Abstract
Aims
Cardiovascular (CV) hospitalization is a predictor of CV mortality and has a negative impact on patients’ quality of life. The primary endpoint of A placebo-controlled, double-blind, parallel-arm Trial to assess the efficacy of dronedarone 400 mg bid for the prevention of cardiovascular Hospitalization or death from any cause in patiENTs with Atrial fibrillation/atrial flutter (ATHENA), a composite of first CV hospitalization or death from any cause, was significantly reduced by dronedarone. This post hoc analysis evaluated the secondary endpoint of CV hospitalization and the clinical benefit of dronedarone on the number and duration of CV hospitalizations in patients with atrial fibrillation (AF).
Methods and results
ATHENA was a double-blind, parallel group study in 4628 patients with a history of paroxysmal/persistent AF and additional risk factors, treated with placebo or dronedarone. Dronedarone treatment significantly reduced the risk of first CV hospitalization (P < 0.0001 vs. placebo), while the risk of first non-CV hospitalization was similar in both groups (P = 0.77). About half of the CV hospitalizations were AF-related, with a median duration of hospital stay of four nights. The risk of any hospitalization for AF [hazard ratio (95% confidence interval) 0.626 (0.546−0.719)] and duration of hospital stay were significantly reduced by dronedarone (P < 0.0001 vs. placebo). Dronedarone treatment reduced total hospitalizations for acute coronary syndrome (P = 0.0105) and the time between the first AF/atrial flutter recurrence and CV hospitalization/death (P = 0.0048). Hospitalization burden was significantly reduced across all levels of care (P < 0.05). Cumulative incidence data indicated that the effects of dronedarone persisted for at least 24 months.
Conclusion
Dronedarone reduced the risk for CV hospitalization and the total hospitalization burden in this patient group.
The trial is registered under ClinicalTrials.gov #NCT 00174785.
Keywords: Anti-arrhythmic drug, Atrial fibrillation, Dronedarone, Hospitalization
Introduction
The lifetime risk of atrial fibrillation (AF) in men and women aged 40 years is one in four.1 The incidence and prevalence of AF increase with age, resulting in a substantial public health burden.2 A community-based cohort study indicated a rise in AF of 13% over the last 2 decades, and if this increase continues, 15.9 million people in the USA will develop AF by the year 2050.2 The increasing occurrence of AF is associated with an increase in mortality,3,4 as well as debilitating stroke5 and heart failure.3,4 Not unexpectedly, these trends are also associated with increased hospitalizations related to AF,6–9 which may, in turn, indicate increased mortality risk.10
In the recent ATHENA [A placebo-controlled, double-blind, parallel-arm Trial to assess the efficacy of dronedarone 400 mg twice a day (bid) for the prevention of cardiovascular Hospitalization or death from any cause in patiENts with Atrial fibrillation/atrial flutter] trial, in 4628 patients with paroxysmal or persistent AF or atrial flutter (AFL), dronedarone treatment significantly decreased the number of first cardiovascular (CV) hospitalizations compared with placebo [hazard ratio (HR)=0.74, P< 0.001], driven mainly by a reduction in the number of hospitalizations for AF.11 Furthermore, results from ATHENA demonstrated that dronedarone treatment increases the time to first hospitalization for CV reasons.
Here we present the results of a post hoc analysis, using data from the ATHENA study to further evaluate the effect of dronedarone on hospitalizations, by examining all hospitalization events and the length of hospital stay in patients with paroxysmal or persistent AF, or AFL.
Methods
Details of the main study protocol have been published previously.11,12 In brief, ATHENA was a randomized, double-blind, placebo-controlled trial conducted in 551 centres in 37 countries. The study was conducted according to the principles of good clinical practice. Patients were recruited between 29 June 2005 and 30 December 2006; subjects were followed up for a minimum of 1 year. The trial was sponsored by sanofi-aventis.
The aim of this post hoc data analysis was to evaluate the number of first hospitalizations per treatment group, the number of hospitalizations after first AF/AFL recurrence, the number of all hospitalizations, the duration of hospital stay, and the hospitalization burden over time.
Patient population
Patients with paroxysmal or persistent AF, or AFL, were eligible for enrollment if one or more of the following risk factors were present: aged ≥70 years, arterial hypertension (ongoing therapy with at least two antihypertensive drugs of different classes), diabetes mellitus, prior stroke or transient ischaemic attack (TIA) or systemic embolism, left atrial diameter ≥50 mm by M-mode echocardiography, or left ventricular ejection fraction ≤40%. For each patient, a 12-lead electrocardiogram (ECG) within 6 months prior to randomization had to be available showing AF or AFL. A second 12-lead ECG within the same period had to show sinus rhythm. During the course of the trial, the inclusion criteria were revised, requiring patients to be aged ≥70 years with one or more of the pre-specified risk factors, or aged ≥75 years regardless of whether they had any previously specified risk factors.
