Abstract
Background:
Pre-B-cell colony-enhancing factor (PBEF) is a potential biomarker for acute lung injury (ALI) in sepsis. We aimed to determine the clinical correlates for elevated plasma PBEF upon ICU admission for severe sepsis and the usefulness of PBEF to predict ALI development and sepsis mortality.
Methods:
This is a prospective cohort of patients admitted to the medical ICU with severe sepsis. Patients without available blood samples or who were not enrolled within 24 h of admission were excluded. Plasma collected within 24 h of ICU admission was measured for PBEF concentrations by enzyme-linked immunosorbent assay. Patients were followed for ALI development as defined by the American-European Consensus Conference and for all-cause hospital mortality.
Results:
Between September 30, 2008, and March 10, 2009, 113 patients were enrolled, and 50 (44%) developed ALI. Elevated PBEF levels significantly correlated with higher APACHE (Acute Physiology and Chronic Health Evaluation) III scores (R2 = 0.08, P = .003) and failure to reach early sepsis goals within 6 h of severe sepsis (P = .003). PBEF did not differ by ALI status (P = .58). The mortality rate was 46%. Nonsurvivors had higher PBEF levels than survivors (2.53 ng/mL; interquartile range [IQR], 1.07-8.16 vs 1.44 ng/mL; IQR, 0.84-2.81; P = .02). After adjusting for severity of illness, PBEF levels were no longer significantly associated with mortality (OR, 1.44 per 10-fold increase; 95% CI, 0.69-3.03, P = .34).
Conclusions:
In this study, elevated PBEF did not correlate with lung injury in sepsis. However, it was associated with sepsis mortality mainly due to its association with greater severity of illness on ICU admission.
Acute lung injury (ALI) and its severe form, ARDS, occurs after some initial injury, such as sepsis, aspiration, trauma, or pneumonia. However, only a minority of patients with such injuries progress to ALI. Sepsis is the leading cause of ALI, but only 38% of patients with sepsis develop ALI.1 Currently, our incomplete understanding of who progresses to ALI and lack of a diagnostic blood test make preventative measures and accurate diagnosis difficult.
Pre-B-cell colony-enhancing factor (PBEF), also known as the intracellular enzyme nicotinamide phosphoribosyl transferase (Nampt) and the adipokine visfatin, is a proinflammatory cytokine that has shown promise as a biomarker in ALI. Initially shown to enhance B-cell maturation in the context of IL-7 and stem cell factor,2 elevated PBEF levels have been associated with inflammatory conditions, such as rheumatoid arthritis,3 inflammatory bowel disease,4 atherosclerosis, and myocardial infarction.5 More recently, PBEF, through its delay of neutrophil apoptosis, appears to enhance the inflammatory response in experimental and clinical sepsis.6
Furthermore, Ye et al7 demonstrated significant PBEF gene expression, in both animal and human models of ALI, indicating PBEF as a possible novel ALI biomarker. PBEF has also been shown as an integral inflammatory mediator in the development and severity of ventilator-induced lung injury.8 Polymorphisms in the PBEF gene have been associated with development of sepsis, ALI/ARDS, and mortality in ARDS.7,9 Elevated PBEF protein levels have also been demonstrated in patients with ALI,7 but the control subjects tended to be healthy individuals and the association has not been validated in other studies. Thus, it is not clear whether an elevated PBEF level is a marker of severe sepsis, predisposing to ALI, or of ALI in sepsis.
Although initial studies are promising, an understanding of the clinical predictors of PBEF in the critically ill is important in further assessing the potential of PBEF as a biomarker for ALI in sepsis. In noncritically ill individuals, PBEF has been associated with diabetes10,11 and obesity12 and found to be decreased with IV insulin therapy.13 These clinical factors have been associated with the development of ALI and variable outcomes in ALI14,15 and sepsis.16 It is not clear whether these findings also extend to a critically ill population. In this study, we aimed to determine the clinical correlates of PBEF in a cohort of ICU patients with severe sepsis and to investigate whether elevated PBEF levels are associated with ALI development and mortality in sepsis.
Materials and Methods
Study Population
Patients admitted to the Medical ICU at Mount Sinai Medical Center, New York, New York, from September 30, 2008, to March 10, 2009, were screened upon ICU admission for severe sepsis, as defined by the American College of Chest Physicians/Society of Critical Care Medicine.17 Patients with severe sepsis were excluded if they could not be screened and enrolled within 24 h of ICU admission, if no clinical blood samples were available within the first 24 h of ICU admission, or if the patient was previously enrolled into the study. A waiver of informed consent for collection of excess clinical samples and data collection was granted by the institution review board of the Mount Sinai School of Medicine (GCO#08-0641).
