Abstract
Thrombotic microangiopathy (TMA) refers to a clinical and pathological syndrome in which endothelial injury results in the manifestations of thrombocytopenia, microangiopathic hemolytic anemia, and renal injury. A host of etiologies may induce endothelial injury and TMA, including enteric bacterial toxins, deficiency or dysfunction of complement regulatory proteins, deficiency or inhibition of von Willebrand factor cleaving proteases, and factors that inhibit endothelial cell proliferation and turnover. This has led specialists to concentrate on these specific inciting factors in terms of designing treatment and management. However, a key and less recognized factor is the underlying level of endothelial health. Indeed, many subjects with hereditary etiologies may remain disease free for years, and may never develop disease. Others with acute inciting events such as E coli O157 enteritis never manifest TMA. Experimental studies document the importance of specific factors such as endothelial nitric oxide levels in helping to protect animals from TMA. This suggests that one might approach the management of TMA not simply with specific treatments aimed at the underlying hereditary cause or inciting event, but rather also at general measures that may improve overall endothelial health. We propose studies to determine if interventions known to improve endothelial health, such as the administration of ACE inhibitors, statins, vitamin C, allopurinol, or nitric oxide-producing drugs may be able to prevent TMA even in subjects with underlying hereditary conditions that would otherwise predispose them to these diseases.
Keywords: hemolytic uremic syndrome, nitric oxide, endothelial cell, vascular endothelial growth factor
Background
Thrombotic microangiopathy refers to a clinical and pathological syndrome characterized by thrombocytopenia, mechanical hemolytic anemia, and renal injury. The underlying pathogenesis of thrombotic microangiopathy is considered to be endothelial cell injury, resulting in the renal lesion of arteriolar injury, endothelial swelling of the glomerular and peritubular capillaries, and intracapillary platelet and fibrin rich thrombi formation. The two most common types of thrombotic microangiopathies are the hemolytic uremic syndrome (HUS) and thrombotic thrombocytopenic purpura (TTP). HUS is a condition in which the renal manifestations are most manifest, and TTP presents more as a systemic disorder in which renal involvement is classically mild.
Case Vignette
A 37-year-old woman presents to the emergency room with epistaxis. She has a history of atypical hemolytic uremic syndrome (aHUS). Her first diagnosis of aHUS was made during the post-partum period of her first and only pregnancy 15 years ago. She responded to multiple rounds of plasma exchange. Her second episode of aHUS occurred 10 years ago around the time of an upper respiratory infection and again responded to multiple rounds of plasma exchange. She maintains that she has been asymptomatic since her last presentation. Her only other medical problem is hypertension for which she takes metoprolol 25 mg twice daily. She has been feeling fatigued and chilled for two days prior to the epistaxis. In the emergency room, serum hematocrit was 25.4%, hemoglobin was 8.5 g/dL, and platelets were 55. LDH level was 1019 and haptoglobin was undetectable. Serum creatinine was 2.5 and BUN was 45. Plasma exchange is initiated and the patient responds after 18 treatments. Serum creatinine and the time of hospital discharge is 1.4. Recurrent aHUS in an uncommon disorder and presents a challenge to the treating physician. Currently there are no direct therapeutic agents that have been shown to prevent recurrent episodes of aHUS. We hypothesize that agents known to improve endothelial health may have a role in preventing aHUS relapses.
Pathogenesis
Over the last decades a variety of etiologies of the thrombotic microangiopathies have been identified (Table 1). The best established diagnoses are TMAs associated with infection due to E Coli 0157:H7, the ADAMTS13 deficiency, or with genetic or acquired deficiencies in complement regulatory proteins. Treatments for these conditions are often tailored for the underlying etiology. For example, TMA caused by E Coli, generally responds to supportive care alone 1, whereas plasma exchange is preferred for patients with ADAMTS13 related disease to either replace the deficient protease or to remove anti-protease antibodies2. Patients with TMA secondary to complement regulatory protein deficiencies or mutations are usually treated with plasma exchange to replace the deficient complement protein. However, long term outcomes in patients with Factor H, Factor I, or C3 mutations remains poor3.
Table 1.
