Abstract
Aims
Cardiac resynchronization therapy (CRT) is associated with reverse left ventricular (LV) remodelling. However, the effects of CRT-induced mechanical remodelling on electrical remodelling, and the occurrence of ventricular arrhythmias have not been clearly established. We studied the relationship between mechanical remodelling, electrical remodelling, and the occurrence of appropriate implantable cardioverter-defibrillator (ICD) therapy 1 year after CRT.
Methods and results
We analysed data from 45 patients who underwent ICD-CRT implantation at our centre. Significant LV reverse remodelling was defined by a minimum 10% decrease in the LV end-diastolic diameter (LVEDd) at 1 year of follow-up. Electrocardiographic indices of dispersion of repolarization [QTc, Tpeak-Tend (Tp-e) and their dispersion] were measured immediately and 1 year post-CRT implantation. The occurrence of appropriate ICD therapy was noted for each patient. Patients with (n= 21) and without (n= 24) significant LV reverse remodelling had similar baseline characteristics. At 1 year of follow-up, patients with mechanical reverse LV remodelling exhibited a significant decrease in QTc (505 ± 42 vs. 485 ± 52 ms, P < 0.05) and Tp–e (107 ± 26 vs. 92 ± 22 ms, P < 0.0001). However, patients without mechanical LV reverse remodelling exhibited a significant increase in QT dispersion (29 ± 43 vs. 98 ± 47 ms, P = 0.002) and Tp–e dispersion (22 ± 21 vs. 54 ± 36 ms, P = 0.0001). Finally patients with mechanical LV reverse remodelling experienced a lower rate of ICD therapy (P = 0.0025) after a mean follow-up of 19 months.
Conclusion
Reverse LV mechanical remodelling is associated with reversal of electrical remodelling and a lower rate of appropriate ICD therapy following CRT.
Keywords: Dispersion of repolarization, Cardiac resynchronization therapy, Remodelling
Introduction
Cardiac resynchronization therapy (CRT) has proven to decrease morbidity and overall mortality in heart failure (HF) patients with cardiac dyssynchrony.1–3 The effects of CRT on left ventricular (LV) reverse mechanical remodelling have been well established.4 However, the role of CRT on reverse electrical remodelling has not been well-characterized. Electrocardiographic indices of dispersion of repolarization (DR), such as Tpeak–Tend (Tp–e), are risk markers for malignant ventricular arrhythmias.5–7 In this study, we analysed the evolution of electrocardiogram (ECG) parameters reflecting DR 1 year after initiation of CRT and the relationship of reverse electrical remodelling to reverse mechanical remodelling. Finally, we studied the relationship of reverse mechanical remodelling to the incidence of ventricular arrhythmias.
Methods
Patients
We analysed data from 45 patients with successful placement of implantable cardioverter-defibrillator (ICD)-CRT devices at our centre between January 2003 and June 2005. All patients had regular follow-ups at our centre including device evaluations at our location. The review of these data was approved by the institutional review board.
Inclusion criteria
The inclusion criteria were:
New York Heart Association (NYHA) functional class III or IV HF symptoms despite optimal medical therapy.
LV ejection fraction (LVEF) ≤35% assessed by echocardiography, angiography, or radionuclide scanning.
QRS duration ≥120 ms or QRS <120 ms, and left intraventricular dyssynchrony8,9 assessed by transthoracic echocardiography with Doppler tissue imaging analysis (difference of at least 60 ms between the timing of the peak systolic velocities of the septum vs. the lateral wall).10
Implantation technique
All patients underwent cardiac resynchronization ICD implantation. An LV lead was implanted via the coronary sinus in all cases in order to achieve permanent epicardial stimulation from a lateral or posterolateral vein. If these veins were not accessible, then the lead was implanted in a diagonal vein, close to the lateral LV wall. The right ventricular lead was implanted in the apex in all patients. All patients in sinus rhythm received an atrio-biventricular pulse generator programmed in DDD(R) mode. Biventricular pacing was assessed on the ECG during the follow-up as the presence of R-wave in lead V1 and presence of Q-wave in lead I. For each patient the AV and VV delay were optimized with echocardiography after CRT implantation. For AV interval optimization the Ritter method was used to determine the optimal A-V interval. This technique involves programming the AV delay to first a short and then a long interval. At each of these settings assessment on end-diastolic filling was performed using several Doppler based on echocardiographic techniques.11 The optimal VV interval was identified by finding the interval at which the stroke volume was determined to be maximal using Doppler echocardiography. The stroke volume was determined using AV VTI assessment. Stroke volume values were compared at simultaneous biventricular pacing and at delays of 20, 40, and 80 ms. Multiple measurements were made using at least three cardiac cycles at each interval assessed. The interval associated with the highest stroke volume was considered ‘Optimal'.12
All the patients included in this study had at least 95% of biventricular pacing assessed by device interrogation during follow-up.
Data collected
The following characteristics for each patient were recorded at baseline and 1 year of follow-up post-CRT implantation: NYHA functional class, electrocardiographic, and echocardiographic parameters [LVEF and LV end-diastolic diameter (LVEDd)]. Patients without appropriate echocardiographic and/or electrocardiographic follow-up were excluded from the study. Then this population represents a subset of UCLA patients implanted with CRT-ICD during the inclusion period.