Exclusion criteria of note for this analysis included a diagnosis of permanent AF, an unstable haemodynamic condition, NYHA class IV congestive heart failure, any severe non-cardiac illness limiting life expectancy, and conditions incompatible with inclusion in a clinical trial.
Patients were randomly assigned to receive dronedarone 400 mg bid or placebo (ratio 1:1). Randomization was stratified by centre and by the presence or absence of AF or AFL at the time of randomization.
The follow-up visit schedule included clinical evaluations at days 7 and 14, and at months 1, 3, 6, 9, 12, and every 3 months thereafter. It was planned for the trial to have a minimum follow-up duration of 12 months and all patients, irrespective of the occurrence of a primary endpoint, were followed until the common study end date of 30 December 2007 or until death, with the exception of two patients in the placebo group who were lost to follow-up.
Reporting of hospitalizations
Any unplanned hospitalization (i.e. admission with an overnight stay in hospital covering at least two consecutive dates) was categorized by the investigator as either CV or non-CV according to pre-specified main reasons.12 The reasons for CV hospitalizations were defined prior to study start as follows: myocardial infarction or unstable angina; stable angina pectoris or atypical chest pain; atherosclerosis related (if not otherwise specified); transcutaneous coronary, cerebrovascular, or peripheral procedure; CV surgery except for cardiac transplantation; AF and other supraventricular rhythm disorders; ventricular arrhythmia or non-fatal cardiac arrest; worsening congestive heart failure (CHF), including pulmonary oedema, or dyspnoea of cardiac origin; cardiac transplantation; syncope; implantation of a pacemaker, implantable cardioverter defibrillator, or any other cardiac device; TIA or stroke (except for intracranial haemorrhage); pulmonary embolism or deep vein thrombosis; blood pressure related (hypotension or hypertension; except for syncope); major bleeding (requiring two or more units of blood or any intracranial haemorrhage); and CV infection. For each hospitalization, the number of nights spent in the hospital was recorded according to the following three categories: intensive care unit or coronary care unit, step-down or medium care unit, and regular ward.
Data for the primary endpoint and for CV hospitalizations separately were also analysed for the following regions: North America, South America, Eastern Europe, Western Europe, Asia, and ‘Other’ (comprising Australia, India, Israel, Morocco, New Zealand, South Africa, and Tunisia).
Statistical analysis
All analyses were performed according to the intention-to-treat principle. A separate analysis was performed for each reason for a CV hospitalization. Time to first event was estimated using the Kaplan–Meier method and compared by means of the log-rank test. All P values were two tailed. The dronedarone-to-placebo HRs with confidence intervals (CIs) were estimated using Cox's proportional hazard model.
Time for all hospitalizations (CV/non-CV) was analysed by plotting the mean number of CV/non-CV hospitalizations over time using Nelson–Aalen non-parametric cumulative incidence functions for each treatment group. The two treatment groups were compared using a two-sided log-rank asymptotic test for repeated event-time data. The HR and associated 95% CIs were estimated within the Andersen–Gill multiplicative intensity regression with treatment group as the only binary variable. The cumulative incidence was described at selected time points (6 months, 12 months, 18 months, 24 months, and 30 months) according to the treatment group and CV hospitalization status, and cumulative incidence for each 6-month period (0–6 months, 6–12 months, 12–18 months, 18–24 months, and 24–30 months) was derived.
The total number of hospitalizations and the number of total hospitalization days were compared using a log-rank test and a Wilcoxon test, respectively.
Results
The ATHENA study enrolled 4628 patients, of whom 2301 were assigned to dronedarone and 2327 to placebo. Patients had a mean age of 71.6 years and included slightly more male than female patients (53 and 47%, respectively); baseline characteristics were similar between treatment groups.11 As previously reported, the mean ± standard deviation follow-up duration for all patients was 21 ± 5 months, with a median of 22 months.11 The minimum follow-up duration was 1 year, and the maximum was 2.5 years. Details of adverse events occurring during the ATHENA trial have been reported previously.11
Overall, the number of first CV hospitalizations was significantly decreased in the dronedarone group compared with placebo [675 patients in the dronedarone group vs. 859 in the placebo group (HR = 0.74, 95% CI, 0.67–0.82, P< 0.001)]. The number of first hospitalizations for each reason is shown in Table 1, evaluated by the treatment group. The number of first non-CV hospitalizations is also shown. Among the first CV hospitalizations, those related to AF and acute coronary syndrome were significantly fewer in the dronedarone group compared with the placebo group (335 vs. 510, P< 0.0001 for AF, and 62 vs. 89, P = 0.03 for acute coronary syndrome, respectively). The numbers of hospitalizations for stroke or TIA and for heart failure were non-significantly reduced: dronedarone 43 stroke/TIA hospitalizations vs. placebo 61 (P = 0.08) and dronedarone 112 heart failure hospitalizations vs. placebo 132 (P = 0.22). There was no difference between the number of first non-CV hospitalizations between groups (dronedarone 516, placebo 533; P = 0.77).
