Abstract
BACKGROUND
Pulmonary function tests (PFTs) predict respiratory complications and mortality after lung resection via thoracotomy. We sought to determine the impact of PFTs upon complications after thoracoscopic lobectomy.
METHODS
A model for morbidity including published preoperative risk factors and surgical approach was developed by multivariable logistic regression. All patients who underwent lobectomy for primary lung cancer between December, 1999 and October, 2007 with preoperative forced expiratory volume in 1 second (FEV1) or diffusion capacity to carbon monoxide (DLCO) ≤ 60% predicted were reviewed. Preoperative, histopathologic, perioperative, and outcome variables were assessed using standard descriptive statistics. Pulmonary complications were defined as atelectasis requiring bronchoscopy, pneumonia, reintubation, and tracheostomy.
RESULTS
During the study period, 340 patients (median age 67) with DLCO or FEV1 ≤ 60% (mean % predicted FEV1 55±1, mean % predicted DLCO 61±1) underwent lobectomy (173 thoracoscopy, 167 thoracotomy). Operative mortality was 5% (17 patients) and overall morbidity was 48% (164 patients). At least one pulmonary complication occurred in 57 patients (17%). Significant predictors of pulmonary complications by multivariable analysis for all patients included DLCO (odds ratio 1.03, p=0.003), FEV1 (odds ratio 1.04, p=0.003) and thoracotomy as surgical approach (odds ratio 3.46,p=0.0007). When patients were analyzed according to operative approach, DLCO and FEV1 remained significant predictors of pulmonary morbidity for patients undergoing thoracotomy but not thoracoscopy.
CONCLUSIONS
In patients with impaired pulmonary function, preoperative pulmonary function tests are predictors of pulmonary complications when lobectomy for lung cancer is performed via thoracotomy but not via thoracoscopy.
Keywords: Lobectomy, Lung Cancer Surgery, Thoracoscopy, Outcomes
Introduction
Lobectomy is considered the oncologically optimal treatment for patients with early-stage non–small cell lung cancer [1-2]. Pre-operative pulmonary function tests (PFTs) are used to assess risk of complications for lobectomy [3-8]. The most useful PFT parameters in assessing risk of post-operative morbidity, pulmonary complications, respiratory failure, and death are the forced expiratory volume in one second (FEV1) and the diffusion capacity to carbon monoxide (DLCO) [9-20]. The risk of complications after lung resection has been shown to be inversely related to both FEV1 and DLCO, with complication rates increasing with lower values of both FEV1 and DLCO [19,21].
Studies that have established the relationship between PFTs and lung resection morbidity have for the most part included only patients who had undergone lung resection via thoracotomy. Several reports have shown that thoracoscopic lobectomy is associated with lower overall complication rates compared to thoracotomy [22-26]. Small series of thoracoscopic pulmonary resections in patients with poor lung function have demonstrated low morbidity and mortality [27,28]. Poor pulmonary function may not be as powerful in predicting pulmonary complications after thoracoscopic lobectomy compared to thoracotomy. The purpose of this study was to test the hypothesis that a thoracoscopic approach to lobectomy nullifies the prognostic impact of preoperative PFTs.
Patients and Methods
After local Institutional Review Board approval was granted, including waiver of the need for patient consent, the Duke University Medical Center Data Center was queried for Current Procedural Terminology codes linked with pulmonary resection by either an open approach or by a thoracoscopic approach between December 1999 and October 2007. Eligibility for this study was restricted to patients who underwent anatomic lobectomy for lung cancer with pulmonary function testing demonstrating either preoperative forced expiratory volume in 1 second (FEV1) or diffusion capacity to carbon monoxide (DLCO) ≤ 60% predicted. The value of 60% predicted was chosen based on previous studies that have demonstrated that patients with FEV1 and DLCO less than this value have increased risk of postoperative complications [13,29].
Retrospective review of an institutional, prospective database maintained on all thoracic surgery patients documented and compared demographics, preoperative functional status, the use of induction therapy, smoking history, significant comorbidities, the histology and stage of disease, intraoperative details, and postoperative course. Chart review was utilized as necessary to complete data collection. Preoperative performance status was based on Zubrod, or Eastern Cooperative Oncology Group, scores that were included in the prospective database [30]. Any postoperative event prolonging or otherwise altering the postoperative course was recorded along with all operative deaths, which were defined as deaths that occurred within 30 days after operation or those that occurred later but during the same hospitalization. Deaths were captured both by chart review and use of the Social Security Death Index Database. The definitions of postoperative events were based on the Society of Thoracic Surgeons General Thoracic Surgery Database [31]. Pulmonary complications were defined as atelectasis requiring bronchoscopy, pneumonia, reintubation, and tracheostomy. Overall morbidity was defined as the occurrence of at least one postoperative event, and pulmonary morbidity was defined as the occurrence of at least one of the defined pulmonary complications.
Thoracoscopic lobectomy was performed without any rib spreading with the thoracoscope placed in the 8th intercostal space in the midaxillary line and a 4-5cm anterior utility incision in the 5th intercostal space [32]. Thoracotomy in most patients was via standard posterolateral approach with sparing of the serratus muscle; one surgeon (34 of 167 cases) utilized an approach where both the latissimus and serratus muscles were spared and notching of the sixth rib was performed. Patients who initially underwent attempted thoracoscopic lobectomy and then were converted to thoracotomy were considered in the statistical analysis to have had a thoracotomy approach for lobectomy. An epidural catheter for postoperative pain relief was offered to all patients regardless of planned operative approach. Postoperative epidural management was conducted by an anesthesia-run pain service in similar fashion for all patients regardless of operative approach. Chest tubes were routinely placed on water seal immediately postoperatively and removed when no air leak was present and drainage over 24 hours was less than 200 cc.
Univariate analyses were performed on all patients relating pulmonary morbidity to the following patient variables: age, history of diabetes, pack-years, pre-operative chemotherapy, pre-operative radiation, previous thoracic surgery, pathologic stage, surgeon, preoperative pulmonary function tests (forced vital capacity (FVC), FEV1, and DLCO), and surgical approach. Unpaired student’s t tests were used to compare continuous data, Fisher’s exact tests for dichotomous data, and χ2 for categoric variables. A two-tailed p value of less than 0.05 was considered significant. The variables that were significant at P < .20 were entered into a multivariable logistic regression with pulmonary morbidity as the dependent variable and significance set at the 0.05 level. This multivariable logistic regression model was repeated separately on the patients by operative approach. Data are presented as mean ± standard error of the mean unless otherwise noted. The SAS 9.2 statistical package (SAS Institute, Cary, North Carolina) was used for statistical analyses.
