Abstract
Background:
Lymph node (LN) status is an important component of staging; it provides valuable prognostic information and influences treatment decisions. However, the prognostic significance of the number of positive LNs in N1 non-small cell lung cancer (NSCLC) remains unclear. In this study we evaluated whether a higher number of positive LNs results in worse survival among patients with N1 disease.
Methods:
The Surveillance, Epidemiology, and End Results database was used to identify 3,399 patients who underwent resection for N1 NSCLC. Subjects were categorized into groups based on the number of positive nodes: one, two to three, four to eight, and more than eight positive LNs. The prognostic significance of the number of positive LNs in relation to survival was evaluated using the Kaplan-Meier method. Stratified and Cox regression analysis were used to evaluate the relationship between the number of positive LNs and survival after adjusting for potential confounders.
Results:
Unadjusted survival analysis showed that a greater number of N1 LNs was associated with worse lung cancer-specific (P < .0001) and overall (P < .0001) survival. Mean lung cancer-specific survival was 8.8, 8.2, 6.0, and 3.9 years for patients with one, two to three, four to eight, and more than eight positive LNs, respectively. Stratified and adjusted analysis also showed the number of N1 LNs was an independent predictor of survival after controlling for potential confounders.
Conclusion:
The number of positive LNs is an independent prognostic factor of survival in patients with N1 NSCLC. This information may be used to further stratify patients with respect to risk of recurrence in order to determine postoperative management.
Lung cancer is the leading cause of cancer mortality for both men and women in the United States.1 The overall 5-year survival rate for the disease is only 16%, but patients with resected non-small cell lung cancer (NSCLC) have a considerably better prognosis.2 Staging is a critical aspect of the diagnostic workup of lung cancer as it influences treatment decisions and allows accurate communication about prognosis to patients and their families. Lymph node (LN) status is a key component of the lung cancer staging classification system and a strong determinant of prognosis.3 According to the seventh edition of the TNM classification,4 nodal status is categorized as N0 (no regional LN involvement), N1 (involvement of ipsilateral intrapulmonary, peribronchial, or hilar LNs), N2 (involvement of ipsilateral mediastinal or subcarinal LNs), or N3 (involvement of contralateral LNs) disease.
Accurate staging is particularly important in patients with N1 NSCLC. Although these patients can be cured with resection, the risk of recurrence is relatively high (40%-60% depending on the level of tumor extension).5,6 Unfortunately, limited factors are available to identify the subgroup of N1 patients with poorer prognosis. Some prior studies have suggested a potential association between the number of positive LNs and survival in these patients.7-12 However, many of these studies were limited to small cohorts of patients from single institutions and only assessed the impact of single vs multiple LN involvement.9-11 Other studies combined patients with N1 and N2 disease, making it difficult to extrapolate their results to N1 disease alone.8,12 Because of these limitations, the number of N1 nodes has not been adopted as a prognostic factor in clinical practice.
In this study, we used data from a population-based cohort of patients with resected N1 NSCLC to assess whether the number of LNs involved with NSCLC is an independent prognostic factor in patients with N1 disease. We hypothesized that a higher number of positive LNs will result in worse outcomes among patients with N1 NSCLC.
Materials and Methods
Study subjects were identified from the Surveillance, Epidemiology, and End Results (SEER) database. SEER is a comprehensive registry of patients with cancer that collects data from population-based registries covering approximately 28% of the US population.13 The study sample consisted of patients with NSCLC who underwent resection (lobectomy or pneumonectomy) for what proved to be pathologic N1 NSCLC between 1988 and 2007. The study was limited to primary lung cancer cases that were not diagnosed from death certificate data or during autopsy.2 Patients who underwent neoadjuvant radiation therapy (which can lead to downstaging of LN involvement) and those with incomplete information on tumor size, tumor extension, and/or extent of LN involvement were also excluded from the study. The maximum number of N1 nodes is intrinsically determined by the number of LNs sampled during surgery.14,15 Thus, in order to adequately estimate the extent of LN involvement, we restricted our analysis to patients who underwent sampling of ≥ 10 regional LNs during surgery (the recommended extent of LN dissection in the literature).15-19
Sociodemographic characteristics (age, sex, race/ethnicity, and marital status) were obtained from the SEER registry. Cases were staged according to the seventh edition of the TNM classification using SEER data on tumor extension, LN involvement, and systemic metastasis.4 SEER contains detailed information regarding the level of LN involvement, the number of positive LNs, and the number of LNs resected during surgery. Based on the distribution of the number of N1 nodes in SEER and prior criteria used in the literature, patients were categorized in to four groups based on the number of positive LNs: one, two to three, four to eight, and more than eight N1 nodes.7 Histology subtypes were classified as adenocarcinoma, squamous cell carcinoma, large cell carcinoma, bronchioalveolar carcinoma, and other.20,21 Information regarding surgical treatment was ascertained from the site-specific surgery codes available in SEER. Using these data, patients were classified as having undergone lobectomy (20-45) or pneumonectomy (50-70). Use of postoperative radiation therapy (external beam radiation) was also obtained from SEER. The primary objective of the study was to evaluate lung cancer prognosis. Thus, we used lung cancer-specific survival as the primary study outcome as it allows for controlling for any unrelated causes of death; secondary analyses used all-cause mortality. To estimate lung cancer-specific survival, patients dying from other causes were classified as censored at the date of death. Cause of death information in SEER is provided by the National Center for Health Statistics and obtained from death certificates. Survival was determined as the interval from the date of diagnosis to the date of death or the last follow-up date available in the SEER registry (December 31, 2007).