Table 1.
Number of events and hazard ratio of first hospitalization according to pre-specified reasons during the on-study period
| Reason for hospitalization | Placebo (n = 2327) | Dronedarone (n = 2301) | HR (95% CI) | Log-rank test P value |
|---|---|---|---|---|
| CV hospitalizations | ||||
| AF and other supraventricular rhythm disorders | 510 | 335 | 0.626 (0.546–0.719) | <0.0001 |
| Worsening CHF, including pulmonary oedema or dyspnoea of cardiac origin | 132 | 112 | 0.855 (0.665–1.100) | 0.2207 |
| Acute coronary syndromea | 89 | 62 | 0.699 (0.506–0.967) | 0.0296 |
| Implantation of a pacemaker, implantable cardioverter defibrillator, or other cardiac device | 81 | 64 | 0.793 (0.572–1.101) | 0.1655 |
| Stable angina pectoris or atypical chest pain | 63 | 56 | 0.898 (0.626–1.287) | 0.5561 |
| TIA or stroke (except for intracranial haemorrhage) | 61 | 43 | 0.708 (0.480–1.047) | 0.0819 |
| Transcutaneous coronary, cerebrovascular, or peripheral procedure | 48 | 44 | 0.925 (0.615–1.393) | 0.7102 |
| CV surgery except for cardiac transplantation | 43 | 35 | 0.820 (0.525–1.281) | 0.3824 |
| Hypotension, hypertension; except for syncope | 38 | 30 | 0.797 (0.494–1.286) | 0.3509 |
| Major bleeding (requiring two or more units of blood or any intracranial haemorrhage) | 33 | 36 | 1.103 (0.688–1.769) | 0.6839 |
| Syncope | 32 | 27 | 0.853 (0.511–1.424) | 0.5418 |
| Atherosclerosis related (if not otherwise specified) | 14 | 16 | 1.151 (0.562–2.358) | 0.7004 |
| Ventricular arrhythmia or non-fatal cardiac arrest | 12 | 13 | 1.091 (0.498–2.391) | 0.8277 |
| Pulmonary embolism or deep vein thrombosis | 6 | 14 | 2.337 (0.898–6.082) | 0.0730 |
| Ventricular extrasystoles | 1 | 1 | 1.007 (0.063–16.106) | 0.9958 |
| Cardiac transplantation | 1 | 0 | NC | NC |
| CV infection | 0 | 4 | NC | NC |
| Non-CV hospitalizations | 533 | 516 | 0.982 (0.870–1.108) | 0.7688 |
NC, not calculable.
aAcute coronary syndrome includes myocardial infarction or unstable angina.
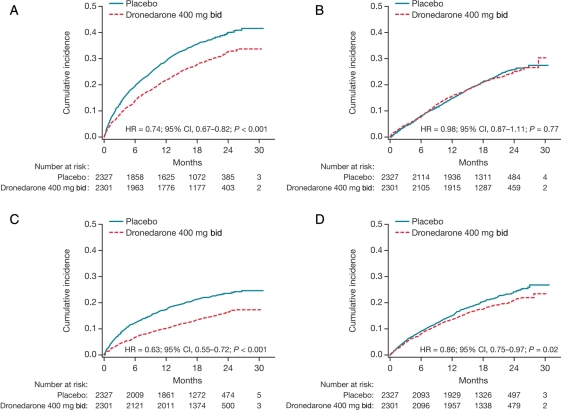
Figure 1 shows Kaplan–Meier curves of time to first CV hospitalization events and non-CV hospitalization events, as well as time to first AF-related hospitalizations and first non-AF-related hospitalizations.
Figure 1.
Kaplan–Meier cumulative incidence curves of time from randomization to first hospitalization for the intention-to-treat population. (A) Cardiovascular hospitalization; (B) non-cardiovascular hospitalization; (C) hospitalization for atrial fibrillation and other supraventricular disorders; (D) non-atrial fibrillation/atrial flutter hospitalization.
