature.68
VEGF also seems to have a direct vasodilatory effect on the systemic vasculature, as infusion of VEGF leads to nitric oxide dependent vasorelaxation in the coronary arteries and other vessels in dogs and humans,69 likely through upregulation of nitric oxide and prostacyclin in vascular endothelial cells.70–71 Suggesting a role for VEGF in control of systemic blood pressure, antagonism of the KDR receptor leads to elevations in mean arterial pressure in mice by a nitric oxide dependent mechanism.72 Most relevant, VEGF inhibition has a biological effect on endothelial function in adult men and women. Side effects of VEGF inhibition in patients undergoing anti-angiogenic cancer therapy are consistent with animal models suggesting VEGF's homeostatic role in the mature vasculature: hypertension, proteinuria, glomerular endothelial damage, hypothyroidism, and in rare cases the reversible posterior leukoencephalopathy syndrome73–78
PlGF, which has approximately 53% homology with VEGF, is expressed at high levels by the human placenta. PlGF homodimers do not bind to the KDR receptor, but do bind to the Flt-1 receptor with high affinity. PlGF has weak mitogenic activity and no effect on vascular permeability in vitro alone, but potentiates the actions of VEGF in cultured endothelial cells and in an in vivo vascular permeability model.54 In contrast to VEGF knockout mice, PlGF null mice have normal vascular development with the exception of subtle defects in the retinal vasculature and in luteal vasculogenesis which do not seem to affect retinal or reproductive functioning.79 However, PlGF null mice do exhibit defects in tumor angiogenesis, post-ischemic retinal and myocardial neovascularization, and wound healing, suggesting that PlGF plays a role in angiogenesis in pathological settings. 79–80 Consistent with this, PlGF stimulates angiogenesis in ischemic myocardium and arterial collateral growth in ischemic limbs. PlGF may act by displacing VEGF from the Flt-1 receptor, allowing it to bind to the more active KDR receptor.54 Other possible mechanisms include direct effects of Flt-1 signaling and the formation of VEGF/PlGF heterodimers.81–82 During pregnancy, the placenta releases PlGF at high amounts into the maternal circulation. Levels increase beginning in the 2nd trimester, peak during weeks 29–32 and decline thereafter. However, because most in vivo investigation of PlGF has been conducted in non-pregnant animals, the function of PlGF in the physiology of normal pregnancy has not been well elucidated.
Transforming growth factor–beta (TGFβ)
The TGFβ family of proteins is made up of ubiquitous growth factors with diverse actions in many cell types. TGFβ is known to be involved in angiogenesis, however the mechanisms are not as well elucidated as those in the VEGF pathway. To initiate intracellular signaling, TGFβ and other proteins in this family must bind to both type I and type II receptors on the cell surface. TGFβ isoforms bind the TGFβ Type II receptor (TGFβII) initially with subsequent binding and activation of type I receptors. Mice null for the TGFβII receptor die at embryonic day 10.5 with defects in hematopoiesis and vasculogenesis, 83 implicating TGFβ in the development of the vasculature. Most cell types, including endothelial cells, express the activin-like kinase type I TGFβ receptor, ALK5, but endothelial cells alone express ALK1 type I receptor.84 Like TGFβ, ALK1 is important in the development of blood vessels, as ALK1 null mice perish by embryonic day 11-12 with growth retardation with markedly reduced number of capillaries and dilation of larger vessels.85 In vitro, TGFβ has differing effects on the activation state of endothelial cells dependent on the dose administered: at low TGFβ doses, ALK1 leads to proliferation and migration of endothelial cells; while at high TGFβ doses, ALK5 inhibits proliferation and migration of endothelial cells.84, 86 Moreover, TGFβ signaling regulates the expression of VEGF, connecting the two pathways and further linking TGFβ to angiogenesis. 87
TGFβ signaling in the vasculature also involves co-receptors which act to modulate TGFβ action. Endoglin is a TGFβ co-receptor expressed in endothelial cells and syncytiotrophoblasts of the placenta. Both human endoglin and ALK1 mutations independently cause hereditary hemorrhagic telangectasia (Osler-Weber-Rendu disease), characterized by multisystemic vascular malformations.88 Endoglin null mice die at embryonic day 10–12 with abnormal vasculature and internal bleeding.89 On a molecular level, endoglin promotes and is required for TGFβ-ALK1 mediated endothelial cell proliferation and migration.86
Besides being involved in angiogenesis in the embryonic phase, TGFβ likely functions in vascular homeostasis in mature vessels. In the mouse brain, TGFβ inhibition in combination with VEGF inhibition resulted in the loss of choroid plexus endothelial fenestrae with formation of periventricular edema detectable on magnetic resonance imaging (MRI).68 Down regulation of TGFβ signaling in the retinal microvasculature leads to decreased perfusion, breakdown of the blood-retinal-barrier, reduced endothelium dependent vasodilation, and increased endothelial cell apoptosis, suggesting a role for TGFβ in the maintenance of the adult vasculature. 90
Anti-angiogenic Factors and Endothelial Dysfunction in Preeclampsia
In 1989 Roberts and Taylor advanced the hypothesis that preeclampsia results from the release of circulating factors by the placenta leading to widespread maternal vascular endothelial dysfunction.91–92 Several lines of evidence continue to support this understanding of the disease. The cardinal signs and symptoms of preeclampsia involve the vasculature, specifically areas of the vasculature with fenestrated endothelia. Furthermore, vessels isolated from the soft tissue of preeclamptic women demonstrate endothelial dysfunction, with impaired endothelium-dependent, but not endothelium-independent dilatation.93–94 Human studies have firmly established the presence of factors released by the injured or activated endothelium in the circulation of women with clinical preeclampsia. These include, among others, endothelin-1,95 fibronectin,96–98 Von Willebrand factor,96, 99 thrombomodulin,100–101 markers of oxidative stress102, and inflammatory cytokines.103 There is also evidence of deficiency of prostacyclin and nitric oxide, vasodilatory factors released by healthy vasculature, in the circulation of women with preeclampsia.104–106 Studies showing that serum from pregnant women with preeclampsia induces endothelial injury and dysfunction in vitro support the theory that a circulating factor causes the aforementioned endothelial dysfunction evident in the disease.92, 102, 107
sFlt-1 and PlGF in the Pathogenesis of Preeclampsia
Because the vascular endothelium relies on pro-angiogenic factors, the release of anti-angiogenic factors by the placenta into the maternal circulation is a plausible cause of the endothelial dysfunction observed in preeclampsia. Investigation by our group and others has characterized two such anti-angiogenic proteins. Soluble fms-like tyrosine kinase (sFlt-1, also referred to as sVEGFR1), an anti-angiogenic protein, is a soluble form of the VEGF/PlGF receptor Flt-1 produced by alternative splicing108. sFlt-1 was initially identified as a product of cultured human endothelial cells and subsequently shown to be produced by the placenta and released into the maternal circulation.108–110 sFlt-1 is a potent inhibitor of VEGF and PlGF activity: recombinant sFlt-1 inhibits endothelial tube formation and blocks the vasodilatory effect of VEGF and PlGF in vitro. 108 Our group identified elevated expression of sFlt-1 by gene expression profiling in placentas delivered from women with preeclampsia.31 Since that time several novel sFlt-1 variant isoforms have been identified and shown to be upregulated in preeclampsia.111–113
Animal data supports a causal role for sFlt-1 in the pathogenesis of the maternal disease. Administration of sFlt-1 to pregnant rats using an adenoviral vector induced hypertension and proteinuria and caused glomerular endotheliosis, the classic renal lesion seen in preeclampsia.31 Other groups have also generated animal models which implicate sFlt-1 in the pathogenesis of preeclampsia. These include rat and baboon models in which uterine hypoxia induced elevated production of sFlt-1, hypertension, and proteinuria,50, 114 as well as a mouse model in which sFlt-1 expression in pregnant mice resulted in hypertension, decreased platelet count, and reduced fetal weight.115
Soluble Endoglin in the Pathogenesis of Preeclampsia
Notably absent from the phenotype of rats administered sFlt-1 is the liver dysfunction and cerebral changes seen in women with severe preeclampsia. Soluble endoglin (sEng) is another anti-angiogenic protein identified by gene expression profiling of placentas from women with preeclampsia. sEng may combine with sFlt-1 to induce features of severe preeclampsia including liver dysfunction, fetal growth restriction, coagulation, and neurologic abnormalities.32, 68 Our group identified the 65kDa soluble endoglin monomer produced by placentas from preeclamptic women at a level 4 fold higher than placentas from women with normal pregnancies. Subsequent in vitro studies demonstrated that sEng reduces the binding of TGFβ1 to its receptor and blocks TGFβ1 induced vasodilation of rat vessels, likely through down regulation of nitric oxide synthase.32 Furthermore, sEng reduced endothelial tube formation in vitro and led to increased capillary permeability in mouse lung, liver, and kidney. Importantly, when pregnant rats were injected with both sFlt-1 and sEng, a condition reminiscent of severe preeclampsia develops with hypertension, nephrotic range proteinuria, low platelet count, elevated liver enyzmes, and reduced fetal weight.32 Thus, most, if not all, clinical manifestations of preeclampsia can be explained by the anti-angiogenic actions of sFlt-1 and sEng on the maternal endothelium. More recent studies have shown that mice injected with both sFlt-1 and sEng expressing adenoviruses, but not those injected with adenoviruses expressing either molecule alone, exhibit not only decreased cerebral perfusion and vascular thrombi but also loss of choroid plexus endothelial fenestrae, choroid plexus endothelial swelling, and cerebral edema on brain MRI.68 While women with eclampsia are known to have brain edema and white matter lesions on MRI, it remains to be seen whether the histopathologic changes in women with preeclampsia with neurologic involvement are similar to those seen in sFlt-1 and sEng injected mice.
