Abstract
Background:
Dysfunction of the interventricular septum has been implicated in right ventricular (RV) failure. However, little is known about the relationship between ventricular septal and RV function in patients without clinical cardiovascular disease. We hypothesized that better septal function would be associated with higher RV ejection fraction and lower RV mass and volume by cardiac MRI.
Methods:
In the Multi-Ethnic Study of Atherosclerosis (MESA), cardiac MRI was performed on community-based participants without clinical cardiovascular disease. Images were analyzed by the harmonic phase method to measure peak circumferential systolic midventricular strain for each wall (anterior, lateral, inferior, and septal). Multivariable linear regression and generalized additive models were used to assess the relationship between septal strain and RV morphology.
Results:
There were 917 participants (45.7% women) with a mean age of 65.7 years. Better septal function was associated with higher RV ejection fraction in a nonlinear fashion after adjustment for all covariates (P = .03). There appeared to be a threshold effect for the contribution of septal strain to RV systolic function, with an almost linear decrement in RV ejection fraction with septal strain from −18% to −10%. Septal function was not related to RV mass or volume.
Conclusions:
Interventricular septal function was linked to RV systolic function independent of other left ventricular regions, even in individuals without clinical cardiovascular disease. This finding confirms animal and human research suggesting the importance of septal function to the right ventricle and implies that changes in septal function could herald RV dysfunction.
Trial registry:
ClinicalTrials.gov; No.: NCT00005487; URL: www.clinicaltrials.gov
Ventricular interdependence is defined by the forces transmitted from one ventricle to the other through the myocardium and pericardium, independent of neurohormonal or circulatory effects.1 Diastolic ventricular interdependence is well recognized; however, systolic ventricular interdependence mediated by the myocardium is less widely appreciated.1-6 Systolic ventricular interdependence has been attributed primarily to the interventricular septum and may have important hemodynamic implications.4,7,8 Systolic ventricular interdependence is believed to be an important mediator in the hemodynamic response to right ventricular (RV) pressure or volume overload and may explain RV failure following cardiac surgery, cardiac transplantation, and left ventricular (LV) assist device placement.1,7-10 Conversely, preservation of septal function may offer a novel therapeutic strategy in conditions such as RV dysplasia.11
Although believed to be important in human pathologic states, the normal physiologic contribution of the septum is more uncertain. Cardiovascular MRI provides reliable and valid measurements of RV function, and MRI tagging enables measurement of systolic function in specific LV segments. Strain is defined as the change in length between MRI tags divided by the original length between the two tags (strain = (L − L0)/L0, where L0 = original length).12 LV strain measured by MRI tagging is the noninvasive standard for the assessment of regional LV function and has been validated against implanted sonomicrometers.13 The identification of a link between septal and RV function, even in disease-free participants, could suggest a method to identify individuals at high risk of RV dysfunction, were they to develop other predisposing conditions, such as obstructive lung disease or congestive heart failure. We hypothesized that better septal function would be associated with higher RV ejection fraction (RVEF) and lower RV end-diastolic mass (RVEDM) and RV end-diastolic volume (RVEDV) in a population free of clinical cardiovascular disease.
Materials and Methods
Multi-Ethnic Study of Atherosclerosis
The Multi-Ethnic Study of Atherosclerosis (MESA) is a multicenter prospective cohort study investigating the prevalence, correlates, and progression of subclinical cardiovascular disease in Caucasians, African Americans, Hispanics, and Chinese.14 In 2000 to 2002, MESA recruited 6,814 men and women aged 45 to 84 years from six US communities: Forsyth County, North Carolina; Northern Manhattan and the Bronx, New York; Baltimore City and Baltimore County, Maryland; St Paul, Minnesota; Chicago, Illinois; and Los Angeles, California. Exclusion criteria included clinical cardiovascular disease (physician diagnosis of heart attack, stroke, transient ischemic attack, heart failure, angina, current atrial fibrillation, or any cardiovascular procedure), weight > 300 lbs, pregnancy, or impediment to long-term participation. The study was approved by University of Pennsylvania Office of Regulatory Affairs Institutional Review Board (protocol number 808374). Informed consent was obtained from all participants.