Statistical Analysis
Differences in the baseline characteristics of patients in the four LN groups were evaluated using the χ2 test. The prognostic significance of the number of positive LNs (N1 nodes) in reference to lung cancer-specific and overall survival was evaluated with the Kaplan-Meier method. Survival figures were calculated up to 15 years after diagnosis to avoid reporting survival estimates based on a small number of observations. We used the log-rank test to compare survival between the four LN groups.
We estimated survival within age, sex, race/ethnicity, histology, tumor site and status, type of surgery, number of LNs sampled (10-14 vs ≥ 14), and radiation therapy use strata to assess if prognostic differences across the four LN groups remained after controlling for these variables. Cox regression analysis was used to assess the association between the number of positive nodes and survival, after adjusting for potential confounders. The assumption of proportionality of hazards was evaluated using log-log plots of survival curves. All analyses were performed with SPSS statistical package (IBM; Chicago, Illinois) using two-sided P values. The study was reviewed by the Mt. Sinai School of Medicine Institutional Review Board and classified as exempt.
Results
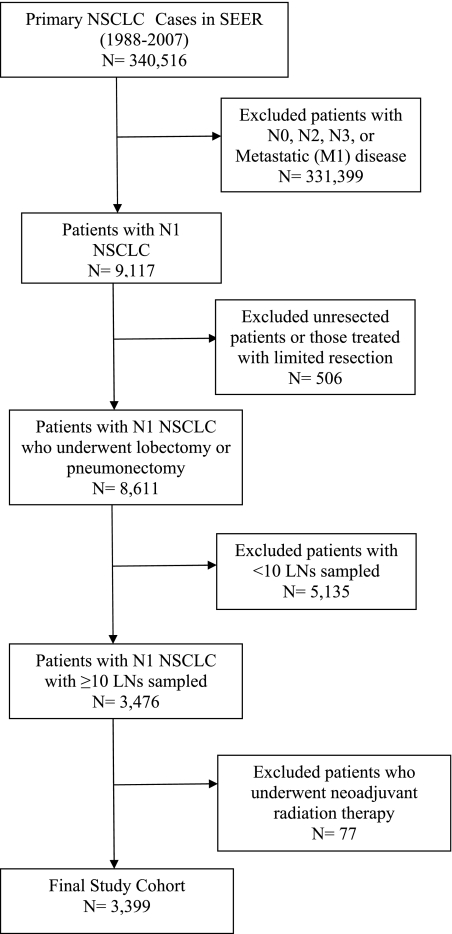
We identified 340,516 patients in SEER with primary NSCLC diagnosed between 1988 and 2007; among these, 9,117 patients had N1 NSCLC. We excluded 506 unresected patients, 5,135 patients who had < 10 regional LNs resected, and 77 patients who underwent neoadjuvant radiation therapy, leaving a final cohort of 3,399 N1 cases (Fig 1). Overall, 40% had one positive LN, 35% had two to three positive LNs, 21% had four to eight positive LNs, and 4% had more than eight positive LNs. The median (interquartile range) of the number of LNs sampled was 14 (12-19). The baseline characteristics of patients in the four LN groups are shown in Table 1. The distribution of age, gender, race/ethnicity, and marital status was similar among patients in the four groups (P > .05 for all comparisons). Tumor histology (P = .03), tumor location (P = .001), tumor status (P < .0001), tumor size (P = .002), type of surgery (P < .0001), and radiation therapy use (P < .0001) were significantly different across groups.
Figure 1.
Cohort selection. LN = lymph node; NSCLC = non-small cell lung cancer; SEER = Surveillance, Epidemiology, and End Results.
Table 1.