Human Studies of sFLT-1, PlGF, and sEng in Preeclampsia
Epidemiologic studies have revealed that blood levels of sFlt-1 and PlGF are altered in women with preeclampsia both during and prior to clinical signs and symptoms of the disease, consistent with a pathogenic role for these angiogenic factors in preeclampsia. sFlt-1 is present at relatively high concentrations in the serum of normal pregnant women at term116, but declines to non-pregnant levels 48 hours after delivery.31 In preeclampsia, sFlt-1 levels begin to rise at least 5 weeks prior to the onset of clinical disease and remain elevated compared to non-affected women.34, 36, 117 Alterations in sFlt1 are more dramatic in patients who have early onset preeclampsia (PE<37 weeks).34 Levels of sFlt-1 also correlate with the severity of the disease 37 In pregnancies afflicted by severe IUGR without preeclampsia, there may also be a modest elevation of sFlt-1 levels.118
Consistent with the pathophysiology suggested by animal models, levels of free PlGF are depressed in women with preeclampsia. In fact, low PlGF levels in the first trimester, prior to the sFlt-1 rise, are a risk factor for subsequent preeclampsia. 34, 119 PlGF can also be measured in the urine of women destined to develop preeclampsia, where levels are depressed compared to normotensive pregnant women beginning at 25 weeks. The degree of suppression of urine PlGF levels is correlated with the severity of the disease.120 In contrast, although circulating free VEGF levels are low in preeclampsia, it is not useful clinically since the majority of patients have levels below the detection limit of the currently available ELISA kits. Of note, the ratio of sFlt-1 to PlGF is a better marker of preeclampsia than either measure alone.121–122 This implies that it is an imbalance of anti- and pro-angiogenic factors rather than the level of either sFlt-1 or PlGF alone leads to preeclampsia.34
Studies of sEng levels in women with and without preeclampsia are consistent with the animal studies, supporting a role for elevated sEng in the pathogenesis of severe preeclampsia. sEng levels in women with normal pregnancies are stable until approximately week 33 of pregnancy, when they rise, peaking at delivery.33 In women with preeclampsia beginning before 37 weeks of gestation (preterm) levels of sEng begin to rise earlier, by 20 weeks gestation and rise more steeply after 33 weeks. Women with preeclampsia beginning after 37 weeks of gestation (term) also have elevated 3rd trimester sEng levels, but a slower rise, beginning at 25 weeks gestation and rising steeply around 33 weeks of gestation.33 The combination of sFlt-1, PlGF and sEng level characterize preeclampsia better than any single analyte, linking the combined action of several angiogenic factors to clinical preeclampsia.33, 123 High circulating levels of both sEng and sFlt-1/PlGF are usually observed before onset of preterm preeclampsia.33–34 Consistent with this, more recent data suggests that alterations in sFlt-1, PlGF, and sEng in women with preeclampsia are associated with maternal vascular dysfunction and impaired nitric oxide formation.106, 35 In preeclampsia-associated placental abruption, sFlt-1, PlGF, and sEng levels have all been shown to be altered.124–125 In eclampsia, sFlt-1, PlGF, and sEng are also altered to a similar degree as in patients with severe preeclampsia, reiterating the combined role of these factors in both of these conditions.126
Other Contributory Factors to the Development of Preeclampsia
Several factors that are upstream to the angiogenic proteins have been tied to the pathogenesis of preeclampsia (see Figure 3). Many of these factors have also been shown to influence sFlt-1 or sEng expression; they may be modulators of their ultimate concentrations in the maternal circulation.
Figure 3. Summary of the pathogenesis of preeclampsia.
AT1-AA, immunological factors, oxidative stress and other factors (such as decreased hemoxygenase expression) may cause placental dysfunction which in turn leads to the release of anti-angiogenic factors (such as sFlt1 and sEng) and other inflammatory mediators to induce preeclampsia
Placental Hypoxia
Because placental hypoxia is thought to be involved in the pathogenesis of preeclampsia (see prior discussion in this review), the effect of hypoxia on sFlt-1 expression has been an area of active investigation. In vitro, culture of cytotrophoblast cells at low oxygen tension has been shown to induce the expression and release of sFlt-1.127–128 sFlt-1 has also been studied in pregnant animal models of preeclampsia in which surgical uteroplacental ischemia is induced and causes hypertension and proteinuria. In both rat114 and baboon50 surgical uterine ischemia has been shown to induce elevated circulating sFlt-1 levels in addition to the preeclampsia-like syndrome. Overexpression of the stabilized form of hypoxia inducible factor 1-α (HIF-1α) during mouse pregnancy is associated with a preeclampsia-like phenotype including elevated levels of sFlt-1 and sEng 129. However, hypoxia studies in animals are associated with enhanced trophoblast invasion130 suggesting that abnormal placentation is the primary event that leads to placental hypoxia which in turn may liberate soluble factors necessary for the maternal syndrome.
Immune Factors
Risk factors for preeclampsia related to paternal antigen exposure suggest that immunologic dysfunction at the fetal-maternal interface may contribute to preeclampsia. Moreover, women without an intact immune system, due to untreated HIV, rarely develop preeclampsia, while HIV treatment and resulting immune reconstitution brings the risk of preeclampsia to levels seen in the general population.131 Normal placentation requires an immune tolerance for fetal antigen which may be altered in preeclampsia, as pathological examination of preeclamptic placentas reveals increased dendritic cell and macrophage infiltration as well as signs of chronic inflammation.41, 132–133,134 Dysregulated complement system has also been proposed as a regulator of placental angiogenesis in animal models135. Decidual NK cells, which promote angiogenesis and are involved in trophoblast invasion may contribute to the abnormal placental development seen in the disease.136 These cells are further implicated by genetic studies finding associations between polymorphisms in killer immunoglobulin receptors (KIR, present on NK cells), HLA-C (KIR ligands present on trophoblasts) and preeclampsia.134 These studies provide compelling evidence that immune dysregulation is involved in preeclampsia pathogenesis, but the mechanisms by which this occurs have so far not been well elucidated.
Renin-Angiotensin Aldosterone Pathway
Normal pregnancy is characterized by resistance to the vasoconstrictive effects of angiotensin II. 124 Levels of renin, angiotensin, and aldosterone are increased despite an overall decrease in systemic vascular resistance.137–139 In pregnancy induced hypertension (preeclampsia or gestational hypertension), this resistance is blunted, resulting in increased sensitivity to angiotensin II when compared to normotensive pregnant women. 124 Circulating angiotensin receptor AT1 activating autoantibodies levels are elevated may explain the hypersensitivity to the effects of angiotensin in preeclampsia140 When injected into pregnant mice, these auto-antibodies lead to hypertension, proteinuria, glomerular endothelial damage, and elevated levels of sFlt-1 and sEng, and thus may contribute to the pathogenesis of preeclampsia.141 Since in some women with a history of preeclampsia auto-antibodies remain elevated, they may also contribute to the development of hypertension in later life.142 Recently, a novel form of circulating oxidized angiotensinogen, which enhances angiotensin formation has been found in the circulation of preeclamptic subjects. 143 However, circulating angiotensin II and aldosterone are suppressed in preeclamptic subjects (and not elevated as one might predict). Studies are needed to evaluate if this oxidized form of angiotensinogen is altered prior to clinical disease.
Alterations in Placental Enzymes
Recently, genetic knockout of the catechol-O-methyltransferase (COMT) enzyme has been shown to recapitulate some signs and symptoms of preeclampsia.144 COMT knockdown leads to a deficiency of 2-methoxyestradiol (2-ME), an inhibitor of the HIF 1α, a transcription factor which acts as a mediator of the cellular response to hypoxia. COMT knockout mice develop placental hypoxia, hypertension, proteinuria and modestly elevated levels of sFlt-1 in contrast to wild type mice. Deficiency of 2-ME is also demonstrable in the serum of women with preeclampsia, thus in some women decreased COMT expression or 2-ME deficiency may be proximal to the elevated levels of sFlt-1. 144 Heme oxygenase-1 (HO-1), a placental enzyme whose product is carbon monoxide (CO), is thought to be a negative regulator of sFlt-1 production. In vitro studies demonstrate that overexpression of HO-1 or CO production inhibits sFlt-1 release from placental explants.145 Consistent with these in vitro studies, end tidal CO levels are lower in women with preeclampsia.146 The suppression of sFlt-1 by CO may also explain the lower risk for preeclampsia in smokers.147
Oxidative Stress/Placental Debris
In preeclampsia, oxidative stress is demonstrable both in the placenta and in the maternal circulation.148–149 Preeclamptic placentas produce greater quantities of superoxide and have less antioxidant capacity compared with normal placentas. Maternal serum from preeclamptic pregnancy shows evidence of oxidative modification of protein and lipoprotein particles. Blood levels of antioxidants have also been reported to be decreased in women with preeclampsia. Unfortunately, large randomized controlled trials did not show an effect of antioxidants vitamin C and vitamin E on the risk of preeclampsia.150–154 The shedding of placental debris has also been suggested to cause elevated oxidative stress and endothelial dysfunction in preeclampsia. Placental abnormalities and uteroplacental ischemia may induce the shedding of placental microparticles into the maternal circulation and these particles may lead to inflammation and vascular damage.155 Consistent with this, women with preeclampsia have elevated circulating levels of placental debris.156–157 Interestingly, these microparticles have been shown to be associated with sFlt-1 in the maternal circulation, and thus may be an additional source of circulating sFlt-1 in preeclampsia.158
Each of these factors may have a role in the regulation and release of anti-angiogenic factors into the maternal circulation, but none has been shown to be primarily responsible for the imbalance of angiogenic proteins found in preeclampsia. It is possible that the dysregulation of these factors has multiple etiologies, with over production of anti-angiogenic factors the final common pathway to preeclampsia. Alternatively, the imbalance in angiogenic proteins may be the primary derangement, since angiogenic factors are intimately involved in early placental development (reviewed in Khankin et al).159 The regulation of the expression of angiogenic proteins in the placenta is an area of active investigation.
