Summary
Tropomyosin (Tm) purified from skeletal and cardiac muscle often contains disulfide bonds due to oxidation of cysteine groups that are in close proximity in the coiled-coil structure. Are these disulfide crosslinks present in the muscle or produced by oxidation during preparation? To answer this question we reacted one part of freshly dissected rabbit psoas muscle fibers, which was permeabilized with Triton X-100, with N-ethyl maleimide (NEM) to block cysteine groups and another part with 5,5′-dithiobis(2-nitro benzoate) (DTNB) to facilitate disulfide bond formation by interchain sulfhydryl-disulfide exchange. We found, by high resolution gradient SDS polyacrylamide gels, that the NEM-treated muscle was only composed of uncrosslinked Tm and the DTNB treated muscle was composed of disulfide-crosslinked Tm. This work indicates that Tm exists in a reduced state in rabbit psoas muscle.
Keywords: Tropomyosin, Muscle, Reduced, Crosslinked
Introduction
Tropomyosin (Tm) is an essential component of the regulatory system of skeletal, cardiac and smooth muscle. It is also found in all cells but the function of non-muscle Tm is not as well understood. Muscle Tm isoform α contains 1 cysteine at position 190 and isoform β contains 2 cysteines at positions 190 and 36. The composition of the coiled-coil dimer in rabbit skeletal Tm is 60% αα and 40% αβ (Lehrer 1975). These cysteines can be oxidized to produce intramolecular disulfide crosslinks either by 5,5′-dithiobis (2-nitro benzoate) (DTNB) via sulfhydryl-disulfide exchange (Lehrer 1975) or by Cu2+-catalyzed oxidation (Johnson and Smillie 1975; Stewart 1975). Because disulfide bond formation requires close proximity between neighboring cysteines, these studies showed that the 2 coiled-coil chains are in register. The DTNB reaction was also used to study the relative stability of the different isoforms of muscle Tm, αα, αβ and ββ (Lehrer and Joseph 1987). The different Tms have similar actin binding and Ca2+-regulation properties in reconstituted systems (Boussouf et al. 2007). When purified from muscle or made by recombinant DNA methods, the Tms often are partially disulfide cross-linked depending on storage conditions. In most studies the crosslinks are usually reduced with reducing agents such as dithiothreitol.
Disulfide-crosslinked Tm has been reported in failing hearts due to activity of reactive oxygen species (ROS) (Canton et al. 2011, and references therein). It is not known if Tm crosslinking contributes to the heart failure or is a result of the failure. The crosslinked and uncrosslinked Tm forms have different thermal and dynamic stabilities which may introduce flexibility to affect its regulatory properties (Lehrer 1978). It is thus important to determine what the state of Tm is in muscle. In this work, with the use of freshly dissected psoas rabbit muscle, we have shown by blocking the SH groups to prevent oxidation and by high resolution gradient SDS-gel electrophoreses that the Tm is in a reduced state.
Methods
Immediately after sacrifice, rabbit psoas muscle strips, each about 3.5–4.0 cm long, 5–10 mm wide, and 2–3 mm thick (total weight ∼8 g), were dissected out and immersed in separate tubes containing 10 ml of either 5 mM NEM or 5 mM DTNB and also contained 1% Triton X-100, 75 mM KCl, 5 mM Kphosphate buffer, pH 7.2, 2 mM EGTA, 2 mM MgCl2, and 0.1 mM phenylmethylsulfonyl fluoride (PMSF). The samples were incubated for 3 h at 4°C. The NEM and DTNB concentrations were increased to 10 mM and the incubation was continued for 1 h at room temperature and overnight at 4°C. The next day the samples were spun to pellet and suspended twice in buffer (10 ml, 0.1 M NaCl, 10 mM HEPES buffer, pH 7.5 containing 0.1 mM PMSF). 5 ml of each sample was homogenized with 1% SDS to extract soluble proteins, spun to remove insoluble material and run on 4–20% SDS-gradient gels (Bio-Rad).
The remaining 5 ml of each sample in buffer (no SDS) was boiled for 1 min. to precipitate denatured proteins and thereby purify soluble Tm, spun to remove aggregated denatured proteins and the supernatants were run on 4–20% SDS-gradient gels.