Data Collection
Baseline demographic information, such as age, history of diabetes, liver disease, alcohol abuse, and clinical variables for calculation of APACHE (Acute Physiology and Chronic Health Evaluation) III score were collected.18 Ventilatory parameters were recorded for determination of Murray Lung Injury Score (LIS)19 within the first 24 h of ICU admission. Documentation of timing for sepsis and onset of organ failure were reviewed to determine whether early sepsis resuscitation goals were met within 6 h of initial organ failure.20 These goals were defined as follows: (1) central venous pressure of 12 to 18 mm Hg, (2) mean arterial pressures ≥ 65 mm Hg, (3) urine output averages ≥ 0.5 mL/kg/h, and (4) central venous oxygen saturation of 70%, if available.21 During their medical ICU course, patients were followed daily for ALI development, as defined by the American-European Consensus Conference.22 A structured ALI tutorial23 was used to establish consensus in the interpretation of chest radiographs for bilateral infiltrates consistent with ALI. Additionally, the cohort was followed for all-cause ICU mortality, hospital mortality, ICU length of stay (LOS), and hospital LOS.
Determination of PBEF in Plasma
Excess plasma from EDTA-containing tubes drawn within 24 h of ICU admission for clinical testing were processed within an hour of collection and stored at –80°C. Thawed samples were analyzed for PBEF concentrations by using a commercially available sandwich enzyme-linked immunosorbent assay (AdipoGen Inc; Seoul, Korea) as per manufacturing instructions. Samples were analyzed in duplicate with an initial 10-fold dilution. Samples with values higher than the highest standard were reanalyzed by serial dilution. Laboratory personnel were blinded to the ALI and outcome status of the patients, and investigators involved in radiologic interpretation of ALI were blinded to PBEF levels.
Statistical Analyses
Continuous data are presented as mean values and standard deviations if normal, median values and interquartile range (IQR) (25th-75th percentiles) if nonnormal. Categorical variables are presented as absolute values and percentages. PBEF levels were logarithmically transformed for normality prior to analyses.
Univariate analyses were performed using Fisher exact tests, Student t tests, Wilcoxon rank-sum tests, or Spearman correlation tests as appropriate. Variables with P value < .1 on univariate analyses were entered into a backward selection algorithm and eliminated if they did not meet a P value ≤ .1. Potential confounders remaining after the selection algorithm were tested for interaction with PBEF with the addition of an interaction term. Because no significant interactions were found (P > .2), interaction terms were not included in the final models. Final multivariate model included results from the backward selection algorithm and plasma PBEF after logarithmic transformation. The P value of < .05 was considered statistically significant. All statistical analyses were performed using SAS, version 9.1.3 (SAS Institute; Cary, North Carolina).
Results
Study Population
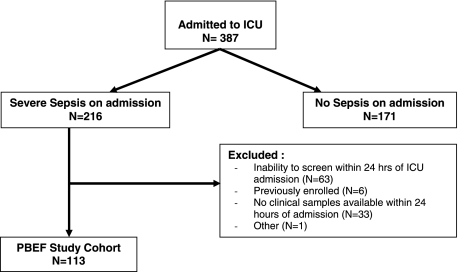
Of the 387 patients admitted to the medical ICU between September 30, 2008, and March 10, 2009, 216 (56%) fulfilled criteria for severe sepsis. Of these patients, 63 could not be screened and enrolled within 24 h of ICU admission, six were previously enrolled during their current hospitalization, 33 did not have clinical samples left over within 24 h, and one patient was excluded for other reasons, leaving a total of 113 eligible patients with severe sepsis and available blood samples for analysis (Fig 1). Compared with those enrolled in the study, patients with sepsis not included in the study were older (62 ± 14 vs 60 ± 18; P = .06). However, they did not differ significantly in gender, race, or hospital mortality (P > .2).
Figure 1.
Enrollment for the PBEF study cohort (N = 113). PBEF = pre-B-cell colony-enhancing factor.