Implications for Diagnosis and Treatment
Currently there is no laboratory test which provides a reliable diagnosis of preeclampsia. This is problematic as hypertension and proteinuria are not specific to preeclampsia and the treatment for preeclampsia (delivery) puts the preterm fetus at risk. Many women present with "atypical" preeclampsia, without either hypertension or proteinuria, and some of these women go on to have unexpected severe disease.28 Since the initial reports that sFlt-1 may be intimately involved in preeclampsia pathogenesis, several studies have demonstrated the ability of the ratio of sFlt-1 and PlGF to distinguish women with and without preeclampsia, using newly developed automated assays with sensitivities and specificities greater than 95% for preterm preeclampsia.121, 160–161 Moreover, the measurement of anti-angiogenic proteins seems to distinguish preeclampsia in women with chronic diseases who may have hypertension or proteinuria for other reasons, including diabetes and systemic lupus erythematosus.162–163 Levels of anti-angiogenic factors similarly differentiate women with HELLP from women with low platelets due to other conditions including thrombotic thrombocytopenic purpura.164 However, not all patients with preeclampsia have been reported to have altered sFlt1 and PlGF.165 Whether these patients presenting with low levels of sFlt1 and signs/symptoms of preeclampsia represent a non-angiogenic form of the disease or are simply misdiagnosed remains unknown. One possibility is that women with underlying vascular disease may develop preeclamptic signs/symptoms at relatively lower levels of sFlt-1.166 Future prospective studies and clinical trials will define how best to use levels of angiogenic proteins in clinical management. The implication of sFlt-1 in the pathogenesis of preeclampsia also opens the possibility for the development of new targeted therapies. Administration of VEGF rescues the phenotype produced by either sFlt-1 administration167–168 or uterine hypo-perfusion in rats,169 suggesting that VEGF itself or compounds which mimic its actions such as PlGF may hold promise as treatments for preeclampsia and related conditions. Levels of anti-angiogenic factors may also serve as a useful intermediate outcome in initial trials of potential preeclampsia therapies in humans and animals.
Implications for Later Cardiovascular Disease
Delivery of the placenta cures preeclampsia, yet affected women continue to have an elevated risk of cardiovascular disease many years postpartum. Large retrospective epidemiologic studies have consistently demonstrated an elevated risk for many types of cardiovascular disease in women with a history of preeclampsia.170–173 According to a recent meta-analysis, the prevalence of hypertension in women with previous preeclampsia is greater than 50% an average of 14 years after pregnancy, 3–4 times the risk found in women without preeclampsia.174 Similarly, the risk of death from cardiovascular disease and cerebrovascular disease is about 2-fold greater in women with a history of preeclampsia. Women who have had preeclampsia before 34 weeks or preeclampsia combined with preterm birth, have even higher risk of death from cardiovascular disease, 4–8 times the risk of women who had a normal pregnancy.172–174
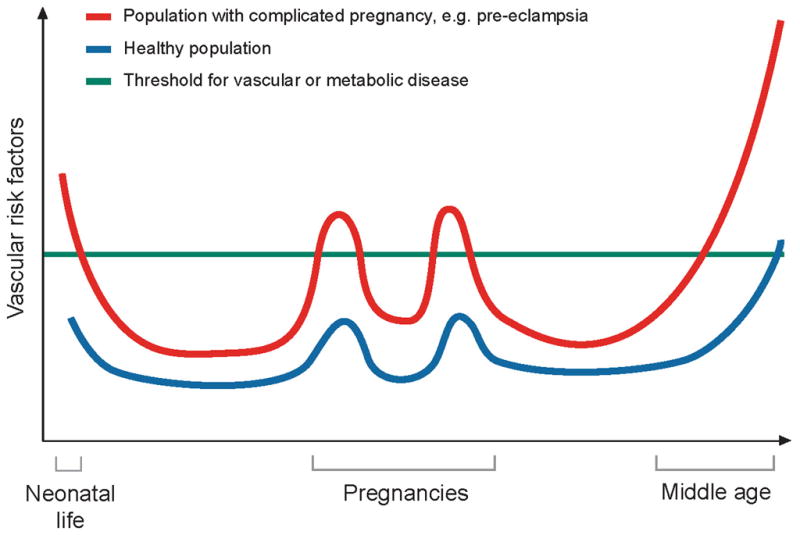
The mechanisms which account for an increased risk of cardiovascular disease in women with a history of preeclampsia are not yet well understood, but endothelial dysfunction, which has been linked to atherosclerosis, persists in formerly preeclamptic women many years after an affected pregnancy. Three months up to at least 3 years postpartum, women with prior preeclampsia demonstrate a decrement in endothelium dependent dilatation.175 Women with a history of preeclampsia also have been reported to be sensitive to angiotensin II and salt.176 In addition, markers of endothelial activation including VCAM-1 and ICAM-1 appear to be higher more than 15 years after pregnancy in women with previous preeclampsia, independent of body mass index and smoking.177 It is possible that shared risk factors may jointly predispose to preeclampsia, endothelial dysfunction, and cardiovascular disease. In this regard, diabetes mellitus, chronic hypertension, and renal disease prior to pregnancy all confer an elevated risk for preeclampsia. Even subclinical insulin resistance and inflammation, well known to elevate the risk of cardiovascular disease, predispose women to preeclampsia and persist up to 30 years after the disease.16, 26, 178–181 Endothelial dysfunction and cardiovascular disease following preeclampsia may be attributable to these preexisting risk factors and others yet unknown. A recent study of cardiovascular risk factors present before and after pregnancy suggests that nearly half of the elevated risk for future hypertension after preeclampsia can be explained by pre-pregnancy risk factors.182 Therefore, pregnancy may be viewed as a stress test that can reveal subclinical cardiovascular disease phenotypes long before overt disease (see Figure 4) However, further investigation will be necessary to determine whether preeclampsia itself may injure the endothelium and thereby increase the risk of atherosclerosis and cardiovascular disease.
Figure 4.
Model for pregnancy as a stress test for long term cardiovascular disease. This model suggests that the women who develop preeclampsia (red line) have greater underlying vascular risk. This manifests during pregnancy (two pregnancies are represented) in the form of preeclampsia as well as later in life in the form of hypertension, heart disease, stroke, and other forms of cardiovascular disease. In women without these underlying vascular risk factors (blue line), pregnancy may increase vascular risk slightly, but not enough to lead to preeclampsia. These women have a lower cardiovascular risk both during pregnancy and later in life. Figure adapted from Sattar et al 183
Although levels of sFlt-1 decline after delivery of the placenta, a persistent and subtle anti-angiogenic milieu may contribute to lasting endothelial dysfunction and an elevated risk of cardiovascular disease in women with a history of preeclampsia. Some176, 179, but not all studies 35, 184–185 have shown that levels of sFlt-1 remained higher in women with a history of preeclampsia compared to those without preeclampsia an average of 18 months postpartum, independent of body mass index, blood pressure, and smoking. The source of sFlt-1 in non pregnant individuals may be peripheral blood mononuclear cells, as monocytes in women with preeclampsia produce elevated levels of sFlt-1 compared to those from control subjects.186–187 Persistent alterations in the levels of anti-angiogenic proteins may explain not only the elevated cardiovascular disease risk but also the observed lower risk of malignancy,174 and higher risk of acquired hypothyroid function188 in women with a history of preeclampsia. The role of anti-angiogenic proteins in the pathophysiology of cardiovascular disease has not been well worked out. In fact, angiogenesis has been viewed as both a pathogenic and therapeutic factor in cardiovascular disease (reviewed by Khurana et al).189 A recent study in 130 patients with chronic kidney disease (CKD), suggests that elevated levels of sFlt-1 in this group of patients may contribute to endothelial dysfunction and cardiovascular disease risk. Serum from these patients had anti-angiogenic activity compared to control serum, which could be attenuated with the administration of an anti-sFlt-1 antibody,187 and sFlt-1 levels were greater in subjects who had a history of myocardial infarction or stroke. Relatively high sFlt-1 levels are also associated with carotid intima-media thickness and progression of atherosclerosis in hypertensive subjects.190 Studies of cardiovascular function and atherogenic potential in animal models expressing high levels of sFlt-1 chronically would add insight as to whether anti-angiogenic molecules are possible contributors to cardiovascular disease in women. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia have also been recently described, however the mechanisms mediating these phenotypes are largely unknown.191
Conclusions
Preeclampsia is a disease which begins in the placenta and ends at the maternal endothelium. As reviewed here, evidence suggests that the maternal disease is attributable to anti-angiogenic factors sFlt-1 and sEng, released by an abnormal placenta. These anti-angiogenic factors antagonize the effects of pro-angiogenic factors VEGF, PlGF, and TGFβ, which are important in the maintenance of the vascular endothelium. While these anti-angiogenic proteins likely cause the maternal disease and may prove to be a useful diagnostic aid, the primary cause of the placental disease is an active area of investigation. Preeclampsia portends future cardiovascular disease, and research into mechanisms by which this increased risk occurs, including the possible role of pro-angiogenic and anti-angiogenic factors, may lead to new insights into the pathogenesis of cardiovascular disease in women.