Results
Lobectomy was performed for lung cancer in 943 patients overall during the study period; 340 of these patients (median age of 67) had either DLCO or FEV1 ≤ 60% (mean % predicted FEV1 55±1, mean % predicted DLCO 61±1). Of these 340 patients, 167 patients had lobectomy via thoracotomy and 173 patients via thoracoscopy. Eight patients who underwent an initial attempt at lobectomy via thoracoscopy (4.4% of all patients who underwent attempted thoracoscopic lobectomy) required conversion to thoracotomy to complete the lobectomy; six conversions were due to bleeding and two were due to adhesions. Demographic, baseline characteristics, pathologic cancer stage, and comorbid conditions are shown in Table 1.
Table 1.
Demographics, Baseline Characteristics, and Comorbid Conditions.
| Characteristic | All (n=340) | Thoracotomy (n=167) |
Thoracoscopy (n=173) |
p |
|---|---|---|---|---|
| Age | 66±1 | 64±1 | 68±1 | 0.001 |
|
| ||||
| Tobacco abuse | 320 (94%) | 160 (96%) | 160 (92%) | 0.3 |
|
| ||||
| Pack years | 54±2 | 52±2 | 55±3 | 0.3 |
|
| ||||
| Smoking within 14 days | 57 (17%) | 27 (16%) | 30 (17%) | 0.9 |
|
| ||||
| Previous Thoracic Surgery | 89 (26%) | 55 (33%) | 34 (20%) | 0.01 |
|
| ||||
| Preoperative Weight Loss | 39 (11%) | 26 (16%) | 13 (8%) | 0.03 |
|
| ||||
| Zubrod Status 0 or 1 | 310 (91%) | 154 (92%) | 156 (90%) | 0.6 |
|
| ||||
| Forced Expiratory Volume in one second (% predicted) |
55±1 | 56±1 | 54±1 | 0.3 |
|
| ||||
| Diffusing Capacity of the Lung for Carbon Monoxide (% predicted) |
61±1 | 62±1 | 60±1 | 0.5 |
|
| ||||
| American Society of Anesthesiology Score | 2.6±0.1 | 2.6±0.1 | 2.6±0.1 | 0.5 |
|
| ||||
| Pre-operative Chemotherapy | 54 (16%) | 44 (26%) | 10 (6%) | <0.0001 |
|
| ||||
| Pre-operative Radiation | 50 (15%) | 39 (23%) | 11 (6%) | <0.0001 |
|
| ||||
| Hypertension | 169 (50%) | 82 (49%) | 87 (50%) | 0.8 |
|
| ||||
| Chronic Obstructive Pulmonary Disease | 154(45%) | 72 (43%) | 82 (47%) | 0.4 |
|
| ||||
| Coronary Artery Disease | 69 (20%) | 27 (12%) | 42 (24%) | 0.08 |
|
| ||||
| Diabetes | 56 (16%) | 30 (18%) | 26 (15%) | 0.6 |
|
| ||||
| Cerebrovascular Disease | 36 (10%) | 20 (12%) | 16 (9%) | 0.5 |
|
| ||||
| Stroke | 11 (3%) | 5 (3%) | 6 (3%) | 1 |
|
| ||||
| Peripheral Vascular Disease | 31 (9%) | 12 (7%) | 19 (11%) | 0.3 |
|
| ||||
| Renal Insufficiency | 18 (5%) | 10 (3%) | 8 (5%) | 0.6 |
|
| ||||
| Congestive Heart Failure | 20 (6%) | 5 (3%) | 15 (9%) | 0.04 |
|
| ||||
| Steroid Use | 18 (5%) | 10 (6%) | 8 (5%) | 0.6 |
|
| ||||
| Pathologic Stage | ||||
| Stage IA | 98 (29%) | 25 (15%) | 73 (42%) | <0.0001 |
| Stage IB | 120 (35%) | 54 (32%) | 66 (38%) | 0.3 |
| Stage IIA | 13 (4%) | 7 (5%) | 6 (3%) | 0.8 |
| Stage IIB | 38 (11%) | 28 (17%) | 10 (6%) | 0.002 |
| Stage IIIA | 35 (10%) | 29 (17%) | 6 (3%) | <0.0001 |
| Stage IIIB | 28 (8%) | 17 (10%) | 11 (6%) | 0.2 |
| Stage IV | 8 (2%) | 7 (4%) | 1 (1%) | 0.03 |
Overall operative mortality was 5% (17 patients) and overall morbidity was 48% (164 patients). The median chest tube duration for all patients was 4 days, and the median hospitalization was 5 days. Postoperative events are listed in Table 2. The most common non-pulmonary complications in the entire group were need for chest tube more than 5 days (76 patients, 22%), atrial arrhythmia (67 patients, 20%), post-operative transfusion (44 patients, 13%), and delirium/mental status changes (23 patients, 7%). At least one pulmonary complication occurred in 57 patients (17%). Individual pulmonary complications were atelectasis requiring bronchoscopy (42 patients, 12%), pneumonia (40 patients, 12%), reintubation (18 patients, 5%), and tracheostomy (6 patients, 2%).
Table 2.
