Abstract
Background:
Serum levels of surfactant protein D (SP-D) have been suggested as reflecting epithelial damage in acute lung injury, COPD, and idiopathic pulmonary fibrosis (IPF). However, little is known about SP-D levels in the setting of lung transplantation.
Methods:
We examined plasma SP-D levels in 104 subjects from a prospective, multicenter cohort study of lung allograft recipients. Plasma SP-D was measured by enzyme-linked immunosorbent assay prior to transplant and daily for 3 days after transplant.
Results:
Subjects undergoing transplant for IPF had higher baseline SP-D levels (median, 325 ng/mL) compared with subjects with cystic fibrosis, COPD, and pulmonary hypertension (median, 100, 80, and 82 ng/mL, respectively; P = .0001). Among subjects with IPF undergoing bilateral transplant, SP-D levels declined rapidly postoperatively. In contrast, SP-D levels in subjects undergoing single lung transplant for IPF remained significantly higher than those of bilateral allograft recipients. Among subjects undergoing single lung transplant for IPF, the development of primary graft dysfunction (PGD) was associated with a subsequent rise in SP-D levels, whereas SP-D levels in IPF subjects undergoing bilateral transplant declined, even in the presence of grade 3 PGD. Importantly, single lung allograft recipients without PGD had higher postoperative SP-D levels than bilateral allograft recipients with PGD.
Conclusions:
Subjects undergoing lung transplant for IPF have significantly higher baseline plasma SP-D levels compared with those with other diagnoses. Plasma SP-D is likely a biomarker of the air-blood barrier integrity in the native IPF lung, but may be less useful as a biomarker of PGD after transplant.
Surfactant protein D (SP-D) is a lung collectin, synthesized and secreted by alveolar type-II cells and Clara cells. The 43-kD monomer assembles into higher-order quaternary structures, predominantly a dodecamer composed of four homotrimers. SP-D is a potent innate immune modulator that enhances clearance of a wide variety of pathogens,1-5 promotes phagocytosis of apoptotic cells,6,7 and, depending on local conditions, either enhances or inhibits proinflammatory cytokine release.4,8,9
Normally, SP-D is localized primarily in the lung, with relatively low serum levels.10 However, elevated serum levels of SP-D have been associated with numerous acute and chronic lung diseases.11-17 Higher serum SP-D levels predict increased mortality and decreased ventilator-free survival in acute lung injury.11 In COPD, SP-D levels are reduced in the lung,18 but higher serum levels predict greater risk of COPD exacerbation and are lowered by treatment with oral corticosteroids.14 In idiopathic pulmonary fibrosis (IPF), higher serum SP-D levels are associated with the extent of radiographic infiltrates, rate of decline in lung function, and mortality.19,20
Despite investigation into numerous lung diseases, very little is known about plasma SP-D levels in the setting of lung transplantation. One study of lung allograft recipients demonstrated that bile acid aspiration is associated with decreased levels of SP-D in the lung allograft,21 but plasma SP-D was not examined. We sought to determine whether plasma SP-D levels among patients undergoing lung transplant would differ by pretransplant diagnosis and whether the presence of primary graft dysfunction (PGD) would influence the expected decline in SP-D levels following lung transplant.
Materials and Methods
Study Design
We performed a prospective cohort study of patients undergoing first lung transplant at eight centers in the United States since 2003 (a full list of investigators and participating centers is included in e-Appendix 1).22-24 The study sample consisted of the first 128 consecutive subjects recruited into the cohort between March 2003 and November 2004, of whom 109 subjects had plasma samples available for analysis. Four subjects with Eisenmenger syndrome and/or congenital heart disease and one subject with lymphangioleiomyomatosis were excluded from analysis, leaving 104 subjects in the final cohort. Clinical variables were categorized and defined using methods published previously.25 Subjects were considered to have PGD if they met the International Society of Heart and Lung Transplantation definition for grade 3 PGD at any time in the first 72 h after transplant.26 The study was approved by the institutional review boards at each of the participating centers (details in e-Appendix 2). Informed consent was obtained from all participants.
Plasma SP-D Measurement
Blood samples were collected within 24 h prior to lung transplant, approximately 6 h after allograft reperfusion, and daily thereafter for 3 days. Samples were centrifuged within 30 min of collection. The separated plasma was stored at −80°C for subsequent batched analysis. Plasma levels of SP-D were measured in duplicate using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Yamasa Corporation; Tokyo, Japan). ELISA was performed according to the manufacturer’s instructions and calibrated to a standard concentration curve. The intraassay coefficient of variation was 5.1%.