Acknowledgments
Funding Sources: C.E.P. is a Howard Hughes Institute Medical Research Training Fellow. R.J.L. receives salary support from the intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. S.A.K is supported by the Howard Hughes Medical Institute, an established investigator award from the American Heart Association and by a Clinical Scientist award from the Burroughs Wellcome Fund.
Footnotes
Disclosure: SAK reports having served as a consultant to Abbott, Beckman Coulter, Roche, and Johnson & Johnson and having been named co-inventor on multiple provisional patents filed by Beth Israel Deaconess Medical Center for the use of angiogenesis-related proteins for the diagnosis and treatment of preeclampsia. These patents have been nonexclusively licensed to several companies. All other authors have nothing to declare.
References
- 1.Acog practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33. Obstet Gynecol. 2002 Jan;99:159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 2.Berg CJ, Mackay AP, Qin C, Callaghan WM. Overview of maternal morbidity during hospitalization for labor and delivery in the united states: 1993-1997 and 2001-2005. Obstet Gynecol. 2009;113:1075–1081. doi: 10.1097/AOG.0b013e3181a09fc0. [DOI] [PubMed] [Google Scholar]
- 3.MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet Gynecol. 2001;97:533–538. doi: 10.1016/s0029-7844(00)01223-0. [DOI] [PubMed] [Google Scholar]
- 4.Liu A, Wen SW, Bottomley J, Walker MC, Smith G. Utilization of health care services of pregnant women complicated by preeclampsia in ontario. Hypertens Pregnancy. 2009;28:76–84. doi: 10.1080/10641950802366252. [DOI] [PubMed] [Google Scholar]
- 5.Thadhani R, Ecker JL, Kettyle E, Sandler L, Frigoletto FD. Pulse pressure and risk of preeclampsia: A prospective study. Obstet Gynecol. 2001;97:515–520. doi: 10.1016/s0029-7844(00)01192-3. [DOI] [PubMed] [Google Scholar]
- 6.Bosio PM, McKenna PJ, Conroy R, O'Herlihy C. Maternal central hemodynamics in hypertensive disorders of pregnancy. Obstet Gynecol. 1999;94:978–984. doi: 10.1016/s0029-7844(99)00430-5. [DOI] [PubMed] [Google Scholar]
- 7.Lafayette RA, Druzin M, Sibley R, Derby G, Malik T, Huie P, Polhemus C, Deen WM, Myers BD. Nature of glomerular dysfunction in pre-eclampsia. Kidney Int. 1998;54:1240–1249. doi: 10.1046/j.1523-1755.1998.00097.x. [DOI] [PubMed] [Google Scholar]
- 8.Stillman IE, Karumanchi SA. The glomerular injury of preeclampsia. J Am Soc Nephrol. 2007;18:2281–2284. doi: 10.1681/ASN.2007020255. [DOI] [PubMed] [Google Scholar]
- 9.Garovic VD, Wagner SJ, Turner ST, Rosenthal DW, Watson WJ, Brost BC, Rose CH, Gavrilova L, Craigo P, Bailey KR, Achenbach J, Schiffer M, Grande JP. Urinary podocyte excretion as a marker for preeclampsia. Am J Obstet Gynecol. 2007;196:320, e321–327. doi: 10.1016/j.ajog.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Rolfes DB, Ishak KG. Liver disease in toxemia of pregnancy. Am J Gastroenterol. 1986;81:1138–1144. [PubMed] [Google Scholar]
- 11.Sibai BM, Ramadan MK, Usta I, Salama M, Mercer BM, Friedman SA. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (hellp syndrome) Am J Obstet Gynecol. 1993;169:1000–1006. doi: 10.1016/0002-9378(93)90043-i. [DOI] [PubMed] [Google Scholar]
- 12.Weinstein L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: A severe consequence of hypertension in pregnancy. Am J Obstet Gynecol. 1982;142:159–167. doi: 10.1016/s0002-9378(16)32330-4. [DOI] [PubMed] [Google Scholar]
- 13.Isler CM, Rinehart BK, Terrone DA, Martin RW, Magann EF, Martin JN., Jr Maternal mortality associated with hellp (hemolysis, elevated liver enzymes, and low platelets) syndrome. Am J Obstet Gynecol. 1999;181:924–928. doi: 10.1016/s0002-9378(99)70343-1. [DOI] [PubMed] [Google Scholar]
- 14.Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin MS, Lamy C, Mas JL, Caplan LR. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 15.Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstetrics and gynecology. 2005;105:402–410. doi: 10.1097/01.AOG.0000152351.13671.99. [DOI] [PubMed] [Google Scholar]
- 16.Wolf M, Sandler L, Munoz K, Hsu K, Ecker JL, Thadhani R. First trimester insulin resistance and subsequent preeclampsia: A prospective study. J Clin Endocrinol Metab. 2002;87:1563–1568. doi: 10.1210/jcem.87.4.8405. [DOI] [PubMed] [Google Scholar]
- 17.Hansen AR, Barnes CM, Folkman J, McElrath TF. Maternal preeclampsia predicts the development of bronchopulmonary dysplasia. J Pediatr. 2010;156:532–536. doi: 10.1016/j.jpeds.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 19.Esplin MS, Fausett MB, Fraser A, Kerber R, Mineau G, Carrillo J, Varner MW. Paternal and maternal components of the predisposition to preeclampsia. N Engl J Med. 2001;344:867–872. doi: 10.1056/NEJM200103223441201. [DOI] [PubMed] [Google Scholar]
- 20.Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: Systematic review of controlled studies. BMJ. 2005;330:565. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tubbergen P, Lachmeijer AM, Althuisius SM, Vlak ME, van Geijn HP, Dekker GA. Change in paternity: A risk factor for preeclampsia in multiparous women? J Reprod Immunol. 1999;45:81–88. doi: 10.1016/s0165-0378(99)00040-6. [DOI] [PubMed] [Google Scholar]
- 22.Skjaerven R, Wilcox AJ, Lie RT. The interval between pregnancies and the risk of preeclampsia. N Engl J Med. 2002;346:33–38. doi: 10.1056/NEJMoa011379. [DOI] [PubMed] [Google Scholar]
- 23.Klonoff-Cohen HS, Savitz DA, Cefalo RC, McCann MF. An epidemiologic study of contraception and preeclampsia. JAMA. 1989;262:3143–3147. [PubMed] [Google Scholar]
- 24.Wang JX, Knottnerus AM, Schuit G, Norman RJ, Chan A, Dekker GA. Surgically obtained sperm, and risk of gestational hypertension and pre-eclampsia. Lancet. 2002;359:673–674. doi: 10.1016/S0140-6736(02)07804-2. [DOI] [PubMed] [Google Scholar]
- 25.Yogev, Chen, Hod, Coustan, Oats, McIntyre, Metzger, Lowe, Dyer, Dooley, Trimble, McCance, Hadden, Persson, Rogers Hyperglycemia and adverse pregnancy outcome (hapo) study: Preeclampsia. Am J Obstet Gynecol. 2010;202:255, e251–257. doi: 10.1016/j.ajog.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf M, Kettyle E, Sandler L, Ecker JL, Roberts J, Thadhani R. Obesity and preeclampsia: The potential role of inflammation. Obstet Gynecol. 2001;98:757–762. doi: 10.1016/s0029-7844(01)01551-4. [DOI] [PubMed] [Google Scholar]
- 27.Conde-Agudelo A, Althabe F, Belizan JM, Kafury-Goeta AC. Cigarette smoking during pregnancy and risk of preeclampsia: A systematic review. Am J Obstet Gynecol. 1999;181:1026–1035. doi: 10.1016/s0002-9378(99)70341-8. [DOI] [PubMed] [Google Scholar]
- 28.Sibai BM, Stella CL. Diagnosis and management of atypical preeclampsia-eclampsia. Am J Obstet Gynecol. 2009;200:481, e481–487. doi: 10.1016/j.ajog.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 29.Thangaratinam S, Ismail KM, Sharp S, Coomarasamy A, Khan KS. Accuracy of serum uric acid in predicting complications of pre-eclampsia: A systematic review. BJOG. 2006;113:369–378. doi: 10.1111/j.1471-0528.2006.00908.x. [DOI] [PubMed] [Google Scholar]
- 30.Lim KH, Friedman SA, Ecker JL, Kao L, Kilpatrick SJ. The clinical utility of serum uric acid measurements in hypertensive diseases of pregnancy. Am J Obstet Gynecol. 1998;178:1067–1071. doi: 10.1016/s0002-9378(98)70549-6. [DOI] [PubMed] [Google Scholar]
- 31.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sflt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D'Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 33.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 34.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. The New England journal of medicine. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 35.Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation. 2010;122:478–87. doi: 10.1161/CIRCULATIONAHA.109.895458. [DOI] [PubMed] [Google Scholar]
- 36.Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, Goncalves L, Gomez R, Edwin S, Mazor M. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 37.Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee Kim Y, Goncalves LF, Gomez R, Edwin S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young investigator award. Am J Obstet Gynecol. 2004;190:1541–1547. doi: 10.1016/j.ajog.2004.03.043. discussion 1547–1550. [DOI] [PubMed] [Google Scholar]
- 38.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95:884–891. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- 39.Matsuo K, Kooshesh S, Dinc M, Sun CC, Kimura T, Baschat AA. Late postpartum eclampsia: Report of two cases managed by uterine curettage and review of the literature. Am J Perinatol. 2007;24:257–266. doi: 10.1055/s-2007-976548. [DOI] [PubMed] [Google Scholar]
- 40.Soto-Wright V, Bernstein M, Goldstein DP, Berkowitz RS. The changing clinical presentation of complete molar pregnancy. Obstet Gynecol. 1995;86:775–779. doi: 10.1016/0029-7844(95)00268-V. [DOI] [PubMed] [Google Scholar]