Electrocardiography methods
Each patient underwent an ECG during biventricular pacing within 24 h and 1 year post-implantation. The ECG was recorded with a standard digital recorder as 12 simultaneous leads at a paper speed of 25 mm/s. Each ECG was scanned and transferred for digital analysis, individual leads were magnified and analysed with a precision of 3 ms.13 Lead analysis per ECG was performed by two different experts, both of whom were blinded on patient follow-up data. The following parameters were measured for each ECG: heart rate, QRS duration, QT, QT dispersion, Tp-e and Tp-e dispersion. Each measurement represented the average of all 12 ECG leads. The QTc interval was calculated using the Bazett formula.14 The QT interval was measured from the beginning of the QRS complex to the end of the T-wave, defined as the tangent to the downslope of the T-wave and the isoelectric line.15 QT dispersion was defined as the difference between the minimum and maximum QT interval of the 12 ECG leads. Tp-e was measured in all derivations and was obtained from the difference between the QT interval and QT peak interval (measured from the beginning of the QRS to the peak of T-wave). In the case of negative or biphasic T-waves, the QT peak was measured to the nadir of the T-wave. If a U wave was present, only the T-wave was considered for measurement. The Tp-e dispersion interval was obtained by calculating the difference between the minimum and maximum Tp-e interval in the precordial leads.
Echocardiography methods
Echocardiography was performed on each patient during biventricular pacing at baseline and 1 year post-implantation. Echocardiographic exams were completed using commercially available ultrasound equipment (Acuson Sequoia C256, Siemens Company, Mountain View, CA, USA) with a multifrequency transducer for harmonic imaging. All images were acquired digitally; and reviewed and measured offline on an Acuson (Siemens) KinetDx digital review station. Measurements were performed by an experienced echocardiographer ( J.S.C.) without knowledge of the clinical or electrophysiological status of each individual patient. Left ventricular volumes and ejection fraction were quantified using the modified Simpson's rule method as per the American Society of Echocardiography (ASE) Committee Recommendations for Chamber Quantification in all patients.16 Similarly, left atrial volumes, which have been shown to be powerful predictors of cardiovascular outcomes including prognosis after myocardial infarction, or, degree of diastolic dysfunction, were quantified at end systole from the apical views by the modified Simpson's rule method.16–18 Colour flow imaging evaluation of mitral regurgitation with a visual qualitative estimate was made per the ASE Echocardiography Report on the Evaluation of Native Valvular Regurgitation.19 A continuous-wave Doppler beam was then directed through the mitral regurgitation jet and the dP/dt of the rate of development of LV pressure as a less load-dependent measure of contractility was quantified.20 Spectral pulsed-wave Doppler and tissue Doppler imaging were used to measure the pulsed-wave Doppler mitral transvalvular flow velocities and lateral basal LV myocardial tissue Doppler imaging velocities, including the pulsed-wave Doppler mitral E and A velocities and mitral valve inflow to inflow time (MVIT), and the E′, A′ and peak systolic (Sa) tissue Doppler imaging velocities.21–23 Data calculated included the E′/A′ ratio as a relatively load-independent index of left atrial pressure.21–26 Left ventricular outflow tract velocities were recorded and LV ejection time (LVET) was measured from the opening to closing of the aortic valve. The LV Myocardial Performance (‘Tei') Index (LVMPI) was calculated from the combination of the LVET and MVIT, where the LVMPI = [MVIT−LVET]/LVET.25–27
Assessment of implantable cardioverter-defibrillator therapy
Patients were evaluated in the outpatient device clinic after biventricular-ICD implantation at 3 months post-implantation, and every 6 months thereafter (with device interrogation at all visits). Patients were also instructed to call the device clinic in the event of new symptoms or ICD discharges. Occurrences of ICD shocks or antitachycardia pacing (ATP) were confirmed in all cases by device interrogation. An electrophysiologist, blinded to the results of DR measurements, confirmed the appropriateness of the ICD therapy. Inappropriate therapy was excluded from our analysis. The ICD data were available for all the patients included in this study.
Definition of groups
The HF population was divided into two groups: (i) patients with evidence of LV reverse remodelling and (ii) patients without evidence of LV reverse remodelling. A patient was considered to have LV reverse remodelling if the LVEDd decreased by at least 10%.28
Statistical analysis
Continuous variables were expressed as median ± interquartile range, and statistical significance was assessed using the Mann–Whitney test. Categorical variables, expressed as numbers or percentages, were analysed with the Fischer's exact test. Differences between immediate and 1 year post-CRT ECG parameters were tested by Wilcoxon signed rank test for continuous variables. A univariate analysis of variables was also performed. Cumulative event rates (appropriate ICD therapy) were calculated with a log-rank test according to the Kaplan–Meier method. All tests were two-tailed, and a P value<0.05 was considered statistically significant.
Results
Baseline characteristics
The overall patient characteristics are described in Table 1. The mean age was 59 ± 13 years. Ischaemic cardiomyopathy and chronic atrial fibrillation was observed in 38 and 13% of our study population, respectively. In 40% of the cases obligate right ventricular pacing was the reason for referral for a biventricular upgrade. The LV lead was placed in a lateral or posterolateral vein in 82% of cases and a diagonal vein leading to the lateral wall in the remaining cases. Four patients with QRS <120 ms and left intraventricular dyssynchrony and 9 patients with QRS between 120 and 150 ms were included in this study.
Table 1.