—Baseline Characteristics of Study Subjects According to the Number of Positive LNs
| No. Positive Nodes |
|||||
| Characteristic | 1 (n = 1,364) | 2-3 (n = 1,200) | 4-8 (n = 694) | > 8 (n = 141) | P Value |
| Age, y | .56 | ||||
| ≤ 50 | 130 (10) | 104 (9) | 57 (8) | 17 (12) | |
| 51-60 | 290 (21) | 276 (23) | 168 (24) | 29 (21) | |
| ≥ 60 | 944 (69) | 820 (68) | 469 (68) | 95 (67) | |
| Female sex | 526 (39) | 455 (38) | 276 (40) | 56 (40) | .87 |
| Race/ethnicity | .38 | ||||
| White | 1,159 (85) | 989 (83) | 564 (81) | 118 (84) | |
| Black | 87 (6) | 75 (6) | 57 (8) | 10 (7) | |
| Hispanic | 49 (4) | 50 (4) | 28 (4) | 4 (3) | |
| Other | 69 (5) | 86 (7) | 45 (7) | 9 (6) | |
| Married | 876 (64) | 787 (66) | 429 (62) | 97 (69) | .27 |
| Histology | .03 | ||||
| Adenocarcinoma | 605 (44) | 526 (44) | 342 (49) | 71 (51) | |
| Squamous cell carcinoma | 544 (40) | 491 (41) | 245 (35) | 40 (28) | |
| Large cell carcinoma | 65 (5) | 72 (6) | 46 (7) | 9 (6) | |
| Bronchioalveolar cell carcinoma | 77 (6) | 63 (5) | 34 (5) | 10 (7) | |
| Other | 73 (5) | 48 (4) | 27 (4) | 11 (8) | |
| Tumor location | .001 | ||||
| Upper lobe | 772 (57) | 640 (53) | 326 (47) | 63 (45) | |
| Middle lobe | 44 (3) | 44 (4) | 19 (3) | 7 (5) | |
| Lower lobe | 428 (31) | 392 (33) | 276 (40) | 59 (42) | |
| Other location | 120 (9) | 124 (10) | 73 (10) | 12 (8) | |
| Tumor status | < .0001 | ||||
| T1A | 158 (12) | 85 (7) | 51 (7) | 8 (6) | |
| T1B | 207 (15) | 158 (13) | 83 (12) | 19 (14) | |
| T2 | 151 (11) | 124 (10) | 68 (10) | 21 (15) | |
| T2A | 429 (31) | 442 (37) | 254 (37) | 44 (31) | |
| T2B | 161 (12) | 177 (15) | 114 (16) | 19 (13) | |
| T3 | 258 (19) | 214 (18) | 124 (18) | 30 (21) | |
| Tumor size | .002 | ||||
| < 2 cm | 207 (15) | 124 (10) | 74 (11) | 12 (8) | |
| 2-3 cm | 324 (24) | 258 (22) | 138 (20) | 38 (27) | |
| 3-5 cm | 483 (35) | 481 (40) | 274 (39) | 49 (35) | |
| 5-7 cm | 203 (15) | 206 (17) | 132 (19) | 23 (16) | |
| ≥ 7 cm | 147 (11) | 131 (11) | 76 (11) | 19 (14) | |
| Type of surgery | < .0001 | ||||
| Lobectomy | 1,012 (74) | 791 (66) | 441 (64) | 87 (62) | |
| Pneumonectomy | 352 (26) | 409 (34) | 253 (36) | 54 (38) | |
| Postoperative radiation therapy | < .0001 | ||||
| Yes | 277 (20) | 352 (29) | 261 (38) | 64 (45) | |
| No | 1,087 (80) | 848 (71) | 433 (62) | 77 (55) | |
Data are presented as No. (%). LN = lymph node.
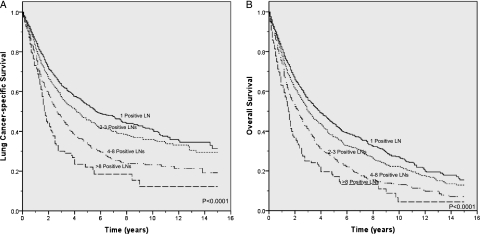
Unadjusted survival analysis showed that a greater number of N1 LNs was associated with worse lung cancer-specific (P < .0001) and overall (P < .0001) survival (Figs 2A, 2B). Mean lung cancer specific-survival was 8.8 years (95% CI, 8.2-9.5), 8.2 years (95% CI, 7.5-8.8), 6.0 years (95% CI, 5.3-6.7) and 3.9 years (95% CI, 2.9-4.9) for patients with one, two to three, four to eight, and more than eight positive LNs, respectively. Stratification by tumor status, an established prognostic factor, also showed a significant association between a higher number of positive LNs and worse lung cancer-specific and overall survival (P < .05 for all comparisons) (e-Figs 1A-1F). Similarly, a higher number of N1 nodes was associated with worse prognosis in analyses stratified by age, sex, race/ethnicity, marital status, tumor size, histology and location, type of surgery, number of LNs sampled, and use of radiation therapy (P < .05 for all comparisons) (Table 2). However, this association was not statistically significant among some subgroups (black race and large cell or bronchioalveolar cell histology).