Statistical Analysis
In bivariable analysis, continuous variables were compared using Student t tests, Wilcoxon rank sum tests, analysis of variance, or Kruskal-Wallis tests, as appropriate. Correlations between continuous variables were assessed using Spearman rank correlation coefficients. Multivariable linear regression was used to determine whether pretransplant diagnosis was associated with baseline SP-D levels and was independent of the effect of other clinical variables. Age, sex, and race were forced into the model. Additional covariates were added one at a time and were considered confounders if they altered the coefficient of association between diagnosis and SP-D levels by ≥ 10%.27 Additional analyses included a nonparametric test for the trend in SP-D levels over time.28 All analyses were conducted using Stata, version 11, software (StataCorp LP; College Station, Texas).
Results
Of the 104 subjects meeting eligibility criteria, 48 (46%) had COPD, 14 (13%) had cystic fibrosis (CF), 38 (37%) had IPF, and four (4%) had idiopathic pulmonary arterial hypertension (IPAH). Demographic and baseline characteristics of these subject groups are detailed in Table 1. Subjects with CF and IPAH were younger than those with COPD or IPF, and subjects with IPF were more likely to be taking oral steroids prior to transplant. The large majority of subjects were white, and there were roughly equal numbers of male and female subjects.
Table 1.
—Subject Characteristics by Diagnosis
| Characteristic | COPD (n = 48) | CF (n = 14) | IPF (n = 38) | IPAH (n = 4) | P Value |
| Age, y | 58 (52-61) | 30 (25-34) | 57 (54-63) | 24 (19-38) | .0001 |
| Female sex | 25 (52) | 8 (57) | 12 (32) | 2 (50) | .2 |
| Race | |||||
| White | 43 (90) | 12 (86) | 33 (87) | 2 (50) | .01 |
| Black | 5 (10) | 0 | 3 (8) | 0 | … |
| Hispanic | 0 | 2 (14) | 1 (3) | 0 | … |
| Asian | 0 | 0 | 0 | 1 (25) | … |
| Other | 0 | 0 | 1 (3) | 1 (25) | … |
| Weight, kg | 75 (60-83) | 55 (49-63) | 80 (67-89) | 56 (49-67) | .0001 |
| Bilateral transplant | 18 (38) | 14 (100) | 20 (53) | 4 (100) | < .001 |
| Cardiopulmonary bypass | 6 (13) | 8 (57) | 15 (39) | 4 (100) | < .0001 |
| Cardiopulmonary bypass time, min | 216 (181-227) | 298 (261-377) | 219 (161-330) | 297 (253-336) | .06 |
| Pretransplant oxygen use | 43 (90) | 13 (93) | 35 (92) | 4 (100) | .8 |
| Pretransplant oral steroid use | 18 (38) | 8 (57) | 28 (74) | 0 | .001 |
| PGD | 4 (9) | 4 (31) | 11 (29) | 2 (50) | .02 |
| Baseline plasma SP-D level, ng/mL | 80 (50-124) | 100 (87-238) | 325 (267-489) | 82 (71-93) | .0001 |
Data are presented as median (interquartile range) for continuous variables and No. (%) for categorical variables. PGD is defined by International Society for Heart and Lung Transplantation guidelines for grade 3 disease within 72 h of transplant.26 CF = cystic fibrosis; IPAH = idiopathic pulmonary arterial hypertension; IPF = idiopathic pulmonary fibrosis; PGD = primary graft dysfunction; SP-D = surfactant protein D.
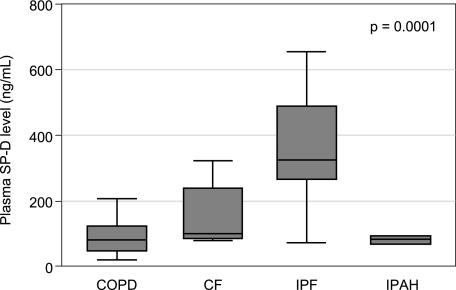
As shown in Figure 1, baseline (pretransplant) plasma SP-D levels were much higher in subjects with IPF relative to all other diagnoses (median, 325 ng/mL vs 100 ng/mL for CF, 80 ng/mL for COPD, and 82 ng/mL for IPAH; P = .0001). In a multivariable model, IPF was independently associated with higher baseline SP-D levels, even after controlling for age, sex, and race (P < .001). Pretransplant oral corticosteroid use, pretransplant oxygen use, and weight did not confound the relationship between IPF and baseline SP-D levels and were not included in the final model. Baseline SP-D levels were not significantly associated with the development of PGD after transplant.