Postoperative Events.
| Event | All (n=340) | Thoracotomy (n=167) |
Thoracoscopy (n=173) |
p |
|---|---|---|---|---|
| Thirty-day Mortality | 17 (5%) | 11 (7%) | 6 (3%) | 0.2 |
| Thirty-day Morbidity | 164 (48%) | 96 (57%) | 68 (39%) | 0.001 |
| Hospital Stay (median) | 5 | 6 | 4 | 0.2 |
| Chest Tube Duration (median) | 4 | 4 | 3 | 0.8 |
| Any Pulmonary Complication | 57 (17%) | 36 (22%) | 21 (12%) | 0.03 |
| Need for chest tube > 5 days | 76 (22%) | 39 (23%) | 37 (21%) | 0.7 |
| Atrial arrhythmia | 67 (20%) | 42 (25%) | 25 (14%) | 0.01 |
| Post-operative transfusion | 44 (13%) | 30 (18%) | 14 (8%) | 0.009 |
| Post-operative bronchoscopy | 42 (12%) | 28 (17%) | 14 (8%) | 0.02 |
| Pneumonia | 40 (12%) | 25 (15%) | 15 (9%) | 0.09 |
| Delirium/Mental Status Changes | 23 (7%) | 17 (10%) | 6 (3%) | 0.02 |
| Unplanned reintubation | 18 (5%) | 10 (6%) | 8 (5%) | 0.6 |
| New Renal Insufficiency | 15 (4%) | 11 (7%) | 4 (2%) | 0.06 |
| Need for enteral nutrition tube | 10 (3%) | 4 (2%) | 6 (4%) | 0.8 |
| Wound Infection/Empyema | 9 (3%) | 7 (4%) | 2 (1%) | 0.1 |
| Need for tracheostomy | 6 (2%) | 4 (2%) | 2 (1%) | 0.4 |
| Re-operation for decortication | 5 (1%) | 3 (2%) | 2 (1%) | 0.7 |
| Re-operation for bleeding | 5 (1%) | 4 (2%) | 1 (0.6%) | 0.2 |
| Vocal cord paralysis | 2 (0.6%) | 1 (0.6%) | 1 (0.6%) | 1 |
| Myocardial infarction | 2 (0.6%) | 1 (0.6%) | 1 (0.6%) | 1 |
| Pulmonary embolism | 2 (0.6%) | 2 (1%) | 0 | 0.2 |
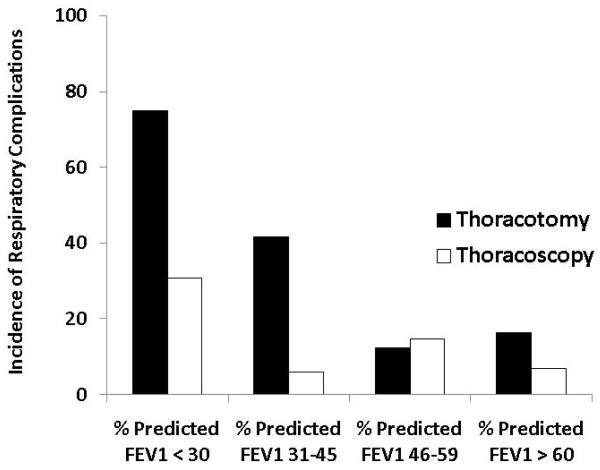
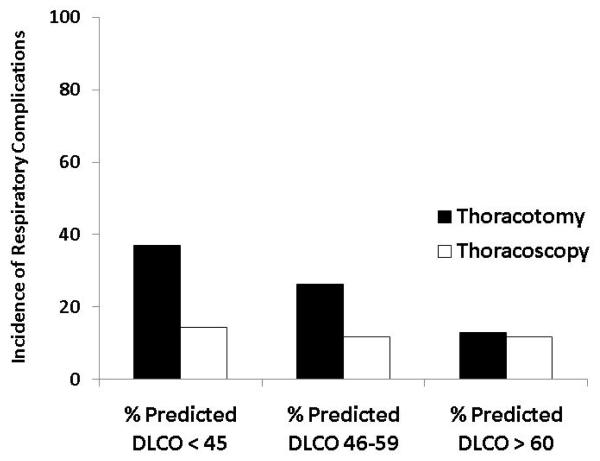
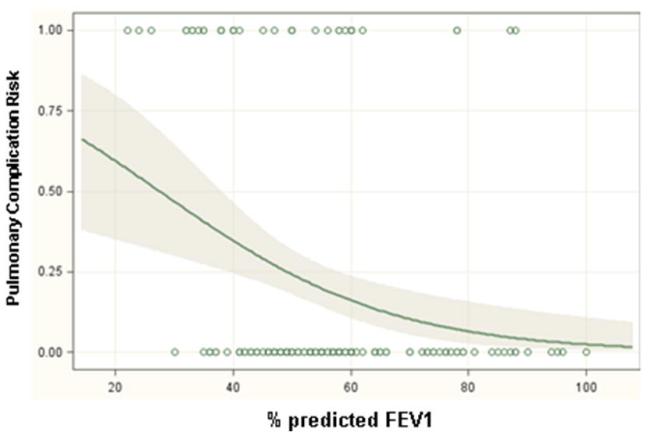
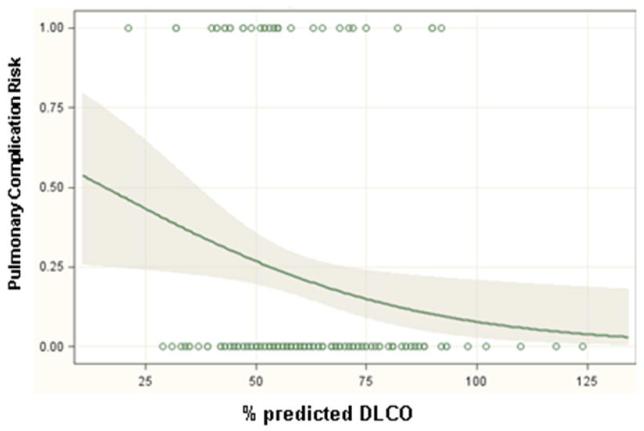
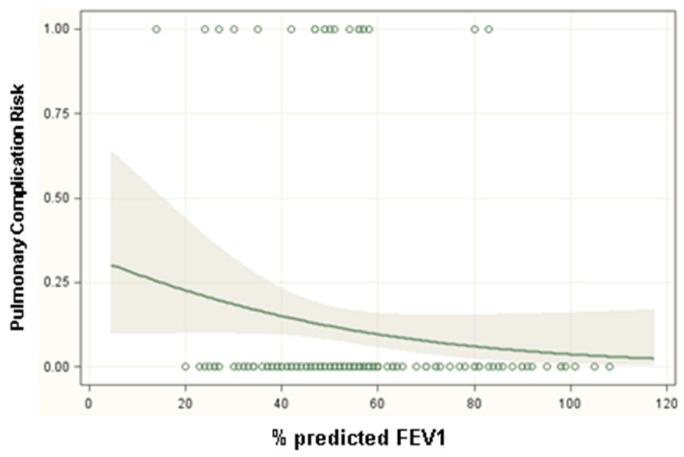
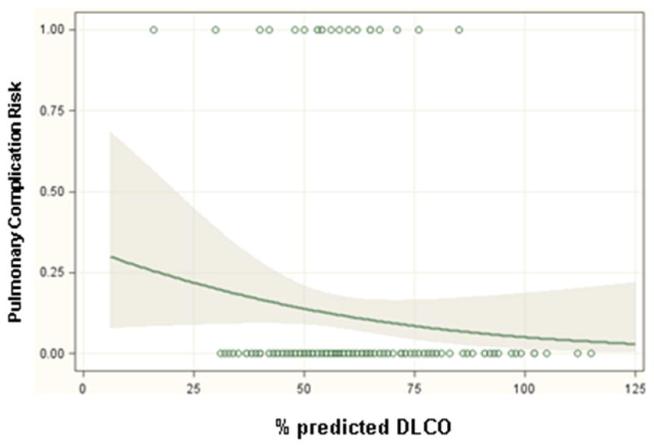
Significant predictors of respiratory complications by multivariable analysis for all patients included DLCO (odds ratio 1.03, p=0.01), FEV1 (odds ratio 1.04, p=0.005) and thoracotomy as surgical approach (odds ratio 3.2, p=0.001) (Table 3). When patients were analyzed separately according to operative approach, DLCO and FEV1 remained significant predictors of respiratory morbidity for patients undergoing thoracotomy but not thoracoscopy (Table 4). Table 5 and figure 1 demonstrate the incidence of pulmonary complications stratified by pre-operative FEV1 and DLCO. Figures 2 and 3 show regression lines for risk of pulmonary complications relative to preoperative % predicted DLCO and FEV1 for both thoracotomy and thoracoscopy approaches. These figures demonstrate that the risk of pulmonary complications was related to the degree of impairment for both FEV1 and DLCO for patients undergoing thoracotomy, but not for patients undergoing thoracoscopy.
Table 3.
Logistic Regression Model of risk factors for respiratory complications.
| Variable | All patients (n=340) | ||
|---|---|---|---|
| Odds Ratio | 95% CI | p | |
| Surgical Approach (Thoracotomy vs Thoracoscopy) | 3.2 | 1.6-6.5 | 0.001 |