Overall, almost a quarter of the patients in each group had >1 hospitalization due to any cause during the study period (dronedarone 20.1%, placebo 24.5%). Multiple CV hospitalizations were reported in 12.3% of dronedarone-treated patients, and 15.9% in the placebo group. Table 2 shows the number of hospitalizations and their duration when all hospitalizations are included in the analyses, regardless of how many hospitalizations each patient had. Among the patients experiencing at least one AF-related hospitalization during the study, 50% remained in hospital for at least four nights and 25% for at least eight nights. The total number of hospitalizations for AF was reduced from 829 with placebo to 514 (P < 0.001) with dronedarone and the number of days in hospital from 4637 to 3132, respectively (P < 0.001). The number of hospitalizations for acute coronary syndrome was reduced from 113 in the placebo group to 71 in the dronedarone group (P = 0.01) and the number of hospitalization days from 1188 to 816, respectively (P = 0.04). Neither the total number nor the duration of non-CV hospitalizations was reduced significantly by dronedarone.
Table 2.
Total number of hospitalizations and hospital days by pre-specified classification
| Total number of hospitalizations |
Total number of hospital days |
|||||
|---|---|---|---|---|---|---|
| Reason for hospitalization | Placebo (n = 2327) | Dronedarone (n = 2301) | Log-rank test P value | Placebo (n = 2327) | Dronedarone (n = 2301) | Wilcoxon test P value |
| CV hospitalizations | ||||||
| AF and other supraventricular rhythm disorders | 829 | 514 | <0.0001 | 4637 | 3132 | <0.0001 |
| Worsening CHF, including pulmonary oedema or dysponea of cardiac origin | 184 | 165 | 0.505 | 1511 | 1486 | 0.202 |
| Acute coronary syndromea | 113 | 71 | 0.01047 | 1188 | 816 | 0.038 |
| Implantation of a pacemaker, implantable cardioverter defibrillator, or other cardiac device | 83 | 65 | NC | 372 | 334 | 0.206 |
| Stable angina pectoris or atypical chest pain | 72 | 69 | NC | 533 | 402 | 0.611 |
| TIA or stroke (except intracranial haemorrhage) | 64 | 46 | NC | 1122 | 439 | 0.079 |
| Transcutaneous coronary, cerebrovascular, or peripheral procedure | 55 | 52 | NC | 165 | 168 | 0.718 |
| CV surgery except cardiac transplantation | 47 | 38 | NC | 436 | 332 | 0.519 |
| Hypotension, hypertension; except syncope | 40 | 30 | NC | 297 | 238 | 0.349 |
| Major bleeding (requiring two or more units of blood or any intracranial haemorrhage) | 33 | 41 | NC | 349 | 423 | 0.687 |
| Syncope | 33 | 32 | NC | 190 | 102 | 0.371 |
| Atherosclerosis related (if not otherwise specified) | 20 | 17 | NC | 269 | 199 | 0.693 |
| Ventricular arrhythmia or non-fatal cardiac arrest | 14 | 16 | NC | 120 | 91 | 0.820 |
| Pulmonary embolism or deep vein thrombosis | 6 | 15 | NC | 121 | 137 | 0.070 |
| Ventricular extrasystoles | 1 | 1 | NC | 13 | 3 | 0.994 |
| Cardiac transplantation | 1 | 0 | NC | 20 | 0 | 0.320 |
| CV infection | 0 | 5 | NC | 0 | 14 | 0.044 |
| Other CV hospitalization | 285 | 254 | 0.2842 | 1870 | 1524 | 0.240 |
| Non-CV hospitalizations | 715 | 676 | 0.480 | 6326 | 5901 | 0.703 |
NC, not calculable.
aAcute coronary syndrome includes myocardial infarction or unstable angina.
In Table 3, it can be seen that a reduction in the total number of nights spent in hospital is observed at all levels of care. In addition, dronedarone decreased the risk of first CV hospitalization in the intensive care unit or cardiological care unit by ∼19% compared with placebo (Table 4). This reduction in risk was not only observed in hospitalizations due to AF (placebo 122 vs. dronedarone 82), but also in hospitalizations due to stroke/TIA (21 vs. 11) and myocardial infarction/unstable angina (31 vs. 24), indicating a beneficial effect of dronedarone beyond AF-related hospitalizations.
Table 3.
