Abstract
β-catenin signaling is required for embryonic tooth morphogenesis and promotes continuous tooth development when activated in embryos. To determine whether activation of this pathway in the adult oral cavity could promote tooth development, we induced mutation of epithelial β-catenin to a stabilized form in adult mice. This caused increased proliferation of the incisor tooth cervical loop, outpouching of incisor epithelium, abnormal morphology of the epithelial-mesenchymal junction, and enhanced expression of genes associated with embryonic tooth development. Ectopic dental-like structures were formed from the incisor region following implantation into immunodeficient mice. Thus, forced activation of β-catenin signaling can initiate an embryonic-like program of tooth development in adult rodent incisor teeth.
Keywords: tooth, development, β-catenin, Wnt, dental
Introduction
The Wnt/β-catenin signaling pathway regulates many aspects of development and disease. Binding of a Wnt ligand to Frizzled (FZ) and LDL-related protein (LRP) 5/6 receptors at the cell surface results in stabilization and accumulation of cytoplasmic β-catenin, its translocation to the nucleus, and transcriptional activation of target genes by complexes of β-catenin with members of the LEF/TCF transcription factor family (Gordon and Nusse, 2006). Wnt/β-catenin signaling activity is observed at the initiation and subsequent stages of embryonic tooth development and is required at multiple stages of this process (Jarvinen et al., 2006; Liu et al., 2008). Forced activation of β-catenin signaling in embryonic oral epithelium results in increased expression of genes required for tooth morphogenesis, continuous initiation of dental development, and ectopic tooth formation (Jarvinen et al., 2006; Kuraguchi et al., 2006; Liu et al., 2008). Analysis of these data identifies β-catenin signaling as a key fate determinant in the embryonic oral ectoderm, and places this pathway upstream of other factors necessary for dental development. However, the functions of β-catenin signaling in controlling dental epithelial stem cell proliferation, and whether activation of this pathway can promote tooth development in the adult, are unknown. To address these questions, we examined the pattern of endogenous Wnt/β-catenin signaling activity in the developing and post-natal incisor tooth cervical loop, a known repository of dental stem cells, and determined the effects of in vivo activation of Wnt/β-catenin signaling in the adult oral cavity by mutation of β-catenin to a constitutively active form.
Materials & Methods
Generation of Mouse Lines and Genotyping
Mice carrying tetO-Cre (Mucenski et al., 2003) and KRT5-rtTA (Diamond et al., 2000) transgenes and Ctnnb1fl(ex3)/+ (Harada et al., 1999) were placed on doxycycline chow (1 mg/kg, Bio-serv, Laurel, MD, USA) to induce β-catenin mutation (Zhang et al., 2008). All animal experiments were performed under University of Pennsylvania IACUC-approved protocols.
Histology, Immunofluorescence, BrdU Incorporation, and in situ Hybridization
Dissected incisors were fixed and decalcified in pH 7.0 2% Formalin, 10% EDTA in PBS at 4°C for 14 days. Histology, immunofluorescence with anti-β-catenin, BrdU assays, and in situ hybridization with digoxygenin-labeled probes were as described previously (Andl et al., 2002; Liu et al., 2007, 2008). To control for specificity of immunofluorescence, we omitted primary antibody.