Results
The SDS-extracted NEM- and DTNB-treated samples and control proteins are shown in Fig. 1A. The NEM- and DTNB-treated SDS-extracted muscle are shown at different loadings. The bands between 25 and 50 kDa can be identified by comparison with the actin (A), rabbit skeletal Tm and troponin T (TnT). The two lower bands of the triplet, just below the 37 kDa marker, have very similar mobility as rabbit skeletal α- and β-Tm. There are no bands in the disulfide crosslinked region near the 75 kDa marker where the disulfide crosslinked Tm would be found. This indicates that the Tm exists in muscle in the reduced form. Treatment with DTNB with the muscle fibers provided further evidence that Tm is reduced in intact muscle. DTNB crosslinks Tm by a process of sulfhydryl-disulfide exchange of proximal cysteines (Lehrer 1975). The formation of disulfide-crosslinked Tm which occurs only after reaction of native Tm with DTNB indicates that the cysteines were in a native conformation in muscle. The reaction was almost quantitative as judged by the lack of remaining bands in the monomer mobility region. Comparison with the TnT control and the DTNB-treated rabbit skeletal Tm (which is only partially crosslinked), identify the dimer band near the 75 kDa marker as crosslinked Tm. Previous studies have shown that DTNB produces two crosslinked forms of rabbit skeletal Tm, α-α and α-β from the two isoforms present α, and β. (Lehrer 1975). These crosslinked bands are seen near the 75 kDa marker. The bands at higher mobility between the 15 kDa ad 25 kDa markers correspond to myosin light chains, troponin I and troponin C. Myosin bands are seen for the NEM-treated fibers near the 250 kDa marker, which are missing in the DTNB-treated fibers. Apparently, the DTNB crosslinked the myosin to produce high molecular weight species, which did not extract with SDS or/and did not enter the gel. The actin bands were light relative to the regulatory protein bands, perhaps due to less efficient extraction with SDS of the polymerized actin.
Fig. 1.
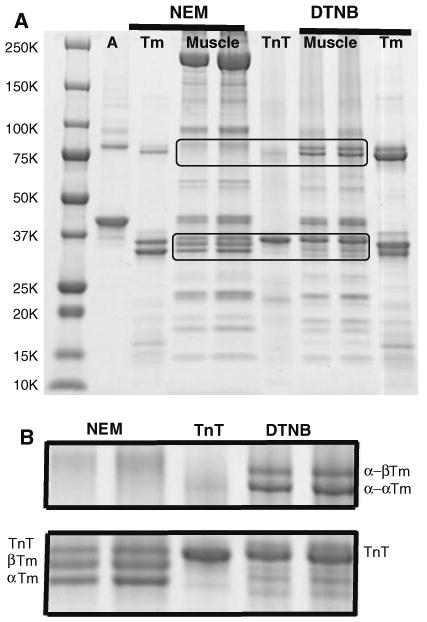
A SDS gradient gel of psoas muscle fibers treated with NEM or DTNB. A rabbit skeletal actin control, Tm rabbit skeletal Tm control; TnT rabbit skeletal troponin T control, Muscle 2 loadings of NEM- or DTNB-treated muscle fibers. The crosslinked Tm region (upper) and the uncrosslinked region (lower) are magnified in B. B Magnified upper and lower regions of the gradient gel of A. Note: (1) presence of α- and β-Tm and absence of crosslinked α-α and α-β Tm for NEM treatment; (2) presence of crosslinked α-α and α-β Tm and absence of uncrosslined α- and β-Tm for DTNB treatment
A magnified image of the dimer region (from upper rectangle) and monomer region (from lower rectangle) shows the assignment more clearly (Fig. 1B).
The supernatant from boiling NEM- and DTNB-treated muscle fibers in buffer was run on SDS-gels (Fig. 2). Most of the actin and myosin and other bands were removed by this treatment. The β-Tm bands were not as well separated from remaining TnT on this gel so the presence or absence of Tm was ascertained by only considering the α-Tm band. As is seen the α-Tm band is present in the NEM-treated muscle and absent in the DTNB-treated fibers. Conversely, the DTNB-treated fibers showed that the Tm was only present in the dimer region as α-α and α-β Tm.
Fig. 2.