Etiologies of Thrombotic Microangiopathy
| E Coli O157:H7 or other shiga toxin producing organisms |
| ADAMTS13 deficiency |
| Complement regulatory protein deficiencies or mutations |
| Drugs (Calcineurin inhibitors, oral contraceptives, chemotherapeutic agents, ticlodopine, clopidogrel, quinine) |
| Pregnancy related |
| Neuraminidase producing organisms leading to T antigen recognition and possible endothelial cell injury |
| Hemodynamic factors including malignant hypertension |
| Idiopathic |
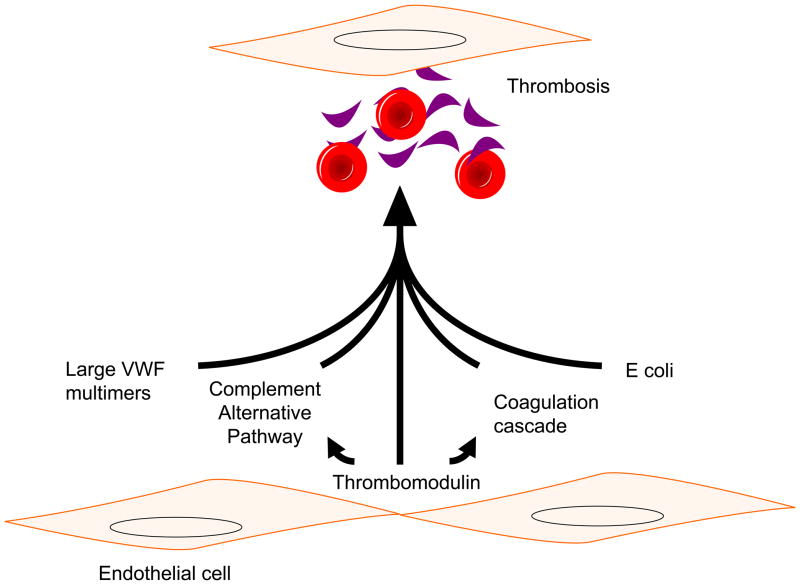
Importantly, there is a notion that there could be a missing factor in TMA, and this is supported by a variety of findings. For example, patients with deficient complement regulation leading to atypical hemolytic uremic syndrome (aHUS) may be asymptomatic for decades prior to the development of disease3, 4 while disease flares in these patients are often initiated by an inciting event such as an infection. There are also case reports of patients with combined ADAMTS13 deficiency and complement protein mutations who had more severe disease5. Some subjects with severe hemorrhagic colitis due to E coli O157 develop HUS whereas others do not. While there is much focus on the mechanisms by which E coli O157 toxins or lack of key complement regulatory proteins may cause TMA, less emphasis has been placed on the underlying health of the endothelium (Figure 1). Thus, it is the contention in this paper that initiating measures to improve endothelial health could provide an ancillary approach to the management of these disorders.
Figure 1.
Pathogenesis of thrombotic microangiopathy. Abbreviation: vWF, von Willebrand factor.
The endothelium is a highly active tissue responsible in part for regulating vascular tone, coagulation, and inflammation. Why the glomerular endothelium is the main target of TMAs is, unfortunately, still unknown. Some have postulated that the fenestrated nature of the glomerular endothelial cell (GEC) leaves it more susceptible to complement activation since the glomerular basement membrane is without its own complement regulators3. Also, the GEC has been shown to be dependent on podocyte produced vascular endothelial growth factor to maintain its health so a process negatively impacting the podocyte may then lead to a weakened endothelial cell6. While direct injury of the endothelium is known to cause TMA, alterations in underlying endothelial function may also have a key predisposing role. We discuss specific functions of the endothelium and how it may modulate TMA. If our notion is true, an important insight will emerge, which is that general measures to maintain a healthy endothelium could be an alternative strategy for both preventing and managing TMA regardless of the underlying etiology.
Recent Advances
Protective Factors for the Endothelium
Endothelial cells express endothelial nitric oxide synthase (eNOS), which produces low levels of nitric oxide (NO) that has a key function in maintaining local vasodilation, protecting endothelial cells from toxicity of circulating cytokines such as tumor necrosis factor-alpha (TNF-α), and reducing the risk for local thrombosis. Endothelial NO, for example, inhibits the exocytosis of endothelial cell vacuoles (Weibel Palade bodies) to release P selectins and von Willebrand factor (vWF), both of which can initiate local inflammation and thrombosis7. Control of endothelial NO levels is governed in part by vascular endothelial growth factor (VEGF), and VEGF also has important roles in maintaining endothelial cell integrity.