| % predicted Forced Expiratory Volume in one second (1 point decrease) |
1.04 | 1.01-1.06 | 0.005 |
| % predicted Diffusing Capacity of the Lung for Carbon Monoxide (1 point decrease) |
1.03 | 1.01-1.05 | 0.01 |
| Diabetes | 2.6 | 0.82-7.93 | 0.10 |
| % predicted Forced Vital Capacity (1 point decrease) | 1.01 | 0.98-1.03 | 0.41 |
| Pre-operative Radiation Therapy | 1.22 | 0.52-2.91 | 0.6 |
Table 4.
Logistic Regression Model of risk factors for respiratory complications by operative approach.
| Variable | Thoracotomy | Thoracoscopy | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P | Odds Ratio | 95% CI | p | |
| % predicted Forced Expiratory Volume in one second (1 point decrease) |
1.05 | 1.01-1.09 | 0.006 | 1.02 | 0.98-1.06 | 0.38 |
| % predicted Diffusing Capacity of the Lung for Carbon Monoxide (1 point decrease) |
1.04 | 1.01-1.06 | 0.01 | 1.03 | 0.99-1.08 | 0.11 |
Table 5.
Incidence of respiratory complications as a function of preoperative pulmonary function.
| % predicted ≤30 |
% predicted 31-45 |
% predicted 46- 59 |
% predicted ≥60 | |||||
|---|---|---|---|---|---|---|---|---|
| N | Resp Comps |
n | Resp Comps |
n | Resp Comps |
n | Resp Comps |
|
| FEV1 | ||||||||
| All | 17 | 7 (41%) | 70 | 17 (24%) | 156 | 21 (13%) | 99 | 12 (12%) |
| Thoracotomy | 4 | 3 (75%) | 36 | 15 (42%) | 74 | 9 (12%) | 55 | 9 (16%) |
| Thoracoscopy | 13 | 4 (31%) | 34 | 2 (6%) | 82 | 12 (15%) | 44 | 3 (7%) |
| DLCO | ||||||||
| All | 4 | 3 (75%) | 51 | 11 (22%) | 125 | 23 (18%) | 162 | 20 (12%) |
| Thoracotomy | 2 | 1 (50%) | 25 | 9 (36%) | 57 | 15 (26%) | 85 | 11 (13%) |
| Thoracoscopy | 2 | 2 (100%) | 26 | 2 (8%) | 68 | 8 (12%) | 77 | 9 (12%) |
Figure 1.
Incidence of respiratory complications as a function of (a) preoperative FEV1 and (b) preoperative DLCO.
Figure 2.
Regression lines showing the predicted probability of pulmonary complications as a function of (a) preoperative % predicted FEV1 and (b) preoperative % predicted DLCO for patients undergoing lobectomy via thoracotomy. The shaded areas surrounding the regression line represent 95 percent confidence limits. The circles at the top and bottom of the plots show the actual observed occurrence (top plot line) or lack of occurrence (bottom plot line) of a pulmonary complication and the pulmonary function measurement for each patient in the study.
Figure 3.
Regression lines showing the predicted probability of pulmonary complications as a function of (a) preoperative % predicted FEV1 and (b) preoperative % predicted DLCO for patients undergoing lobectomy via thoracoscopy. The shaded areas surrounding the regression line represent 95 percent confidence limits. The circles at the top and bottom of the plots show the actual observed occurrence (top plot line) or lack of occurrence (bottom plot line) of a pulmonary complication and the pulmonary function measurement for each patient in the study.