- 41.Salafia CM, Pezzullo JC, Lopez-Zeno JA, Simmens S, Minior VK, Vintzileos AM. Placental pathologic features of preterm preeclampsia. Am J Obstet Gynecol. 1995;173:1097–1105. doi: 10.1016/0002-9378(95)91333-5. [DOI] [PubMed] [Google Scholar]
- 42.Damsky CH, Fitzgerald ML, Fisher SJ. Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J Clin Invest. 1992;89:210–222. doi: 10.1172/JCI115565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Y, Damsky CH, Chiu K, Roberts JM, Fisher SJ. Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J Clin Invest. 1993;91:950–960. doi: 10.1172/JCI116316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest. 1997;99:2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 2005;46:1077–1085. doi: 10.1161/01.HYP.0000187899.34379.b0. [DOI] [PubMed] [Google Scholar]
- 46.Genbacev O, Joslin R, Damsky CH, Polliotti BM, Fisher SJ. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest. 1996;97:540–550. doi: 10.1172/JCI118447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palmer SK, Moore LG, Young D, Cregger B, Berman JC, Zamudio S. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in colorado. Am J Obstet Gynecol. 1999;180:1161–1168. doi: 10.1016/s0002-9378(99)70611-3. [DOI] [PubMed] [Google Scholar]
- 48.North RA, Ferrier C, Long D, Townend K, Kincaid-Smith P. Uterine artery doppler flow velocity waveforms in the second trimester for the prediction of preeclampsia and fetal growth retardation. Obstet Gynecol. 1994;83:378–386. [PubMed] [Google Scholar]
- 49.Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, Bennett W. Reduced uterine perfusion pressure (rupp) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med. 2006;122:383–392. doi: 10.1385/1-59259-989-3:381. [DOI] [PubMed] [Google Scholar]
- 50.Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, Waugh R, McKenzie P, Kirwan P, Hennessy A. Uteroplacental ischemia results in proteinuric hypertension and elevated sflt-1. Kidney Int. 2007;71:977–984. doi: 10.1038/sj.ki.5002175. [DOI] [PubMed] [Google Scholar]
- 51.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 52.Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT. Vascular permeability factor, an endothelial cell mitogen related to pdgf. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 53.Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico MG. Isolation of a human placenta cdna coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci U S A. 1991;88:9267–9271. doi: 10.1073/pnas.88.20.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park JE, Chen HH, Winer J, Houck KA, Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to flt-1 but not to flk-1/kdr. J Biol Chem. 1994;269:25646–25654. [PubMed] [Google Scholar]
- 55.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of kdr and flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- 56.Keyt BA, Nguyen HV, Berleau LT, Duarte CM, Park J, Chen H, Ferrara N. Identification of vascular endothelial growth factor determinants for binding kdr and flt-1 receptors. Generation of receptor-selective vegf variants by site-directed mutagenesis. J Biol Chem. 1996;271:5638–5646. doi: 10.1074/jbc.271.10.5638. [DOI] [PubMed] [Google Scholar]
- 57.Clauss M, Weich H, Breier G, Knies U, Rockl W, Waltenberger J, Risau W. The vascular endothelial growth factor receptor flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem. 1996;271:17629–17634. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]
- 58.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 59.Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci U S A. 1998;95:9349–9354. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chappell JC, Taylor SM, Ferrara N, Bautch VL. Local guidance of emerging vessel sprouts requires soluble flt-1. Dev Cell. 2009;17:377–386. doi: 10.1016/j.devcel.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the vegf gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 62.Maharaj AS, Saint-Geniez M, Maldonado AE, D'Amore PA. Vascular endothelial growth factor localization in the adult. Am J Pathol. 2006;168:639–648. doi: 10.2353/ajpath.2006.050834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol. 1998;140:947–959. doi: 10.1083/jcb.140.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O'Brien SM, Davis RB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ, McDonald DM. Vegf-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 65.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of vegf-a expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. Vegf is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- 67.LeCouter J, Moritz DR, Li B, Phillips GL, Liang XH, Gerber HP, Hillan KJ, Ferrara N. Angiogenesis-independent endothelial protection of liver: Role of vegfr-1. Science. 2003;299:890–893. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- 68.Maharaj AS, Walshe TE, Saint-Geniez M, Venkatesha S, Maldonado AE, Himes NC, Matharu KS, Karumanchi SA, D'Amore PA. Vegf and tgf-beta are required for the maintenance of the choroid plexus and ependyma. J Exp Med. 2008;205:491–501. doi: 10.1084/jem.20072041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ku DD, Zaleski JK, Liu S, Brock TA. Vascular endothelial growth factor induces edrf-dependent relaxation in coronary arteries. Am J Physiol. 1993;265:H586–592. doi: 10.1152/ajpheart.1993.265.2.H586. [DOI] [PubMed] [Google Scholar]
- 70.He H, Venema VJ, Gu X, Venema RC, Marrero MB, Caldwell RB. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/kdr activation of c-src. J Biol Chem. 1999;274:25130–25135. doi: 10.1074/jbc.274.35.25130. [DOI] [PubMed] [Google Scholar]
- 71.Wheeler-Jones C, Abu-Ghazaleh R, Cospedal R, Houliston RA, Martin J, Zachary I. Vascular endothelial growth factor stimulates prostacyclin production and activation of cytosolic phospholipase a2 in endothelial cells via p42/p44 mitogen-activated protein kinase. FEBS Lett. 1997;420:28–32. doi: 10.1016/s0014-5793(97)01481-6. [DOI] [PubMed] [Google Scholar]
- 72.Facemire CS, Nixon AB, Griffiths R, Hurwitz H, Coffman TM. Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension. 2009;54:652–658. doi: 10.1161/HYPERTENSIONAHA.109.129973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Glusker P, Recht L, Lane B. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;354:980–982. doi: 10.1056/NEJMc052954. discussion 980–982. [DOI] [PubMed] [Google Scholar]
- 74.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 75.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 76.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patel TV, Morgan JA, Demetri GD, George S, Maki RG, Quigley M, Humphreys BD. A preeclampsia-like syndrome characterized by reversible hypertension and proteinuria induced by the multitargeted kinase inhibitors sunitinib and sorafenib. Journal of the National Cancer Institute. 2008;100:282–284. doi: 10.1093/jnci/djm311. [DOI] [PubMed] [Google Scholar]
- 78.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE. Vegf inhibition and renal thrombotic microangiopathy. The New England journal of medicine. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, Vandendriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 80.Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, Nagy JA, Hooper A, Priller J, De Klerck B, Compernolle V, Daci E, Bohlen P, Dewerchin M, Herbert JM, Fava R, Matthys P, Carmeliet G, Collen D, Dvorak HF, Hicklin DJ, Carmeliet P. Revascularization of ischemic tissues by plgf treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-flt1. Nat Med. 2002;8:831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 81.Autiero M, Waltenberger J, Communi D, Kranz A, Moons L, Lambrechts D, Kroll J, Plaisance S, De Mol M, Bono F, Kliche S, Fellbrich G, Ballmer-Hofer K, Maglione D, Mayr-Beyrle U, Dewerchin M, Dombrowski S, Stanimirovic D, Van Hummelen P, Dehio C, Hicklin DJ, Persico G, Herbert JM, Shibuya M, Collen D, Conway EM, Carmeliet P. Role of plgf in the intra- and intermolecular cross talk between the vegf receptors flt1 and flk1. Nat Med. 2003;9:936–943. doi: 10.1038/nm884. [DOI] [PubMed] [Google Scholar]
- 82.Cao Y, Chen H, Zhou L, Chiang MK, Anand-Apte B, Weatherbee JA, Wang Y, Fang F, Flanagan JG, Tsang ML. Heterodimers of placenta growth factor/vascular endothelial growth factor. Endothelial activity, tumor cell expression, and high affinity binding to flk-1/kdr. J Biol Chem. 1996;271:3154–3162. doi: 10.1074/jbc.271.6.3154. [DOI] [PubMed] [Google Scholar]
- 83.Oshima M, Oshima H, Taketo MM. Tgf-beta receptor type ii deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol. 1996;179:297–302. doi: 10.1006/dbio.1996.0259. [DOI] [PubMed] [Google Scholar]