Baseline characteristics of the study subjects (n = 45)
| Age (year) | 59 ± 13 |
| Male (%) | 78 |
| Hypertension (%) | 36 |
| Dyslipidemia (%) | 33 |
| Smokers (%) | 16 |
| Diabetes (%) | 11 |
| NYHA class (n) | 3.1 ± 0.3 |
| AF (%) | 13 |
| LVEF (%) | 21.8 ± 6.0 |
| LVEDd (mm) | 66 ± 8 |
| Prior rhythm pacing (%) | 40 |
| Lateral LV lead position (%) | 82 |
| Ischaemic cardiomyopathy (%) | 38 |
| Pre-implantation QRS duration (ms) | 164 ± 37 |
| ACEI or ARB (%) | 87 |
| Beta-blockers (%) | 84 |
| Diuretics (%) | 82 |
| Digoxin (%) | 42 |
| Amiodarone (%) | 31 |
NYHA, New York Heart Association classification; AF, atrial fibrillation, LVEF, left ventricle ejection fraction; LVEDd, left ventricle end-diastolic dimension; LV, left ventricle; ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers.
One-year follow-up
At 1 year of follow-up 21 (47%) patients demonstrated significant LV reverse mechanical remodelling, whereas 24 (53%) patients did not. The characteristics of patients with or without LV reverse remodelling are shown in Table 2. No significant differences in cardiovascular risk factors and HF drug therapy were observed between these two groups. Baseline transthoracic echocardiography patterns (LVEF and LVEDd) were similar in both groups (20.9 ± 6.2% vs. 22.7 ± 5.3%; 67 ± 8 mm vs. 66 ± 9 mm, P = NS). New York Heart Association functional class and pre-implantation QRS duration (163 ± 39 vs. 160 ± 45 ms, P = NS) were comparable in patients with or without LV reverse remodelling.
Table 2.
Baseline characteristics of patients with or without mechanical reverse left ventricular remodelling
| Reverse remodelling (n = 21) | No Reverse remodelling (n = 24) | P | |
|---|---|---|---|
| Mean±SD | Mean±SD | ||
| Age (year) | 58 ± 15 | 60 ± 13 | 0.68 |
| Male (%) | 76 | 79 | 0.99 |
| Hypertension (%) | 33 | 37 | 0.99 |
| Dyslipidemia (%) | 29 | 33 | 0.76 |
| Smokers (%) | 10 | 20 | 0.42 |
| Diabetes (%) | 10 | 12 | 0.99 |
| NYHA class (n) | 3.1 ± 0.3 | 3.2 ± 0.4 | 0.49 |
| AF (n, %) | 10 | 17 | 0.67 |
| LVEF (%) | 20.9 ± 6.2 | 22.7 ± 5.3 | 0.29 |
| LVEDd (mm) | 67 ± 8 | 66 ± 9 | 0.55 |
| Prior rhythm pacing (%) | 24 | 42 | 0.34 |
| Pre-implantation QRS duration (ms) | 163 ± 39 | 160 ± 45 | 0.83 |
| Primary prevention indication (%) | 67 | 63 | 0.99 |
| Lateral LV lead position (%) | 76 | 88 | 0.44 |
| Ischaemic cardiomyopathy (%) | 29 | 46 | 0.36 |
| ACEI or ARB (%) | 95 | 79 | 0.19 |
| Beta-blockers (%) | 81 | 87 | 0.69 |
| Diuretics (%) | 81 | 83 | 0.99 |
| Digoxin (%) | 52 | 33 | 0.24 |
| Amiodarone (%) | 24 | 37 | 0.35 |
NYHA, New York Heart Association classification; AF, atrial fibrillation; LVEF, left ventricle ejection fraction; LVEDd, left ventricle end diastolic dimension; LV, left ventricle; ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers.
The comparison of ECG parameters in patients with or without reverse LV remodelling immediately after CRT showed no differences for heart rate, QRS duration, QTc, QT dispersion, Tp-e and Tp-e dispersion in the two groups. At 1 year of follow-up, QRS duration, QTc, and Tp-e dispersion were similar in both groups. Moreover heart rate was identical in patients with or without LV reverse remodelling. However, QT dispersion (68 ± 36 vs. 98 ± 47 ms, P = 0.02) and Tp-e (92 ± 22 vs. 111 ± 26 ms, P = 0.01) were significantly lower in patients with LV reverse mechanical remodelling.
Finally at 1 year, patients with LV reverse remodelling experienced a significant decrease in NYHA class (3.1 ± 0.3 vs. 2.0 ± 0.6, P < 0.0001) and an increase in LVEF (20.9 ± 6.2% vs. 30.1 ± 11.1%, P = 0.0002). Similarly, a significant reduction in NYHA class was shown in patients without LV reverse remodelling (3.2 ± 0.4 vs. 2.7 ± 0.6, P < 0.0001), although no changes in LVEF were observed for this group (22.7 ± 5.3 vs. 23.8 ± 7.8%, P = NS) 1 year after CRT implantation.
Effects of implantable cardioverter-defibrillator on echocardiographic remodelling parameters
As shown in Table 3 patients with mechanical LV reverse remodelling showed significant decrease in LV end diastolic (264 ± 111 vs. 182 ± 51 ml, P = 0.01) and systolic volume (230 ± 117 vs. 133 ± 54 ml, P = 0.009). Moreover, they experienced a marked reduction in MVIT (505 ± 75 vs. 442 ± 41 ms, P = 0.02) and LVMPI (1.02 ± 0.33 vs. 0.65 ± 0.26, P = 0.02); and an increase in LVET (251 ± 41 vs. 272 ± 38 ms, P = 0.05). Conversely, patients without LV mechanical reverse remodelling (Table 3), as defined in our study, had no changes in LV end-diastolic volume, end-systolic volume, and other remodelling parameters at 1 year after CRT.