Incisor Implantation and Micro-CT Analysis
Apical ends of incisors dissected from bone under sterile conditions (Akintoye et al., 2006) were implanted in duplicate into dorsal subcutaneous incisions in 8-week-old female nude mice (NIH-III-nu, Charles River Laboratories, Wilmington, MA, USA). Micro-CT imaging of samples in 70% ethanol was performed with an eXplore Locus SP scanner (GE Healthcare Techologies, London, Ontario, Canada), with the following parameters: 80 kVp, 80 µA, 250-µm Al filter, and 4 frame averages. Images were acquired at an isotropic resolution of 16 µm (16 µm × 16 µm × 16 µm cubic voxels) with 2 hrs of scan time, 760 views in 0.5° steps with 1.7 sec of exposure and a 2x2 detector bin mode. Raw data were reconstructed by a modified Feldkamp algorithm (Feldkamp et al., 1984), with 16-bit gray-scale apparent density units. Reconstructed image data were viewed with MicroView (GE Healthcare) and ImageJ (http://rsbweb.nih.gov/ij/). Multi-planar reformatting at arbitrary oblique slices, maximum intensity projection, and volume rendering techniques were performed with OsiriX (www.osirix-viewer.com). For color volume rendering, we chose a color palette representative of bones and muscles and a non-linear logarithmic inverse opacity function to enhance subtle differences in grayscale. This allowed for the identification of enamel as distinct from soft tissue, dentin, and bone.
Results
Rodent molar teeth, like human teeth, are not replaced in adult life. However, the rodent incisor tooth grows continuously, relying on a pool of epithelial stem cells in the labial cervical loop at the tooth base that constantly generates enamel-secreting ameloblasts. To examine Wnt/β-catenin signaling activity in incisor and cervical loop development, we used 3 independent Wnt reporter transgenic lines: BATgal, TOPGAL, and Axin2lacZ (DasGupta and Fuchs, 1999; Jho et al., 2002; Maretto et al., 2003; Yu et al., 2005). Expression was similar in all 3 lines and was detected throughout incisor epithelium at E12.5 (Appendix Fig. 1a), in the enamel knot at E15 (Appendix Fig. 1b), and in mesenchymal cells adjacent to the enamel knot and developing cervical loop (Appendix Figs. 1b-1e, 1j, 1k, 1q). Expression of the Wnt10a and Wnt10b ligands localized to both mesenchymal and epithelial cells (Appendix Figs. 1f-1i, 1o, 1p). However, in incisor epithelial cells, Wnt reporter activity was down-regulated after E15 (Appendix Fig. 1c-1e, 1j, 1k, 1q), and nuclear localized β-catenin was not observed at P0.5 (Appendix Figs. 1l-1n). Wnt activity was not strongly present in the wild-type epithelial cervical loop, either during embryonic development (Appendix Figs. 1b-1e, 1j) or post-natally (Appendix Figs. 1k, 1q).
To determine the effects of inducible forced activation of epithelial β-catenin signaling in the adult oral cavity, we utilized Keratin5 (KRT5)-rtTA tetO-Cre Ctnnb1fl(ex3)/+ mice in which the gene encoding β-catenin, Ctnnb1, can be mutated to a stabilized, constitutively active form in KRT5-promoter-active epithelial cells by dosage with oral doxycycline (Zhang et al., 2008). Immunofluorescence revealed KRT5 expression in basal oral ectodermal cells and incisor epithelia, including stellate reticulum cells of the cervical loop, but not in mesenchymal cells (Appendix Fig. 2). No dental abnormalities were observed in uninduced Keratin5-rtTA tetO-Cre Ctnnb1fl(ex3)/+ mice (data not shown). In adult mutants examined 5 days after the initiation of doxycycline induction, β-catenin protein levels were increased in oral and dental epithelia, including the incisor cervical loop (Figs. 1a, 1b). Serial sectioning, histological analysis, and analysis of BrdU incorporation revealed marked thickening of the stellate reticulum layer (Fig. 1d, arrowhead), expansion and outpouching of the labial cervical loop (Fig. 1d, arrows), and increased proliferation of stellate reticulum cells (Figs. 1e, 1f, arrowhead). Proliferation of dental papilla mesenchymal cells adjacent to the inner dental epithelium (IDE) of the cervical loop was also up-regulated (Figs. 1e, 1f, arrow).
Figure 1.