Comment
Preoperative PFTs are probably the most important tool available to surgeons in evaluating the risk of patients under consideration for major lung resection [3-8]. FEV1 and DLCO are the PFT parameters most useful in assessing risk of post-operative morbidity, pulmonary complications, respiratory failure, and death, although there is no general agreement on the individual predictive value of the parameters or the limits beyond which lung resection should not be performed [9-20,33,34]. Although specific absolute pre-operative limitations for parameters such as FEV1 have been quoted for various procedures, the absolute numbers for PFTs are dependent on the patient’s body surface area, age, and gender. Therefore, the percent predicted value is much more useful when determining a patient’s ability to tolerate lung resection. Both pre-operative values and predicted post-operative values that take into account the planned extent of lung resection are useful in stratifying patients according to their risk of operative morbidity. Patients with pre-operative FEV1 and DLCO less than 60% predicted have been shown to have increased risk of postoperative complications [13,29]. Predicted post-operative values of less than 40 percent predicted for either FEV1 or DLCO also indicate that patients have increased risk of morbidity and mortality with surgery [15]. The predicted post-operative DLCO is probably the most useful parameter in assessing risk of post-operative morbidity, pulmonary complications, respiratory failure, and death [9-15].
However, the studies that have established the relationship between PFTs and the operative morbidity of lung resection involved patients who almost exclusively underwent thoracotomy to accomplish their pulmonary resection. Thoracoscopic lobectomy has been shown in several studies to be associated with lower overall complication rates compared to thoracotomy, though this approach is used in only 12-20% of lobectomies performed in the United States [22-26,35]. Several small series have suggested that lobectomies could be accomplished via a thoracoscopic approach in patients with impaired pulmonary function as indicated by a reduced FEV1 with outcomes similar to what would be expected in patients who did not have a low FEV1 [27,28]. These studies, though, have involved a small number of patients undergoing thoracoscopic lobectomy (19 patients or less) and did not attempt to correlate complications with PFT parameters.
This current study reviewed 340 patients undergoing lobectomy for lung cancer with pre-operative PFTs indicating impaired pulmonary function. Although the range of DLCO and FEV1 varied in the group of patients, all had at least one value which would suggest that they had an increased risk of complications after lung resection. Despite the patients’ impaired pulmonary function and significant comorbid conditions listed in Table 1, lobectomy was accomplished with acceptable overall morbidity and mortality (48% and 5%, respectively). The majority of patients did not experience a postoperative complication, and most complications were non-life threatening.
The analysis of our entire cohort demonstrated that, similar to what has been shown in several studies, the risk of pulmonary complications increased with lower values of DLCO and FEV1 [13,29]. However, we also found that surgical approach independently predicted the occurrence of pulmonary complications in this higher risk group. By examining patients separately by operative approach, we showed that PFTs predicted pulmonary complications for patients undergoing thoracotomy but not thoracoscopy. Although the results of this study are not able to explain the reasons for this observation, the difference likely results from both reduced pain and better preservation of chest wall mechanics in patients undergoing thoracoscopy that allows better pulmonary function immediately post-operatively [36,37]. Indeed, the observed FEV1 in the immediate postoperative period after lobectomy has recently been shown to be both significantly lower and much more predictive of cardiopulmonary complications than the predicted post-operative values [38,39].
The main strength of this study is that, compared to other studies that have attempted to correlate PFTs and the operative morbidity of lung resections, a significant number in this series underwent a minimally invasive approach, allowing assessment of its effect on outcomes. This allowed creation of a multivariable model of complications using previously published prognostic factors as well as an adequately-powered approach variable. The main limitations of this study are the retrospective nature and the fact that only patients at a single institution were examined. The procedures in this report were also performed by several different surgeons, which introduces potential confounders such as different selection criteria for surgery, surgical techniques, and postoperative management.
In conclusion, lobectomy can be performed safely for lung cancer in patients with impaired pulmonary function, with reduced morbidity when a thoracoscopic approach is used in these higher risk patients. PFTs do not have the same predictive ability for complications for patients with marginal lung function when they are undergoing thoracoscopy compared to thoracotomy. Patients with lung cancer and poor lung function often do not have a treatment modality available to them with equal efficacy as surgical resection. Given that the present study demonstrates that a thoracoscopic approach reduces morbidity, the survival advantages of lobectomy over lesser resection for lung cancer may be able to be conferred to patients with marginal lung function when this approach is utilized. When technically possible, we believe that thoracoscopy should be strongly considered as the approach of choice in these patients.
Discussion
11. Pulmonary Function Tests Do Not Predict
Pulmonary Complications after Thoracoscopic
Lobectomy. Paper presented by Mark F.
Berry, Durham, NC.
berry037@mc.duke.edu
Discussion by Mark J. Krasna, M.D.,
Maryland
markkrasna@catholichealth.net
Dr. M. Krasna (Towson, MD):
Dr. Mack, Dr. Cerfolio, members and guests.
First, I wish to congratulate Dr. Berry on an excellent presentation and thank him and his colleagues for sending me the manuscript well in advance. The title of his talk, PFTs Do Not Predict Pulmonary Complication, is almost scintillating. It hints that what we have here is a revolutionary concept that goes against years of thoracic surgical lore. We should not be surprised that even an old-fashioned bastion of conservative surgery, like Duke, would produce such a manuscript. His colleagues, led by Tommy D’Amico, have helped revolutionize how we treat patients with early stage lung cancer by instituting a standard, simplified version of the VATS lobectomy. Over the years, the use of VATS has led us to question other aspects of lung resection such as the need for routine chest X-rays by Dr. Tom Rice, labs by Dr. McKenna, chest tubes or use of suction by Dr. Cerfolio. It is with this overall reassessment of our approach to lung cancer that we should consider the current paper. On the surface, these data seem to fly in the face of the life’s work of two of our present members, Dr. Ferguson and President-Elect Naunheim, who have carefully documented the utility and the value of various PFTs in identifying patients who are at high risk for lung resection. So let’s look at the data.
Nine hundred forty-three patients were resected over eight years; 340 of these had low PFTs, defined as less than 50%, although there was one graph where you had a median of 55%; you will have to explain that later. I agree, by the way, that using the percent predicted is the best way to use the PFTs in this setting. Of these, roughly half the patients, about 167, had a standard thoracotomy and the rest had a VATS lobectomy.
The authors reported a 5% mortality, which seems a bit high, and an overall morbidity of 48%, which is to be expected with such a high-risk group. This leads to my first question. What was the mortality of the other 603 lobectomy patients who were low risk?
11. Pulmonary Function Tests Do Not Predict Pulmonary Complications after Thoracoscopic Lobectomy. Response by Mark F. Berry, Durham, NC.
DR. BERRY: I don’t know that number off the top of my head, but I think it is probably around 3%. But that is just a very rough guess.