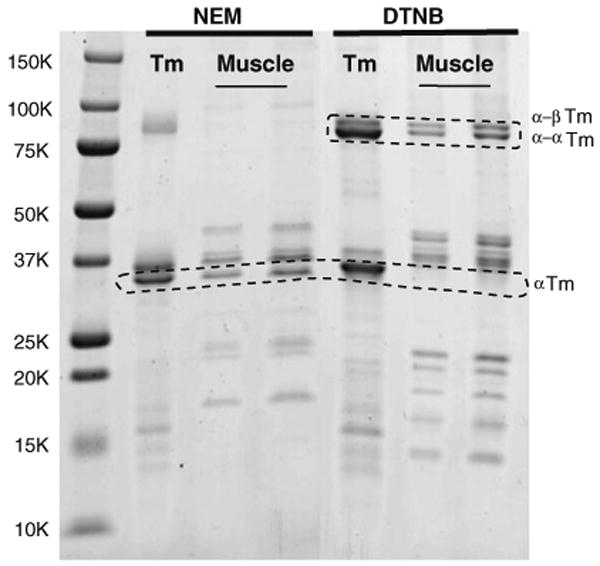
SDS gradient gel of supernatants after boiling the NEM and DTNB samples to purify the Tm. Note: (1) presence of α-Tm and absence of crosslinked Tm species for NEM treatment; (2) absence of α-Tm and presence of crosslinked Tm for DTNB treatment; (3) loss of myosin heavy chain, actin and some low molecular weight species
Discussion
In the absence of reducing agents such as dithiothreitol or β-mercaptoethanol, Tm prepared from muscle tissue or by recombinant DNA methods often contains a variable amount of disulfide crosslinks at position 190 caused by oxidation; e.g., disulfide crosslinks were present in the crystal structure of an 119 residue peptide of Tm (Brown et al. 2005). We showed that DTNB can react with Tm slowly to quantitatively produce disulfide bonds facilitated by protein dynamics which briefly exposes the SH groups (Lehrer 1975). Although the environment in the muscle cell is presumed to be reducing, e.g., due to the presence of reduced glutathione, it never had previously been shown that skeletal Tm is reduced. By reacting the SH groups of Tm in fresh permeabilized muscle fibers with the blocking reagent NEM, this study has shown that Tm is reduced in psoas muscle and can therefore is likely to be reduced in other muscle tissue. The use of DTNB to purposely crosslink Tm was a control that showed that the Tm was native and accessible to reaction with small reagents over the long incubation time. SDS-PAGE on gradient gels of SDS-extracted muscle fibers allowed separation of most of the components and the monomeric and dimeric cross-linked Tm species to be identified. Several studies have indicated that Tm in normal hearts appears to be reduced (Canton et al. 2011, and references therein). Using a somewhat different approach we show that Tm is reduced in skeletal muscle.
Acknowledgments
We appreciate the critical reading of the manuscript by Dr. Zenon Grabarek and support from National Institutes of Health (HL91162).
Contributor Information
Sherwin S. Lehrer, Email: Lehrer@BBRI.org, Cardiovascular Program, Boston Biomedical Research Institute, 64 Grove Street, Watertown, MA 02472, USA; Department of Neurology, Harvard Medical School, Boston, MA, USA.
Socheata Ly, Cardiovascular Program, Boston Biomedical Research Institute, 64 Grove Street, Watertown, MA 02472, USA.
Franklin Fuchs, Cardiovascular Program, Boston Biomedical Research Institute, 64 Grove Street, Watertown, MA 02472, USA.
References
- Boussouf SE, Maytum R, et al. Role of tropomyosin isoforms in the calcium sensitivity of striated muscle thin filaments. J Muscle Res Cell Motil. 2007;28:49–58. doi: 10.1007/s10974-007-9103-z. [DOI] [PubMed] [Google Scholar]
- Brown JH, Zhou Z, et al. Structure of the mid-region of tropomyosin: bending and binding sites for actin. Proc Natl Acad Sci USA. 2005;102:18878–18883. doi: 10.1073/pnas.0509269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canton M, Menazza S, et al. Oxidation of myofibrillar proteins in human heart failure. J Am Coll Cardiol. 2011;57:300–309. doi: 10.1016/j.jacc.2010.06.058. [DOI] [PubMed] [Google Scholar]
- Johnson F, Smillie LB. Rabbit skeletal alpha-tropomyosin chains are in register. Biochem Biophys Res Commun. 1975;64:1316–1322. doi: 10.1016/0006-291x(75)90836-0. [DOI] [PubMed] [Google Scholar]
- Lehrer SS. Intramolecular crosslinking of tropomyosin via disulfide bond formation: evidence for chain register. Proc Natl Acad Sci USA. 1975;72:3377–3381. doi: 10.1073/pnas.72.9.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer SS. Effects of an interchain disulfide bond on tropomyosin structure: intrinsic fluorescence and circular dichroism studies. J Mol Biol. 1978;118:209–226. doi: 10.1016/0022-2836(78)90413-8. [DOI] [PubMed] [Google Scholar]
- Lehrer SS, Joseph D. Differences in local conformation around cysteine residues in αα, αβ and ββ rabbit skeletal tropomyosin. Arch Biochem Biophys. 1987;256:1–9. doi: 10.1016/0003-9861(87)90419-x. [DOI] [PubMed] [Google Scholar]
- Stewart M. Tropomyosin: evidence for no stagger between chains. FEBS Lett. 1975;53:5–7. doi: 10.1016/0014-5793(75)80668-5. [DOI] [PubMed] [Google Scholar]