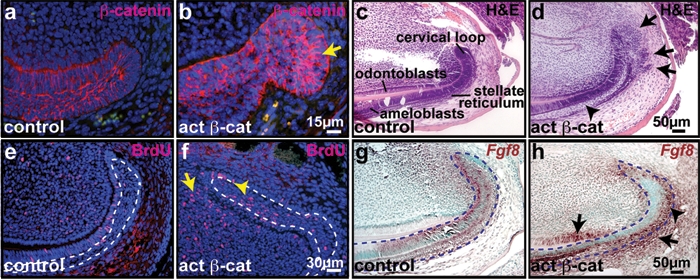
Forced activation of β-catenin signaling caused expansion of the incisor tooth cervical loop. Sagittal sections of control or KRT5-rtTA tetO-Cre Ctnnb1fl(ex3)/+ decalcified upper incisors at post-natal day (P) 41, 5 days after the initiation of doxycycline induction. (a,b) β-catenin protein (red) was elevated in mutant epithelial cells (arrow). (c,d) Hematoxylin and eosin (H&E) staining revealed expansion of mutant stellate reticulum (arrowhead) and multiple outpouchings of the cervical loop (arrows). (e,f) BrdU incorporation (red) was increased in mutant stellate reticulum (f, arrowhead) and dental pulp (f, arrow). (g,h) Increased and expanded Fgf8 RNA expression (purple-brown) in mutant stellate reticulum (arrowhead), odontoblasts, and mesenchymal cells adjacent to the ODE (arrows). White dashed lines in (e,f) outline the stellate reticulum region. Blue dashed lines outline the epithelium in (g,h). Sections in (a,b,e,f) were counterstained with DAPI (blue). Sections in (g,h) were counterstained with methyl green.
To investigate the molecular mechanisms underlying the effects of activated epithelial β-catenin in the cervical loop, we examined expression of genes required for the development and maintenance of this structure, including Fgf10, Fgf3, Fgf4, and Bmp4 (Harada et al., 2002; Wang et al., 2007). In situ hybridization did not reveal obvious changes in the expression patterns or levels of these transcripts in activated β-catenin mutants induced for 5 days compared with controls (data not shown). We therefore investigated the effects of activated β-catenin on the expression of Fgf8, which is normally expressed in embryonic dental lamina and competes with Bmp4, expressed in adjacent epithelium, to specify the locations of dental precursor vs. intervening non-dental cells at the initiation of embryonic dental development (Neubuser et al., 1997; St Amand et al., 2000). In control adult incisors, Fgf8 mRNA was present in IDE cells of the cervical loop and pre-ameloblasts, and was weakly expressed in the stellate reticulum and in dental papilla mesenchyme adjacent to the IDE (Fig. 1g). In mutant mice, Fgf8 expression was up-regulated in stellate reticulum (Fig. 1h, arrowhead), and was ectopically activated in dental follicle mesenchyme adjacent to the outer dental epithelium (ODE) and in pre-odontoblasts (Fig. 1h, arrows). These results indicate that forced activation of β-catenin up-regulates Fgf8 in dental epithelium and mesenchyme. Since β-catenin is mutated only in epithelial cells, expression of Fgf8 in mutant mesenchyme is likely a secondary effect of β-catenin activation.
To determine the longer-term effects of β-catenin activation, we examined adult mutant and littermate control mice 20 days after initiating doxycycline induction. Histological analysis revealed ectopic outpouchings of incisor tooth tissue in the cervical loop and from more anterior labial and, to a lesser extent, lingual incisor regions (Appendix Figs. 3a, 3b; Figs. 2a-2d). Although superficially similar in appearance to embryonic tooth buds, the outpouchings lacked a clear histological structure resembling an enamel knot. In some cases, we observed ectopic elongated epithelial and mesenchymal cells that were separated by a layer of acellular material (Figs. 2a-2d) and were positive for the ameloblast marker Amelogenin and the ameloblast and odontoblast marker Dentin Sialophosphoprotein (Dspp), respectively (Figs. 2e-2h; Appendix Figs. 3c-3f). Mutant epithelium displayed elevated expression of β-catenin protein (Appendix Figs. 3g-3j) and the Wnt target gene c-myc (Appendix Figs. 3k, 3l). Proliferation was increased in the mutant cervical loop, invaginating epithelium, and adjacent mesenchymal cells compared with controls (Appendix Figs. 3m, 3n). No obvious abnormalities in the molar teeth or the diastema region were observed after 20 days of doxycycline induction. Mice died approximately 3 wks after the initiation of induction, precluding analysis of the possible effects of β-catenin activation in molar tooth and diastema regions at later stages.