Table 3.
Comparison of immediate and one year post-implantable cardioverter-defibrillator echocardiography data for patients with or without left ventricular reverse remodelling
| Variable | LV reverse remodelling (n = 21) |
No LV reverse remodelling (n = 24) |
||||
|---|---|---|---|---|---|---|
| Immediate post-CRT | One year after CRT | P | Immediate post-CRT | One year after CRT | P | |
| LVEDV volume (ml) | 264 ± 111 | 182 ± 51 | 0.01* | 189 ± 66 | 192 ± 64 | 0.96 |
| LVESV volume (ml) | 230 ± 117 | 133 ± 54 | 0.009* | 141 ± 53 | 140 ± 75 | 0.62 |
| LVET (ms) | 251 ± 41 | 272 ± 38 | 0.05 | 254 ± 33 | 268 ± 46 | 0.69 |
| MVIT (ms) | 505 ± 75 | 442 ± 41 | 0.02* | 460 ± 86 | 457 ± 39 | 0.87 |
| LVMPI | 1.02 ± 0.33 | 0.65 ± 0.26 | 0.02* | 0.83 ± 0.31 | 0.75 ± 0.33 | 0.90 |
| LA volume (ml) | 90 ± 36 | 63 ± 24 | 0.08 | 102 ± 36 | 84 ± 20 | 0.10 |
| E′/A′ ratio | 0.7 ± 0.2 | 0.8 ± 0.4 | 0.59 | 1.2 ± 0.7 | 1.2 ± 0.9 | 0.79 |
| Mitral valve Sa (cm/s) | 9 ± 2 | 13 ± 7 | 0.24 | 9 ± 1 | 10 ± 2 | 0.68 |
LVEDV, left ventricular end diastolic volume; LVESV, left ventricular end systolic volume; LVET, left ventricle ejection time; MVIT, mitral valve inflow to inflow time; LVMPI, left ventricle myocardial performance index; LA, left atrium; Sa, systolic amplitude.
*P < 0.05.
Effects of implantable cardioverter-defibrillator on reverse electrical remodelling (Figure 1)
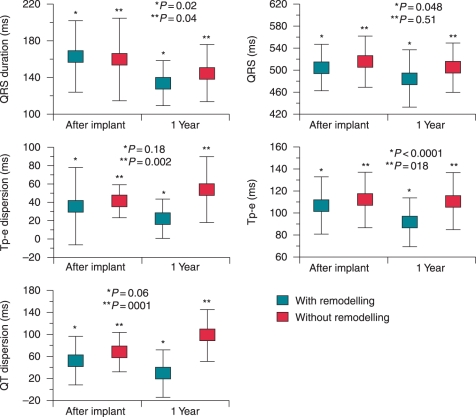
Figure 1.
Evolution of electrocardiographic parameters immediately and one year post-implantable cardioverter-defibrillator in patients with (n = 21) and without (n = 24) LV reverse remodeling. Results are shown as mean ± SD. QTc = QT corrected measurement.
For the patient group with LV reverse mechanical remodelling, QRS duration (151 ± 28 vs. 134 ± 24 ms, P = 0.02), QTc (505 ± 42 vs.485 ± 52 ms, P = 0.048) and Tp-e (107 ± 26 vs. 92 ± 22 ms, P < 0.0001) were reduced after 1 year of biventricular pacing. However, QT dispersion and Tp-e dispersion were similar immediately and 1 year after CRT.
For the patient group without LV reverse mechanical remodelling, QRS duration declined moderately after 1 year of biventricular pacing (159 ± 30 vs.145 ± 31 ms, P = 0.04). However QTc and Tp-e did not demonstrate any changes. Finally QT dispersion (29 ± 43 vs.98 ± 47 ms, P = 0.0001) and Tp-e dispersion (22 ± 21 vs.54 ± 36 ms, P = 0.002) were significantly increased. Figure 2 displays an example of a patient with LV reverse mechanical remodelling. Electrocardiograms of the patient immediately and at one year post-CRT are shown.
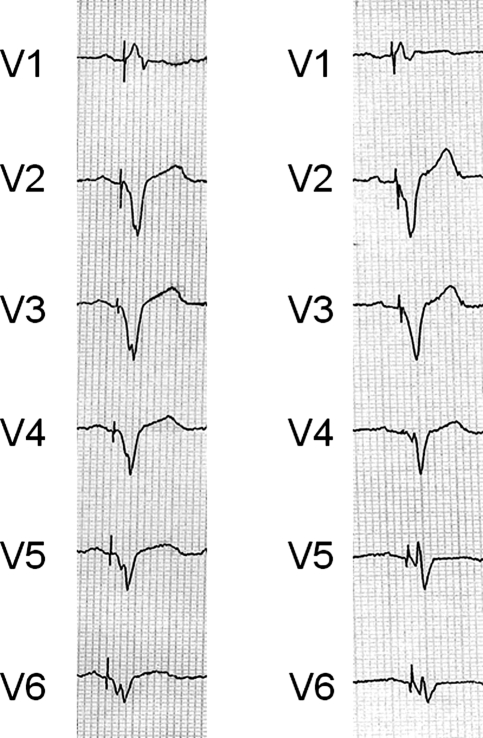
Figure 2.