The importance of endothelial NO in TMA is becoming increasingly recognized. For example, mice deficient in endothelial NO develop a TMA-like lesion with aging that is associated with glomerular capillary deposition of vWF and elevated circulating P selectin levels consistent with unimpaired release of endothelial –derived Weibel Palade bodies8. Preeclampsia, which is another form of TMA, has also been associated with low circulating nitric oxide levels even following recovery of disease9. Compatibly, recent evidence documented that soluble endoglin, which can inactivate eNOS by inhibiting TGF-beta signaling10, increases the risk for preeclampsia11, 12. Finally, hemolysis, such as occurs in TMA, may further lower NO levels due to the ability of free hemoglobin to consume NO13.
A reduction in local VEGF in the podocyte can also induce a TMA-like lesion in the kidney14. Since VEGF is a major factor to regulate endothelial NO production, a potential mechanisms for this TMA could be low NO level due to a lack of VEGF stimulation. Likewise, inhibition of circulating VEGF due to the production of a soluble VEGF receptor (sFLlt-1) has been shown to have a role in preeclampsia11. Similarly, inhibition of VEGF with the monoclonal VEGF inhibitor bevacizumab may cause a TMA-like lesion with proteinuria and hypertension in patients6.
Recently, it has been found that approximately 5% of patients with atypical HUS may carry mutations in the gene for thrombomodulin, a glycoprotein that may help regulate both clotting and complement activation on the endothelial cell surface 15. When thrombomodulin activity is altered, the endothelial cell surface is at further risk for injury.
While a lack of these factors may predispose to TMA, there is also evidence that replacing these factors may confer benefit in TMA. For example, NO has been shown to be protective in animal models of HUS suggesting a role for vasodilation and platelet inhibition16. Administration of VEGF can also rescue or protect renal injury in animal models of TMA 17, 18. This suggests that stimulation or maintenance of endothelial NO or VEGF could be protective from pathogenic mechanisms driving TMA.
Factors Disrupting Endothelial Function
Malignant hypertension may cause TMA via injury to the vascular endothelium. van den Born et al demonstrated increased amounts of VWF and reduced levels of ADAMTS13 in a group of patients with malignant hypertension and TMA19. Endothelial NO levels are also low in this condition, likely due to consumption by local oxidative stress20. Indeed, Vaziri et al have reported that the injection of an inhibitor of the intracellular glutathione antioxidant pathway results in acute oxidative stress, a loss of nitric oxide, and the development of malignant hypertension in rats21.
Conditions that disrupt the normal anticoagulant function of the endothelium can increase the risk for TMA. For example, pregnancy is a well-known hypercoagulable state, especially for subjects at term and post-delivery. The coagulation factors I, II, VII, VIII, IX, and X all increase along with a decrease in protein S levels and an increase in acquired resistance to activated protein C (APC) 22, 23. Interestingly, increases in plasma thrombomodulin, plasminogen activator inhibitor-1, and von Willebrand factor with a corresponding decrease in ADAMTS13 levels24, all of which occur in pregnancy, indicate the possibility of loss of endothelial cell membrane integrity and decreased fibrinolytic activity, respectively25. It is thus not surprising that approximately 10% of cases of TMA occur with pregnancy or in the post-partum period24, 26. Pregnancy has also been reported to precipitate TMA in subjects with hereditary TTP-HUS24.
Estrogen in OCPs also have prothrombotic effects including inducing APC resistance, increasing the plasma levels of Protein C and protein C inhibitors, and decreasing APC independent anticoagulation activity of protein S and the plasma levels of total Protein S and C4b binding protein27. Similarly, subjects on birth control pills (OCPs) have been reported to be at increased risk for developing idiopathic HUS28, 29. In turn, estrogen is able to protect endothelial cell by stimulating endothelial NO production30. Hence, it is possible that the prothrombotic effects of estrogen could be blocked by a concomitant increase in endothelial NO in the presence of healthy endothelium whereas it might be unmasked in the setting where endothelial NO levels are reduced (such as in subjects with obesity or metabolic syndrome).