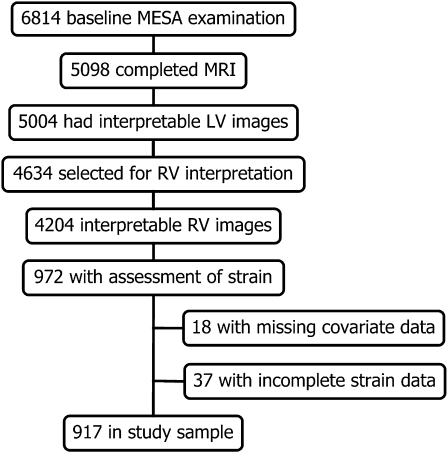
MRI scans were performed on MESA participants eligible for MRI (ie, without metal implants, devices, or fragments). Of the 6,814 in the cohort, 5,098 underwent MRI, and LV function could be interpreted in 5,004. A separate ancillary study performed strain analysis on a subset of 1,099 of these 5,004 participants. The MESA Right Ventricle project selected 4,634 scans and successfully read 4,204 scans from the 5,004 interpretable for LV function. The study population (N = 972) consisted of the intersection of the 1,099 scans selected for strain measurements and the 4,204 with interpretable RV function (Fig 1).
Figure 1.
Study sample. LV = left ventricular; MESA = Multi-Ethnic Study of Atherosclerosis; RV = right ventricular.
MRI Protocol
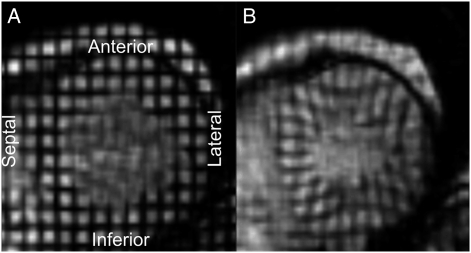
The cardiac MRI protocol has been described previously.15 All imaging was performed on 1.5-T magnets with a four-element, phased-array surface coil positioned anteriorly and posteriorly and ECG gating. Imaging consisted of fast gradient echo cine images with temporal resolution ≤ 50 milliseconds. After completing the standard imaging protocol, three tagged short-axis slices (base to apex) were obtained. Parallel striped tags (Fig 2) were prescribed in two orthogonal orientations using ECG-triggered fast gradient echo sequence with spatial modulation of magnetizations.16 The parameters for tagged MRI were as follows: field of view, 40 cm; slice thickness, 8 to 10 mm; repetition time, 3.5 to 7.2 milliseconds; echo time, 2.0 to 4.2 milliseconds; flip angle, 10° to 12°; matrix size, 256 × 96 to 140; temporal resolution, 20 to 40 milliseconds; and tag spacing, 7 mm.
Figure 2.
Example of short-axis myocardial tagging images from one study participant. A, end diastole. B, end systole.
MRI Data Analysis
Methods for interpretation of LV and RV parameters have been reported previously.17,18 Briefly, RV image analysis was performed by two independent analysts on Windows workstations using QMASS software, version 4.2 (Medis; Leiden, The Netherlands). The endocardial and epicardial borders of the RV were traced manually on the short-axis cine images at the end-systolic and end-diastolic phase. Papillary muscles and trabeculae were included in the RV volumes and excluded from RVEDM.19,20 RV end-systolic volume (RVESV) and RVEDV were calculated using the Simpson rule by summation of areas on each slice multiplied by the sum of slice thickness and image gap. RVEDM was determined at the end-diastolic phase as the difference between end-diastolic epicardial and endocardial volumes multiplied by the specific gravity of the heart (1.05 g/cm3).15 RV stroke volume (RVSV) was calculated by subtracting RVESV from RVEDV. RVEF was calculated by dividing RVSV by RVEDV. The intraclass correlation coefficients from random, blinded, interreader re-reads of 240 scans for RVEDM and RVEDV were 0.89 and 0.96, respectively, and 0.80 for RVEF.