Example of ECG for a patient with LV reverse remodeling immediately (left ECG) and one year after implantable cardioverter-defibrillator (right ECG).
Occurrence of appropriate therapy and left ventricular reverse mechanical remodelling
After a mean follow-up of 19 months, 10 patients experienced appropriate therapy (5 ATP alone and 5 shocks). Eight patients (18%) experienced inappropriate therapy due to sinus tachycardia in 3 patients, atrial fibrillation or flutter in 4 patients and device problems (noise, double counting) in 1 patient. As shown in Figure 3, patients with LV reverse mechanical remodelling had a notably lower rate of appropriate ICD-therapy in comparison to patients without LV reverse mechanical remodelling (P = 0.0025).
Figure 3.
Kaplan–Meier curves of ICD therapy incidence in patients with and without LV reverse mechanical remodelling.
Discussion
Major findings
Our study shows that patients with LV reverse mechanical remodelling demonstrated a significant decrease in DR parameters such as Tp-e, after one year of biventricular pacing. Further, these patients experienced a lower rate of appropriate ICD therapy.
Effects of implantable cardioverter-defibrillator on mechanical remodelling
Systolic HF triggers progressive LV dilatation and adverse remodelling.29 Implantable cardioverter-defibrillator improves the quality of life, HF symptoms and mortality in patients with systolic HF and ventricular dyssynchrony.1–3 Previous clinical studies have also shown the beneficial effects of CRT on LV function, including reversal of LV remodelling.4,30,31 Left ventricular reverse mechanical remodelling at 3–6 months after CRT implantation, which was sustained at one year of follow-up, has been demonstrated.31 Our population exhibited a significant degree of LV reverse mechanical remodelling in 47% of cases after one year of biventricular pacing. However, other studies have found a higher percentage of LV reverse mechanical remodelling (60%).31 The definition used for LV reverse mechanical remodelling may account for this discrepancy. For example, Sutton and colleagues defined LV reverse mechanical remodelling as any decrease in LV volume.31 However, our study criterion was more rigid, requiring a reduction of at least 10% in the LV diameter to qualify as LV reverse mechanical remodelling. Consistent with our study, Cappola et al.28 found a 10% reduction of LV volume in 37% of their population 6 months after CRT implantation. Finally, in order to fully confirm LV reverse mechanical remodelling; our study demonstrated the benefits in the evolution of other echocardiographic parameters, such as MVIT, LV volume or LVMPI.
Effects of implantable cardioverter-defibrillator on electrical remodelling
We demonstrated that LV reverse mechanical remodelling after CRT was associated with beneficial ECG modification. Of particular note, CRT induced a reduction of DR parameters in patients with significant LV reverse mechanical remodelling.
However, the effect of CRT on reverse electrical remodelling is controversial.
Various studies demonstrated a reduction in spontaneous QRS duration late after CRT implantation.32,33 Moreover, Dizon et al.34 reported a loss of spontaneous left bundle branch block in a patient after biventricular pacing. Other studies did not demonstrate significant changes of paced QRS duration several months after CRT.33,35 Differences in methods of analysis may account for the discrepancies observed between former studies and our results. For example, our study used a very sensitive technique to measure ECG parameters. Furthermore, the population under study in our retrospective analysis had distinguishable differences in comparison to other studies. For instance, Ricci et al.33 studied a population with LV lead placement in the lateral wall in only 37% of cases compared with 82% in our population. Finally, our study design differed from the above-mentioned studies in that we divided our population according to the occurrence of LV reverse mechanical remodelling.
The mechanism of decrease in QRS duration and DR parameters in patients with LV reverse mechanical remodelling requires further investigation. Ventricular remodelling during HF includes myocyte apoptosis and inflammation,36,37 leading to an increase in ventricular fibrosis and subsequent impairment of the normal electrical conduction system.38,39 Chakir et al. demonstrated that CRT reversed LV remodelling by reducing myocytes apoptosis and activating different kinases.40 Additionally as demonstrated by Aiba et al.41 experimentally, CRT could induce electrophysiological changes by reducing action potential duration heterogeneity and early depolarization occurrence. It is therefore conceivable that by reversing mechanical LV remodelling, CRT could contribute to the improvement of intraventricular conduction.
However our results could also be explained by the fact that the reverse electrical remodelling induced by CRT could be the cause of reverse mechanical remodelling. Indeed electrical remodelling in dyssynchronous hearts includes downregulation of K currents and alterations of depolarizing Na current and Ca homeostasis. CRT has been shown to reverse both electrical and mechanical remodelling.42 Immediate effects of CRT may include electrical changes in QRS duration and repolarization dispersion13 that could be the origin of reverse mechanical remodelling. Further studies are required to explore this hypothesis.
Effects of implantable cardioverter-defibrillator on ventricular arrhythmias
The effects of CRT on ventricular arrhythmias43,44 are controversial. For example, the COMPANION study suggested that the addition of an ICD to CRT was necessary to reduce global mortality and sudden cardiac death.45,46 A meta-analysis of randomized trials of CRT revealed that although biventricular pacing reduces death due to heart pump failure, other causes of death (including sudden cardiac death) might be increased.47 Recently, the CARE-HF study3 demonstrated no significant reduction of sudden cardiac death in patients undergoing CRT at ∼2 years of follow-up. The CARE-HF follow-up was later extended to 3 years, at which point a beneficial effect of CRT on sudden cardiac death was observed.48 The mechanism for the delayed beneficial effect of CRT on sudden cardiac death is unknown. However, our results indicate a marked reduction in DR parameters (Tp-e, QTc) at one year post-CRT in patients with LV reverse remodelling. Of note patients without LV reverse remodelling experienced an increase in these parameters. These data could partly explain why these patients have a higher risk of appropriate therapy.