The production of complement regulatory proteins by the endothelium may help to prevent activation of the coagulation cascade. The membrane attack complex attracts and activates platelets and causes the release of VWF from endothelial cells while the complement fragment proteins C3a and C5a induce platelet activation and aggregation, up regulate plasminogen activator inhibitor-1, and trigger the release of cytokines31. C5a also has been shown to cause the release of the anticoagulant molecule heparan sulfate from endothelial cells further increasing the thrombogenic potential on the endothelial cell surface32. C4b binding protein also complexes with Protein S, thereby decreasing its anticoagulant effects. Thus in areas of endothelial cell inflammation local complement activation could increase the risk for local clot formation. This likely explains the relationship of HUS to hereditary conditions in which complement regulatory protein mutations occur, but also suggests that any condition that leads to local intravascular complement activation could predispose to the development of an HUS like syndrome.
Direct Endothelial Toxins
The calcineurin inhibitors (CNI) cyclosporine and tacrolimus are also associated with TMA in both renal transplant and non-renal transplant patients. In renal transplantation, a multiple hit etiology is easily implicated as a number of additional factors have been linked to post-transplant de novo TMA, including marginal kidneys, viral infections, other medications, and malignancy33. CNIs directly injure endothelial cells, decrease the production of prostacyclin and NO while increasing thromboxane A2 and endothelin synthesis, and reduce the formation of activated protein C 34–37. Dysregulation of complement regulatory proteins by CNI may contribute to cyclosporine-induced TMA 38. Agents that block mTOR, such as sirolimus, may increase the risk of TMA in subjects receiving CNI also in part by delaying repair of injured endothelium and by causing a local down regulation of VEGF39
Chemotherapy agents may also increase the risk for TMA by inducing endothelial injury. Examples include mitomycin-C, gemcitabine and quinine 40, 41, 42 The mechanism of injury is not well understood but in part may relate to the development of antibodies to tumors 43, and with quinine to the development of autoantibodies to normal cells such as platelets, granulocytes, and endothelial cells42.
E coli associated HUS
Shiga toxin, released by E Coli O157:H7, is the primary cause of diarrhea associated HUS. Shiga toxin acts in part by inducing endothelial cells to secrete ultra large VWF, by impairing the activity of ADAMTS13, and by increasing the expression of P-selectin and platelet endothelial cell adhesion molecule 144. Shiga toxin also binds and prevents the activity of the complement regulatory protein, Factor H45, and may explain why activation of systemic complement occurs in cases of HUS induced by E coli O15746.
Despite the significant toxicity of Shiga toxin to the endothelium, during outbreaks of HUS caused by E Coli only a small percentage of exposed individuals end of developing HUS. This may be partially explained by virulence factors such as inoculum size, or host factors such as age, gastric acidity, immune status, antibiotic use and immune response47. However, the underlying health of the endothelium likely has a major role. This could explain why subjects with underlying defects in complement regulation who develop TMA after exposure to Shiga toxin appear to develop particularly severe disease48 It may also explain why mice deficient in ADAMTS13 do not develop TMA unless they are stimulated to do so with shiga toxin, epinephrine, or collagen 49, 50.
Summary
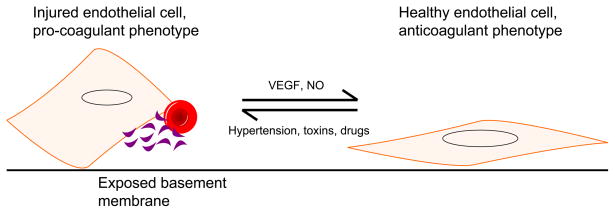
We therefore propose that the underlying health of the endothelium may have a large role in governing the manifestations of disease following insult to the endothelium (Figure 2). When considered this way, treatments aimed at improving endothelial function might be useful in protecting individuals from developing TMA from both exogenous and hereditary mechanisms.
Figure 2.
Pathways to endothelial cell injury. Abbreviations: NO, nitric oxide; VEGF, vascular endothelial growth factor
Table 2 shows agents that are known to improve endothelial function and theoretically could have a role in preventing TMA. For example, statins, in addition to their lipid lowering qualities, increase the expression of endothelial NO and decrease the inflammatory and thrombogenic potential of endothelial cells51–53. Angiotensin converting enzyme inhibitors and to a lesser extent, angiotensin receptor blockers, also increase endothelial NO. Xanthine oxidase inhibitors have also been shown to improve endothelial function in a number of clinical trials54,55. We propose future studies, particularly in subjects with hereditary causes of TMA, to see if the use of these agents may be able to reduce the episodes of TMA.
Table 2.