Figure 1.
Subjects with IPF had significantly higher plasma levels of SP-D relative to other diagnoses. The P value reflects group comparison by Kruskal-Wallis test. CF = cystic fibrosis; IPAH = idiopathic pulmonary arterial hypertension; IPF = idiopathic pulmonary fibrosis; SP-D = surfactant protein D.
After transplant, plasma SP-D levels in subjects with IPF declined, becoming similar to those of other diagnoses by day 3 posttransplant (Fig 2). However, the trend in plasma SP-D levels after transplant for IPF was different for single and bilateral allograft recipients. Among subjects with IPF undergoing bilateral lung transplant (n = 20, 53% of subjects with IPF), plasma SP-D levels declined rapidly in the immediate postoperative period to a median of 132 ng/mL on the day of transplant (P = .002 for comparison with baseline) and continued to decline steadily thereafter (median, 82 ng/mL, 69 ng/mL, and 57 ng/mL on posttransplant days 1 through 3, respectively; P < .001 for trend from baseline). In contrast, postoperative SP-D levels in the 18 subjects undergoing single lung transplant for IPF remained elevated on the day of transplant (median, 249 ng/mL; P = .38 for comparison with baseline) and were significantly higher than those of bilateral allograft recipients throughout the postoperative period (Fig 3). Although plasma SP-D levels in bilateral allograft recipients for IPF continued to decline after posttransplant day 1 (P = .04 by nonparametric test for trend), they did not decline significantly after day 1 among single allograft recipients for IPF (P = .79 for trend). Although the use of cardiopulmonary bypass was significantly more frequent among bilateral allograft recipients (54% vs 6% of single organ recipients, P < .0001), the first plasma SP-D levels after allograft reperfusion were not significantly associated with the use of cardiopulmonary bypass (median SP-D level, 69 ng/mL vs 71 ng/mL for those who did not undergo bypass; P = .71) or with the duration of cardiopulmonary bypass among bilateral organ recipients (Spearman correlation, −0.30; P = .26).
Figure 2.
After transplant, plasma SP-D levels in subjects with IPF declined steadily, becoming similar to those of patients with other diagnoses by day 3 posttransplant. See Figure 1 legend for expansion of abbreviations.
Figure 3.
In subjects with IPF, plasma SP-D levels remain higher after single lung transplant compared with double lung transplant. Day zero SP-D levels are postoperative on the day of transplant. P values reflect pairwise comparisons by Wilcoxon rank sum test. See Figure 1 legend for expansion of abbreviations.
Among subjects undergoing single lung transplant for IPF, the development of PGD was associated with a subsequent rise in plasma SP-D levels (P = .02 for upward trend beginning day 1 posttransplant), whereas SP-D levels in subjects undergoing bilateral transplant for IPF remained low, even in the presence of grade 3 PGD (Fig 4, P = .62 for trend beginning day 1 posttransplant). This difference was observed regardless of whether subjects developed PGD on the first, second, or third posttransplant day. Interestingly, within the IPF group, single lung allograft recipients without PGD had higher postoperative plasma SP-D levels than bilateral lung allograft recipients with PGD (median postoperative SP-D levels, 144 ng/mL vs 80 ng/mL, respectively; P = .0004). This difference was not driven entirely by the marked difference on the day of transplant (day zero) because it remained significant even if SP-D levels on day zero were excluded (median SP-D levels, 125 ng/mL vs 79 ng/mL, respectively; P = .004).
Figure 4.
In subjects undergoing single lung transplant for IPF, PGD was associated with a subsequent rise in plasma SP-D levels. In contrast, plasma SP-D levels declined after bilateral lung transplant for IPF, regardless of the development of PGD. PGD = primary graft dysfunction. See Figure 1 legend for expansion of other abbreviations.