PBEF Concentration and Its Clinical Correlates
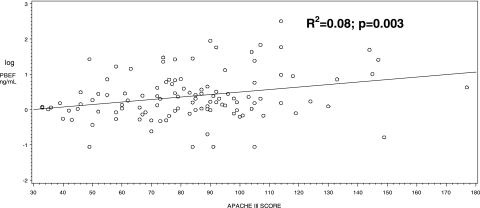
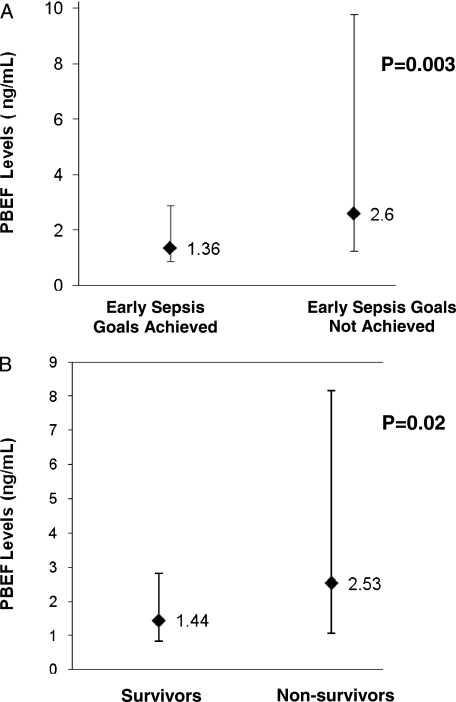
Plasma was collected a median of 4.9 h (IQR, 1.75-11.35) from ICU admission. The median PBEF concentration was 1.72 ng/mL (IQR, 0.96-5.34). Significantly higher plasma PBEF levels were found for patients with higher severity of illness (R2 = 0.08, P = .003) (Fig 2) and for patients who failed to reach early sepsis resuscitation goals within 6 h of severe sepsis (2.60 [IQR, 1.25-9.78], P = .003) (Fig 3). Those who failed to reach sepsis resuscitation goals also had significantly higher APACHE III scores than those patients who did reach the goals within 6 h of severe sepsis (95.24 ± 29.2 vs 75.32 ± 21.3; P = .0003). PBEF levels did not correlate with age (R2 = 0.0007, P = .78) or BMI (R2 = 0.002, P = .63). We found no significant associations between PBEF levels and clinical variables related to metabolism and obesity, such as diabetes, insulin use, and waist-hip ratio, or history of inflammatory or chronic vascular disorders, such as inflammatory bowel disease, rheumatoid arthritis, atherosclerosis, and myocardial infarction (Table 1).
Figure 2.
Scatterplot of PBEF levels (logarithmically scaled) and APACHE III score. Statistical analyses were performed using the Spearman correlation test. APACHE = Acute Physiology and Chronic Health Evaluation. See Figure 1 legend for expansion of other abbreviation.
Figure 3.
Significant predictors of PBEF plasma levels (ng/mL). A, Plasma PBEF levels shown as median with error bars indicating interquartile range for patients who did and did not achieve early sepsis resuscitation goals within 6 h of severe sepsis. B, Plasma PBEF levels shown as median with error bars indicating interquartile range for survivors and nonsurvivors with sepsis in the cohort. See Figure 1 legend for expansion of abbreviation.
Table 1.
—Univariate Analyses to Determine Clinical Correlates of PBEF Plasma Levels
| Variable | Plasma PBEF Levels (N = 113) | P Value | |
| Gender | .34 | ||
| Male, n = 63 | 2.06 (0.88-6.84) | ||
| Female, n = 50 | 1.47 (0.97-3.72) | ||
| Racea | .48 | ||
| White, n = 55 | 1.62 (0.68-7.16) | ||
| Non-white, n = 56 | 2.06 (1.12-4.94) | ||
| Diabetes history | .29 | ||
| Yes, n = 33 | 2.07 (0.95-3.13) | ||
| No, n = 80 | 1.66 (0.98-7.25) | ||
| Corticosteroid use prior to ICU admission | .83 | ||
| Yes, n = 28 | 2.06 (1.23-5.60) | ||
| No, n = 85 | 1.63 (0.93-4.54) | ||
| Source of sepsis | .44 | ||
| Pulmonary source, n = 45 | 2.06 (1.08-5.86) | ||
| Extrapulmonary source, n = 68 | 1.63 (0.81-4.94) | ||
| History of inflammatory or chronic vascular disease | .57 | ||
| Yes, n = 21 | 1.54 (0.77-2.85) | ||
| No, n = 92 | 1.95 (1.03-5.97) | ||
| Alcohol abuse | .84 | ||
| Yes, n = 9 | 1.72 (0.48-3.13) | ||
| No, n = 104 | 1.75 (0.97-5.60) | ||
| Early sepsis resuscitation goals achieved within 6 h of severe sepsis, No. (%)b | .003 | ||
| Yes, n = 65 | 1.36 (0.88-2.88) | ||
| No, n = 45 | 2.60 (1.25-9.78) | ||
| Mortality | .02 | ||
| Nonsurvivors, n = 52 | 2.53 (1.07-8.16) | ||
| Survivors, n = 61 | 1.44 (0.84-2.81) |
Data are presented as median (IQR), ng/mL. PBEF = pre-B-cell colony-enhancing factor.
Race data were missing in two patients.
Achievement of early sepsis resuscitation goals was available in 110 out of 113 patients.
PBEF and Development of ALI
Fifty out of 113 patients (44%) developed ALI. ALI development was significantly associated with higher BMI (P = .01) and pulmonary source of sepsis (P = .007) (Table 2). Compared with those without ALI, patients with ALI had significantly higher LIS on admission to the ICU (2.36 ± 0.8 in patients with ALI vs 1.11 ± 0.8 in patients without ALI, P < .0001) and longer ICU LOS (9.5 [IQR, 3-13] in patients with ALI vs 2.0 [IQR, 1-4] in patients without ALI, P < .0001).