Strain was defined as the change in length between tags divided by the original length.12 Figure 2 illustrates the tagging used to measure strain. During systole, the tags move closer together. A more negative value reflects more shortening and enhanced contraction. Peak regional circumferential strain was determined in four LV walls (anterior, lateral, inferior, and septal) in the midwall layer. The tagged images were analyzed by the harmonic phase method. Harmonic phase (Diagnosoft, Inc; Palo Alto, California) enables fast determination of strain with excellent interobserver and intraobserver agreement that has been validated against other well-established methods of strain analysis.21,22
Covariates
Race/ethnicity was self-reported during the baseline MESA examination according to the 2000 US Census criteria as race (Caucasian, African American, etc) and ethnicity (Hispanic or non-Hispanic). Participants self-identifying as Hispanic were categorized as Hispanic. Standard questionnaires were used to ascertain smoking status (classified as never, former, or current) and pack-years smoked. Participants self-reported intentional exercise levels, which were measured in metabolic equivalent minutes per week. Resting BP was measured three times using the Dinamap Monitor PRO 100 automated oscillometric device (Critikon, Inc; Tampa, Florida), and the average of the last two measurements was used. Hypertension was defined as systolic BP ≥ 140 mm Hg, as diastolic BP ≥ 90 mm Hg, or by self-report of hypertension and current use of antihypertensive medication. Presence of diabetes mellitus was based on self-reported physician diagnosis, use of medication for hyperglycemia, or a fasting glucose level ≥ 126 mg/dL, the latter measured by rate reflectance spectrophotometry (Johnson & Johnson Ortho-Clinical Diagnostics, Inc; Rochester, New York). Fasting glucose between 100 mg/dL and 125 mg/dL was considered impaired fasting glucose. Fasting blood samples were drawn and sent to a central laboratory for measurement of glucose.
Statistical Analysis
Continuous variables are expressed as means and SDs or ranges. Categorical variables are expressed as percentages. Bivariable and multivariable linear regression models were used to assess the relationship of circumferential septal strain (independent variable) with RVEDM, RVEDV, RVSV, and RVEF (dependent variables). Generalized additive models were used if there was significant nonlinearity of the association between regional LV function and RV morphology. We did not include LV ejection fraction as a confounder, instead adjusting for LV circumferential strain in the remaining regions (anterior, lateral, and inferior) to account for the expected association between global LV and RV function. Covariates were chosen on the basis of known associations with ventricular size and heart disease, including demographics and anthropometric variables, as well as with variables reflecting comorbidities, such as smoking, hypertension, and diabetes mellitus. Adjustment for height and weight in all analyses avoided the assumptions made in indexing the RV measures to certain parameters of body size (eg, body surface area) while achieving the same end of accounting for differences in body size between participants. A sensitivity analysis was performed excluding patients with conduction defects (QRS complex > 120 milliseconds). Analyses were performed using STATA, version 10.0 (StataCorp; College Station, Texas) statistical software.
Results
There were 972 participants with measurements of RV function and LV regional strain. Thirty-seven were excluded because of incomplete regional function data, and 18 were excluded because they had missing data for clinical variables, leaving 917 in the final study sample (Fig 1).
The mean age of the study sample was 65.7 years, and 54.3% were men; 32.5% were Caucasian, 28.0% were African American, 31.5% were Hispanic, and 8.0% were Chinese (Table 1). The mean BMI was 28.0 kg/m2. Those included were somewhat more likely to be men, Hispanic, and nonsmokers and less likely to be African American than those excluded.
Table 1.
—Characteristics of the Study Sample Compared With Those Patients Excluded
| Characteristics | Study Sample (n = 917) | Excluded (n = 55) |
| Age, y | 65.7 ± 9.7 | 64.5 ± 10.0 |
| Male sex | 54.3 | 45.6 |
| Race/ethnicity | ||
| Caucasian | 32.5 | 32.7 |
| African American | 28.0 | 40.0 |
| Hispanic | 31.5 | 18.2 |
| Chinese | 8.0 | 9.1 |
| Educational attainment | ||
| No high school degree | 19.7 | 22.0 |
| High school degree | 20.9 | 18.0 |
| Some college | 26.7 | 34.0 |
| College degree | 14.6 | 12.0 |
| Higher than bachelor degree | 18.0 | 14.0 |
| Height, cm | 166.3 ± 10.2 | 165.3 ± 10.0 |
| Weight, kg | 77.4 ± 14.7 | 75.0 ± 15.1 |
| BMI, kg/m2 | 28.0 ± 4.6 | 27.4 ± 4.4 |
| Cigarette smoking status | ||
| Never | 51.5 | 30.0 |
| Former | 37.3 | 52.0 |
| Current | 11.2 | 18.0 |
| Hypertension | 48.3 | 43.6 |
| Systolic BP, mm Hg | 128.0 ± 20.0 | 130.0 ± 24.2 |
| Diastolic BP, mm Hg | 71.9 ± 10.2 | 74.2 ± 10.3 |
| Diabetes mellitus (treated or untreated) | 14.3 | 13.0 |
Data are presented as mean ± SD or %.