Figure 2.
A, Lung cancer-specific survival according to the number of N1 LNs. B, Overall survival according to the number of N1 LNs. See Figure 1 legend for expansion of abbreviation.
Table 2.
—Stratified Analysis Comparing Mean Lung Cancer-Specific Survival According to the Number of Positive LNs
| Mean Lung Cancer-Specific Survival per No. Positive Nodes, y |
|||||
| Characteristic | 1 | 2-3 | 4-8 | > 8 | P Value |
| Age, y | |||||
| ≤ 50 | 10.9 | 11.3 | 7.7 | 4.1 | .01 |
| 51-60 | 9.8 | 8.1 | 7.0 | 3.5 | < .0001 |
| ≥ 60 | 7.8 | 7.7 | 4.7 | 3.9 | < .0001 |
| Female gender | 9.1 | 8.4 | 5.7 | 3.1 | < .0001 |
| Race/ethnicity | |||||
| White | 8.7 | 8.0 | 5.8 | 4.0 | < .0001 |
| Black | 7.6 | 9.0 | 7.6 | 2.9 | .05 |
| Hispanic | 10.1 | 7.1 | 2.6 | 3.1 | < .0001 |
| Other | 6.6 | 8.2 | 4.3 | 2.3 | .003 |
| Married | 8.7 | 8.3 | 6.6 | 4.1 | < .0001 |
| Histology | |||||
| Adenocarcinoma | 8.3 | 7.1 | 5.1 | 2.4 | < .0001 |
| Squamous cell carcinoma | 9.7 | 9.7 | 6.1 | 5.1 | < .0001 |
| Large cell carcinoma | 4.5 | 6.1 | 6.3 | 4.6 | .88 |
| Bronchioalveolar cell carcinoma | 8.0 | 5.7 | 4.2 | 3.0 | .30 |
| Other | 10.0 | 7.6 | 4.5 | 4.3 | .01 |
| Tumor location | |||||
| Upper lobe | 9.3 | 8.6 | 6.7 | 5.3 | < .0001 |
| Middle lobe | 11.6 | 4.9 | 3.7 | 2.5 | .003 |
| Lower lobe | 7.3 | 7.6 | 4.5 | 2.8 | < .0001 |
| Other location | 6.4 | 7.0 | 5.5 | 2.7 | .04 |
| Tumor status | |||||
| T1A | 10.2 | 10.2 | 6.3 | 4.1 | .02 |
| T1B | 8.2 | 7.5 | 6.8 | 2.1 | < .0001 |
| T2 | 10.0 | 7.9 | 7.6 | 3.1 | .002 |
| T2A | 9.7 | 8.5 | 5.0 | 4.1 | < .0001 |
| T2B | 6.8 | 6.5 | 4.1 | 2.3 | < .0001 |
| T3 | 5.0 | 8.0 | 5.0 | 3.1 | .003 |
| Tumor size | |||||
| < 2 cm | 11.7 | 8.8 | 6.6 | 4.6 | .02 |
| 2-3 cm | 8.4 | 8.4 | 7.6 | 2.2 | < .0001 |
| 3-5 cm | 9.2 | 8.5 | 5.0 | 4.0 | < .0001 |
| 5-7 cm | 6.3 | 6.8 | 4.3 | 2.6 | .001 |
| ≥ 7 cm | 5.7 | 6.8 | 4.3 | 2.7 | < .0001 |
| Type of surgery | |||||
| Lobectomy | 8.9 | 7.8 | 6.0 | 4.8 | < .0001 |
| Pneumonectomy | 8.1 | 8.7 | 5.9 | 2.3 | < .0001 |
| No. of LNs resected | |||||
| 10-14 | 8.4 | 7.8 | 5.2 | 2.4 | < .0001 |
| > 14 | 9.4 | 8.5 | 6.3 | 4.4 | < .0001 |
| Postoperative radiation therapy | |||||
| Yes | 7.7 | 8.4 | 6.6 | 3.4 | < .0001 |
| No | 9.4 | 8.0 | 5.4 | 4.4 | < .0001 |
See Table 1 legend for expansion of abbreviation.
Adjusted analysis using Cox regression also showed that the number of N1 LNs was an independent predictor of lung cancer-specific and overall survival after controlling for potential confounders. Compared with patients with one positive LN, the hazard of lung cancer mortality was 1.16 (95% CI, 1.03-1.30), 1.53 (95% CI, 1.34-1.75), and 2.25 (95% CI, 1.80-2.81) for patients in the two to three, four to eight, and more than eight N1 LN categories, respectively (Table 3). Other predictors of lung cancer-specific survival included younger age (hazard ratio [HR], 1.03; 95% CI, 1.02-1.04), female sex (HR, 0.79; 95% CI, 0.71-0.88), being married (HR, 0.90; 95% CI, 0.81-1.00), and squamous cell histology (HR, 0.66; 95% CI, 0.59-0.75). Similar patterns were observed in analyses using overall survival as an outcome.