Among subjects undergoing transplant for COPD, CF, or IPAH, plasma SP-D levels declined in the postoperative period and were not affected by the development of PGD (data not shown). Bilateral lung transplant for these diagnoses was associated with declining plasma SP-D levels regardless of the presence of PGD, in keeping with our findings in IPF. However, we were unable to analyze formally whether PGD was associated with rising plasma SP-D levels after single lung transplant for non-IPF indications because all subjects with CF and IPAH underwent bilateral lung transplant and only two subjects developed PGD after single lung transplant for COPD. In these two COPD subjects, there was no rise in posttransplant plasma SP-D levels.
Discussion
Among patients awaiting lung transplant, IPF is associated with markedly higher plasma levels of SP-D than are other common pretransplant diagnoses, independent of multiple potential confounders. In addition, patients with IPF who receive single lung allografts have significantly higher plasma SP-D levels than those receiving bilateral lung allografts, even in the presence of grade 3 PGD. These observations offer insight into the probable mechanism of elevated levels of plasma SP-D and its use as a biomarker in IPF.
The presence of SP-D in the circulation was first described by Honda and colleagues,16 and the plasma level in healthy adults is approximately 60 ng/mL.10 Circulating levels of SP-D are increased in a variety of lung diseases,11-17,19 but IPF is associated with substantially higher SP-D levels than are all other diseases investigated, with the exception of pulmonary alveolar proteinosis.10 Our study similarly found that IPF was associated with higher plasma levels of SP-D relative to other diseases in a cohort of patients awaiting lung transplant. Further, we have established that the higher plasma levels of SP-D seen in IPF are independent of confounding by differences in age, sex, race, weight, or use of oxygen and/or steroids.
After transplant for IPF, plasma SP-D levels decline, but both the rate and extent of decline in plasma SP-D levels is highly influenced by single vs bilateral lung transplant. Importantly, although acute lung injury has been associated with elevated plasma levels of SP-D,11 PGD was not associated with elevations in plasma SP-D in our study. In fact, among subjects with IPF, single lung allograft recipients without PGD had significantly higher plasma SP-D levels than bilateral lung allograft recipients with PGD, indicating that plasma SP-D levels in the posttransplant setting are likely determined predominantly by events in the inflamed native lung rather than the allograft. Additionally, PGD was not associated with any increase in plasma SP-D levels among subjects with COPD, CF, or IPAH. For these reasons, we propose that circulating SP-D is a biomarker of the air-blood barrier integrity in the native IPF lung, but may be less useful as a biomarker of PGD after transplant.
Why SP-D should act as a biomarker for acute lung injury in the general population, but not for PGD after lung transplant, is unclear. One possibility is that the donor epithelium is unable to upregulate SP-D production in the immediate posttransplant period. Immunosuppressive medications would be unlikely to result in impaired SP-D expression because corticosteroids are used routinely in this setting and are known to induce SP-D expression.29 Another possibility is that oxidative and/or nitrosative stress associated with ischemia reperfusion in the allograft results in posttranslational modifications to SP-D, rendering it undetectable by ELISA and obscuring any increase in plasma levels. Both oxidative30 and nitrosative31 stress have been demonstrated to induce alterations in SP-D structure, and whether these altered forms of SP-D are detectable by the commercial antibody used for our ELISA is unknown. In addition, although PGD is a form of acute lung injury, its pathophysiology is primarily that of ischemia reperfusion injury and may be distinct from the mechanisms of acute lung injury in other settings. Whatever the mechanism, our data suggest that plasma SP-D as measured by ELISA may not be a useful diagnostic biomarker of PGD after lung transplant. Whether it may serve as a biomarker of outcomes of PGD, however, remains to be investigated.
In contrast, circulating SP-D levels appear to predict clinically relevant outcomes in IPF.19,20 The mechanisms responsible for higher circulating levels of SP-D in IPF remain unclear. Given that extensive surveys have found no evidence of significant SP-D production in the vascular compartment,32 elevated circulating levels of SP-D must result from either increased translocation into the plasma from other compartments or decreased elimination from the plasma. The marked differences we observed in plasma SP-D levels after single vs bilateral lung transplant strongly suggest that in the context of IPF, SP-D enters the plasma by translocation from the inflamed native lung. Thus, the removal of both native lungs during bilateral transplant is associated with a rapid decline in plasma SP-D levels, whereas single lung transplant leaves one native lung in place as a source for continued SP-D translocation. This interpretation of the data is corroborated by our observation that plasma SP-D levels rise in single lung allograft recipients with IPF who develop PGD, whereas they remain low in bilateral lung allograft recipients regardless of the development of PGD, suggesting that plasma SP-D levels are acting as a biomarker of inflammation or endothelial dysfunction in the native lung, not PGD in the allograft. Whether PGD in the allograft could induce inflammation in the contralateral native lung is speculative, but PGD is known to be associated with increased circulating markers of endothelial dysfunction,24 suggesting that PGD could lead to increased vascular permeability in the native lung and thus increased translocation of SP-D into the circulation.