Duration of first hospitalization during the on-study period according to the level of care
| Placebo (n = 2327) | Dronedarone (n = 2301) | P value | |
|---|---|---|---|
| All CV hospitalizations | |||
| Patients with at least one CV hospitalization | 859 (36.9%) | 675 (29.3%) | |
| Total number of nights | 5807 | 4792 | <0.001 |
| Nights in intensive care/coronary care unit | 888 | 482 | 0.027 |
| Nights in step-down unit or medium care | 922 | 724 | 0.010 |
| Nights on regular ward | 3997 | 3586 | 0.002 |
| AF hospitalizations | |||
| Patients with at least one AF hospitalization | 510 (21.9%) | 335 (14.6%) | |
| Total number of nights | 2837 | 2092 | <0.001 |
| Nights in intensive care/coronary care unit | 358 | 175 | 0.007 |
| Nights in step-down unit or medium care | 518 | 292 | <0.001 |
| Nights on regular ward | 1961 | 1625 | <0.001 |
Table 4.
Survival analysis: time from randomization to first CV hospitalization in intensive care/coronary care unit during the study period
| Placebo (n = 2327) | Dronedarone (n = 2301) | |
|---|---|---|
| Number of events | 270 (11.6%) | 218 (9.5%) |
| Cumulative incidence of events (95% CI) | ||
| At 6 months | 0.057 (0.047–0.066) | 0.041 (0.033–0.050) |
| At 1 year | 0.082 (0.071–0.094) | 0.068 (0.058–0.079) |
| At 2 years | 0.127 (0.112–0.142) | 0.107 (0.093–0.121) |
| Log-rank test P value | 0.0174 | |
| HR (95% CI)a | 0.806 (0.674–0.963) | |
aDetermined from Cox regression model.
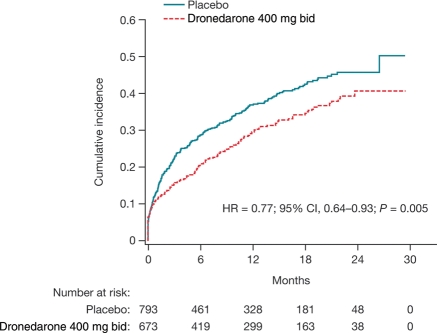
Figure 2 shows the time from first AF/AFL recurrence (based on ECGs and cardioversion) to first CV hospitalization or death from any cause during the on-study period. This analysis includes all randomized patients with a first AF/AFL recurrence (based on ECGs and cardioversion) and without presence of AF/AFL as a stratification factor. The time between recurrence and CV hospitalization/death was significantly reduced by dronedarone [hazard ratio (HR) (95% CI) = 0.771 (0.643–0.925), P = 0.0048].
Figure 2.
Kaplan–Meier cumulative incidence curves of time from first atrial fibrillation/atrial flutter recurrence (based on electrocardiograms and cardioversion) to first cardiovascular hospitalization during the on-study period. Data are for all randomized patients with a first atrial fibrillation/atrial flutter recurrence and without presence of atrial fibrillation/atrial flutter as per stratification factor.
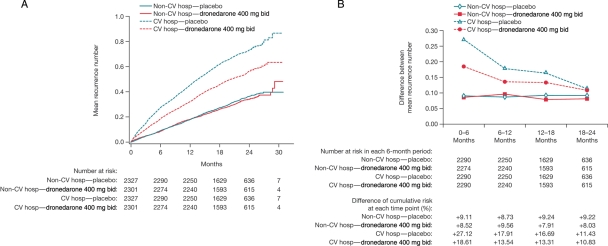
The Nelson–Aalen cumulative incidence of all CV and non-CV hospitalizations is shown in Figure 3A. Repeated CV hospitalizations were reduced with dronedarone, but non-CV hospitalizations were not. Figure 3B shows the hospitalization burden (cumulative incidence of hospitalizations) of CV and non-CV hospitalization during the first 2 years after randomization. The hospitalization burden for CV hospitalization decreased over time and remained lower with dronedarone treatment.
Figure 3.
Incidence of cardiovascular and non-cardiovascular hospitalizations. (A) Summary of Nelson–Aalen cumulative incidence curves of all cardiovascular and non-cardiovascular hospitalizations during the on-study period (intention-to-treat population); (B) curve and summary of cumulative incidence difference between each time point for all cardiovascular and non-cardiovascular hospitalizations during the on-study period (intention-to-treat population).
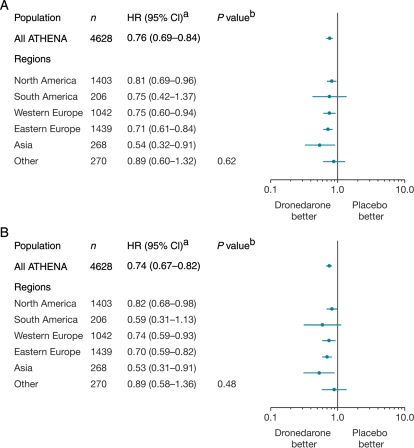
The HR estimates and 95% CIs for the primary endpoint and time to first CV hospitalization events are shown in Figure 4 for the different regions. Results were consistent with the overall population, with all risk estimates favouring dronedarone treatment (P value for interaction=0.62). Although the duration of CV hospitalizations varied across regions according to regional clinical habits, the effect of dronedarone on the reduction of CV hospitalizations was consistent across regions, as shown in Figure 4B (P = 0.48).