Factors thought to be supportive of endothelial cell health or those thought to trigger endothelial cell dysfunction and thrombosis
| Factors associated with anticoagulation and endothelial cell health | Factors associated with procoagulation and endothelial cell dysfunction |
|---|---|
| Statins51–53 | Complement mutations |
| Angiotensin converting enzyme inhibitors, angiotensin receptor blockers56, 57 | ADAMTS13 related |
| Polyphenols58, 59 | Pregnancy, increased estrogen |
| Ascorbic acid60 | Decreased NO |
| Allopurinol61–63 | Drugs, toxins |
Acknowledgments
Supported in part by NIH DK-52121 (RJJ, TN) DK-076690 (JMT)
Footnotes
Disclosure:
Ryan J. Goldberg-none
Takahiko Nakagawa-none
Richard J. Johnson has patents on the use of VEGF to treat thrombotic microangiopathy and also to treat preeclampsia.
Joshua M. Thurman is a stockholder in and consultant for Taligen Therapeutics, Inc.
References
- 1.Bitzan M. Treatment options for HUS secondary to Escherichia coli O157:H7. Kidney Int Suppl. 2009 Feb;(112):S62–66. doi: 10.1038/ki.2008.624. [DOI] [PubMed] [Google Scholar]
- 2.George JN. The thrombotic thrombocytopenic purpura and hemolytic uremic syndromes: evaluation, management, and long-term outcomes experience of the Oklahoma TTP-HUS Registry, 1989–2007. Kidney Int Suppl. 2009 Feb;(112):S52–54. doi: 10.1038/ki.2008.622. [DOI] [PubMed] [Google Scholar]
- 3.Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009 Oct 22;361(17):1676–1687. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez-Corral P, Perez-Caballero D, Huarte O, et al. Structural and functional characterization of factor H mutations associated with atypical hemolytic uremic syndrome. Am J Hum Genet. 2002 Dec;71(6):1285–1295. doi: 10.1086/344515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noris M, Bucchioni S, Galbusera M, et al. Complement factor H mutation in familial thrombotic thrombocytopenic purpura with ADAMTS13 deficiency and renal involvement. J Am Soc Nephrol. 2005 May;16(5):1177–1183. doi: 10.1681/ASN.2005010086. [DOI] [PubMed] [Google Scholar]
- 6.Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008 Mar 13;358(11):1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsushita K, Morrell CN, Cambien B, et al. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell. 2003 Oct 17;115(2):139–150. doi: 10.1016/s0092-8674(03)00803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakayama TSW, Yoshimura A, Zhang L, Kosugi T, Campbell-Thompson M, Kojima H, Croker BP, Nakagawa T. Endothelial von Willebrand Factor release due to eNOS deficiency predisposes to thrombotic microangiopathy in mouse aging kidney. Am J Pathol. 2010 doi: 10.2353/ajpath.2010.090316. (In press.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Germain AM, Romanik MC, Guerra I, et al. Endothelial dysfunction: a link among preeclampsia, recurrent pregnancy loss, and future cardiovascular events? Hypertension. Jan. 2007;49(1):90–95. doi: 10.1161/01.HYP.0000251522.18094.d4. [DOI] [PubMed] [Google Scholar]
- 10.Foster RR. The importance of cellular VEGF bioactivity in the development of glomerular disease. Nephron Exp Nephrol. 2009;113(1):e8–e15. doi: 10.1159/000228078. [DOI] [PubMed] [Google Scholar]
- 11.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003 Mar;111(5):649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006 Jun;12(6):642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 13.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005 Apr 6;293(13):1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 14.Eremina V, Sood M, Haigh J, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003 Mar;111(5):707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delvaeye M, Noris M, De Vriese A, et al. Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N Engl J Med. 2009 Jul 23;361(4):345–357. doi: 10.1056/NEJMoa0810739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dran GI, Fernandez GC, Rubel CJ, et al. Protective role of nitric oxide in mice with Shiga toxin-induced hemolytic uremic syndrome. Kidney Int. 2002 Oct;62(4):1338–1348. doi: 10.1111/j.1523-1755.2002.kid554.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim YG, Suga SI, Kang DH, et al. Vascular endothelial growth factor accelerates renal recovery in experimental thrombotic microangiopathy. Kidney Int. 2000 Dec;58(6):2390–2399. doi: 10.1046/j.1523-1755.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- 18.Suga S, Kim YG, Joly A, et al. Vascular endothelial growth factor (VEGF121) protects rats from renal infarction in thrombotic microangiopathy. Kidney Int. 2001 Oct;60(4):1297–1308. doi: 10.1046/j.1523-1755.2001.00935.x. [DOI] [PubMed] [Google Scholar]
- 19.van den Born BJ, van der Hoeven NV, Groot E, et al. Association between thrombotic microangiopathy and reduced ADAMTS13 activity in malignant hypertension. Hypertension. 2008 Apr;51(4):862–866. doi: 10.1161/HYPERTENSIONAHA.107.103127. [DOI] [PubMed] [Google Scholar]
- 20.Patterson ME, Mouton CR, Mullins JJ, Mitchell KD. Interactive effects of superoxide anion and nitric oxide on blood pressure and renal hemodynamics in transgenic rats with inducible malignant hypertension. Am J Physiol Renal Physiol. 2005 Oct;289(4):F754–759. doi: 10.1152/ajprenal.00419.2004. [DOI] [PubMed] [Google Scholar]
- 21.Zhou XJ, Vaziri ND, Wang XQ, Silva FG, Laszik Z. Nitric oxide synthase expression in hypertension induced by inhibition of glutathione synthase. J Pharmacol Exp Ther. 2002 Mar;300(3):762–767. doi: 10.1124/jpet.300.3.762. [DOI] [PubMed] [Google Scholar]
- 22.Stirling Y, Woolf L, North WR, Seghatchian MJ, Meade TW. Haemostasis in normal pregnancy. Thromb Haemost. 1984 Oct 31;52(2):176–182. [PubMed] [Google Scholar]
- 23.Brenner B. Haemostatic changes in pregnancy. Thromb Res. 2004;114(5–6):409–414. doi: 10.1016/j.thromres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 24.George JN. The association of pregnancy with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Curr Opin Hematol. 2003 Sep;10(5):339–344. doi: 10.1097/00062752-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 25.de Moerloose P, Mermillod N, Amiral J, Reber G. Thrombomodulin levels during normal pregnancy, at delivery and in the postpartum: comparison with tissue-type plasminogen activator and plasminogen activator inhibitor-1. Thromb Haemost. 1998 Mar;79(3):554–556. [PubMed] [Google Scholar]
- 26.Fakhouri F, Roumenina L, Provot F, et al. Pregnancy-associated hemolytic uremic syndrome revisited in the era of complement gene mutations. J Am Soc Nephrol. May;21(5):859–867. doi: 10.1681/ASN.2009070706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kemmeren JM, Algra A, Meijers JC, et al. Effect of second-and third-generation oral contraceptives on the protein C system in the absence or presence of the factor VLeiden mutation: a randomized trial. Blood. 2004 Feb 1;103(3):927–933. doi: 10.1182/blood-2003-04-1285. [DOI] [PubMed] [Google Scholar]
- 28.Hauglustaine D, Vanrenterghem Y, Michielsen OP, van Damme B. Oestrogen containing oral contraceptives, decreased prostacyclin production, and haemolytic uraemic syndrome. Lancet. 1981 Feb 7;1(8215):328–329. doi: 10.1016/s0140-6736(81)91943-7. [DOI] [PubMed] [Google Scholar]
- 29.Hauglustaine D, Van Damme B, Vanrenterghem Y, Michielsen P. Recurrent hemolytic uremic syndrome during oral contraception. Clin Nephrol. 1981 Mar;15(3):148–153. [PubMed] [Google Scholar]
- 30.Tolbert T, Oparil S. Cardiovascular effects of estrogen. Am J Hypertens. 2001 Jun;14(6 Pt 2):186S–193S. doi: 10.1016/s0895-7061(01)02087-8. [DOI] [PubMed] [Google Scholar]