Table 3.
—Adjusted Association Between the Number of Positive LNs and Survival
| Variable | Lung Cancer-Specific Survival Hazard Ratio (95% CI) | Overall Survival Hazard Ratio (95% CI) |
| No. of positive LNs | ||
| 1 | Reference | Reference |
| 2-3 | 1.16 (1.03-1.30) | 1.13 (1.03-1.26) |
| 4-8 | 1.53 (1.34-1.75) | 1.46 (1.30-1.64) |
| > 8 | 2.25 (1.80-2.81) | 2.17 (1.78-2.65) |
| Age, y | 1.03 (1.02-1.04) | 1.03 (1.03-1.04) |
| Female gender | 0.79 (0.71-0.88) | 0.78 (0.71-0.85) |
| Race/ethnicity | ||
| White | Reference | Reference |
| Black | 0.91 (0.74-1.12) | 0.96 (0.81-1.15) |
| Hispanic | 1.00 (0.77-1.30) | 1.08 (0.86-1.35) |
| Other | 0.95 (0.77-1.17) | 0.98 (0.82-1.17) |
| Married | 0.90 (0.81-1.00) | 0.87 (0.79-0.95) |
| Histology | ||
| Adenocarcinoma | Reference | Reference |
| Squamous cell carcinoma | 0.66 (0.59-0.75) | 0.76 (0.69-0.84) |
| Large cell carcinoma | 1.01 (0.83-1.24) | 1.12 (0.94-1.33) |
| Bronchioalveolar carcinoma | 0.91 (0.73-1.13) | 0.97 (0.80-1.18) |
| Other | 0.75 (0.59-0.96) | 0.82 (0.67-1.01) |
| Tumor status | ||
| T1A | Reference | Reference |
| T1B | 1.42 (1.12-1.80) | 1.43 (1.17-1.75) |
| T2 | 1.29 (1.00-1.65) | 1.30 (1.05-1.61) |
| T2A | 1.48 (1.20-1.83) | 1.46 (1.22-1.75) |
| T2B | 1.94 (1.54-2.45) | 1.79 (1.47-2.19) |
| T3 | 2.21 (1.76-2.77) | 1.97 (1.62-2.40) |
| Tumor location | ||
| Upper lobe | Reference | Reference |
| Middle lobe | 1.24 (0.94-1.64) | 1.21 (0.96-1.54) |
| Lower lobe | 1.17 (1.05-1.30) | 1.12 (1.02-1.23) |
| Other location | 1.20 (1.01-1.43) | 1.22 (1.05-1.42) |
| Type of surgery | ||
| Lobectomy | Reference | Reference |
| Pneumonectomy | 1.12 (1.00-1.26) | 1.11 (1.01-1.23) |
| Postoperative radiation therapy | 1.11 (0.99-1.23) | 1.11 (1.01-1.22) |
See Table 1 legend for expansion of abbreviation.
Discussion
Accurate staging is important in order to select the best treatment and communicate prognostic information to patients with lung cancer. In this study, we showed that the number of positive LNs is an independent prognostic factor in a large, population-based cohort of patients with N1 NSCLC. Adjusted analyses showed a higher risk of death with each increasing category of positive LNs. Moreover, the increased hazard of death conferred by more than eight positive LNs is equivalent to that of a finding in T3 disease. If validated, this prognostic factor can be incorporated into future lung cancer staging systems. This information may help identify patients with high risk of recurrence who would be candidates for more aggressive postoperative treatment.
Prior studies have evaluated whether N1 patients can be further stratified in terms of prognosis based on the extent of LN involvement. Some of these studies have shown that multiple, compared with single, LN metastasis is associated with worse prognosis.9-11 Research evaluating the number of positive LNs as a new prognostic factor in N1 NSCLC has shown conflicting results.5,7,8,12,22 Using similar groupings as ours, Marra et al7 found an association, in univariate but not in adjusted analysis, between the number of positive nodes and survival in a cohort of patients with resected N1 NSCLC from a single referral center. Similarly, a study including patients with N1 and N2 NSCLC showed that a higher number of positive LNs is associated with worse survival.12 Conversely, Martini et al5 did not find an association between the specific nodes involved or the number of nodes affected and survival among 75 patients with N1 NSCLC. A Japanese study also failed to find a relationship between survival and the number of N1 LNs.22 These studies were conducted among cases from single referral centers and, in general, included small numbers of patients. Additionally, some studies were conducted in heterogeneous populations (N1 and N2 disease) and did not control for the number of LNs sampled, factors that may explain the variability of results. Our data show a strong association between the number of positive LNs and survival in a cohort of unselected patients with N1 disease. Given the large number of patients in our study, the broad geographic representation, and long-term follow-up data in SEER, these results strongly suggest that the number of positive LNs is an independent prognostic factor among patients with resected N1 NSCLC.