Figure 4.
Hazard ratio (dronedarone 400 mg bd vs. placebo) estimates with confidence intervals according to region. (A) Primary endpoint (first cardiovascular hospitalization or death from any cause); (B) cardiovascular hospitalization. Data are for all randomized patients. aDetermined from Cox regression model. bP values are for interaction between regions and treatment based on Cox regression model.
Discussion
The main finding of this ATHENA post hoc analysis is that dronedarone reduces the number of CV hospitalizations and reduces the length of hospital stay for CV reasons. The reduction in first hospitalizations in intensive care/cardiac care units suggests a reduction in CV hospitalizations associated with potentially life-threatening conditions. Since CV hospitalization is a valid surrogate endpoint for mortality in studies of AF,10 this is an important finding. The overall reduction of hospitalization burden observed in the current analysis, including reduction of CV events, indicates that dronedarone may reduce the severity of CV disease in this patient population.
Anti-arrhythmic drugs approved for maintenance of sinus rhythm in patients with AF have all been documented to prevent or delay the recurrence of AF.13 ATHENA is the only study powered to demonstrate a clinical benefit beyond maintenance of sinus rhythm specifically in patients with AF.
A post hoc analysis of the AF Follow-up Investigation of Rhythm Management (AFFIRM) study also investigated CV hospitalization as an alternative endpoint for death.14 In that analysis, patients treated with a rhythm-control strategy were hospitalized for CV reasons at a higher rate than patients treated with a rate-control strategy (46 vs. 36%, P < 0.001). However, when hospitalizations related to treatment (i.e. cardioversion) were excluded, rates were similar in both cohorts (24 vs. 27%, respectively). A further analysis of the data found significant associations between CV hospitalization and death in both treatment arms.14
The AF–CHF study was conducted in a patient population with more and worse heart failure than either ATHENA or AFFIRM, but the hospitalization results of AF–CHF were similar to those of AFFIRM.15 Patients in the rhythm-control cohort were hospitalized at significantly higher rates during the 1 year than patients in the rate-control cohort (46 vs. 39%, respectively; P = 0.001). Furthermore, hospitalizations for AF were significantly higher in the rhythm-control cohort than the rate-control cohort (14 vs. 9%, respectively; P = 0.001).
Although a reduction in hospitalizations was also demonstrated in patients with AF for dofetilide in the two Danish Investigations of Arrhythmias and Mortality ON Dofetilide (DIAMOND),16 the primary populations of those studies were patients with heart failure or recent myocardial infarction. A pooled post hoc analysis of the 506 patients with AF at baseline in the DIAMOND studies demonstrated a reduction in hospitalizations with dofetilide treatment, accompanied by a more frequent conversion to sinus rhythm.16
The reduced number and duration of hospitalizations in the dronedarone group vs. placebo seen in the current ATHENA analysis may also be related to maintenance of sinus rhythm. In accordance with this finding, hospitalizations related to AF were markedly reduced in number and duration. This reduction in AF-related hospitalizations was not simply due to fewer cardioversions, but also to a decrease in the severity of recurrent AF episodes.
It is also noteworthy that hospitalizations related to acute coronary syndromes were reduced. While hospitalizations related to acute coronary syndromes could be reduced by less frequent AF, one might speculate that the heart-rate-lowering effect of dronedarone may be important. Dronedarone slows the heart rate not only during AF but also during sinus rhythm.17 This may be the result of a class IV effect; however, like the benzocycloalkane ivabradine, dronedarone has been shown to inhibit the If-current of the sinus node, inducing a selective reduction in heart rate.18,19 The potential importance of heart rate lowering has recently been strengthened by the results of the Systolic Heart failure treatment with the If inhibitor ivabradine Trial (SHIFT), where the heart-rate-lowering effect of ivabradine reduced CV mortality in patients with heart failure.20
Although the number of hospitalizations due to acute coronary syndrome and AF was significantly reduced by dronedarone, hospitalizations due to other CV causes were not significantly different from placebo. There was a noticeable trend towards reduced hospitalization days due to stroke in the dronedarone group (439 vs. 1122 in the placebo group), and although this was not significant (P = 0.079), possibly due to the small number of patients hospitalized due to stroke (43 dronedarone patients vs. 61 placebo patients), it nevertheless provides additional support for the benefits of dronedarone treatment in this patient population. Interestingly, a previous ATHENA subanalysis that took into account not only hospitalizations due to stroke, but also death due to stroke, and strokes reported only as AEs, demonstrated that the incidence of stroke was significantly reduced with dronedarone treatment, from 1.8% per year with placebo to 1.2% per year [HR = 0.66 (95% CI 0.46–0.96), P = 0.027].21
In this post hoc analysis, the data for the six regions for the primary outcome and for CV hospitalizations alone were consistent with the overall study population.