Figure 2.
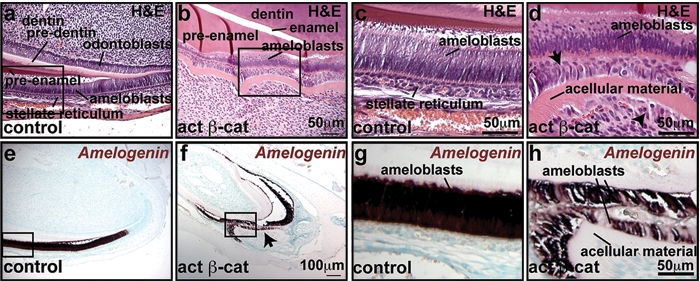
Prolonged activation of β-catenin signaling causes formation of ectopic dental structures. Sagittal sections of control or KRT5-rtTA tetO-Cre Ctnnb1fl(ex3)/+ decalcified upper incisors at post-natal day (P) 41, 20 days after the initiation of doxycycline treatment. (a-d) H&E staining reveals outgrowth of mutant dental epithelial and mesenchymal tissue. Mutant stellate reticulum is expanded, and stellate reticulum cells (d, arrow) and underlying mesenchymal cells (d, arrowhead) are elongated in the mutant compared with the control. A layer of acellular material separates these 2 layers. (c,d) Magnifications of the boxed areas in (a,b), respectively. (e-h) Differentiation of ectopic dental tissues in the mutant (f, h, arrow), indicated by in situ hybridization for the ameloblast marker amelogenin (e-h) (purple-brown signals). (g,h) Magnifications of the boxed areas in (e,f), respectively. Sections in (e-h) were counterstained with methyl green.
To determine whether prolonged induced epithelial β-catenin activation alters expression of additional markers and regulators of embryonic tooth development in incisor teeth, we examined expression of dental regulators 20 days after the initiation of induction. Expression of Fgf8 mRNA remained dramatically up-regulated in mutant cervical loop and adjacent mesenchymal cells (Appendix Figs. 4a, 4b). Fgf8 was also expressed in the epithelium and mesenchyme of ectopic tooth-like structures (Appendix Figs. 4c, 4d). Mesenchymal FGF3 is a key regulator of dental epithelial stem cell proliferation, while mesenchymal BMP4 actively represses FGF3 expression, thus negatively controlling epithelial stem cell and transient amplifying cell proliferation (Wang et al., 2007). At 20 days, expression of Fgf3 mRNA was ectopically activated in all layers of the cervical loop in a scattered manner and was also up-regulated in dental mesenchyme adjacent to the IDE (Figs. 3a, 3b), as well as in the epithelium and mesenchyme of ectopic tooth-like structures (Appendix Figs. 4e-4h). The relatively late effects of β-catenin mutation on Fgf3 expression, and its induction in the mesenchyme, suggest that Fgf3 up-regulation is an indirect consequence of β-catenin activation. Expression of Bmp4 was down-regulated in both epithelium and mesenchyme of mutant incisors compared with controls (Figs. 3c, 3d), and nuclear localized phosphorylated Smad1,5,8, an indicator of active BMP signaling, was decreased in mutant epithelium and mesenchyme (Figs. 3e, 3f).
Figure 3.