Translocation of proteins from the lung into the plasma is a known phenomenon. Evolving evidence suggests that even under normal conditions there is a bidirectional flow of macromolecules across the air-blood barrier in the lung, most likely via passive diffusion through water-filled pores near the tight junctions of the alveolar epithelium.10 Additionally, murine models have shown that tracer proteins of various sizes instilled into the trachea are found in greater levels in the plasma after administration of endotoxin33 and during experimental sepsis,34 suggesting that inflammation increases the translocation of proteins and other macromolecules across the air-blood barrier. Further, inflammation may also lead to increased production of SP-D in the lung,35 increasing the concentration gradient driving its translocation into the plasma.
Although it is also possible that circulating SP-D is the result of translocation into the plasma from organs other than the lung, the vast majority of SP-D production occurs in the lung, making it the most likely source. In addition, it would be difficult to explain why single and bilateral lung transplant would differentially affect translocation from an extrapulmonary site. Decreased plasma clearance of SP-D could also explain the elevated SP-D levels seen in IPF, although our data again argue that this is not the predominant mechanism. Given that single and bilateral lung allograft recipients are treated similarly in the postoperative period and receive the same immunosuppressive regimens, it is unlikely that they would have marked differences in their ability to eliminate SP-D from the plasma. It is conceivable that SP-D is cleared from the circulation by cardiopulmonary bypass rather than by an endogenous process. However, in our cohort, subjects undergoing bypass did not have lower plasma SP-D levels in the immediate postoperative period relative to subjects not undergoing bypass. Similarly, among bilateral allograft recipients, there was no significant correlation between the duration of cardiopulmonary bypass and the first posttransplant plasma SP-D level. Thus, we propose that the differences in plasma SP-D noted between recipients of single and bilateral lung allografts for IPF in our study are unlikely to be due to differences in plasma clearance and are far more likely to be explained by differences in translocation of protein into the plasma.
Another possibility is that SP-D is secreted directly into the plasma by the native lung. Under normal conditions, Clara cells and type-II alveolar cells secrete SP-D into the airway lumen. However, recent evidence in SP-D-overexpressing mice suggests that when SP-D is expressed at high levels, plasma levels of SP-D increase, even in the absence of lung injury or loss of air-blood barrier integrity.36 Although this is not direct evidence of basolateral SP-D secretion, it certainly supports the hypothesis that epithelial damage and type-II pneumocyte hyperplasia associated with IPF may induce high levels of SP-D expression, resulting in basolateral secretion into plasma. It is also plausible that PGD in the allograft enhances this effect, either by inducing further increases in SP-D expression or by affecting the air-blood barrier integrity in the native lung. However, the fact that PGD was only associated with increased plasma SP-D levels in single lung allograft recipients and not bilateral allograft recipients again suggests that this phenomenon would be isolated to the native lung. As such, the usefulness of plasma SP-D as a biomarker of PGD is still uncertain at best. Although further investigation could find a role for SP-D as a biomarker of PGD in single lung allograft recipients with IPF, investigation of other surfactant proteins may be more productive. For example, surfactant protein A has a considerably smaller quaternary structure, and plasma levels may correlate better with the air-blood barrier integrity and possibly PGD.
Conclusions
The results of our study support the hypothesis that elevated plasma levels of SP-D in patients with IPF reflect increased translocation from the native lung into the circulation. In addition, the differential effect of single and bilateral lung transplant for IPF on circulating levels of SP-D strengthens the argument that plasma levels of SP-D reflect events in the native lung. Given that plasma SP-D levels also appear to predict meaningful clinical outcomes in IPF, we propose that plasma SP-D will likely prove to be a very useful biomarker of disease activity in this population. In contrast, plasma SP-D may be less useful as a biomarker of PGD after lung transplant.