The additional information provided by the number of N1 LNs can be used to better discriminate prognosis and to evaluate disease aggressiveness. Our study shows that patients with a higher number of positive LNs are at a high risk of recurrence; thus, these data can help identify patients who need close monitoring or who should receive aggressive postoperative treatment. Current treatment guidelines for N1 NSCLC recommend surgical resection followed by adjuvant platinum-based chemotherapy.23 However, acute toxicity of adjuvant chemotherapy is not uncommon and the long-term adverse effects can be severe.24 Thus, more accurate risk assessment should help target chemotherapy to the subgroup of patients with worse prognosis, particularly among individuals who may be at higher risk for chemotherapy-related toxicity due to comorbidities or advanced age. Additionally, the prognostic value of the number of LNs can help physicians provide more accurate disease information to assist patients and their families in making treatment decisions and long-term planning. Our results also suggest that cancers with a higher number of LN metastases show a more aggressive behavior compared with cancers of the same size and tumor extension but single N1 involvement. Thus, further research evaluating differences in the genetic and molecular characteristics of these tumors may help us to understand some of the mechanisms leading to lung cancer progression.
The LN ratio has been proposed as an alternative prognostic factor in N1 NSCLC. The maximum number of positive LNs is limited by the total number of nodes dissected. In order to circumvent this problem, the LN ratio is calculated as the ratio of positive to total number of LNs sampled. A prior study showed that the LN ratio is also a significant prognostic indicator of survival in N1 NSCLC.15 The use of the LN ratio has also been validated in other organ systems.14,25-28 Although the LN ratio may be applied among patients with a low number of LNs sampled, current data suggest that staging based on < 10 LNs is not accurate.15-19 Additionally, the number of positive LNs is a more direct and intuitive measure of the extent of LN involvement, and thus may be more readily adapted into clinical practice.
Although the number of positive nodes and the LN ratio have shown to be valid prognostic factors of N1 NSCLC, both measures are sensitive to the total number of LNs evaluated during surgery. LN assessment during surgery for NSCLC is a contentious issue. Although several studies have shown that evaluation of ≥ 10 LNs is beneficial for staging purposes,15,18,19 results have been conflicting regarding the value of performing systematic LN dissection compared with LN sampling. Because of the lack of consensus regarding the extent of lymphadenectomy, there is still considerable practice variability among surgeons.18,19 The increasing use of video-assisted thoracoscopy (VATS) for lung cancer surgery has been another source of controversy in this field. Short-term outcomes after lung resection from the American College of Surgeons Oncology Group Z0030 clinical trial have shown that patients who underwent VATS lobectomy and lymphadenectomy had a similar number of mediastinal nodes retrieved with fewer complications than those who underwent open thoracotomy.29 Conversely, a retrospective study found that patients undergoing VATS had a lower number of LNs evaluated.30 These conflicting results show that further research is necessary to establish guidelines regarding the best strategy to evaluate hilar and mediastinal LNs during lung cancer resection.
Several strengths and limitations of our study should be noted. The SEER registry is a comprehensive, population-based registry of cancer cases. Thus, the information in SEER is less affected by selection bias or unique practice patterns specific to particular institutions. The large number of patients with resected N1 disease in the registry allowed us to assess the importance of the number of positive nodes while focusing on patients with adequate LN sampling. Additionally, the relatively large study cohort allowed us to perform stratified analysis within key subgroups (tumor status, surgery type, and use of radiation therapy, among others) to show that the number of positive LNs is independently associated with survival after controlling for these established prognostic factors.
The SEER database abstracts cause of death from death certificates; thus, there is a potential for misclassification. However, the underlying cause of death for patients dying from lung cancer progression was found to be accurate in a study using a large registry.31 Additionally, stage-specific lung cancer-specific survival rates in SEER are similar to those reported in studies using other sources of data.3,15 Moreover, we also confirmed our results in secondary analyses using all-cause mortality, an outcome that should not be subject to misclassification. SEER does not include information on comorbidities or use of neoadjuvant chemotherapy. However, we excluded patients who had undergone neoadjuvant radiation therapy, which is usually given in combination with chemotherapy, thus decreasing the likelihood that these patients, who received chemotherapy before surgery, were included in our cohort.
In summary, our study shows that the number of positive LNs is an independent prognostic factor in patients with resected N1 NSCLC. Patients with a higher number of positive LNs have worse lung cancer-specific and overall survival and should be considered for more aggressive treatment. If validated, this prognostic factor should be incorporated into future lung cancer staging classifications to allow for a more accurate assessment of prognosis.