- 84.Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct tgf-beta type i receptors. EMBO J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, Li L, Miyazono K, ten Dijke P, Kim S, Li E. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci U S A. 2000;97:2626–2631. doi: 10.1073/pnas.97.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M, Mummery C, Arthur HM, ten Dijke P. Endoglin promotes endothelial cell proliferation and tgf-beta/alk1 signal transduction. EMBO J. 2004;23:4018–4028. doi: 10.1038/sj.emboj.7600386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shao ES, Lin L, Yao Y, Bostrom KI. Expression of vascular endothelial growth factor is coordinately regulated by the activin-like kinase receptors 1 and 5 in endothelial cells. Blood. 2009;114:2197–2206. doi: 10.1182/blood-2009-01-199166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J. Endoglin, a tgf-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994;8:345–351. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- 89.Bourdeau A, Dumont DJ, Letarte M. A murine model of hereditary hemorrhagic telangiectasia. J Clin Invest. 1999;104:1343–1351. doi: 10.1172/JCI8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walshe TE, Saint-Geniez M, Maharaj AS, Sekiyama E, Maldonado AE, D'Amore PA. Tgf-beta is required for vascular barrier function, endothelial survival and homeostasis of the adult microvasculature. PLoS One. 2009;4:e5149. doi: 10.1371/journal.pone.0005149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: An endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 92.Rodgers GM, Taylor RN, Roberts JM. Preeclampsia is associated with a serum factor cytotoxic to human endothelial cells. Am J Obstet Gynecol. 1988;159:908–914. doi: 10.1016/s0002-9378(88)80169-8. [DOI] [PubMed] [Google Scholar]
- 93.McCarthy AL, Woolfson RG, Raju SK, Poston L. Abnormal endothelial cell function of resistance arteries from women with preeclampsia. Am J Obstet Gynecol. 1993;168:1323–1330. doi: 10.1016/0002-9378(93)90389-z. [DOI] [PubMed] [Google Scholar]
- 94.Ashworth JR, Warren AY, Baker PN, Johnson IR. Loss of endothelium-dependent relaxation in myometrial resistance arteries in pre-eclampsia. Br J Obstet Gynaecol. 1997;104:1152–1158. doi: 10.1111/j.1471-0528.1997.tb10939.x. [DOI] [PubMed] [Google Scholar]
- 95.Nova A, Sibai BM, Barton JR, Mercer BM, Mitchell MD. Maternal plasma level of endothelin is increased in preeclampsia. Am J Obstet Gynecol. 1991;165:724–727. doi: 10.1016/0002-9378(91)90317-k. [DOI] [PubMed] [Google Scholar]
- 96.Deng L, Bremme K, Hansson LO, Blomback M. Plasma levels of von willebrand factor and fibronectin as markers of persisting endothelial damage in preeclampsia. Obstet Gynecol. 1994;84:941–945. [PubMed] [Google Scholar]
- 97.Taylor RN, Crombleholme WR, Friedman SA, Jones LA, Casal DC, Roberts JM. High plasma cellular fibronectin levels correlate with biochemical and clinical features of preeclampsia but cannot be attributed to hypertension alone. Am J Obstet Gynecol. 1991;165:895–901. doi: 10.1016/0002-9378(91)90435-t. [DOI] [PubMed] [Google Scholar]
- 98.Stubbs TM, Lazarchick J, Horger EO., 3rd Plasma fibronectin levels in preeclampsia: A possible biochemical marker for vascular endothelial damage. Am J Obstet Gynecol. 1984;150:885–887. doi: 10.1016/0002-9378(84)90468-x. [DOI] [PubMed] [Google Scholar]
- 99.Thorp JM, Jr, White GC, 2nd, Moake JL, Bowes WA., Jr Von willebrand factor multimeric levels and patterns in patients with severe preeclampsia. Obstet Gynecol. 1990;75:163–167. [PubMed] [Google Scholar]
- 100.Minakami H, Takahashi T, Izumi A, Tamada T. Increased levels of plasma thrombomodulin in preeclampsia. Gynecol Obstet Invest. 1993;36:208–210. doi: 10.1159/000292631. [DOI] [PubMed] [Google Scholar]
- 101.Hsu CD, Iriye B, Johnson TR, Witter FR, Hong SF, Chan DW. Elevated circulating thrombomodulin in severe preeclampsia. Am J Obstet Gynecol. 1993;169:148–149. doi: 10.1016/0002-9378(93)90151-8. [DOI] [PubMed] [Google Scholar]
- 102.Musci TJ, Roberts JM, Rodgers GM, Taylor RN. Mitogenic activity is increased in the sera of preeclamptic women before delivery. Am J Obstet Gynecol. 1988;159:1446–1451. doi: 10.1016/0002-9378(88)90572-8. [DOI] [PubMed] [Google Scholar]
- 103.Cackovic M, Buhimschi CS, Zhao G, Funai EF, Norwitz ER, Kuczynski E, Lockwood CJ, Buhimschi IA. Fractional excretion of tumor necrosis factor-alpha in women with severe preeclampsia. Obstet Gynecol. 2008;112:93–100. doi: 10.1097/AOG.0b013e31817c4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mills JL, DerSimonian R, Raymond E, Morrow JD, Roberts LJ, 2nd, Clemens JD, Hauth JC, Catalano P, Sibai B, Curet LB, Levine RJ. Prostacyclin and thromboxane changes predating clinical onset of preeclampsia: A multicenter prospective study. JAMA. 1999;282:356–362. doi: 10.1001/jama.282.4.356. [DOI] [PubMed] [Google Scholar]
- 105.Fitzgerald DJ, Entman SS, Mulloy K, FitzGerald GA. Decreased prostacyclin biosynthesis preceding the clinical manifestation of pregnancy-induced hypertension. Circulation. 1987;75:956–963. doi: 10.1161/01.cir.75.5.956. [DOI] [PubMed] [Google Scholar]
- 106.Sandrim VC, Palei AC, Metzger IF, Gomes VA, Cavalli RC, Tanus-Santos JE. Nitric oxide formation is inversely related to serum levels of antiangiogenic factors soluble fms-like tyrosine kinase-1 and soluble endogline in preeclampsia. Hypertension. 2008;52:402–407. doi: 10.1161/HYPERTENSIONAHA.108.115006. [DOI] [PubMed] [Google Scholar]
- 107.Myers J, Mires G, Macleod M, Baker P. In preeclampsia, the circulating factors capable of altering in vitro endothelial function precede clinical disease. Hypertension. 2005;45:258–263. doi: 10.1161/01.HYP.0000153461.58298.a4. [DOI] [PubMed] [Google Scholar]
- 108.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kendall RL, Wang G, Thomas KA. Identification of a natural soluble form of the vascular endothelial growth factor receptor, flt-1, and its heterodimerization with kdr. Biochem Biophys Res Commun. 1996;226:324–328. doi: 10.1006/bbrc.1996.1355. [DOI] [PubMed] [Google Scholar]
- 110.Clark DE, Smith SK, He Y, Day KA, Licence DR, Corps AN, Lammoglia R, Charnock-Jones DS. A vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulation. Biol Reprod. 1998;59:1540–1548. doi: 10.1095/biolreprod59.6.1540. [DOI] [PubMed] [Google Scholar]
- 111.Thomas CP, Andrews JI, Raikwar NS, Kelley EA, Herse F, Dechend R, Golos TG, Liu KZ. A recently evolved novel trophoblast-enriched secreted form of fms-like tyrosine kinase-1 variant is up-regulated in hypoxia and preeclampsia. J Clin Endocrinol Metab. 2009;94:2524–2530. doi: 10.1210/jc.2009-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sela S, Itin A, Natanson-Yaron S, Greenfield C, Goldman-Wohl D, Yagel S, Keshet E. A novel human-specific soluble vascular endothelial growth factor receptor 1: Cell-type-specific splicing and implications to vascular endothelial growth factor homeostasis and preeclampsia. Circ Res. 2008;102:1566–1574. doi: 10.1161/CIRCRESAHA.108.171504. [DOI] [PubMed] [Google Scholar]
- 113.Heydarian M, McCaffrey T, Florea L, Yang Z, Ross MM, Zhou W, Maynard SE. Novel splice variants of sflt1 are upregulated in preeclampsia. Placenta. 2009;30:250–255. doi: 10.1016/j.placenta.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 114.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension. 2007;50:1142–1147. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 115.Lu F, Longo M, Tamayo E, Maner W, Al-Hendy A, Anderson GD, Hankins GD, Saade GR. The effect of over-expression of sflt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. Am J Obstet Gynecol. 2007;196:396, e391–397. doi: 10.1016/j.ajog.2006.12.024. discussion 396 e397. [DOI] [PubMed] [Google Scholar]
- 116.Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, Taketani Y. Elevated serum soluble vascular endothelial growth factor receptor 1 (svegfr-1) levels in women with preeclampsia. J Clin Endocrinol Metab. 2003;88:2348–2351. doi: 10.1210/jc.2002-021942. [DOI] [PubMed] [Google Scholar]
- 117.Hertig A, Berkane N, Lefevre G, Toumi K, Marti HP, Capeau J, Uzan S, Rondeau E. Maternal serum sflt1 concentration is an early and reliable predictive marker of preeclampsia. Clin Chem. 2004;50:1702–1703. doi: 10.1373/clinchem.2004.036715. [DOI] [PubMed] [Google Scholar]
- 118.Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, Schaaps JP, Cabrol D, Frankenne F, Foidart JM. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: Pathophysiological consequences. J Clin Endocrinol Metab. 2003;88:5555–5563. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- 119.Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, Ecker J, Karumanchi SA. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab. 2004;89:770–775. doi: 10.1210/jc.2003-031244. [DOI] [PubMed] [Google Scholar]
- 120.Levine RJ, Thadhani R, Qian C, Lam C, Lim KH, Yu KF, Blink AL, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Urinary placental growth factor and risk of preeclampsia. JAMA. 2005;293:77–85. doi: 10.1001/jama.293.1.77. [DOI] [PubMed] [Google Scholar]