Table 2 shows the RV parameters of the study sample. The mean RVEF was 70.3% ± 6.4%. RVEF was < 55% in 12 participants. The mean LVEF was 68.4% ± 7.5%. Strain was greatest in the lateral region (−20.5%) and less in the anterior, septal, and inferior regions (−18.2%, −16.1%, and −13.8%, respectively). Seventy-six percent had septal strain between −10% and −20%.
Table 2.
—Cardiac MRI Characteristics of the Study Sample
| Characteristics | Study Sample (n = 917) |
| RVEDV, mL | 123.7 ± 30.7 |
| RVEDM, g | 20.3 ± 4.1 |
| RV stroke volume, mL | 80.3 ± 20.4 |
| RVEF, % | 70.3 ± 6.4 |
| LV ejection fraction, % | 68.4 ± 7.5 |
| LV systolic circumferential myocardial strain, % | |
| Anterior | −18.2 ± 4.0 |
| Lateral | −20.5 ± 3.8 |
| Inferior | −13.8 ± 4.4 |
| Septal | −16.1 ± 3.9 |
Data are presented as mean ± SD. LV = left ventricular; RV = right ventricular; RVEDM = right ventricular end-diastolic mass; RVEDV = right ventricular end-diastolic volume; RVEF = right ventricular ejection fraction.
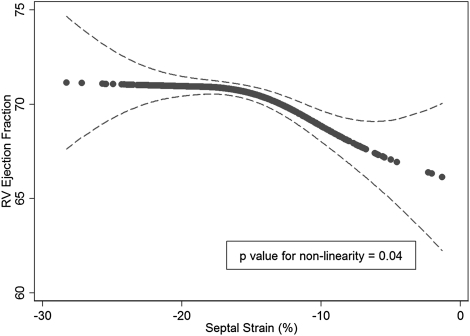
Better LV septal function (more negative strain value) was associated with greater RVEF in a nonlinear fashion in an unadjusted generalized additive model (P = .03) and when fully adjusted for function of the remaining regions and all covariates (P = .04) (Fig 3, Table 3). RVEF appeared stable across estimates of septal strain beyond −18%, whereas there was an almost linear decrement in RVEF, with septal strain from −18% to −10%, suggesting a threshold effect for the contribution of septal strain to RV systolic function. Similarly, better septal function was associated with greater RVSV (Table 3). The results were unchanged when participants with ventricular conduction defects were excluded (data not shown). In the unadjusted model, better septal function was associated with decreased RVEDM and RVEDV (P < .05); however, these associations were not statistically significant after adjustment for covariates (Table 3).
Figure 3.
Generalized additive model (GAM) of septal strain and RV ejection fraction. Solid line represents fully adjusted smooth GAM function (dashed lines, 95% CI). As ventricular septal function worsens (strain approaches 0), there is an apparent threshold at ∼ 18% where decreased septal function is associated with decreased RV function. See Figure 1 legend for expansion of the abbreviation.
Table 3.
—Bivariable and Multivariable Models Describing the Relationship Between Ventricular Septal Function and RV Morphology and Function
| Dependent Variable | βa (95% CI) | P Value |
| RVEF | ||
| Unadjusted | … | .03b |
| Adjusted model 1 | … | .05b |
| Adjusted model 2 | … | .04b |
| RV stroke volume | ||
| Unadjusted | −0.01 (−1.35, −1.33) | .99 |
| Adjusted model 1 | −1.66 (−2.83, −0.50) | .01 |
| Adjusted model 2 | −1.45 (−2.62, −0.29) | .02 |
| RVEDV | ||
| Unadjusted | −2.04 (0.03, 4.05) | .03 |
| Adjusted model 1 | −1.08 (−2.68, 0.52) | .19 |
| Adjusted model 2 | −0.83 (−2.43, 0.78) | .31 |
| RVEDM | ||
| Unadjusted | 0.30 (0.03, 0.56) | .03 |
| Adjusted model 1 | −0.13 (−0.35, 0.09) | .24 |
| Adjusted model 2 | −0.11 (−0.33, 0.12) | .34 |
Adjusted model 1 includes anterior, lateral, and inferior strain; age; sex; race/ethnicity; height; and weight. Adjusted model 2 includes all covariates from adjusted model 1 with the addition of systolic BP, diastolic BP, hypertension, diabetes, total cholesterol, smoking status, pack-years smoked, and exercise. See Table 2 legend for expansion of abbreviations.