Table 2.
—Baseline Characteristics of Study Population (N = 113)
| Characteristic | PBEF Study Cohort (N = 113) | ALI Group (n = 50) | Non-ALI Group (n = 63) | P Value |
| Age, y | 59.70 ± 18.0 | 57.60 ± 19.1 | 61.37 ± 17.1 | .27 |
| Male sex | 63 (56) | 24 (48) | 39 (62) | .18 |
| White racea | 55 (49) | 24 (48) | 31 (49) | .83 |
| BMIb | 27.83 ± 8.4 | 30.19 ± 10.0 | 25.99 ± 6.5 | .01 |
| Diabetes history | 33 (29) | 13 (26) | 20 (32) | .54 |
| Source of sepsis | ||||
| Pulmonary source | 45 (40) | 27 (54) | 18 (29) | .007 |
| Extrapulmonary source | 68 (60) | 23 (46) | 45 (71) | |
| Alcohol abuse | 9 (8) | 6 (12) | 3 (5) | .18 |
| LIS | 1.66 ± 1.0 | 2.36 ± 0.8 | 1.11 ± 0.8 | < .0001 |
| APACHE III score | 83.51 ± 27.3 | 85.2 ± 30.9 | 82.19 ± 24.4 | .57 |
| ICU LOS | 4 (2-10) | 9.5 (3-13) | 2 (1-4) | < .0001 |
| Hospital LOS | 14.5 (7-31) | 18 (7-39.5) | 12 (7-27) | .28 |
| Hospital mortality | 52 (46) | 27 (54) | 25 (40) | .18 |
| Plasma PBEF levels | 1.72 (0.96-5.34) | 1.71 (0.93-7.16) | 1.17 (0.97-4.29) | .58 |
Data are presented as No. (%), mean ± SD, or median (IQR). ALI = acute lung injury; APACHE = Acute Physiology and Chronic Health Evaluation; LIS 5 Murray Lung Injury Score; LOS = length of stay. See Table 1 legend for expansion of other abbreviation.
Race data were missing in two patients.
BMI data missing in one patient.
Upon univariate analyses, PBEF levels did not differ by ALI status (P = .58) or LIS on admission (R2 = 0.02, P = .20). On multivariate analysis, after adjusting for pulmonary source of sepsis and BMI, plasma PBEF was not significantly associated with ALI (OR, 0.78 per 10-fold increase; 95% CI, 0.43-1.41, P = .41).
PBEF and Mortality in Sepsis
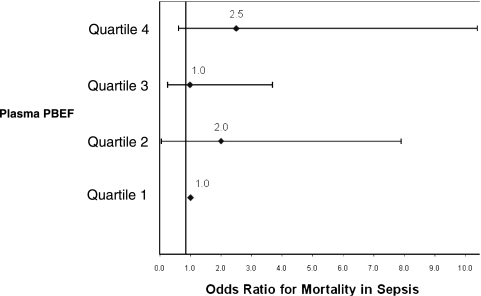
The overall hospital mortality for the study was 46% (52 out of 113). Compared with survivors, nonsurvivors tended to have a higher incidence of ALI (52% in nonsurvivors vs 38% in survivors, P = .18), statistically higher LIS (1.91 ± 0.89 in nonsurvivors vs 1.46 ± 0.4 in survivors, P = .02), and shorter hospital LOS (9.5 [IQR, 4-26] in nonsurvivors vs 22 [IQR, 10-40] in survivors, P = .001) (Table 3). Nonsurvivors were older (P = .02), were more often treated with corticosteroids prior to ICU admission (P = .02), less likely to meet sepsis resuscitation goals within 6 h (P = .004), and had greater severity of illness by APACHE III (P < .0001). Nonsurvivors had significantly higher PBEF levels than survivors (2.53 ng/mL [IQR, 1.07-8.16] in nonsurvivors vs 1.44 ng/mL [IQR, 0.84-2.81] in survivors, P = .02) (Fig 3). However, after adjusting for age, corticosteroid use prior to the ICU, and severity of illness, PBEF was no longer significantly associated with mortality (OR, 1.44 per 10-fold increase; 95% CI, 0.69-3.03; P = .34) (Table 4). Additionally, patients were stratified according to quartiles of plasma PBEF. Compared with the lowest quartile of PBEF measured, increasing quartiles of PBEF were neither significantly nor consistently associated with mortality in sepsis (Fig 4).
Table 3.