Our study also showed a significant reduction of Tp-e (a marker of ventricular arrhythmias) by a mean of 10 ms at 1 year in the LV reverse remodelling population. Therefore, the delayed beneficial effects of CRT in relation to mechanical reverse remodelling could counterbalance it's potentially proarrhythmic effects immediately after implantation.49 Such opposing mechanisms could explain the delayed beneficial effects of CRT on ventricular arrhythmias and sudden cardiac death. However, the mechanism by which reverse mechanical remodelling is associated with a decrease in ventricular arrhythmias (and appropriate ICD therapy) remains unclear. Experimental studies have highlighted LV dilatation during ventricular remodelling as an important contributor to the heterogeneous reduction in ventricular action potential duration and refractoriness,50,51 leading to ventricular arrhythmias.52
Limitations of the study
Certain limitations with regard to this study should be considered. Our study is retrospective by nature, and is composed of a small sample size. Therefore, our results need to be confirmed on a larger prospective study. Moreover we observed no ICD therapy in the reverse mechanical remodelling group of patients. However, our mean follow-up was relatively short (19 months). Then it is conceivable that with longer follow-up patients with mechanical reverse remodelling would also experience more ICD therapy.
Additionally some degree of fusion between intrinsic QRS and biventricular paced QRS could have interfered with the measurement of ECG parameters. However, we do not believe that this bias was significant as QRS morphology was similar with the presence of R in lead V1 and presence Q wave in lead I indicating biventricular pacing during the follow-up. Moreover due to medication such as beta-blockers or progression of the disease this fusion should tend to decrease with time.
Also our population was quiet heterogeneous including patients with narrow QRS who were implanted before recent publication showing the absence of benefit of CRT in this population.53 However we believe that this heterogeneity makes the finding remarkable, because the correlation between electrical and mechanical remodelling was still present regardless of wide or narrow QRS patients.
Finally further studies are required to define the mechanism of electrical reverse remodelling.
Conclusion
Patients who underwent CRT had significant LV reverse mechanical remodelling. These patients demonstrated significant reverse electrical remodelling. This population also had a lower rate of appropriate ICD therapy.
Funding
Supported by a grant from the NHLBI, R01HL084261.
Conflict of interest: none to declare.
References
- 1.Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873–80. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 2.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 3.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators . The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 4.St John Sutton MG, Plappert T, Abraham WT, Smith AL, DeLurgio DB, Leon AR, et al. Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation. 2003;107:1985–90. doi: 10.1161/01.CIR.0000065226.24159.E9. [DOI] [PubMed] [Google Scholar]
- 5.Perkiomaki JS, Koistinen MJ, Yli-Mayry S, Huikuri HV. Dispersion of QT interval in patients with and without susceptibility to ventricular tachyarrhythmias after previous myocardial infarction. J Am Coll Cardiol. 1995;26:174–9. doi: 10.1016/0735-1097(95)00122-g. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu M, Ino H, Okeie K, Yamaguchi M, Nagata M, Hayashi K, et al. T-peak to T-end interval may be a better predictor of high-risk patients with hypertrophic cardiomyopathy associated with a cardiac troponin I mutation than QT dispersion. Clin Cardiol. 2002;25:335–9. doi: 10.1002/clc.4950250706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe N, Kobayashi Y, Tanno K, Miyoshi F, Asano T, Kawamura M, et al. Transmural dispersion of repolarization and ventricular tachyarrhythmias. J Electrocardiol. 2004;37:191–200. doi: 10.1016/j.jelectrocard.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Achilli A, Sassara M, Ficili S, Pontillo D, Achilli P, Alessi C, et al. Long-term effectiveness of cardiac resynchronization therapy in patients with refractory heart failure and “narrow” QRS. J Am Coll Cardiol. 2003;42:2117–24. doi: 10.1016/j.jacc.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Gasparini M, Mantica M, Galimberti P, Marconi M, Genovese L, Faletra F, et al. Beneficial effects of biventricular pacing in patients with a “narrow” QRS. Pacing Clin Electrophysiol. 2003;26:169–74. doi: 10.1046/j.1460-9592.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 10.Bax JJ, Molhoek SG, van Erven L, Bootsma M, Boersma E, Steendijk P, et al. Usefulness of myocardial tissue Doppler echocardiography to evaluate left ventricular dyssynchrony before and after biventricular pacing in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2003;91:94–7. doi: 10.1016/s0002-9149(02)03009-6. [DOI] [PubMed] [Google Scholar]