Per SD (3.9%) increment of septal strain.
P value for nonlinearity from the generalized additive model.
Discussion
We have shown that interventricular septal function was associated with global RV systolic function independent of the other LV functional regions and a variety of potential confounders in individuals free of clinical cardiovascular disease. This association was nonlinear, with an apparent threshold effect at a cutoff (∼ 18%) that was close to the mean circumferential septal strain value (16%) in our data. It may be surprising that even in this cardiovascular disease-free cohort, strain levels below 18% were associated with decrements in RVEF. Although the effect estimates appear small (∼ 3% to 4% difference in RVEF for a 5% absolute difference in septal strain), the magnitude of these effects is similar to (or greater than) the decrements in LV ejection fraction seen in active smokers or patients with diabetes in MESA.23 There was a corresponding association between septal strain and RVSV. We found that regional LV function was not related to RVEDM and other volumes.
Several lines of investigation in animals have demonstrated the importance of the septum to RV function. Early studies revealed that near-complete destruction of the RV free wall led to little perturbation in hemodynamics.24,25 A study using an isolated heart preparation showed that RV systolic pressure varied with filling of the LV and that the effect was likely mediated by the interventricular septum.26 Other groups have shown that the LV contribution to RV-generated pressure is greater than the RV free wall component.27 Finally, decreasing LV volume with LV assist device placement in dogs leads to ipsilateral shift and dysfunction of the interventicular septum, resulting in decreased RV contractility.8,28
Human studies further enlighten the role of the interventricular septum in RV function. Historically, the septum has been regarded as a functional part of the LV because it moves toward the LV free wallin systole; however, this view of the septum may be incomplete. Banka et al29 demonstrated that the septum thickens and moves into both the LV and the RV cavities during systole, thus contributing to biventricular function. Further, LV assist device implantation causes clinical RV dysfunction in approximately 25% of patients, which is attributed to septal shift as seen in animal studies.9,30 Therefore, septal function may contribute significantly to RV function.
In addition to supporting a septum-mediated mechanism of subclinical RV dysfunction, the present study’s findings challenge the traditional view that septal dysfunction is an effect of RV failure and establishes the importance of septal function to RV systolic function even in the absence of clinical heart or lung disease. Puwanant et al31 measured septal strain in patients with significant pulmonary hypertension (PH) and excluded those with LV disease. They found that circumferential septal strain was reduced in patients with PH compared to control subjects but that there were also decrements in LV lateral circumferential strain. In patients with PH, longitudinal septal strain also was reduced, but LV lateral strain was normal compared to control subjects. Our findings suggest that baseline differences in septal function even in “normal” individuals may contribute to the interindividual differences seen in RV responses to increased afterload.
Given the importance of effective septal function to the right ventricle in both health and disease, therapies aimed at restoration of synchronous septal contraction could hold promise. Abnormal septal motion is evident on the presentation echocardiogram of > 90% of patients with pulmonary arterial hypertension and may be an important therapeutic target, considering the effect on RV systolic function across the spectrum from health to disease.32,33 An animal model of RV failure showed that significant dysynchrony was present in RV failure and that pacing not only improved RV systolic function, but also ameliorated adverse diastolic ventricular interaction.34 Our findings provide further mechanistic support for this therapeutic approach.