Acknowledgments
Author contributions: Ms Jonnalagadda and Dr Wisnivesky are the guarantors of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article.
Ms Jonnalagadda: contributed to study conception and design, analysis and interpretation of data, and drafting of the article.
Dr Smith: contributed to study concept and design, critical revision of the article for important intellectual content, and final approval of the article.
Ms Mhango: contributed to analysis and interpretation of the data, revision of the manuscript, and administrative, technical, or logistic support.
Dr Wisnivesky: contributed to study conception and design, analysis and interpretation of the data, statistical expertise, drafting of the article, and final approval of the article.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Wisnivesky is a member of the EHE research board and has received lecture honorarium from Novartis Pharmaceuticals and a COPD research grant from GlaxoSmithKline. Mss Jonnalagadda and Mhango and Dr Smith have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors of the study had no role in the study design, data collection, analysis, and interpretation or writing of the report.
Additional information: The e-Figure can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/140/2/433/suppl/DC1.
Abbreviations
- LN
lymph node
- NSCLC
non-small cell lung cancer
- SEER
Surveillance, Epidemiology, and End Results
- VATS
video-assisted thoracoscopy
Footnotes
Funding/Support: This study was supported by the Doris Duke Charitable Foundation for Clinical Research and, in part, by the National Cancer Institute [Grant 5R01CA131348-03].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Jemal A, Seigel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Wisnivesky JP, Henschke CI, Swanson S, et al. Limited resection for the treatment of patients with stage IA lung cancer. Ann Surg. 2010;251(3):550–554. doi: 10.1097/SLA.0b013e3181c0e5f3. [DOI] [PubMed] [Google Scholar]
- 3.Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997;111(6):1718–1723. doi: 10.1378/chest.111.6.1718. [DOI] [PubMed] [Google Scholar]
- 4.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- 5.Martini N, Flehinger BJ, Nagasaki F, Hart B. Prognostic significance of N1 disease in carcinoma of the lung. J Thorac Cardiovasc Surg. 1983;86(5):646–653. [PubMed] [Google Scholar]
- 6.Miyoshi K, Mimura T, Iwanaga K, Adachi S, Tsubota N, Okada M. Surgical treatment of clinical N1 non-small cell lung cancer: ongoing controversy over diagnosis and prognosis. Surg Today. 2010;40(5):428–432. doi: 10.1007/s00595-008-4072-4. [DOI] [PubMed] [Google Scholar]
- 7.Marra A, Hillejan L, Zaboura G, Fujimoto T, Greschuchna D, Stamatis G. Pathologic N1 non-small cell lung cancer: correlation between pattern of lymphatic spread and prognosis. J Thorac Cardiovasc Surg. 2003;125(3):543–553. doi: 10.1067/mtc.2003.322. [DOI] [PubMed] [Google Scholar]
- 8.Kang CH, Ra YJ, Kim YT, Jheon S-H, Sung SW, Kim JH. The impact of multiple metastatic nodal stations on survival in patients with resectable N1 and N2 nonsmall-cell lung cancer. Ann Thorac Surg. 2008;86(4):1092–1097. doi: 10.1016/j.athoracsur.2008.06.056. [DOI] [PubMed] [Google Scholar]
- 9.Martini N, Burt ME, Bains MS, McCormack PM, Rusch VW, Ginsberg RJ. Survival after resection of stage II non-small cell lung cancer. Ann Thorac Surg. 1992;54(3):460–466. doi: 10.1016/0003-4975(92)90435-7. [DOI] [PubMed] [Google Scholar]
- 10.Sayar A, Turna A, Kiliçgün A, Solak O, Urer N, Gürses A. Prognostic significance of surgical-pathologic multiple-station N1 disease in non-small cell carcinoma of the lung. Eur J Cardiothorac Surg. 2004;25(3):434–438. doi: 10.1016/j.ejcts.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Osaki T, Nagashima A, Yoshimatsu T, Tashima Y, Yasumoto K. Survival and characteristics of lymph node involvement in patients with N1 non-small cell lung cancer. Lung Cancer. 2004;43(2):151–157. doi: 10.1016/j.lungcan.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 12.Ueda K, Kaneda Y, Sakano H, et al. Independent predictive value of the overall number of metastatic N1 and N2 stations in lung cancer. Jpn J Thorac Cardiovasc Surg. 2003;51(7):297–301. doi: 10.1007/BF02719381. [DOI] [PubMed] [Google Scholar]