—Univariate Analyses to Determine Predictors of Hospital Mortality (N = 113)
| Characteristic | Survivors (n = 61) | Nonsurvivors (n = 52) | P Value |
| Age, y | 56.13 ± 17.9 | 63.88 ± 17.4 | .02 |
| Male sex | 34 (56) | 29 (56) | .99 |
| White racea | 30 (49) | 24 (46) | .85 |
| BMIb | 27.50 ± 8.9 | 28.22 ± 7.9 | .66 |
| Diabetes history | 18 (30) | 15 (29) | 1.00 |
| Corticosteroid use prior to ICU admission | 10 (16) | 18 (35) | .02 |
| Pulmonary source of sepsis | 27 (44) | 18 (35) | .34 |
| Alcohol abuse | 3 (5) | 6 (12) | .30 |
| ALI development | 23 (38) | 27 (52) | .18 |
| Early sepsis resuscitation goals achieved within 6 h of severe sepsisc | 43 (72)d | 22 (44)e | .004 |
| LIS | 1.46 ± 1.0 | 1.91 ± 0.9 | .02 |
| APACHE III score | 70.16 ± 21.1 | 99.17 ± 25.6 | < .0001 |
| ICU LOS | 4 (2-10) | 3 (1-10.5) | .20 |
| Hospital LOS | 22 (10-40) | 9.5 (4-26) | .001 |
| Plasma PBEF levels, ng/mL | 1.44 (0.84-2.81) | 2.53 (1.07-8.16) | .02 |
Data are presented as No. (%), mean ± SD, or median (IQR). See Tables 1 and 2 legends for expansion of abbreviations.
Race data were missing in two patients.
BMI data missing in one patient.
Achievement of early sepsis resuscitation goals was available in 110 out of 113 patients.
60 out of 61 survivors.
50 out of 52 nonsurvivors.
Table 4.
—Multivariable Logistic Regression Demonstrating ORs for Effect on Hospital Mortality
| Characteristic | OR | 95% CI | P Value |
| Age, y | 1.03 | 1.00-1.06 | .07 |
| APACHE III score | 1.06 | 1.03-1.09 | < .0001 |
| Corticosteroid use prior to ICU | 3.72 | 1.16-11.97 | .03 |
| Plasma PBEF (per 10-fold unit increase) | 1.44 | 0.69-3.03 | .34 |
Figure 4.
OR for mortality in sepsis after stratifying plasma PBEF levels into quartiles. Quartile 1 (< 0.86 ng/mL) is used as reference. See Figure 1 legend for expansion of abbreviation.
Discussion
PBEF is believed to play a key role in ALI pathogenesis, with biomarker potential for this devastating condition.7,24 In this study of patients with severe sepsis admitted to the ICU, plasma PBEF levels were not associated with ALI/ARDS or LIS. However, PBEF was correlated with APACHE III score, failure to achieve early sepsis resuscitation goals, and mortality in sepsis. This study has several strengths. To our knowledge, this is the largest study of PBEF in critically ill patients with sepsis. All samples were collected within 24 h of ICU admission and processed within 1 h of collection, minimizing temporal changes over the course of critical illness and preanalytic factors that could have influenced the determination of plasma PBEF levels. All outcomes, including development of ALI, were collected prospectively, minimizing misclassification. Laboratory personnel performing the assay for PBEF were blinded to patient outcomes, minimizing bias. PBEF was also measured by enzyme-linked immunosorbent assay, which has been found to be more reliable than less-precise quantification assays,25 such as Western blot with densitometric quantification.7
In this study, many of the clinical predictors found to be associated with ALI/ARDS or mortality in sepsis were consistent with other reports in different populations. As expected, ALI and/or LIS were associated with longer ICU LOS and hospital mortality. Consistent with prior studies,15,26-30 this study demonstrated that those patients with a pulmonary source of infection, higher BMI, and alcohol abuse had higher rates of development of ALI, whereas ALI developed less frequently in those with diabetes, although not all of the associations were statistically significant given the sample size. Patients with corticosteroid therapy prior to ICU admission had a significantly higher rate of death on both univariate and multivariate analysis, likely reflecting previously reported associations between increased mortality and chronic immunosupression.31 In addition, our findings that older age, failure to achieve resuscitation goals within 6 h of sepsis, lung injury, and APACHE III score were significantly associated with mortality in sepsis is also consistent with prior reports.32-36
PBEF is believed to play an important role in the development of ALI. Li et al24 suggested PBEF as a facilitator of pulmonary inflammation and alveolar epithelial barrier dysfunction via its regulation of other inflammatory cytokines (eg, IL-8). Also, increased expression of PBEF may mediate the vascular permeability characteristic of ALI.37,38 In their initial report of the association of PBEF with ALI, Ye et al7 demonstrated significantly increased PBEF transcription in lung tissue from critically ill patients and elevated PBEF protein levels in a small cohort (n = 8) with nonspecified cause of ALI when compared with healthy control subjects. In addition, specific PBEF gene polymorphisms have been linked to an increased risk of ARDS development and ICU mortality among those at risk.9 But it is not clear whether PBEF was associated with the initial injury, such as severe sepsis, leading to ALI or with ALI itself.