- 31.Bossi F, Bulla R, Tedesco F. Endothelial cells are a target of both complement and kinin system. Int Immunopharmacol. 2008 Feb;8(2):143–147. doi: 10.1016/j.intimp.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Platt JL, Dalmasso AP, Lindman BJ, Ihrcke NS, Bach FH. The role of C5a and antibody in the release of heparan sulfate from endothelial cells. Eur J Immunol. 1991 Nov;21(11):2887–2890. doi: 10.1002/eji.1830211135. [DOI] [PubMed] [Google Scholar]
- 33.Ponticelli C, Banfi G. Thrombotic microangiopathy after kidney transplantation. Transpl Int. 2006 Oct;19(10):789–794. doi: 10.1111/j.1432-2277.2006.00354.x. [DOI] [PubMed] [Google Scholar]
- 34.Brown Z, Neild GH. Cyclosporine inhibits prostacyclin production by cultured human endothelial cells. Transplant Proc. 1987 Feb;19(1 Pt 2):1178–1180. [PubMed] [Google Scholar]
- 35.Garcia-Maldonado M, Kaufman CE, Comp PC. Decrease in endothelial cell-dependent protein C activation induced by thrombomodulin by treatment with cyclosporine. Transplantation. 1991 Mar;51(3):701–705. doi: 10.1097/00007890-199103000-00030. [DOI] [PubMed] [Google Scholar]
- 36.Burke GW, Ciancio G, Cirocco R, et al. Microangiopathy in kidney and simultaneous pancreas/kidney recipients treated with tacrolimus: evidence of endothelin and cytokine involvement. Transplantation. 1999 Nov 15;68(9):1336–1342. doi: 10.1097/00007890-199911150-00020. [DOI] [PubMed] [Google Scholar]
- 37.Burdmann EA, Andoh TF, Yu L, Bennett WM. Cyclosporine nephrotoxicity. Semin Nephrol. 2003 Sep;23(5):465–476. doi: 10.1016/s0270-9295(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 38.Goldberg RJRB, Amura C, Ferreira VP, Cortes C, Pangburn MK, Tomlinson S, Holers M. Cyclosporine Disrupts Complement Regulation on Endothelial Cells. Paper presented at: American Society of Nephrology; 2009; San Diego, CA. [Google Scholar]
- 39.Fortin MC, Raymond MA, Madore F, et al. Increased risk of thrombotic microangiopathy in patients receiving a cyclosporin-sirolimus combination. Am J Transplant. 2004 Jun;4(6):946–952. doi: 10.1111/j.1600-6143.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- 40.Valavaara R, Nordman E. Renal complications of mitomycin C therapy with special reference to the total dose. Cancer. 1985 Jan 1;55(1):47–50. doi: 10.1002/1097-0142(19850101)55:1<47::aid-cncr2820550108>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 41.Proia ADHE, Silberman HR. Mitomycin-induced hemolytic uremic syndrome. Arch Pathol Lab Med. 1984;108(12):959–962. [PubMed] [Google Scholar]
- 42.Maguire RB, Stroncek DF, Campbell AC. Recurrent pancytopenia, coagulopathy, and renal failure associated with multiple quinine-dependent antibodies. Ann Intern Med. 1993 Aug 1;119(3):215–217. doi: 10.7326/0003-4819-119-3-199308010-00006. [DOI] [PubMed] [Google Scholar]
- 43.Cantrell JE, Jr, Phillips TM, Schein PS. Carcinoma-associated hemolytic-uremic syndrome: a complication of mitomycin C chemotherapy. J Clin Oncol. 1985 May;3(5):723–734. doi: 10.1200/JCO.1985.3.5.723. [DOI] [PubMed] [Google Scholar]
- 44.Zheng XL, Sadler JE. Pathogenesis of thrombotic microangiopathies. Annu Rev Pathol. 2008;3:249–277. doi: 10.1146/annurev.pathmechdis.3.121806.154311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orth D, Khan AB, Naim A, et al. Shiga toxin activates complement and binds factor H: evidence for an active role of complement in hemolytic uremic syndrome. J Immunol. 2009 May 15;182(10):6394–6400. doi: 10.4049/jimmunol.0900151. [DOI] [PubMed] [Google Scholar]
- 46.Thurman JM, Marians R, Emlen W, et al. Alternative Pathway of Complement in Children with Diarrhea-Associated Hemolytic Uremic Syndrome. Clin J Am Soc Nephrol. 2009 Oct 9; doi: 10.2215/CJN.02730409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karmali MA. Host and pathogen determinants of verocytotoxin-producing Escherichia coli-associated hemolytic uremic syndrome. Kidney Int Suppl. 2009 Feb;(112):S4–7. doi: 10.1038/ki.2008.608. [DOI] [PubMed] [Google Scholar]