- 13.Bethesda, MD: National Cancer Institute; 2010. SEER. Cancer statistics review 1975-2010. http://seer.cancer.gov/data/options.html. Updated 2010 with 2009 data. Accessed July 2010. [Google Scholar]
- 14.Greenstein AJ, Litle VR, Swanson SJ, Divino CM, Packer S, Wisnivesky JP. Prognostic significance of the number of lymph node metastases in esophageal cancer. J Am Coll Surg. 2008;206(2):239–246. doi: 10.1016/j.jamcollsurg.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Bria E, Milella M, Sperduti I, et al. A novel clinical prognostic score incorporating the number of resected lymph-nodes to predict recurrence and survival in non-small-cell lung cancer. Lung Cancer. 2009;66(3):365–371. doi: 10.1016/j.lungcan.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 16.Sawyer TE, Bonner JA, Gould PM, et al. Factors predicting patterns of recurrence after resection of N1 non-small cell lung carcinoma. Ann Thorac Surg. 1999;68(4):1171–1176. doi: 10.1016/s0003-4975(99)00678-5. [DOI] [PubMed] [Google Scholar]
- 17.Gajra A, Newman N, Gamble GP, Kohman LJ, Graziano SL. Effect of number of lymph nodes sampled on outcome in patients with stage I non-small-cell lung cancer. J Clin Oncol. 2003;21(6):1029–1034. doi: 10.1200/JCO.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Varlotto JM, Recht A, Nikolov M, Flickinger JC, Decamp MM. Extent of lymphadenectomy and outcome for patients with stage I nonsmall cell lung cancer. Cancer. 2009;115(4):851–858. doi: 10.1002/cncr.23985. [DOI] [PubMed] [Google Scholar]
- 19.Ludwig MS, Goodman M, Miller DL, Johnstone PAS. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest. 2005;128(3):1545–1550. doi: 10.1378/chest.128.3.1545. [DOI] [PubMed] [Google Scholar]
- 20.Edwards BK, Johnson CH, Adamo M, Peace S, Fritz A, Percy-Laurry A. Histology coding and multiple primary rules. NAACR Narrative, Winter 2005 Edition. 2005;(12) [Google Scholar]
- 21.Percy-Laurry AL, Johnson CH, Reichman ME, Adamo M, Lewis DR, Peace S. Revising the multiple primary and histology coding rule. J Registry Manag. 2007;34(3):81–86. [Google Scholar]
- 22.Nakagawa T, Okumura N, Kokado Y, Miyoshi K, Matsuoka T, Kameyama K. Retrospective study of patients with pathologic N1-stage II non-small cell lung cancer. Interact Cardiovasc Thorac Surg. 2007;6(4):474–478. doi: 10.1510/icvts.2007.154641. [DOI] [PubMed] [Google Scholar]
- 23.The NCCN clinical practice guidelines in oncology: non-small cell lung cancer. Version V.I.2010. National Comprehensive Cancer Network Web site. http://nccn.org/professionals/physicians-glslf_guidelines.asp. Accessed August 2010.
- 24.Arriagada R, Dunant A, Pignon J-P, et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol. 2010;28(1):35–42. doi: 10.1200/JCO.2009.23.2272. [DOI] [PubMed] [Google Scholar]
- 25.Herr HW. Superiority of ratio based lymph node staging for bladder cancer. J Urol. 2003;169(3):943–945. doi: 10.1097/01.ju.0000032474.22093.06. [DOI] [PubMed] [Google Scholar]
- 26.Berger AC, Sigurdson ER, LeVoyer T, et al. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol. 2005;23(34):8706–8712. doi: 10.1200/JCO.2005.02.8852. [DOI] [PubMed] [Google Scholar]
- 27.Lee H-Y, Choi H-J, Park K-J, et al. Prognostic significance of metastatic lymph node ratio in node-positive colon carcinoma. Ann Surg Oncol. 2007;14(5):1712–1717. doi: 10.1245/s10434-006-9322-3. [DOI] [PubMed] [Google Scholar]
- 28.Nitti D, Marchet A, Olivieri M, et al. Ratio between metastatic and examined lymph nodes is an independent prognostic factor after D2 resection for gastric cancer: analysis of a large European monoinstitutional experience. Ann Surg Oncol. 2003;10(9):1077–1085. doi: 10.1245/aso.2003.03.520. [DOI] [PubMed] [Google Scholar]
- 29.Scott WJ, Allen MS, Darling G, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg. 2010;139(4):976–983. doi: 10.1016/j.jtcvs.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 30.Denlinger CE, Fernandez F, Meyers BF, et al. Lymph node evaluation in video-assisted thoracoscopic lobectomy versus lobectomy by thoracotomy. Ann Thorac Surg. 2010;89(6):1730–1736. doi: 10.1016/j.athoracsur.2010.02.094. [DOI] [PubMed] [Google Scholar]
- 31.Percy C, Stanek E, III, Gloeckler L. Accuracy of cancer death certificates and its effect on cancer mortality statistics. Am J Public Health. 1981;71(3):242–250. doi: 10.2105/ajph.71.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]