In this study of critically ill patients with sepsis, PBEF was not associated with lung injury score on admission or with subsequent development of ALI. This may be because PBEF is a marker of disease severity and poor outcome in sepsis rather than a specific marker for ALI development. Alternatively, increased PBEF may be found within cells, not necessarily reflected in the peripheral blood in sepsis. Along with its inflammatory role in innate immunity, PBEF functions within the cell as a Nampt, the rate-limiting step in nicotinamide adenine dinucleotide biosynthesis, which initiates the respiratory burst and generates reactive oxygen species crucial in antimicrobial defenses.39 Jia et al6 found that although PBEF, as a secreted cytokine, is necessary for inhibiting neutrophil apoptosis in sepsis, this process also requires endogenous PBEF, suggesting a complementary role for its intracellular Nampt activity in this setting. Thus, plasma PBEF may not fully reflect the role of PBEF in sepsis and lung injury.
PBEF has also been shown to play a key role in the pathogenesis of sepsis,6 the most common underlying cause of ALI. Through its inhibition of neutrophil apoptosis, facilitation of nicotinamide adenine dinucleotide-dependent immune processes, and stimulation of proinflammatory cytokines, higher PBEF levels may underlie more extensive immune and endothelial dysfunction in sepsis, translating into worsening illness and outcome. In this study, elevated plasma PBEF was associated with mortality in severe sepsis. However, after taking severity of illness into account, PBEF was no longer significantly associated with mortality. Rather than a biomarker for ALI or sepsis, PBEF may serve as an indicator of severity of illness. Indeed, the interaction between PBEF and severity of illness was also found in a prior study of a different cohort where the T-1001G variant allele in the PBEF gene was associated with development of ALI, except among those who were most severely ill.9
Although critically ill patients with sepsis who did not meet resuscitative goals within 6 h of ICU admission had higher levels of PBEF, increased PBEF levels may just reflect higher severity of illness. Patients who failed to achieve early sepsis resuscitation goals also had higher APACHE III scores after admission to the ICU, and after adjusting for severity of illness, ability to reach early resuscitation goals was no longer associated with mortality in sepsis.
PBEF, also known as visfatin, an adipocytokine with high expressivity in visceral fat,40 has been associated with obesity,12 diabetes,10,11 hyperglycemia,13 and insulin therapy13 in noncritically ill individuals in some but not all studies.12,41,42 Among critically ill patients, obesity, diabetes, hyperglycemia, and intensive insulin therapy have been associated with variable development of or outcomes in ARDS and sepsis.14,15,29,30,43,44 However, plasma PBEF was not associated with obesity, diabetes, or insulin therapyin our cohort of critically ill patients with sepsis. Our finding suggests that the adipokine PBEF may not explain the previously reported relationships between these metabolic conditions and outcomes in sepsis.
There are several limitations to this study. This is a single-center study of sepsis; therefore, our findings’ generalizability of the results to other patients with sepsis has not been demonstrated. However, the consistency of the clinical predictors for ALI and mortality in sepsis between this study and other published cohorts suggests that the study population is similar to others, and our results may be applicable to other patients with sepsis. Furthermore, PBEF was not collected over several days, so no inferences about the temporal changes in PBEF over the course of sepsis can be made. Although all samples were collected within 24 h of ICU admission, with the majority of plasma collected within 12 h, we cannot exclude the possibility that PBEF levels may vary within the first 24 h of critical illness. In addition, this study focused on critically ill patients with sepsis. The clinical correlates of PBEF in other critical illnesses may differ. Last, although this study consists of a well-characterized cohort of critically ill patients with severe sepsis and is larger than prior studies, the power may still be limited in detecting a small difference in PBEF levels between patients with and without ALI.
In summary, we have shown elevated PBEF levels do not correlate with diabetes, obesity, insulin use, and development of lung injury in sepsis, but PBEF was associated with greater severity of illness and inability to achieve resuscitation goals within 6 h. Although PBEF was not predictive for the development of ALI, the importance of PBEF in sepsis presentation and outcome was confirmed in this study. PBEF was associated with mortality in sepsis, mainly due to its association with greater severity of illness on admission to the ICU. Further exploration of PBEF’s role in critical illness holds the potential for better understanding of the pathophysiology underlying sepsis and ALI.
Acknowledgments
Author contributions: Dr Lee: contributed to the study design, data collection and analyses, and manuscript preparation.
Dr Gong: contributed to the study design, data collection and analyses, and manuscript preparation.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: We thank Daniel Ceusters, BA, for research administration; Gary August, BA, for data management; John Frederick, MD, David Fogelman, MD, Jessica Jeffries, MD, Nadine Spring, MPH, and Adam Wright, BS, for patient enrollment and data collection; medical ICU nurses and staff for sample collection; and Johnny Loke, MS, Tom Marron, MS, and Taia Wang, MS, for laboratory collaboration. Work was performed at Mount Sinai Medical Center, New York, New York.