- 121.Verlohren S, Galindo A, Schlembach D, Zeisler H, Herraiz I, Moertl MG, Pape J, Dudenhausen JW, Denk B, Stepan H. An automated method for the determination of the sflt-1/pigf ratio in the assessment of preeclampsia. Am J Obstet Gynecol. 2010;202:161, e161–161, e111. doi: 10.1016/j.ajog.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 122.Buhimschi CS, Norwitz ER, Funai E, Richman S, Guller S, Lockwood CJ, Buhimschi IA. Urinary angiogenic factors cluster hypertensive disorders and identify women with severe preeclampsia. Am J Obstet Gynecol. 2005;192:734–741. doi: 10.1016/j.ajog.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 123.Kusanovic JP, Romero R, Chaiworapongsa T, Erez O, Mittal P, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Edwin SS, Gomez R, Yeo L, Conde-Agudelo A, Hassan SS. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med. 2009;22:1021–1038. doi: 10.3109/14767050902994754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Signore C, Mills JL, Qian C, Yu K, Lam C, Epstein FH, Karumanchi SA, Levine RJ. Circulating angiogenic factors and placental abruption. Obstet Gynecol. 2006;108:338–344. doi: 10.1097/01.AOG.0000216014.72503.09. [DOI] [PubMed] [Google Scholar]
- 125.Signore C, Mills JL, Qian C, Yu KF, Rana S, Karumanchi SA, Levine RJ. Circulating soluble endoglin and placental abruption. Prenat Diagn. 2008;28:852–858. doi: 10.1002/pd.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vaisbuch E, Whitty J, Hassan S, Romero R, Kusanovic J, Cotton D, Sorokin Y, Karumanchi SA. Circulating angiogenic and anti-angiogenic factors in women with eclampsia. Am J Obstet Gynecol. 2010 doi: 10.1016/j.ajog.2010.08.049. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nagamatsu T, Fujii T, Kusumi M, Zou L, Yamashita T, Osuga Y, Momoeda M, Kozuma S, Taketani Y. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: An implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004;145:4838–4845. doi: 10.1210/en.2004-0533. [DOI] [PubMed] [Google Scholar]
- 128.Gerber HP, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not flk-1/kdr, is up-regulated by hypoxia. J Biol Chem. 1997;272:23659–23667. doi: 10.1074/jbc.272.38.23659. [DOI] [PubMed] [Google Scholar]
- 129.Tal R, Shaish A, Barshack I, Polak-Charcon S, Afek A, Volkov A, Feldman B, Avivi C, Harats D. Effects of hypoxia-inducible factor-1{alpha} overexpression in pregnant mice. Possible implications for preeclampsia and intrauterine growth restriction. Am J Pathol. 2010 doi: 10.2353/ajpath.2010.090800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rosario GX, Konno T, Soares MJ. Maternal hypoxia activates endovascular trophoblast cell invasion. Dev Biol. 2008;314:362–375. doi: 10.1016/j.ydbio.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wimalasundera RC, Larbalestier N, Smith JH, de Ruiter A, Mc GTSA, Hughes AD, Poulter N, Regan L, Taylor GP. Pre-eclampsia, antiretroviral therapy, and immune reconstitution. Lancet. 2002;360:1152–1154. doi: 10.1016/s0140-6736(02)11195-0. [DOI] [PubMed] [Google Scholar]
- 132.Huang SJ, Chen CP, Schatz F, Rahman M, Abrahams VM, Lockwood CJ. Pre-eclampsia is associated with dendritic cell recruitment into the uterine decidua. J Pathol. 2008;214:328–336. doi: 10.1002/path.2257. [DOI] [PubMed] [Google Scholar]
- 133.Lockwood CJ, Matta P, Krikun G, Koopman LA, Masch R, Toti P, Arcuri F, Huang ST, Funai EF, Schatz F. Regulation of monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha and interleukin-1beta in first trimester human decidual cells: Implications for preeclampsia. Am J Pathol. 2006;168:445–452. doi: 10.2353/ajpath.2006.050082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hiby SE, Walker JJ, O'Shaughnessy KM, Redman CW, Carrington M, Trowsdale J, Moffett A. Combinations of maternal kir and fetal hla-c genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200:957–965. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med. 2006;203:2165–2175. doi: 10.1084/jem.20061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O. Decidual nk cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 137.Rinsler MG, Rigby B. Function of aldosterone in the metabolism of sodium and water in pregnancy. Br Med J. 1957;2:966–969. doi: 10.1136/bmj.2.5051.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Weir RJ, Paintin DB, Robertson JI, Tree M, Fraser R, Young J. Renin, angiotensin and aldosterone relationships in normal pregnancy. Proc R Soc Med. 1970;63:1101–1102. doi: 10.1177/003591577006311P110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Weir RJ, Brown JJ, Fraser R, Kraszewski A, Lever AF, McIlwaine GM, Morton JJ, Robertson JI, Tree M. Plasma renin, renin substrate, angiotensin ii, and aldosterone in hypertensive disease of pregnancy. Lancet. 1973;1:291–294. doi: 10.1016/s0140-6736(73)91540-7. [DOI] [PubMed] [Google Scholar]
- 140.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin at1 receptor. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14:855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hubel CA, Wallukat G, Wolf M, Herse F, Rajakumar A, Roberts JM, Markovic N, Thadhani R, Luft FC, Dechend R. Agonistic angiotensin ii type 1 receptor autoantibodies in postpartum women with a history of preeclampsia. Hypertension. 2007;49:612–617. doi: 10.1161/01.HYP.0000256565.20983.d4. [DOI] [PubMed] [Google Scholar]
- 143.Zhou A, Carrell RW, Murphy MP, Wei Z, Yan Y, Stanley PL, Stein PE, Broughton Pipkin F, Read RJ. A redox switch in angiotensinogen modulates angiotensin release. Nature. 2010;468:108–111. doi: 10.1038/nature09505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kanasaki K, Palmsten K, Sugimoto H, Ahmad S, Hamano Y, Xie L, Parry S, Augustin HG, Gattone VH, Folkman J, Strauss JF, Kalluri R. Deficiency in catechol-o-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453:1117–1121. doi: 10.1038/nature06951. [DOI] [PubMed] [Google Scholar]
- 145.Cudmore M, Ahmad S, Al-Ani B, Fujisawa T, Coxall H, Chudasama K, Devey LR, Wigmore SJ, Abbas A, Hewett PW, Ahmed A. Negative regulation of soluble flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115:1789–1797. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- 146.Baum M, Schiff E, Kreiser D, Dennery PA, Stevenson DK, Rosenthal T, Seidman DS. End-tidal carbon monoxide measurements in women with pregnancy-induced hypertension and preeclampsia. Am J Obstet Gynecol. 2000;183:900–903. doi: 10.1067/mob.2000.109047. [DOI] [PubMed] [Google Scholar]
- 147.Karumanchi SA, Levine RJ. How does smoking reduce the risk of preeclampsia? Hypertension. 2010;55:1100–1101. doi: 10.1161/HYPERTENSIONAHA.109.148973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Raijmakers MT, Dechend R, Poston L. Oxidative stress and preeclampsia: Rationale for antioxidant clinical trials. Hypertension. 2004;44:374–380. doi: 10.1161/01.HYP.0000141085.98320.01. [DOI] [PubMed] [Google Scholar]
- 149.Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med. 1999;222:222–235. doi: 10.1177/153537029922200305. [DOI] [PubMed] [Google Scholar]
- 150.Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, Pearson GD, Wapner RJ, Varner MW, Thorp JM, Jr, Mercer BM, Peaceman AM, Ramin SM, Carpenter MW, Samuels P, Sciscione A, Harper M, Smith WJ, Saade G, Sorokin Y, Anderson GB. Vitamins c and e to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362:1282–1291. doi: 10.1056/NEJMoa0908056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS. Vitamins c and e and the risks of preeclampsia and perinatal complications. N Engl J Med. 2006;354:1796–1806. doi: 10.1056/NEJMoa054186. [DOI] [PubMed] [Google Scholar]
- 152.Spinnato JA, 2nd, Freire S, Pinto ESJL, Cunha Rudge MV, Martins-Costa S, Koch MA, Goco N, Santos Cde B, Cecatti JG, Costa R, Ramos JG, Moss N, Sibai BM. Antioxidant therapy to prevent preeclampsia: A randomized controlled trial. Obstet Gynecol. 2007;110:1311–1318. doi: 10.1097/01.AOG.0000289576.43441.1f. [DOI] [PubMed] [Google Scholar]
- 153.Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH. Vitamin c and vitamin e in pregnant women at risk for pre-eclampsia (vip trial): Randomised placebo-controlled trial. Lancet. 2006;367:1145–1154. doi: 10.1016/S0140-6736(06)68433-X. [DOI] [PubMed] [Google Scholar]
- 154.Villar J, Purwar M, Merialdi M, Zavaleta N, Thi Nhu Ngoc N, Anthony J, De Greeff A, Poston L, Shennan A. World health organisation multicentre randomised trial of supplementation with vitamins c and e among pregnant women at high risk for pre-eclampsia in populations of low nutritional status from developing countries. BJOG. 2009;116:780–788. doi: 10.1111/j.1471-0528.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- 155.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 156.Knight M, Redman CW, Linton EA, Sargent IL. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1998;105:632–640. doi: 10.1111/j.1471-0528.1998.tb10178.x. [DOI] [PubMed] [Google Scholar]
- 157.Chua S, Wilkins T, Sargent I, Redman C. Trophoblast deportation in pre-eclamptic pregnancy. Br J Obstet Gynaecol. 1991;98:973–979. doi: 10.1111/j.1471-0528.1991.tb15334.x. [DOI] [PubMed] [Google Scholar]