There are some limitations in the present study. Data on LV diastolic function in our cohort would have been interesting; however, subclinical LV diastolic dysfunction would not account for our findings because diastolic function is not known to preferentially affect the septum. Likewise, the overall correlation between LV and RV function does not explain our findings because we adjusted for strain in the other LV regions (accounting for overall correlations between LV and RV function). We measured circumferential strain; however, longitudinal strain may contribute more to RV function.7 Our results, therefore, may actually underestimate the septal contribution to RV function. Newer MRI sequences may be more precise (steady-state free precession imaging); however, potential error in MRI measurements should bias toward the null hypothesis, so the associations may be even stronger than we have shown. Factors affecting myocardial contractility may simultaneously affect septal strain and RVEF. Subclinical atherosclerotic disease, BP, and tobacco use have been associated with reduction in regional ventricular function, but none of these factors have been shown to differentially affect the ventricular septum.35-37 In addition, we adjusted for these and other potential confounders. Finally, this study is cross-sectional, so causality cannot be confirmed.
In summary, our findings support the importance of the ventricular septum to RV function. The impact of hemodynamics, diastolic function, valvular disease, and the three-dimensional shape of the RV on the relationships we have documented is an area for further research. Future studies assessing prediction, prevention, and treatment of RV failure should focus on the function of the ventricular septum.
Acknowledgments
Author contributions: Dr Dibble had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Dibble: contributed to the study concept and design, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content.
Dr Lima: contributed to the study concept and design, acquisition and interpretation of MRI data, and critical revision of the manuscript for important intellectual content.
Dr Bluemke: contributed to the study concept and design, acquisition and interpretation of MRI data, and critical revision of the manuscript for important intellectual content.
Dr Chirinos: contributed to the study concept and design, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content.
Dr Chahal: contributed to the acquisition and interpretation of MRI data and critical revision of the manuscript for important intellectual content.
Dr Bristow: contributed to the study concept and design and critical revision of the manuscript for important intellectual content.
Dr Kronmal: contributed to the study concept and design, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content.
Dr Barr: contributed to the study concept and design, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content.
Dr Ferrari: contributed to the study concept and design and critical revision of the manuscript for important intellectual content.
Dr Propert: contributed to the study concept and design, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content.
Dr Kawut: contributed to the study concept and design, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: NHLBI staff participated in the design of the MESA study and routinely monitored study performance. NHLBI staff also participated in the internal review of this manuscript prior to publication.
Other contributions: This manuscript has been reviewed by the MESA investigators for scientific content and consistency of data interpretation with previous MESA publications, and significant comments have been incorporated prior to submission for publication. We thank the other investigators, staff, and participants of the MESA for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Abbreviations
- LV
left ventricular
- MESA
Multi-Ethnic Study of Atherosclerosis
- PH
pulmonary hypertension
- RV
right ventricular
- RVEDM
right ventricular end-diastolic mass
- RVEDV
right ventricular end-diastolic volume
- RVEF
right ventricular ejection fraction
- RVESV
right ventricular end-systolic volume
Funding/Support: This work was supported by the National Institutes of Health [R01-HL066075, R01-HL086719, N01-HC95159 through HC95165, and T32-HL-007891].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Santamore WP, Dell’Italia LJ. Ventricular interdependence: significant left ventricular contributions to right ventricular systolic function. Prog Cardiovasc Dis. 1998;40(4):289–308. doi: 10.1016/s0033-0620(98)80049-2. [DOI] [PubMed] [Google Scholar]