DR. KRASNA: Okay. The length of stay and the chest tube duration were five and four days respectively, and, again, this was a bit high, but given the population we are talking about, this seems reasonable.
My other questions are as follows, and I guess I will ask them both and I will let you answer them both subsequently. Were the 10% of patients who were stage IIIA and IIIB predicted before resection, and, if so, did they undergo a VATS lobectomy after chemoradiation; was that in fact any factor when you did your multivariate analysis?
And finally, how do you explain the difference between the two groups? Is this simply an issue of less pain with less early splinting and atelectasis or are there actual changes in the mechanics that take place after a VATS lobectomy versus after a thoracotomy? If you think it is the latter, I would encourage you to study the data in a more detailed fashion or a prospective analysis using plethysmography or MRIs, as Dr. Cooper has done, to determine the answer. It also implies that we should be doing many more lobar rather than sub-lobar resections for these high-risk patients. So, by the way, Tommy, have you stopped using PFTs at Duke?
Thank you again for an excellent presentation and I thank the Association for the privilege of discussing this paper.
DR. BERRY: First, for the PFTs, we chose the value of 60% predicted for either DLCO or FEV1 so somebody could have a preserved DLCO and a low FEV1 or vice versa. That is proably why the means for these parameters are right around 50% to 60%.
Second, I do want to acknowledge all the work that has been done in establishing pulmonary function tests as the risk model for pulmonary resection. In fact, I think that our results support all that data. Our results for thoracotomy fall right in line with what has been published in the past in regards to complication risk and pulmonary function tests.
For the stage IIIA patients, the overwhelming majority of those patients had been identified pre-lobectomy as being stage IIIA and they received induction therapy. Earlier in the series, induction therapy was a relative contraindication to a VATS approach so there was a little bit of a skew such that a greater percentage of the patients undergoing thoracotomy having received preoperative chemotherapy and/or radiation. Induction therapy was not found to be a significant factor in our multivariable analysis, but it might be that the analysis was not sufficiently powered to be able to evaluate induction therapy as a risk factor.
Finally, in trying to speculate why we found a difference between the two approaches, I think the difference likely relates to something that somebody made a comment on in one of the earlier presentations today. I think there is less pain and better preservation of chest wall mechanics early in the postoperative procedure for patients who have a VATS procedure versus a thoracotomy. There have been a few studies from a group in Italy recently that has shown that, of all the pulmonary function tests that we use to try to assess operative risk, probably the most important one is the actual pulmonary function test parameters immediately postoperatively, which turns out to be much lower than what you would predict based on the amount of lung that has been removed. A patient’s ability to utilize the maximum of their pulmonary function might be limited by the morbidity secondary to their incision. That is my speculation of why we see different results between approaches, but we do not have data that actually supports that hypothesis.
DR. CERFOLIO: You are referring to Dr. Brunelli’s paper and we will talk about that in a minute.
11. Pulmonary Function Tests Do Not Predict Pulmonary Complications after Thoracoscopic Lobectomy. Paper presented by Mark F. Berry, Durham, NC. berry037@mc.duke.edu Discussion by Kevin D. Accola, M.D., Florida kaccola@cvsorlando.com Dr. K. Accola (Orlando, FL):
Thank you and one quick question. I know this was a retrospective review, but were these limited muscle-sparing thoracotomies or regular complete muscle-splitting thoracotomies? I think the thoracotomy technique and approach would have an impact on your proposed conclusions.
11. Pulmonary Function Tests Do Not Predict Pulmonary Complications after Thoracoscopic Lobectomy. Response by Mark F. Berry, Durham, NC.
DR. BERRY: For the thoracotomies, Approximately a third of the thoracotomies were completely muscle-sparing where both the latissimus and the serratus were spared with notching of a rib to allow performance of the procedure.
11. Pulmonary Function Tests Do Not Predict
Pulmonary Complications after Thoracoscopic
Lobectomy. Paper presented by Mark F.
Berry, Durham, NC.
berry037@mc.duke.edu
Discussion by Robert J. Cerfolio, M.D.,
Alabama
robert.cerfolio@ccc.uab.edu
Dr. R. Cerfolio (Birmingham, AL):
Are you sure of that, was the intercostal nerve spared? Was the rib not cut or notched, was it really a rib sparing, nerve sparing procedure with holes drilled in the lower rib?
11. Pulmonary Function Tests Do Not Predict Pulmonary Complications after Thoracoscopic Lobectomy. Response by Mark F. Berry, Durham, NC.
DR. BERRY: Drilling of the lower rib was done based on surgeon preference, and probably occurred in about half of the cases. Overall, about a third of the thoracotomies spared both muscles, and the latissimus was divided but the serratus was spared in about two-thirds of the thoracotomies. The serratus was spared in almost every thoracotomy.
11. Pulmonary Function Tests Do Not Predict
Pulmonary Complications after Thoracoscopic
Lobectomy. Paper presented by Mark F.
Berry, Durham, NC.
berry037@mc.duke.edu
Discussion by Todd L. Demmy, M.D.,
New York
todd.demmy@roswellpark.org
Dr. T. Demmy (Buffalo, NY):
I just want to compliment you on your paper and state that we are going to be presenting our data in Florida in February at the Academic Surgical Conference. We seem to have found similar results as yours. We reviewed 59 patients with diffusing capacity or FEV1 less than 40% who had good outcomes and a mortality rate less than 2%.
The one question I have for you is, what is the objective test that you use preoperatively to exclude a patient from surgery? We are using an MVO2 less than 10. What would you use?
11. Pulmonary Function Tests Do Not Predict Pulmonary Complications after Thoracoscopic Lobectomy. Response by Mark F. Berry, Durham, NC.
DR. BERRY: To answer this question and to comment on something that Dr. Krasna had said earlier, I will say that we have not given up on doing pulmonary function tests on patients at Duke. Everyone still gets them before resection.
The ultimate decision on whether somebody is an operative candidate is made as a combination of the pulmonary function tests and a more qualitative evaluation of the patient’s functional status, based on what they can do in just their general activities of daily living, which is probably a surrogate for looking at exercise testing as you said you do.
11. Pulmonary Function Tests Do Not Predict
Pulmonary Complications after Thoracoscopic
Lobectomy. Paper presented by Mark F.