Abbreviations
- ALI
acute lung injury
- APACHE
Acute Physiology and Chronic Health Evaluation
- IQR
interquartile range
- LIS
Murray Lung Injury Score
- LOS
length of stay
- Nampt
nicotinamide phosphoribosyl transferase
- PBEF
pre-B-cell colony-enhancing factor
Footnotes
Funding/Support: Supported by a Doris Duke Clinical Research Fellowship for Medical Students and the National Heart, Lung, and Blood Institute [Grants HL60197, HL084060, and HL086667].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Pepe PE, Potkin RT, Reus DH, Hudson LD, Carrico CJ. Clinical predictors of the adult respiratory distress syndrome. Am J Surg. 1982;144(1):124–130. doi: 10.1016/0002-9610(82)90612-2. [DOI] [PubMed] [Google Scholar]
- 2.Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14(2):1431–1437. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brentano F, Schorr O, Ospelt C, et al. Pre-B cell colony-enhancing factor/visfatin, a new marker of inflammation in rheumatoid arthritis with proinflammatory and matrix-degrading activities. Arthritis Rheum. 2007;56(9):2829–2839. doi: 10.1002/art.22833. [DOI] [PubMed] [Google Scholar]
- 4.Moschen AR, Kaser A, Enrich B, et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178(3):1748–1758. doi: 10.4049/jimmunol.178.3.1748. [DOI] [PubMed] [Google Scholar]
- 5.Dahl TB, Yndestad A, Skjelland M, et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization. Circulation. 2007;115(8):972–980. doi: 10.1161/CIRCULATIONAHA.106.665893. [DOI] [PubMed] [Google Scholar]
- 6.Jia SH, Li Y, Parodo J, et al. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Invest. 2004;113(9):1318–1327. doi: 10.1172/JCI19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye SQ, Simon BA, Maloney JP, et al. Pre-B-cell colony-enhancing factor as a potential novel biomarker in acute lung injury. Am J Respir Crit Care Med. 2005;171(4):361–370. doi: 10.1164/rccm.200404-563OC. [DOI] [PubMed] [Google Scholar]
- 8.Hong SB, Huang Y, Moreno-Vinasco L, et al. Essential role of pre-B-cell colony enhancing factor in ventilator-induced lung injury. Am J Respir Crit Care Med. 2008;178(6):605–617. doi: 10.1164/rccm.200712-1822OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bajwa EK, Yu CL, Gong MN, Thompson BT, Christiani DC. Pre-B-cell colony-enhancing factor gene polymorphisms and risk of acute respiratory distress syndrome. Crit Care Med. 2007;35(5):1290–1295. doi: 10.1097/01.CCM.0000260243.22758.4F. [DOI] [PubMed] [Google Scholar]
- 10.Chen MP, Chung FM, Chang DM, et al. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006;91(1):295–299. doi: 10.1210/jc.2005-1475. [DOI] [PubMed] [Google Scholar]
- 11.Retnakaran R, Youn BS, Liu Y, et al. Correlation of circulating full-length visfatin (PBEF/NAMPT) with metabolic parameters in subjects with and without diabetes: a cross-sectional study. Clin Endocrinol (Oxf) 2008;69(6):885–893. doi: 10.1111/j.1365-2265.2008.03264.x. [DOI] [PubMed] [Google Scholar]
- 12.Varma V, Yao-Borengasser A, Rasouli N, et al. Human visfatin expression: relationship to insulin sensitivity, intramyocellular lipids, and inflammation. J Clin Endocrinol Metab. 2007;92(2):666–672. doi: 10.1210/jc.2006-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haider DG, Schaller G, Kapiotis S, Maier C, Luger A, Wolzt M. The release of the adipocytokine visfatin is regulated by glucose and insulin. Diabetologia. 2006;49(8):1909–1914. doi: 10.1007/s00125-006-0303-7. [DOI] [PubMed] [Google Scholar]
- 14.Moss M, Guidot DM, Steinberg KP, et al. Diabetic patients have a decreased incidence of acute respiratory distress syndrome. Crit Care Med. 2000;28(7):2187–2192. doi: 10.1097/00003246-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Gong MN, Bajwa EK, Thompson BT, Christiani DC. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax. 2010;65(1):44–50. doi: 10.1136/thx.2009.117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 17.Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992;101(6):1481–1483. doi: 10.1378/chest.101.6.1481. [DOI] [PubMed] [Google Scholar]
- 18.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 19.Murray JR, Matthay MA, Luce JM, et al. Pulmonary perspectives: an expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138(3):720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 20.Dellinger RP, Levy MM, Carlet JM, et al. International Surviving Sepsis Campaign Guidelines Committee American Association of Critical-Care Nurses American College of Chest Physicians American College of Emergency Physicians Canadian Critical Care Society European Society of Clinical Microbiology and Infectious Diseases European Society of Intensive Care Medicine European Respiratory Society International Sepsis Forum Japanese Association for Acute Medicine Japanese Society of Intensive Care Medicine Society of Critical Care Medicine Society of Hospital Medicine Surgical Infection Society World Federation of Societies of Intensive and Critical Care Medicine Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296–327. [Google Scholar]
- 21.Rivers E, Nguyen B, Havstad S, et al. Early Goal-Directed Therapy Collaborative Group Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 22.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 23.Tutorial: improving the radiographic diagnosis of acute lung injury. University of Washington Web site. http://depts.washington.edu/kclip/about.shtml. Accessed November 2008.