- 2.Yamaguchi S, Tsuiki K, Miyawaki H, et al. Effect of left ventricular volume on right ventricular end-systolic pressure-volume relation. Resetting of regional preload in right ventricular free wall. Circ Res. 1989;65(3):623–631. doi: 10.1161/01.res.65.3.623. [DOI] [PubMed] [Google Scholar]
- 3.Karunanithi MK, Michniewicz J, Young JA, Feneley MP. Effect of acutely increased left ventricular afterload on work output from the right ventricle in conscious dogs. J Thorac Cardiovasc Surg. 2001;121(1):116–124. doi: 10.1067/mtc.2001.110683. [DOI] [PubMed] [Google Scholar]
- 4.Feneley MP, Olsen CO, Glower DD, Rankin JS. Effect of acutely increased right ventricular afterload on work output from the left ventricle in conscious dogs. Systolic ventricular interaction. Circ Res. 1989;65(1):135–145. doi: 10.1161/01.res.65.1.135. [DOI] [PubMed] [Google Scholar]
- 5.Elzinga G, Piene H, de Jong JP. Left and right ventricular pump function and consequences of having two pumps in one heart. A study on the isolated cat heart. Circ Res. 1980;46(4):564–574. doi: 10.1161/01.res.46.4.564. [DOI] [PubMed] [Google Scholar]
- 6.Oboler AA, Keefe JF, Gaasch WH, Banas JS, Jr, Levine HJ. Influence of left ventricular isovolumic pressure upon right ventricular pressure transients. Cardiology. 1973;58(1):32–44. doi: 10.1159/000169617. [DOI] [PubMed] [Google Scholar]
- 7.Saleh S, Liakopoulos OJ, Buckberg GD. The septal motor of biventricular function. Eur J Cardiothorac Surg. 2006;29(suppl 1):S126–S138. doi: 10.1016/j.ejcts.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 8.Moon MR, Bolger AF, DeAnda A, et al. Septal function during left ventricular unloading. Circulation. 1997;95(5):1320–1327. doi: 10.1161/01.cir.95.5.1320. [DOI] [PubMed] [Google Scholar]
- 9.Santamore WP, Gray LA., Jr Left ventricular contributions to right ventricular systolic function during LVAD support. Ann Thorac Surg. 1996;61(1):350–356. doi: 10.1016/0003-4975(95)01056-4. [DOI] [PubMed] [Google Scholar]
- 10.Ochiai Y, McCarthy PM, Smedira NG, et al. Predictors of severe right ventricular failure after implantable left ventricular assist device insertion: analysis of 245 patients. Circulation. 2002;106(12) suppl 1:I198–I202. [PubMed] [Google Scholar]
- 11.Buckberg GD. RESTORE Group The ventricular septum: the lion of right ventricular function, and its impact on right ventricular restoration. Eur J Cardiothorac Surg. 2006;29(suppl 1):S272–S278. doi: 10.1016/j.ejcts.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Mirsky I, Parmley WW. Assessment of passive elastic stiffness for isolated heart muscle and the intact heart. Circ Res. 1973;33(2):233–243. doi: 10.1161/01.res.33.2.233. [DOI] [PubMed] [Google Scholar]
- 13.Yeon SB, Reichek N, Tallant BA, et al. Validation of in vivo myocardial strain measurement by magnetic resonance tagging with sonomicrometry. J Am Coll Cardiol. 2001;38(2):555–561. doi: 10.1016/s0735-1097(01)01397-3. [DOI] [PubMed] [Google Scholar]
- 14.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 15.Natori S, Lai S, Finn JP, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186(6) suppl 2:S357–S365. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 16.Axel L, Dougherty L. MR imaging of motion with spatial modulation of magnetization. Radiology. 1989;171(3):841–845. doi: 10.1148/radiology.171.3.2717762. [DOI] [PubMed] [Google Scholar]
- 17.Chahal HJC, Tandri H, Jain A, et al. Relation of cardiovascular risk factors in relationship to the right ventricular structure and function by magnetic resonance imaging (results from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2010;106(1):110–116. doi: 10.1016/j.amjcard.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52(25):2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogel-Claussen J, Finn JP, Gomes AS, et al. Left ventricular papillary muscle mass: relationship to left ventricular mass and volumes by magnetic resonance imaging. J Comput Assist Tomogr. 2006;30(3):426–432. doi: 10.1097/00004728-200605000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Sievers B, Kirchberg S, Bakan A, Franken U, Trappe HJ. Impact of papillary muscles in ventricular volume and ejection fraction assessment by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2004;6(1):9–16. doi: 10.1081/jcmr-120027800. [DOI] [PubMed] [Google Scholar]