Acknowledgments
Author contributions: Dr M. W. Sims: contributed to all statistical analyses and the writing of the manuscript.
Dr Beers: contributed to the provision of expertise in the biology of SP-D, interpretation of results, and editing the manuscript.
Dr Ahya: contributed to subject recruitment, sample collection, supervision of clinical assessments, and editing the manuscript.
Dr Kawut: contributed to subject recruitment, sample collection, the supervision of clinical assessments, and editing the manuscript.
Dr K. D. Sims: contributed to the interpretation of results and editing the manuscript.
Dr Lederer: contributed to subject recruitment, sample collection, supervision of clinical assessments, and editing the manuscript.
Dr Palmer: contributed to subject recruitment, sample collection, supervision of clinical assessments, and editing the manuscript.
Dr Wille: contributed to subject recruitment, sample collection, supervision of clinical assessments, and editing the manuscript.
Dr Lama: contributed to subject recruitment, sample collection, supervision of clinical assessments, and editing the manuscript.
Dr Shah: contributed to subject recruitment, sample collection, supervision of clinical assessments, and editing the manuscript.
Dr Orens: contributed to subject recruitment, sample collection, supervision of clinical assessments, and editing the manuscript.
Dr Bhorade: contributed to subject recruitment, sample collection, supervision of clinical assessments, and editing the manuscript.
Dr Crespo: contributed to subject recruitment, sample collection, supervision of clinical assessments, and editing the manuscript.
Dr Weinacker: contributed to subject recruitment, sample collection, supervision of clinical assessments, and editing the manuscript.
Ms Demissie: contributed to data collection, coordination across study centers, and editing the manuscript.
Dr Bellamy: contributed to the provision of expertise on statistical methodology and editing the manuscript.
Dr Christie: contributed to the supervision of the statistical analysis, interpretation of results, and editing the manuscript.
Dr Ware: contributed to the coordination of the performance of all plasma SP-D assays and editing the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr M. W. Sims has received research funding from BioMarck Pharmaceuticals and Spiration, Inc. Dr Kawut has received conference funding from Gilead Sciences and Actelion and funding for consulting and for sitting on steering committees from Gilead. Dr Orens serves on a lung transplant study data safety monitoring board and receives very little financial compensation. The study is run by APT pharmaceuticals. He does not otherwise consult for the company or any other company. Dr Bellamy receives approximately 80% salary support through various National Institutes of Health grants. Dr Ware has received research funding from Sirius Genomics and served on an advisory board for GlaxoSmithKline. The remaining authors have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Additional information: The e-Appendices can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/140/2/489/suppl/DC1.
Abbreviations
- CF
cystic fibrosis
- ELISA
enzyme-linked immunosorbent assay
- IPAH
idiopathic pulmonary arterial hypertension
- IPF
idiopathic pulmonary fibrosis
- PGD
primary graft dysfunction
- SP-D
surfactant protein D
Footnotes
Funding/Support: This work was supported by the National Institutes of Health [Grants HL093303, HL081332, HL088263, HL081619, and HL087115].
A complete list of study participants is located in e-Appendix 1.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Ferguson JS, Voelker DR, Ufnar JA, Dawson AJ, Schlesinger LS. Surfactant protein D inhibition of human macrophage uptake of Mycobacterium tuberculosis is independent of bacterial agglutination. J Immunol. 2002;168(3):1309–1314. doi: 10.4049/jimmunol.168.3.1309. [DOI] [PubMed] [Google Scholar]
- 2.Hartshorn KL, Crouch E, White MR, et al. Pulmonary surfactant proteins A and D enhance neutrophil uptake of bacteria. Am J Physiol. 1998;274(6 Pt 1):L958–L969. doi: 10.1152/ajplung.1998.274.6.L958. [DOI] [PubMed] [Google Scholar]