- 158.Lok CA, Boing AN, Sargent IL, Sooranna SR, van der Post JA, Nieuwland R, Sturk A. Circulating platelet-derived and placenta-derived microparticles expose flt-1 in preeclampsia. Reprod Sci. 2008;15:1002–1010. doi: 10.1177/1933719108324133. [DOI] [PubMed] [Google Scholar]
- 159.Khankin EV, Royle C, Karumanchi SA. Placental vasculature in health and disease. Semin Thromb Hemost. 2010;36:309–320. doi: 10.1055/s-0030-1253453. [DOI] [PubMed] [Google Scholar]
- 160.Sunderji S, Gaziano E, Wothe D, Rogers LC, Sibai B, Karumanchi SA, Hodges-Savola C. Automated assays for svegf r1 and plgf as an aid in the diagnosis of preterm preeclampsia: A prospective clinical study. Am J Obstet Gynecol. 2010;202:40, e41–47. doi: 10.1016/j.ajog.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 161.Ohkuchi A, Hirashima C, Suzuki H, Takahashi K, Yoshida M, Matsubara S, Suzuki M. Evaluation of a new and automated electrochemiluminescence immunoassay for plasma sflt-1 and plgf levels in women with preeclampsia. Hypertens Res. 2010;33:422–427. doi: 10.1038/hr.2010.15. [DOI] [PubMed] [Google Scholar]
- 162.Qazi U, Lam C, Karumanchi SA, Petri M. Soluble fms-like tyrosine kinase associated with preeclampsia in pregnancy in systemic lupus erythematosus. J Rheumatol. 2008;35:631–634. [PubMed] [Google Scholar]
- 163.Cohen A, Lim KH, Lee Y, Rana S, Karumanchi SA, Brown F. Circulating levels of the antiangiogenic marker soluble fms-like tyrosine kinase 1 are elevated in women with pregestational diabetes and preeclampsia: Angiogenic markers in preeclampsia and preexisting diabetes. Diabetes Care. 2007;30:375–377. doi: 10.2337/dc06-1514. [DOI] [PubMed] [Google Scholar]
- 164.Young B, Levine RJ, Salahuddin S, Qian C, Lim KH, Karumanchi SA, Rana S. The use of angiogenic biomarkers to differentiate non-hellp related thrombocytopenia from hellp syndrome. J Matern Fetal Neonatal Med. 2010;23:366–370. doi: 10.1080/14767050903184207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Powers RW, Roberts JM, Cooper KM, Gallaher MJ, Frank MP, Harger GF, Ness RB. Maternal serum soluble fms-like tyrosine kinase 1 concentrations are not increased in early pregnancy and decrease more slowly postpartum in women who develop preeclampsia. Am J Obstet Gynecol. 2005;193:185–191. doi: 10.1016/j.ajog.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 166.Levine RJ, Qian C, Maynard SE, Yu KF, Epstein FH, Karumanchi SA. Serum sflt1 concentration during preeclampsia and mid trimester blood pressure in healthy nulliparous women. Am J Obstet Gynecol. 2006;194:1034–1041. doi: 10.1016/j.ajog.2005.10.192. [DOI] [PubMed] [Google Scholar]
- 167.Li Z, Zhang Y, Ying Ma J, Kapoun AM, Shao Q, Kerr I, Lam A, O'Young G, Sannajust F, Stathis P, Schreiner G, Karumanchi SA, Protter AA, Pollitt NS. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension. 2007;50:686–692. doi: 10.1161/HYPERTENSIONAHA.107.092098. [DOI] [PubMed] [Google Scholar]
- 168.Bergmann A, Ahmad S, Cudmore M, Gruber AD, Wittschen P, Lindenmaier W, Christofori G, Gross V, Gonzalves A, Grone HJ, Ahmed A, Weich HA. Reduction of circulating soluble flt-1 alleviates preeclampsia-like symptoms in a mouse model. J Cell Mol Med. 2010;14:1857–1867. doi: 10.1111/j.1582-4934.2009.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension. 2010;55:380–385. doi: 10.1161/HYPERTENSIONAHA.109.141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: A retrospective cohort study of 129,290 births. Lancet. 2001;357:2002–2006. doi: 10.1016/S0140-6736(00)05112-6. [DOI] [PubMed] [Google Scholar]
- 171.Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, Smith WC. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: Results from cohort study. BMJ. 2003;326:845. doi: 10.1136/bmj.326.7394.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (champs): Population-based retrospective cohort study. Lancet. 2005;366:1797–1803. doi: 10.1016/S0140-6736(05)67726-4. [DOI] [PubMed] [Google Scholar]
- 173.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53:944–951. doi: 10.1161/HYPERTENSIONAHA.109.130765. [DOI] [PubMed] [Google Scholar]
- 174.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chambers JC, Fusi L, Malik IS, Haskard DO, De Swiet M, Kooner JS. Association of maternal endothelial dysfunction with preeclampsia. JAMA. 2001;285:1607–1612. doi: 10.1001/jama.285.12.1607. [DOI] [PubMed] [Google Scholar]
- 176.Saxena AR, Karumanchi SA, Brown NJ, Royle CM, McElrath TF, Seely EW. Increased sensitivity to angiotensin ii is present postpartum in women with a history of hypertensive pregnancy. Hypertension. 2010;55:1239–1245. doi: 10.1161/HYPERTENSIONAHA.109.147595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sattar N, Ramsay J, Crawford L, Cheyne H, Greer IA. Classic and novel risk factor parameters in women with a history of preeclampsia. Hypertension. 2003;42:39–42. doi: 10.1161/01.HYP.0000074428.11168.EE. [DOI] [PubMed] [Google Scholar]
- 178.Parretti E, Lapolla A, Dalfra M, Pacini G, Mari A, Cioni R, Marzari C, Scarselli G, Mello G. Preeclampsia in lean normotensive normotolerant pregnant women can be predicted by simple insulin sensitivity indexes. Hypertension. 2006;47:449–453. doi: 10.1161/01.HYP.0000205122.47333.7f. [DOI] [PubMed] [Google Scholar]
- 179.Wolf M, Hubel CA, Lam C, Sampson M, Ecker JL, Ness RB, Rajakumar A, Daftary A, Shakir AS, Seely EW, Roberts JM, Sukhatme VP, Karumanchi SA, Thadhani R. Preeclampsia and future cardiovascular disease: Potential role of altered angiogenesis and insulin resistance. J Clin Endocrinol Metab. 2004;89:6239–6243. doi: 10.1210/jc.2004-0548. [DOI] [PubMed] [Google Scholar]
- 180.Laivuori H, Tikkanen MJ, Ylikorkala O. Hyperinsulinemia 17 years after preeclamptic first pregnancy. J Clin Endocrinol Metab. 1996;81:2908–2911. doi: 10.1210/jcem.81.8.8768850. [DOI] [PubMed] [Google Scholar]
- 181.Hubel CA, Powers RW, Snaedal S, Gammill HS, Ness RB, Roberts JM, Arngrimsson R. C-reactive protein is elevated 30 years after eclamptic pregnancy. Hypertension. 2008;51:1499–1505. doi: 10.1161/HYPERTENSIONAHA.108.109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Romundstad PR, Magnussen EB, Smith GD, Vatten LJ. Hypertension in pregnancy and later cardiovascular risk. Common antecedents? Circulation. 2010;122:579–84. doi: 10.1161/CIRCULATIONAHA.110.943407. [DOI] [PubMed] [Google Scholar]
- 183.Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: Opportunities for intervention and screening? BMJ. 2002;325:157–160. doi: 10.1136/bmj.325.7356.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Germain AM, Romanik MC, Guerra I, Solari S, Reyes MS, Johnson RJ, Price K, Karumanchi SA, Valdes G. Endothelial dysfunction: A link among preeclampsia, recurrent pregnancy loss, and future cardiovascular events? Hypertension. 2007;49:90–95. doi: 10.1161/01.HYP.0000251522.18094.d4. [DOI] [PubMed] [Google Scholar]
- 185.Yinon Y, Kingdom JC, Odutayo A, Moineddin R, Drewlo S, Lai V, Cherney DZ, Hladunewich MA. Vascular dysfunction in women with a history of preeclampsia and intrauterine growth restriction. Insights into future vascular risk. Circulation. 2010;122:1846–53. doi: 10.1161/CIRCULATIONAHA.110.948455. [DOI] [PubMed] [Google Scholar]
- 186.Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, Thadhani R, Wolf M, Harger G, Markovic N. Extra-placental expression of vascular endothelial growth factor receptor-1, (flt-1) and soluble flt-1 (sflt-1), by peripheral blood mononuclear cells (pbmcs) in normotensive and preeclamptic pregnant women. Placenta. 2005;26:563–573. doi: 10.1016/j.placenta.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 187.Di Marco GS, Reuter S, Hillebrand U, Amler S, Konig M, Larger E, Oberleithner H, Brand E, Pavenstadt H, Brand M. The soluble vegf receptor sflt1 contributes to endothelial dysfunction in ckd. J Am Soc Nephrol. 2009;20:2235–2245. doi: 10.1681/ASN.2009010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Levine RJ, Vatten LJ, Horowitz GL, Qian C, Romundstad PR, Yu KF, Hollenberg AN, Hellevik AI, Asvold BO, Karumanchi SA. Pre-eclampsia, soluble fms-like tyrosine kinase 1, and the risk of reduced thyroid function: Nested case-control and population based study. BMJ. 2009;339:b4336. doi: 10.1136/bmj.b4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Khurana R, Simons M, Martin JF, Zachary IC. Role of angiogenesis in cardiovascular disease: A critical appraisal. Circulation. 2005;112:1813–1824. doi: 10.1161/CIRCULATIONAHA.105.535294. [DOI] [PubMed] [Google Scholar]
- 190.Shin S, Lee SH, Park S, Kang SM, Chung N, Shim WH, Cho SY, Manabe I, Jang Y. Soluble fms-like tyrosine kinase-1 and the progression of carotid intima-media thickness - 24-month follow-up study. Circ J. 2010;74:2211–2215. doi: 10.1253/circj.cj-10-0432. [DOI] [PubMed] [Google Scholar]
- 191.Jayet PY, Rimoldi SF, Stuber T, Salmon CS, Hutter D, Rexhaj E, Thalmann S, Schwab M, Turini P, Sartori-Cucchia C, Nicod P, Villena M, Allemann Y, Scherrer U, Sartori C. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation. 2010;122:488–494. doi: 10.1161/CIRCULATIONAHA.110.941203. [DOI] [PubMed] [Google Scholar]