Berry, Durham, NC.
berry037@mc.duke.edu
Further discussion by Robert J. Cerfolio,
M.D.,
Alabama
robert.cerfolio@ccc.uab.edu
Dr. R. Cerfolio (Birmingham, AL):
Mark, one last question from me. What percent of your patients had both the DLCO and the FEV1 less than 50%? Your mean FEV1 for the series was 61% which is surprising to me. So what percent of patients had both and did you look at the morbidity in that group of patients?
11. Pulmonary Function Tests Do Not Predict Pulmonary Complications after Thoracoscopic Lobectomy. Response by Mark F. Berry, Durham, NC.
DR. BERRY: I don’t know exactly, but it was probably about half the patients who had low values for both parameters.
DR. CERFOLIO: Did you do a sub-analysis of those patients?
DR. BERRY: We did not.
DR. CERFOLIO: I would encourage you to do that.
11. Pulmonary Function Tests Do Not Predict
Pulmonary Complications after Thoracoscopic
Lobectomy. Paper presented by Mark F.
Berry, Durham, NC.
berry037@mc.duke.edu
Discussion by Michael Mack, M.D.,
Texas
mmack@csant.com
Dr. M. Mack (Dallas, TX):
Dr. Cerfolio, now I am totally confused. I read a paper a couple of months ago by Dr. Rusch that finds that VATS lobectomy is good and then we hear in the last session it is bad and now we hear again it is good. Can you very briefly and concisely and articulately give us the bottom line about VATS lobectomy?
DR. CERFOLIO: I think VATS lobectomy is fine in the correct hands, and I think, no offense to the paper we heard earlier, but when you hear a nine-day length of stay for a VATS lobe and a nine-day length of stay for an open lobe, you know there is something wrong with that data. I think VATS lobectomy is fine when it is done in the right hands and if all the nodes are taken, which I think is the hardest part of that operation. However, if sampling lymph nodes is as good as removal as we are going to hear is the case from the national study coming out, then it may not matter. And then the next question is, is there a benefit for robotic lobectomy, and we will find out in the next few years.
11. Pulmonary Function Tests Do Not Predict
Pulmonary Complications after Thoracoscopic
Lobectomy. Paper presented by Mark F.
Berry, Durham, NC.
berry037@mc.duke.edu
Discussion by Daniel L. Miller, M.D.,
Georgia
daniel.miller@emoryhealthcare.org
Dr. D. Miller (Atlanta, GA):
If you think they’re so great, why don’t you do them?
DR. CERFOLIO: I do some, but I don’t think they are any better when a thoracotomy is done by someone who actually does a thoracotomy correctly. I think very few people do thoracotomies the way you and I do them, Dr. Miller, where very little muscle is cut, the nerve is spared, the rib is spared. So we do them, we do some VATS lobectomies, but I still prefer a rib-sparing, nerve-sparing, muscle-sparing thoracotomy. In fact, I have patients that ask for that over a VATS lobe.
Footnotes
To be presented at the Southern Thoracic Surgical Association’s 56th Annual Meeting, Marco Island, Florida, November 5, 2009
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ettinger DS, Bepler G, Bueno R, et al. Non-small cell lung cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2006;4:548–582. doi: 10.6004/jnccn.2006.0046. [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–622. doi: 10.1016/0003-4975(95)00537-u. discussion 622-613. [DOI] [PubMed] [Google Scholar]
- 3.Myrdal G, Gustafsson G, Lambe M, Horte LG, Stahle E. Outcome after lung cancer surgery. Factors predicting early mortality and major morbidity. Eur J Cardiothorac Surg. 2001;20:694–699. doi: 10.1016/s1010-7940(01)00875-2. [DOI] [PubMed] [Google Scholar]
- 4.Patel RL, Townsend ER, Fountain SW. Elective pneumonectomy: factors associated with morbidity and operative mortality. Ann Thorac Surg. 1992;54:84–88. doi: 10.1016/0003-4975(92)91145-y. [DOI] [PubMed] [Google Scholar]
- 5.Bernard A, Deschamps C, Allen MS, et al. Pneumonectomy for malignant disease: factors affecting early morbidity and mortality. J Thorac Cardiovasc Surg. 2001;121:1076–1082. doi: 10.1067/mtc.2001.114350. [DOI] [PubMed] [Google Scholar]
- 6.Wahi R, McMurtrey MJ, DeCaro LF, et al. Determinants of perioperative morbidity and mortality after pneumonectomy. Ann Thorac Surg. 1989;48:33–37. doi: 10.1016/0003-4975(89)90172-0. [DOI] [PubMed] [Google Scholar]
- 7.Kearney DJ, Lee TH, Reilly JJ, DeCamp MM, Sugarbaker DJ. Assessment of operative risk in patients undergoing lung resection. Importance of predicted pulmonary function. Chest. 1994;105:753–759. doi: 10.1378/chest.105.3.753. [DOI] [PubMed] [Google Scholar]
- 8.Pierce RJ, Copland JM, Sharpe K, Barter CE. Preoperative risk evaluation for lung cancer resection: predicted postoperative product as a predictor of surgical mortality. Am J Respir Crit Care Med. 1994;150:947–955. doi: 10.1164/ajrccm.150.4.7921468. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Olak J, Ultmann RE, Ferguson MK. Assessment of pulmonary complications after lung resection. Ann Thorac Surg. 1999;67:1444–1447. doi: 10.1016/s0003-4975(99)00255-6. [DOI] [PubMed] [Google Scholar]
- 10.Yano T, Yokoyama H, Fukuyama Y, Takai E, Mizutani K, Ichinose Y. The current status of postoperative complications and risk factors after a pulmonary resection for primary lung cancer. A multivariate analysis. Eur J Cardiothorac Surg. 1997;11:445–449. doi: 10.1016/s1010-7940(96)01097-4. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson MK, Reeder LB, Mick R. Optimizing selection of patients for major lung resection. J Thorac Cardiovasc Surg. 1995;109:275–281. doi: 10.1016/S0022-5223(95)70389-6. [DOI] [PubMed] [Google Scholar]
- 12.Markos J, Mullan BP, Hillman DR, et al. Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am Rev Respir Dis. 1989;139:902–910. doi: 10.1164/ajrccm/139.4.902. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson MK, Little L, Rizzo L, et al. Diffusing capacity predicts morbidity and mortality after pulmonary resection. J Thorac Cardiovasc Surg. 1988;96:894–900. [PubMed] [Google Scholar]