- 3.LeVine AM, Elliott J, Whitsett JA, et al. Surfactant protein-d enhances phagocytosis and pulmonary clearance of respiratory syncytial virus. Am J Respir Cell Mol Biol. 2004;31(2):193–199. doi: 10.1165/rcmb.2003-0107OC. [DOI] [PubMed] [Google Scholar]
- 4.Limper AH, Crouch EC, O’Riordan DM, et al. Surfactant protein-D modulates interaction of Pneumocystis carinii with alveolar macrophages. J Lab Clin Med. 1995;126(5):416–422. [PubMed] [Google Scholar]
- 5.Madan T, Eggleton P, Kishore U, et al. Binding of pulmonary surfactant proteins A and D to Aspergillus fumigatus conidia enhances phagocytosis and killing by human neutrophils and alveolar macrophages. Infect Immun. 1997;65(8):3171–3179. doi: 10.1128/iai.65.8.3171-3179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark H, Palaniyar N, Hawgood S, Reid KB. A recombinant fragment of human surfactant protein D reduces alveolar macrophage apoptosis and pro-inflammatory cytokines in mice developing pulmonary emphysema. Ann N Y Acad Sci. 2003;1010:113–116. doi: 10.1196/annals.1299.019. [DOI] [PubMed] [Google Scholar]
- 7.Vandivier RW, Ogden CA, Fadok VA, et al. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J Immunol. 2002;169(7):3978–3986. doi: 10.4049/jimmunol.169.7.3978. [DOI] [PubMed] [Google Scholar]
- 8.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5(1):58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 9.Borron PJ, Crouch EC, Lewis JF, Wright JR, Possmayer F, Fraher LJ. Recombinant rat surfactant-associated protein D inhibits human T lymphocyte proliferation and IL-2 production. J Immunol. 1998;161(9):4599–4603. [PubMed] [Google Scholar]
- 10.Hermans C, Bernard A. Lung epithelium-specific proteins: characteristics and potential applications as markers. Am J Respir Crit Care Med. 1999;159(2):646–678. doi: 10.1164/ajrccm.159.2.9806064. [DOI] [PubMed] [Google Scholar]
- 11.Eisner MD, Parsons P, Matthay MA, Ware L, Greene K. Acute Respiratory Distress Syndrome Network Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax. 2003;58(11):983–988. doi: 10.1136/thorax.58.11.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greene KE, Wright JR, Steinberg KP, et al. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med. 1999;160(6):1843–1850. doi: 10.1164/ajrccm.160.6.9901117. [DOI] [PubMed] [Google Scholar]
- 13.Ohnishi H, Yokoyama A, Kondo K, et al. Comparative study of KL-6, surfactant protein-A, surfactant protein-D, and monocyte chemoattractant protein-1 as serum markers for interstitial lung diseases. Am J Respir Crit Care Med. 2002;165(3):378–381. doi: 10.1164/ajrccm.165.3.2107134. [DOI] [PubMed] [Google Scholar]
- 14.Lomas DA, Silverman EK, Edwards LD, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints study investigators Serum surfactant protein D is steroid sensitive and associated with exacerbations of COPD. Eur Respir J. 2009;34(1):95–102. doi: 10.1183/09031936.00156508. [DOI] [PubMed] [Google Scholar]
- 15.Krane M, Griese M. Surfactant protein D in serum from patients with allergic bronchopulmonary aspergillosis. Eur Respir J. 2003;22(4):592–595. doi: 10.1183/09031936.03.00060603. [DOI] [PubMed] [Google Scholar]
- 16.Honda Y, Kuroki Y, Matsuura E, et al. Pulmonary surfactant protein D in sera and bronchoalveolar lavage fluids. Am J Respir Crit Care Med. 1995;152(6 Pt 1):1860–1866. doi: 10.1164/ajrccm.152.6.8520747. [DOI] [PubMed] [Google Scholar]
- 17.Janssen R, Sato H, Grutters JC, et al. Study of Clara cell 16, KL-6, and surfactant protein-D in serum as disease markers in pulmonary sarcoidosis. Chest. 2003;124(6):2119–2125. doi: 10.1378/chest.124.6.2119. [DOI] [PubMed] [Google Scholar]
- 18.Sims MW, Tal-Singer RM, Kierstein S, et al. Chronic obstructive pulmonary disease and inhaled steroids alter surfactant protein D (SP-D) levels: a cross-sectional study. Respir Res. 2008;9:13. doi: 10.1186/1465-9921-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene KE, King TE, Jr, Kuroki Y, et al. Serum surfactant proteins-A and -D as biomarkers in idiopathic pulmonary fibrosis. Eur Respir J. 2002;19(3):439–446. doi: 10.1183/09031936.02.00081102. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi H, Fujishima T, Koba H, et al. Serum surfactant proteins A and D as prognostic factors in idiopathic pulmonary fibrosis and their relationship to disease extent. Am J Respir Crit Care Med. 2000;162(3 Pt 1):1109–1114. doi: 10.1164/ajrccm.162.3.9910080. [DOI] [PubMed] [Google Scholar]