- 11.Ritter P, Padeletti L, Gillio-Meina L, Gaggini G. Determination of the optimal atrioventricular delay in DDD pacing: comparison between echo and peak endocardial acceleration measurements. Europace. 1999;1:126–30. doi: 10.1053/eupc.1998.0032. [DOI] [PubMed] [Google Scholar]
- 12.Pitzalis MV, Iacoviello M, Romito R, Massari F, Rizzon B, Luzzi G, et al. Cardiac resynchronization therapy tailored by echocardiographic evaluation of ventricular asynchrony. J Am Coll Cardiol. 2002;40:1615–22. doi: 10.1016/s0735-1097(02)02337-9. [DOI] [PubMed] [Google Scholar]
- 13.Lellouche N, De Diego C, Akopyan G, Boyle NG, Mahajan A, Cesario DA, et al. Changes and predictive value of dispersion of repolarization parameters for appropriate therapy in patients with biventricular implantable cardioverter-defibrillators. Heart Rhythm. 2007;4:1274–83. doi: 10.1016/j.hrthm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Bazett H. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353–70. [Google Scholar]
- 15.Castro Hevia J, Antzelevitch C, Tornes Barzaga F, Dorantes Sánchez M, Dorticós Balea F, Zayas Molina R, et al. Tpeak–Tend and Tpeak–Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J Am Coll Cardiol. 2006;47:1828–34. doi: 10.1016/j.jacc.2005.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang RM, Bierig M, Devereux RB, Foster E, Pellikka PA, Picard MH, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, et al. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–63. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 18.Beinart R, Boyko V, Schwammenthal E, Kuperstein R, Sagie A, Hod H, et al. Long-term prognostic significance of left atrial volume in acute myocardial infarction. J Am Coll Cardiol. 2004;44:327–34. doi: 10.1016/j.jacc.2004.03.062. [DOI] [PubMed] [Google Scholar]
- 19.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 20.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–50. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 21.Yu CM, Sanderson JE, Marwick TH, Oh JK. Tissue Doppler imaging a new prognosticator for cardiovascular diseases. J Am Coll Cardiol. 2007;49:1903–14. doi: 10.1016/j.jacc.2007.01.078. [DOI] [PubMed] [Google Scholar]
- 22.Ommen SR, Nishimura RA. A clinical approach to the assessment of left ventricular diastolic function by Doppler echocardiography: update 2003. Heart. 2003;89(Suppl 3):iii18–23. doi: 10.1136/heart.89.suppl_3.iii18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–94. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 24.Powell BDER, Yu C-K, Oh JK. Tissue Doppler imaging, strain imaging and dyssynchrony assessment. In: Oh JK, Sewad JB, Tajik AJ, editors. The Echo Manual. 3rd ed. Philaldelphia: Lippincott Williams & Wilkins; 2007. pp. 80–98. [Google Scholar]
- 25.Tei C, Dujardin KS, Hodge DO, Kyle RA, Tajik AJ, Seward JB. Doppler index combining systolic and diastolic myocardial performance: clinical value in cardiac amyloidosis. J Am Coll Cardiol. 1996;28:658–64. doi: 10.1016/0735-1097(96)00202-1. [DOI] [PubMed] [Google Scholar]
- 26.Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, et al. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function—a study in normals and dilated cardiomyopathy. J Cardiol. 1995;26:357–66. [PubMed] [Google Scholar]
- 27.Tei C, Nishimura RA, Seward JB, Tajik AJ. Noninvasive Doppler-derived myocardial performance index: correlation with simultaneous measurements of cardiac catheterization measurements. J Am Soc Echocardiogr. 1997;10:169–78. doi: 10.1016/s0894-7317(97)70090-7. [DOI] [PubMed] [Google Scholar]
- 28.Cappola TP, Harsch MR, Jessup M, Abraham WT, Young JB, Petersen-Stejskal S, et al. Predictors of remodelling in the CRT era: influence of mitral regurgitation, BNP, and gender. J Card Fail. 2006;12:182–8. doi: 10.1016/j.cardfail.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Cohn JN, Ferrari R, Sharpe N. Cardiac remodelling concepts and clinical implications: a consensus paper from an international forum on cardiac remodelling. Behalf of an International Forum on Cardiac Remodelling. J Am Coll Cardiol. 2000;35:569–82. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 30.Delnoy PP, Ottervanger JP, Luttikhuis HO, Nicastia DM, Elvan A, Misier AR, et al. Sustained benefit of cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2007;18:298–302. doi: 10.1111/j.1540-8167.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- 31.Sutton MG, Plappert T, Hilpisch KE, Abraham WT, Hayes DL, Chinchoy E. Sustained reverse left ventricular structural remodelling with cardiac resynchronization at one year is a function of etiology: quantitative Doppler echocardiographic evidence from the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) Circulation. 2006;113:266–72. doi: 10.1161/CIRCULATIONAHA.104.520817. [DOI] [PubMed] [Google Scholar]
- 32.Henrikson CA, Spragg DD, Cheng A, Capps M, Devaughn K, Marine JE, et al. Evidence for electrical remodelling of the native conduction system with cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2007;30:591–5. doi: 10.1111/j.1540-8159.2007.00717.x. [DOI] [PubMed] [Google Scholar]