- 14.Bousamra M, 2nd, Presberg KW, Chammas JH, et al. Early and late morbidity in patients undergoing pulmonary resection with low diffusion capacity. Ann Thorac Surg. 1996;62:968–974. doi: 10.1016/0003-4975(96)00476-6. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson MK. Preoperative assessment of pulmonary risk. Chest. 1999;115(5 Suppl):58S–63S. doi: 10.1378/chest.115.suppl_2.58s. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson MK, Siddique J, Karrison T. Modeling major lung resection outcomes using classification trees and multiple imputation techniques. Eur J Cardiothorac Surg. 2008;34:1085–1089. doi: 10.1016/j.ejcts.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson MK, Durkin AE. A comparison of three scoring systems for predicting complications after major lung resection. Eur J Cardiothorac Surg. 2003;23:35–42. doi: 10.1016/s1010-7940(02)00675-9. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Olak J, Ferguson MK. Diffusing capacity predicts operative mortality but not long-term survival after resection for lung cancer. J Thorac Cardiovasc Surg. 1999;117:581–586. doi: 10.1016/s0022-5223(99)70338-7. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson MK, Vigneswaran WT. Diffusing capacity predicts morbidity after lung resection in patients without obstructive lung disease. Ann Thorac Surg. 2008;85:1158–1164. doi: 10.1016/j.athoracsur.2007.12.071. [DOI] [PubMed] [Google Scholar]
- 20.Brunelli A, Refai MA, Salati M, Sabbatini A, Morgan-Hughes NJ, Rocco G. Carbon monoxide lung diffusion capacity improves risk stratification in patients without airflow limitation: evidence for systematic measurement before lung resection. Eur J Cardiothorac Surg. 2006;29:567–570. doi: 10.1016/j.ejcts.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Wright CD, Gaissert HA, Grab JD, O’Brien SM, Peterson ED, Allen MS. Predictors of prolonged length of stay after lobectomy for lung cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk-adjustment model. Ann Thorac Surg. 2008;85:1857–1865. doi: 10.1016/j.athoracsur.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 22.Park BJ, Zhang H, Rusch VW, Amar D. Video-assisted thoracic surgery does not reduce the incidence of postoperative atrial fibrillation after pulmonary lobectomy. J Thorac Cardiovasc Surg. 2007;133:775–779. doi: 10.1016/j.jtcvs.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 23.Cattaneo SM, Park BJ, Wilton AS, et al. Use of video-assisted thoracic surgery for lobectomy in the elderly results in fewer complications. Ann Thorac Surg. 2008;85:231–235. doi: 10.1016/j.athoracsur.2007.07.080. [DOI] [PubMed] [Google Scholar]
- 24.Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg. 2009;138:419–425. doi: 10.1016/j.jtcvs.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Berry MF, Hanna J, Tong BC, et al. Risk factors for morbidity after lobectomy for lung cancer in elderly patients. Ann Thorac Surg. 2009;88:1093–1099. doi: 10.1016/j.athoracsur.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS Database. J Thorac Cardiovasc Surg. 2009 doi: 10.1016/j.jtcvs.2009.08.026. (In Press) [DOI] [PubMed] [Google Scholar]
- 27.Garzon JC, Ng CS, Sihoe AD, et al. Video-assisted thoracic surgery pulmonary resection for lung cancer in patients with poor lung function. Ann Thorac Surg. 2006;81:1996–2003. doi: 10.1016/j.athoracsur.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 28.Demmy TL, Curtis JJ. Minimally invasive lobectomy directed toward frail and high-risk patients: a case-control study. Ann Thorac Surg. 1999;68:194–200. doi: 10.1016/s0003-4975(99)00467-1. [DOI] [PubMed] [Google Scholar]
- 29.Larsen K Richter, Svendsen UG, Milman N, Brenoe J, Petersen BN. Exercise testing in the preoperative evaluation of patients with bronchogenic carcinoma. Eur Respir J. 1997;10:1559–1565. doi: 10.1183/09031936.97.10071559. [DOI] [PubMed] [Google Scholar]
- 30.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 31.Society of Thoracic Surgeons [Accessed March 5, 2009];2009 http://www.sts.org/sections/stsnationaldatabase.
- 32.Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg. 2006;244:420–425. doi: 10.1097/01.sla.0000234892.79056.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schuurmans MM, Diacon AH, Bolliger CT. Functional evaluation before lung resection. Clin Chest Med. 2002;23:159–172. doi: 10.1016/s0272-5231(03)00066-2. [DOI] [PubMed] [Google Scholar]
- 34.Nakahara K, Ohno K, Hashimoto J, et al. Prediction of postoperative respiratory failure in patients undergoing lung resection for lung cancer. Ann Thorac Surg. 1988;46:549–552. doi: 10.1016/s0003-4975(10)64694-2. [DOI] [PubMed] [Google Scholar]
- 35.Boffa DJ, Allen MS, Grab JD, Gaissert HA, Harpole DH, Wright CD. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg. 2008;135:247–254. doi: 10.1016/j.jtcvs.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 36.Nakata M, Saeki H, Yokoyama N, Kurita A, Takiyama W, Takashima S. Pulmonary function after lobectomy: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 2000;70:938–941. doi: 10.1016/s0003-4975(00)01513-7. [DOI] [PubMed] [Google Scholar]
- 37.Nomori H, Horio H, Naruke T, Suemasu K. What is the advantage of a thoracoscopic lobectomy over a limited thoracotomy procedure for lung cancer surgery? Ann Thorac Surg. 2001;72:879–884. doi: 10.1016/s0003-4975(01)02891-0. [DOI] [PubMed] [Google Scholar]
- 38.Varela G, Brunelli A, Rocco G, et al. Predicted versus observed FEV1 in the immediate postoperative period after pulmonary lobectomy. Eur J Cardiothorac Surg. 2006;30:644–648. doi: 10.1016/j.ejcts.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Varela G, Brunelli A, Rocco G, et al. Measured FEV1 in the first postoperative day, and not ppoFEV1, is the best predictor of cardio-respiratory morbidity after lung resection. Eur J Cardiothorac Surg. 2007;31:518–521. doi: 10.1016/j.ejcts.2006.11.036. [DOI] [PubMed] [Google Scholar]