- 21.D’Ovidio F, Mura M, Ridsdale R, et al. The effect of reflux and bile acid aspiration on the lung allograft and its surfactant and innate immunity molecules SP-A and SP-D. Am J Transplant. 2006;6(8):1930–1938. doi: 10.1111/j.1600-6143.2006.01357.x. [DOI] [PubMed] [Google Scholar]
- 22.Christie JD, Robinson N, Ware LB, et al. Association of protein C and type 1 plasminogen activator inhibitor with primary graft dysfunction. Am J Respir Crit Care Med. 2007;175(1):69–74. doi: 10.1164/rccm.200606-827OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christie JD, Shah CV, Kawut SM, et al. Lung Transplant Outcomes Group Plasma levels of receptor for advanced glycation end products, blood transfusion, and risk of primary graft dysfunction. Am J Respir Crit Care Med. 2009;180(10):1010–1015. doi: 10.1164/rccm.200901-0118OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Covarrubias M, Ware LB, Kawut SM, et al. Lung Transplant Outcomes Group Plasma intercellular adhesion molecule-1 and von Willebrand factor in primary graft dysfunction after lung transplantation. Am J Transplant. 2007;7(11):2573–2578. doi: 10.1111/j.1600-6143.2007.01981.x. [DOI] [PubMed] [Google Scholar]
- 25.Christie JD, Kotloff RM, Pochettino A, et al. Clinical risk factors for primary graft failure following lung transplantation. Chest. 2003;124(4):1232–1241. doi: 10.1378/chest.124.4.1232. [DOI] [PubMed] [Google Scholar]
- 26.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. ISHLT Working Group on Primary Lung Graft Dysfunction Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24(10):1454–1459. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 27.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 28.Cuzick JA. A Wilcoxon-type test for trend. Stat Med. 1985;4(1):87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 29.Dulkerian SJ, Gonzales LW, Ning Y, Ballard PL. Regulation of surfactant protein D in human fetal lung. Am J Respir Cell Mol Biol. 1996;15(6):781–786. doi: 10.1165/ajrcmb.15.6.8969273. [DOI] [PubMed] [Google Scholar]
- 30.Starosta V, Griese M. Oxidative damage to surfactant protein D in pulmonary diseases [published correction appears in Free Radic Res. 2006;40(6):665] Free Radic Res. 2006;40(4):419–425. doi: 10.1080/10715760600571248. [DOI] [PubMed] [Google Scholar]
- 31.Guo CJ, Atochina-Vasserman EN, Abramova E, et al. S-nitrosylation of surfactant protein-D controls inflammatory function. PLoS Biol. 2008;6(11):e266. doi: 10.1371/journal.pbio.0060266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madsen J, Kliem A, Tornoe I, Skjodt K, Koch C, Holmskov U. Localization of lung surfactant protein D on mucosal surfaces in human tissues. J Immunol. 2000;164(11):5866–5870. doi: 10.4049/jimmunol.164.11.5866. [DOI] [PubMed] [Google Scholar]
- 33.Folkesson HG, Weström BR, Pierzynowski SG, Karlsson BW. Lung to blood passage of different-sized molecules during lung inflammation in the rat. J Appl Physiol. 1991;71(3):1106–1111. doi: 10.1152/jappl.1991.71.3.1106. [DOI] [PubMed] [Google Scholar]
- 34.Hansson L, Folkesson HG, Andersson R, et al. Increased passage of bovine serum albumin over the respiratory tract after intratracheal instillation during septic shock in rats. Eur Surg Res. 1992;24(1):45–53. doi: 10.1159/000129187. [DOI] [PubMed] [Google Scholar]
- 35.McIntosh JC, Swyers AH, Fisher JH, Wright JR. Surfactant proteins A and D increase in response to intratracheal lipopolysaccharide. Am J Respir Cell Mol Biol. 1996;15(4):509–519. doi: 10.1165/ajrcmb.15.4.8879185. [DOI] [PubMed] [Google Scholar]
- 36.Kingma PS, Haaning KL, Mierke SK. Secretion of pulmonary surfactant protein D into plasma in the absence of lung injury. Am J Respir Crit Care Med. 2011;183(1):A5162. [Google Scholar]