- 33.Ricci R, Pignalberi C, Ansalone G, Jannone E, Vaccaro MV, Denaro A, et al. Early and late QRS morphology and width in biventricular pacing: relationship to lead site and electrical remodeling. J Interv Card Electrophysiol. 2002;6:279–85. doi: 10.1023/a:1019570022647. [DOI] [PubMed] [Google Scholar]
- 34.Dizon J, Horn E, Neglia J, Medina N, Garan H. Loss of left bundle branch block following biventricular pacing therapy for heart failure: evidence for electrical remodelling? J Interv Card Electrophysiol. 2004;10:47–50. doi: 10.1023/B:JICE.0000011484.61659.b1. [DOI] [PubMed] [Google Scholar]
- 35.Linde C, Leclercq C, Rex S, Garrigue S, Lavergne T, Cazeau S, et al. Long-term benefits of biventricular pacing in congestive heart failure: results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol. 2002;40:111–8. doi: 10.1016/s0735-1097(02)01932-0. [DOI] [PubMed] [Google Scholar]
- 36.Finkel MS, Oddis CV, Jacob TD, Watkins SC, Hattler BG, Simmons RL. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992;257:387–9. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- 37.Hegewisch S, Weh HJ, Hossfeld DK. TNF-induced cardiomyopathy. Lancet. 1990;335:294–5. doi: 10.1016/0140-6736(90)90115-l. [DOI] [PubMed] [Google Scholar]
- 38.Shamim W, Yousufuddin M, Cicoria M, Gibson DG, Coats AJ, Henein MY. Incremental changes in QRS duration in serial ECGs over time identify high risk elderly patients with heart failure. Heart. 2002;88:47–51. doi: 10.1136/heart.88.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada T, Fukunami M, Ohmori M, Iwakura K, Kumagai K, Kondoh N, et al. New approach to the estimation of the extent of myocardial fibrosis in patients with dilated cardiomyopathy: use of signal-averaged electrocardiography. Am Heart J. 1993;126:626–31. doi: 10.1016/0002-8703(93)90413-4. [DOI] [PubMed] [Google Scholar]
- 40.Chakir K, Daya SK, Tunin RS, Helm RH, Byrne MJ, Dimaano VL, et al. Reversal of global apoptosis and regional stress kinase activation by cardiac resynchronization. Circulation. 2008;117:1369–77. doi: 10.1161/CIRCULATIONAHA.107.706291. [DOI] [PubMed] [Google Scholar]
- 41.Aiba T, Hesketh GG, Barth AS, Liu T, Daya S, Chakir K, et al. Electrophysiological consequences of dyssynchronous heart failure and its restoration by resynchronization therapy. Circulation. 2009;119:1220–30. doi: 10.1161/CIRCULATIONAHA.108.794834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aiba T, Tomaselli GF. Electrical remodeling in the failing heart. Curr Opin Cardiol. 2010;25:29–36. doi: 10.1097/HCO.0b013e328333d3d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ermis C, Seutter R, Zhu AX, Benditt LC, VanHeel L, Sakaguchi S, et al. Impact of upgrade to cardiac resynchronization therapy on ventricular arrhythmia frequency in patients with implantable cardioverter-defibrillators. J Am Coll Cardiol. 2005;46:2258–63. doi: 10.1016/j.jacc.2005.04.067. [DOI] [PubMed] [Google Scholar]
- 44.Lin G, Rea RF, Hammill SC, Hayes DL, Brady PA. Effect of cardiac resynchronization therapy on occurrence of ventricular arrhythmia in patients with implantable cardioverter defibrillators undergoing upgrade to cardiac resynchronization therapy devices. Heart. 2008;94:186–90. doi: 10.1136/hrt.2007.118372. [DOI] [PubMed] [Google Scholar]
- 45.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 46.Carson P, Anand I, O'Connor C, Jaski B, Steinberg J, Lwin A, et al. Mode of death in advanced heart failure: the Comparison of Medical, Pacing, and Defibrillation Therapies in Heart Failure (COMPANION) trial. J Am Coll Cardiol. 2005;46:2329–34. doi: 10.1016/j.jacc.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 47.Bradley DJ, Bradley EA, Baughman KL, Berger RD, Calkins H, Goodman SN, et al. Cardiac resynchronization and death from progressive heart failure: a meta-analysis of randomized controlled trials. JAMA. 2003;289:730–40. doi: 10.1001/jama.289.6.730. [DOI] [PubMed] [Google Scholar]
- 48.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. Longer-term effects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization-Heart Failure (CARE-HF) trial extension phase] Eur Heart J. 2006;27:1928–32. doi: 10.1093/eurheartj/ehl099. [DOI] [PubMed] [Google Scholar]
- 49.Fish JM, Brugada J, Antzelevitch C. Potential proarrhythmic effects of biventricular pacing. J Am Coll Cardiol. 2005;46:2340–7. doi: 10.1016/j.jacc.2005.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franz MR, Cima R, Wang D, Profitt D, Kurz R. Electrophysiological effects of myocardial stretch and mechanical determinants of stretch-activated arrhythmias. Circulation. 1992;86:968–78. doi: 10.1161/01.cir.86.3.968. [DOI] [PubMed] [Google Scholar]
- 51.Lab MJ. Mechanically dependent changes in action potentials recorded from the intact frog ventricle. Circ Res. 1978;42:519–28. doi: 10.1161/01.res.42.4.519. [DOI] [PubMed] [Google Scholar]
- 52.Reiter MJ. Effects of mechano-electrical feedback: potential arrhythmogenic influence in patients with congestive heart failure. Cardiovasc Res. 1996;32:44–51. [PubMed] [Google Scholar]
- 53.Beshai JF, Grimm RA, Nagueh SF, Baker JH, 2nd, Beau SL, Greenberg SM, et al. RethinQ Study Investigators. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med. 2007;357:2461–71. doi: 10.1056/NEJMoa0706695. [DOI] [PubMed] [Google Scholar]