It should be noted that the results of the current study cannot be extrapolated to other anti-arrhythmic drugs. In the meta-analysis by Lafuente-Lafuente,22 although the efficacies of commonly used anti-arrhythmic drugs appeared similar, there were some specific differences between them, which could result in important differences in outcomes for patients. Flecainide and propafenone do not lower heart rate and may thereby be less efficacious in reducing hospitalizations. Sotalol has a dual effect of being a beta-blocker and a class III anti-arrhythmic drug, but it may cause torsades de pointes tachycardia and it had an uncertain safety profile in the meta-analysis. Amiodarone appeared to be the most effective of the drugs evaluated by Lafuente-Lafuente in preventing recurrences of AF, while producing fewer AEs and having less associated mortality than beta-blockers.22 In comparison with dronedarone, amiodarone may be more efficacious for maintenance of sinus rhythm,23 but it has a poorer side-effect profile, particularly with long-term use.
In conclusion, treatment of patients with paroxysmal or persistent AF or AFL with dronedarone is associated with a marked reduction in the number and duration of CV hospitalizations, particularly those associated with potentially life-threatening conditions.
Funding
The ATHENA trial was sponsored by Sanofi-Aventis. Funding to pay the Open Access publication charges for this article was provided by Sanofi-Aventis.
Conflict of interest : C.T.-P. has received consulting fees from sanofi-aventis and Cardiome, research grants from sanofi-aventis, and lecture fees from sanofi-aventis and Cardiome. H.J.G.M.C. has received consulting fees from Medacorp, AstraZeneca, sanofi-aventis, and Cardiome, research grants from AstraZeneca and St Jude Medical, Inc., and lecture fees from AstraZeneca, sanofi-aventis, and Chugai Pharmaceuticals. C.G. is an employee of sanofi-aventis and owns shares in the company. R.L.P. has received consulting fees from sanofi-aventis. S.J.C. has received consulting fees and research grants from sanofi-aventis. S.H.H. has received consulting fees from sanofi-aventis, Cardiome, ARYx Therapeutics, and Bristol-Myers Squibb, research grants from sanofi-aventis and St Jude Medical, Inc., and lecture fees from sanofi-aventis, St Jude Medical Inc., and Bristol-Myers Squibb. No other potential conflicts of interest relevant to this article were reported.
Acknowledgements
The authors would like to acknowledge the contribution of M. van Eickels, MD, an employee of sanofi-aventis, Frankfurt, Germany, during the time the ATHENA study was conducted. T.-P. and his co-authors were responsible for drafting the article and critical revision. Editorial support was provided by Sally Mitchell, PhD, of PAREXEL, and was funded by sanofi-aventis.