- 21.Garot J, Bluemke DA, Osman NF, et al. Fast determination of regional myocardial strain fields from tagged cardiac images using harmonic phase MRI. Circulation. 2000;101(9):981–988. doi: 10.1161/01.cir.101.9.981. [DOI] [PubMed] [Google Scholar]
- 22.Castillo E, Osman NF, Rosen BD, et al. Quantitative assessment of regional myocardial function with MR-tagging in a multi-center study: interobserver and intraobserver agreement of fast strain analysis with Harmonic Phase (HARP) MRI. J Cardiovasc Magn Reson. 2005;7(5):783–791. doi: 10.1080/10976640500295417. [DOI] [PubMed] [Google Scholar]
- 23.Heckbert SR, Post W, Pearson GDN, et al. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48(11):2285–2292. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kagan A. Dynamic responses of the right ventricle following extensive damage by cauterization. Circulation. 1952;5(6):816–823. doi: 10.1161/01.cir.5.6.816. [DOI] [PubMed] [Google Scholar]
- 25.Starr I, Jeffers WA, Meade RH., Jr The absence of conspicuous increments of venous pressure after severe damage to the right ventricle of the dog, with a discussion of the relation between clinical congestive failure and heart disease. Am Heart J. 1943;26(3):291–301. [Google Scholar]
- 26.Santamore WP, Lynch PR, Meier G, Heckman J, Bove AA. Myocardial interaction between the ventricles. J Appl Physiol. 1976;41(3):362–368. doi: 10.1152/jappl.1976.41.3.362. [DOI] [PubMed] [Google Scholar]
- 27.Damiano RJ, Jr, La Follette P, Jr, Cox JL, Lowe JE, Santamore WP. Significant left ventricular contribution to right ventricular systolic function. Am J Physiol. 1991;261(5 pt 2):H1514–H1524. doi: 10.1152/ajpheart.1991.261.5.H1514. [DOI] [PubMed] [Google Scholar]
- 28.Moon MR, Castro LJ, DeAnda A, et al. Right ventricular dynamics during left ventricular assistance in closed-chest dogs. Ann Thorac Surg. 1993;56(1):54–66. doi: 10.1016/0003-4975(93)90402-4. [DOI] [PubMed] [Google Scholar]
- 29.Banka VS, Agarwal JB, Bodenheimer MM, Helfant RH. Interventricular septal motion: biventricular angiographic assessment of its relative contribution to left and right ventricular contraction. Circulation. 1981;64(5):992–996. doi: 10.1161/01.cir.64.5.992. [DOI] [PubMed] [Google Scholar]
- 30.Kavarana MN, Pessin-Minsley MS, Urtecho J, et al. Right ventricular dysfunction and organ failure in left ventricular assist device recipients: a continuing problem. Ann Thorac Surg. 2002;73(3):745–750. doi: 10.1016/s0003-4975(01)03406-3. [DOI] [PubMed] [Google Scholar]
- 31.Puwanant S, Park M, Popović ZB, et al. Ventricular geometry, strain, and rotational mechanics in pulmonary hypertension. Circulation. 2010;121(2):259–266. doi: 10.1161/CIRCULATIONAHA.108.844340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bossone E, Duong-Wagner TH, Paciocco G, et al. Echocardiographic features of primary pulmonary hypertension. J Am Soc Echocardiogr. 1999;12(8):655–662. doi: 10.1053/je.1999.v12.a99069. [DOI] [PubMed] [Google Scholar]
- 33.Dubin AM, Feinstein JA, Reddy VM, Hanley FL, Van Hare GF, Rosenthal DN. Electrical resynchronization: a novel therapy for the failing right ventricle. Circulation. 2003;107(18):2287–2289. doi: 10.1161/01.CIR.0000070930.33499.9F. [DOI] [PubMed] [Google Scholar]
- 34.Handoko ML, Lamberts RR, Redout EM, et al. Right ventricular pacing improves right heart function in experimental pulmonary arterial hypertension: a study in the isolated heart. Am J Physiol Heart Circ Physiol. 2009;297(5):H1752–H1759. doi: 10.1152/ajpheart.00555.2009. [DOI] [PubMed] [Google Scholar]
- 35.Rosen BD, Saad MF, Shea S, et al. Hypertension and smoking are associated with reduced regional left ventricular function in asymptomatic: individuals the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;47(6):1150–1158. doi: 10.1016/j.jacc.2005.08.078. [DOI] [PubMed] [Google Scholar]
- 36.Rosen BD, Cushman M, Nasir K, et al. Relationship between C-reactive protein levels and regional left ventricular function in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2007;49(5):594–600. doi: 10.1016/j.jacc.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 37.Edvardsen T, Detrano R, Rosen BD, et al. Coronary artery atherosclerosis is related to reduced regional left ventricular function in individuals without history of clinical cardiovascular disease: the Multiethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26(1):206–211. doi: 10.1161/01.ATV.0000194077.23234.ae. [DOI] [PubMed] [Google Scholar]