- 24.Li H, Liu P, Cepeda J, et al. Augmentation of pulmonary epithelial cell IL-8 expression and permeability by pre-B-cell colony enhancing factor. J Inflamm (Lond) 2008;5:15. doi: 10.1186/1476-9255-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Körner A, Garten A, Blüher M, Tauscher R, Kratzsch J, Kiess W. Molecular characteristics of serum visfatin and differential detection by immunoassays. J Clin Endocrinol Metab. 2007;92(12):4783–4791. doi: 10.1210/jc.2007-1304. [DOI] [PubMed] [Google Scholar]
- 26.Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005;33(6):1191–1198. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 27.Bersten AD, Edibam C, Hunt T, Moran J. Australian and New Zealand Intensive Care Society Clinical Trials Group Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian States. Am J Respir Crit Care Med. 2002;165(4):443–448. doi: 10.1164/ajrccm.165.4.2101124. [DOI] [PubMed] [Google Scholar]
- 28.Moss M, Parsons PE, Steinberg KP, et al. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med. 2003;31(3):869–877. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- 29.Iscimen R, Cartin-Ceba R, Yilmaz M, et al. Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Crit Care Med. 2008;36(5):1518–1522. doi: 10.1097/CCM.0b013e31816fc2c0. [DOI] [PubMed] [Google Scholar]
- 30.Esper AM, Moss M, Martin GS. The effect of diabetes mellitus on organ dysfunction with sepsis: an epidemiological study. Crit Care. 2009;13(1):R18. doi: 10.1186/cc7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poutsiaka DD, Davidson LE, Kahn KL, Bates DW, Snydman DR, Hibberd PL. Risk factors for death after sepsis in patients immunosuppressed before the onset of sepsis. Scand J Infect Dis. 2009;41(6-7):469–479. doi: 10.1080/00365540902962756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34(1):15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 33.Vincent JL, Sakr Y, Sprung CL, et al. Sepsis Occurrence in Acutely Ill Patients Investigators Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 34.Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38(4):1045–1053. doi: 10.1097/CCM.0b013e3181cc4824. [DOI] [PubMed] [Google Scholar]
- 35.Khwannimit B, Bhurayanontachai R. The epidemiology of, and risk factors for, mortality from severe sepsis and septic shock in a tertiary-care university hospital setting. Epidemiol Infect. 2009;137(9):1333–1341. doi: 10.1017/S0950268809002027. [DOI] [PubMed] [Google Scholar]
- 36.Alberti C, Brun-Buisson C, Goodman SV, et al. European Sepsis Group Influence of systemic inflammatory response syndrome and sepsis on outcome of critically ill infected patients. Am J Respir Crit Care Med. 2003;168(1):77–84. doi: 10.1164/rccm.200208-785OC. [DOI] [PubMed] [Google Scholar]
- 37.Liu P, Li H, Cepeda J, et al. Critical role of PBEF expression in pulmonary cell inflammation and permeability. Cell Biol Int. 2009;33(1):19–30. doi: 10.1016/j.cellbi.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye SQ, Zhang LQ, Adyshev D, et al. Pre-B-cell-colony-enhancing factor is critically involved in thrombin-induced lung endothelial cell barrier dysregulation. Microvasc Res. 2005;70(3):142–151. doi: 10.1016/j.mvr.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Sheppard FR, Kelher MR, Moore EE, McLaughlin NJ, Banerjee A, Silliman CC. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J Leukoc Biol. 2005;78(5):1025–1042. doi: 10.1189/jlb.0804442. [DOI] [PubMed] [Google Scholar]
- 40.Fukuhara A, Matsuda M, Nishizawa M, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin [retracted in Science. 2007;318(5850):565] Science. 2005;307(5708):426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 41.Berndt J, Klöting N, Kralisch S, et al. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes. 2005;54(10):2911–2916. doi: 10.2337/diabetes.54.10.2911. [DOI] [PubMed] [Google Scholar]
- 42.Chang YC, Chang TJ, Lee WJ, Chuang LM. The relationship of visfatin/pre-B-cell colony-enhancing factor/nicotinamide phosphoribosyltransferase in adipose tissue with inflammation, insulin resistance, and plasma lipids. Metabolism. 2010;59(1):93–99. doi: 10.1016/j.metabol.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 43.Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78(12):1471–1478. doi: 10.4065/78.12.1471. [DOI] [PubMed] [Google Scholar]
- 44.Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]