References
- 1.Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–46. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 2.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 3.Stewart S, Hart CL, Hole DJ, McMurray JJ. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart. 2001;86:516–21. doi: 10.1136/heart.86.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98:476–84. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 5.Steger C, Pratter A, Martinek-Bregel M, Avanzini M, Valentin A, Slany J, et al. Stroke patients with atrial fibrillation have a worse prognosis than patients without: data from the Austrian Stroke registry. Eur Heart J. 2004;25:1734–40. doi: 10.1016/j.ehj.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 6.Wattigney WA, Mensah GA, Croft JB. Increasing trends in hospitalization for atrial fibrillation in the United States, 1985 through 1999: implications for primary prevention. Circulation. 2003;108:711–16. doi: 10.1161/01.CIR.0000083722.42033.0A. [DOI] [PubMed] [Google Scholar]
- 7.Stewart S, MacIntyre K, MacLeod MM, Bailey AE, Capewell S, McMurray JJ. Trends in hospital activity, morbidity and case fatality related to atrial fibrillation in Scotland, 1986–1996. Eur Heart J. 2001;22:693–701. doi: 10.1053/euhj.2000.2511. [DOI] [PubMed] [Google Scholar]
- 8.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Seward JB, et al. Changing trends of hospital utilization in patients after their first episode of atrial fibrillation. Am J Cardiol. 2008;102:568–72. doi: 10.1016/j.amjcard.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friberg J, Buch P, Scharling H, Gadsbphioll N, Jensen GB. Rising rates of hospital admissions for atrial fibrillation. Epidemiology. 2003;14:666–72. doi: 10.1097/01.ede.0000091649.26364.c0. [DOI] [PubMed] [Google Scholar]
- 10.Friberg L, Rosenqvist M. Cardiovascular hospitalization as a surrogate endpoint for mortality in studies of atrial fibrillation: report from the Stockholm Cohort Study of Atrial Fibrillation. Europace. 2011 doi: 10.1093/europace/eur001. advance access publication 6 April 2011, doi: 10.1093/Europace/eur100 doi:10.1093/Europace/eur001. [DOI] [PubMed] [Google Scholar]
- 11.Hohnloser SH, Crijns HJ, van Eickels M, Gaudin C, Page RL, Torp-Pedersen C, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009;360:668–78. doi: 10.1056/NEJMoa0803778. [DOI] [PubMed] [Google Scholar]
- 12.Hohnloser SH, Connolly SJ, Crijns HJ, Page RL, Seiz W, Torp-Petersen C. Rationale and design of ATHENA: A Placebo-controlled, Double-blind, Parallel arm Trial to assess the efficacy of dronedarone 400 mg bid for the prevention of cardiovascular Hospitalization or death from any cause in patiENts with Atrial fibrillation/atrial flutter. J Cardiovasc Electrophysiol. 2008;19:69–73. doi: 10.1111/j.1540-8167.2007.01016.x. [DOI] [PubMed] [Google Scholar]
- 13.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Europace. 2010;10:1360–420. doi: 10.1093/europace/euq350. [DOI] [PubMed] [Google Scholar]
- 14.Wyse DG, Slee A, Epstein AE, Gersh BJ, Rocco T, Jr, Vidaillet H, et al. AFFIRM Investigators. Alternative endpoints for mortality in studies of patients with atrial fibrillation: the AFFIRM study experience. Heart Rhythm. 2004;1:531–7. doi: 10.1016/j.hrthm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, et al. Atrial Fibrillation and Congestive Heart Failure Investigators. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–77. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen OD, Bagger H, Keller N, Marchant B, Kober L, Torp-Pedersen C. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish Investigations of Arrhythmia and Mortality ON Dofetilide (DIAMOND) substudy. Circulation. 2001;104:292–6. doi: 10.1161/01.cir.104.3.292. [DOI] [PubMed] [Google Scholar]
- 17.Manning A, Thisse V, Hodeige D, Richard J, Heyndrickx JP, Chatelain P. SR 33589, a new amiodarone-like antiarrhythmic agent: electrophysiological effects in anesthetized dogs. J Cardiovasc Pharmacol. 1995;25:252–61. doi: 10.1097/00005344-199502000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Fox K, Ford I, Steg PG, Tendera M, Ferrari R. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:807–16. doi: 10.1016/S0140-6736(08)61170-8. [DOI] [PubMed] [Google Scholar]
- 19.Gögelein H, Ruetten H. Effect of dronedarone, a new multichannel blocking agent, on sodium and HCN4 channels in vitro. Clin Res Cardiol. 2009;98(Suppl 2):P329. [Google Scholar]
- 20.Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, et al. SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–85. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 21.Connolly SJ, Crijns HJ, Torp-Pedersen C, van Eickels M, Gaudin C, Page RL, et al. ATHENA Investigators. Analysis of stroke in ATHENA: A Placebo-controlled, Double-blind, Parallel Arm Trial to assess the efficacy of dronedarone 400 mg BID for the prevention of cardiovascular hospitalization or death from any cause in patients with atrial fibrillation/atrial flutter. Circulation. 2009;120:1174–80. doi: 10.1161/CIRCULATIONAHA.109.875252. [DOI] [PubMed] [Google Scholar]
- 22.Lafuente-Lafuente C, Mouly S, Longas-Tejero MA, Mahe I, Bergmann JF. Antiarrhythmic drugs for maintaining sinus rhythm after cardioversion of atrial fibrillation: a systematic review of randomized controlled trials. Arch Intern Med. 2006;166:719–28. doi: 10.1001/archinte.166.7.719. [DOI] [PubMed] [Google Scholar]
- 23.Le Heuzey JY, De Ferrari GM, Radzik D, Santini M, Zhu J, Davy JM. A Short-term, Randomized, Double-blind, Parallel-group Study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: the DIONYSOS Study. J Cardiovasc Electrophysiol. 2010;21:597–605. doi: 10.1111/j.1540-8167.2010.01764.x. [DOI] [PubMed] [Google Scholar]