- 48.Fang CJ, Fremeaux-Bacchi V, Liszewski MK, et al. Membrane cofactor protein mutations in atypical hemolytic uremic syndrome (aHUS), fatal Stx-HUS, C3 glomerulonephritis, and the HELLP syndrome. Blood. 2008 Jan 15;111(2):624–632. doi: 10.1182/blood-2007-04-084533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Banno F, Kokame K, Okuda T, et al. Complete deficiency in ADAMTS13 is prothrombotic, but it alone is not sufficient to cause thrombotic thrombocytopenic purpura. Blood. 2006 Apr 15;107(8):3161–3166. doi: 10.1182/blood-2005-07-2765. [DOI] [PubMed] [Google Scholar]
- 50.Motto DG, Chauhan AK, Zhu G, et al. Shigatoxin triggers thrombotic thrombocytopenic purpura in genetically susceptible ADAMTS13-deficient mice. J Clin Invest. 2005 Oct;115(10):2752–2761. doi: 10.1172/JCI26007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.John S, Schlaich M, Langenfeld M, et al. Increased bioavailability of nitric oxide after lipid-lowering therapy in hypercholesterolemic patients: a randomized, placebo-controlled, double-blind study. Circulation. 1998 Jul 21;98(3):211–216. doi: 10.1161/01.cir.98.3.211. [DOI] [PubMed] [Google Scholar]
- 52.Blanco-Colio LM, Tunon J, Martin-Ventura JL, Egido J. Anti-inflammatory and immunomodulatory effects of statins. Kidney Int. 2003 Jan;63(1):12–23. doi: 10.1046/j.1523-1755.2003.00744.x. [DOI] [PubMed] [Google Scholar]
- 53.O’Driscoll G, Green D, Taylor RR. Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation. 1997 Mar 4;95(5):1126–1131. doi: 10.1161/01.cir.95.5.1126. [DOI] [PubMed] [Google Scholar]
- 54.Doehner W, Schoene N, Rauchhaus M, et al. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation. 2002 Jun 4;105(22):2619–2624. doi: 10.1161/01.cir.0000017502.58595.ed. [DOI] [PubMed] [Google Scholar]
- 55.Mercuro G, Vitale C, Cerquetani E, et al. Effect of hyperuricemia upon endothelial function in patients at increased cardiovascular risk. Am J Cardiol. 2004 Oct 1;94(7):932–935. doi: 10.1016/j.amjcard.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 56.Koh KK, Bui MN, Hathaway L, et al. Mechanism by which quinapril improves vascular function in coronary artery disease. Am J Cardiol. 1999 Feb 1;83(3):327–331. doi: 10.1016/s0002-9149(98)00862-5. [DOI] [PubMed] [Google Scholar]
- 57.Koh KK, Quon MJ, Han SH, Chung WJ, Lee Y, Shin EK. Anti-inflammatory and metabolic effects of candesartan in hypertensive patients. Int J Cardiol. 2006 Mar 22;108(1):96–100. doi: 10.1016/j.ijcard.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 58.Nagaya N, Yamamoto H, Uematsu M, et al. Green tea reverses endothelial dysfunction in healthy smokers. Heart. 2004 Dec;90(12):1485–1486. doi: 10.1136/hrt.2003.026740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vita JA. Polyphenols and cardiovascular disease: effects on endothelial and platelet function. Am J Clin Nutr. 2005 Jan;81(1 Suppl):292S–297S. doi: 10.1093/ajcn/81.1.292S. [DOI] [PubMed] [Google Scholar]
- 60.Beckman JA, Goldfine AB, Gordon MB, Creager MA. Ascorbate restores endothelium-dependent vasodilation impaired by acute hyperglycemia in humans. Circulation. 2001 Mar 27;103(12):1618–1623. doi: 10.1161/01.cir.103.12.1618. [DOI] [PubMed] [Google Scholar]
- 61.Yiginer O, Ozcelik F, Inanc T, et al. Allopurinol improves endothelial function and reduces oxidant-inflammatory enzyme of myeloperoxidase in metabolic syndrome. Clin Res Cardiol. 2008 May;97(5):334–340. doi: 10.1007/s00392-007-0636-3. [DOI] [PubMed] [Google Scholar]
- 62.Butler R, Morris AD, Belch JJ, Hill A, Struthers AD. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension. 2000 Mar;35(3):746–751. doi: 10.1161/01.hyp.35.3.746. [DOI] [PubMed] [Google Scholar]
- 63.George J, Carr E, Davies J, Belch JJ, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006 Dec 5;114(23):2508–2516. doi: 10.1161/CIRCULATIONAHA.106.651117. [DOI] [PubMed] [Google Scholar]