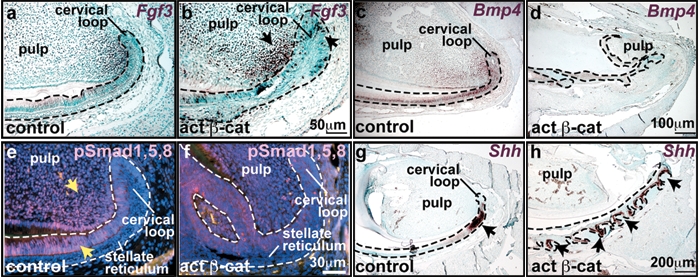
Prolonged activation of epithelial β-catenin caused altered expression of multiple signaling molecules. Upper incisors were dissected from P70 control or mutant mice following 20 days of doxycycline treatment, decalcified, and sectioned sagittally. (a,b) In situ hybridization for Fgf3 (purple-brown) reveals expression of Fgf3 in dental pulp adjacent to the cervical loop in controls. In mutants, Fgf3 is up-regulated in this area, and is also elevated in the mutant cervical loop (arrow). (c-f) Bmp4 transcripts (purple-brown) (c,d) and pSMAD1,5,8 protein (red) (e,f) localize to control ameloblasts, odontoblasts, and pulp cells adjacent to the cervical loop, and are reduced in mutant tissue. (g,h) In situ hybridization reveals Shh expression in control pre-ameloblasts (g, arrow). In the mutant, Shh expression is induced in epithelial outgrowths (h, arrows).
Shh signaling regulates growth and morphogenesis of the embryonic tooth, including the invagination of dental epithelium (Dassule et al., 2000; Gritli-Linde et al., 2002; Cobourne et al., 2004). Expression of Shh mRNA was limited to pre-ameloblasts adjacent to the cervical loop in control incisors. In mutant incisors, the domain of Shh mRNA expression was expanded to include the multiple epithelial invaginations into the mesenchyme (Figs. 3g, 3h). Like Shh, Wnt10b is normally expressed from early stages of embryonic dental development, first appearing in dental placodes, and subsequently localizing to the enamel knot. Wnt10b expression was elevated in ameloblasts and in the epithelium of mutant ectopic dental structures, in a pattern similar to that of Shh (Appendix Figs. 4i-4n). Thus, induced activation of β-catenin in the adult incisor enhances and/or expands the expression of multiple markers and regulators of embryonic tooth development and results in the formation of ectopic differentiating dental structures. Analysis of these data suggests that the mechanisms underlying β-catenin-mediated ectopic dental development may partially mimic those operating in embryonic tooth development.
To test whether ectopic dental structures can become fully mineralized, we dissected the apical ends of incisors from adult mutant mice 10 days after initiating induction and implanted these subcutaneously into nude mice for 8 wks. While control implants maintained their original shapes, mutant implants gave rise to cyst-like structures (Appendix Figs. 5a, 5b). Visualization of the three-dimensional distribution of mineralization by micro-computed tomography (micro-CT) (Ritman, 2002, 2004) showed that the cyst-like structures in mutant implants possessed dentin-like radiopacity, with patchy regions of higher radiopacity resembling that of enamel in control implants (Figs. 4a, 4b; Appendix Figs. 5c, 5d, arrows). Histological analysis of decalcified samples revealed that the cysts contained numerous dental structures at various stages of development, pre-enamel, dentin, and open spaces between ameloblasts and dentin, indicative of enamel (Appendix Figs. 5e-5j). In situ hybridization for the ameloblast markers Ameloblastin and Amelogenin confirmed that differentiating ameloblasts were formed throughout the ectopic dental structures in mutant implants (Figs. 4c-4f and data not shown). Ectopic dentin tubules and enamel-like structures could be identified in non-decalcified ground sections of implants (Figs. 4g-4j; Appendix Figs. 5k-5m). Thus, ectopic dental structures generated by the activation of β-catenin signaling can differentiate to produce mineralized dentin and enamel.
Figure 4.
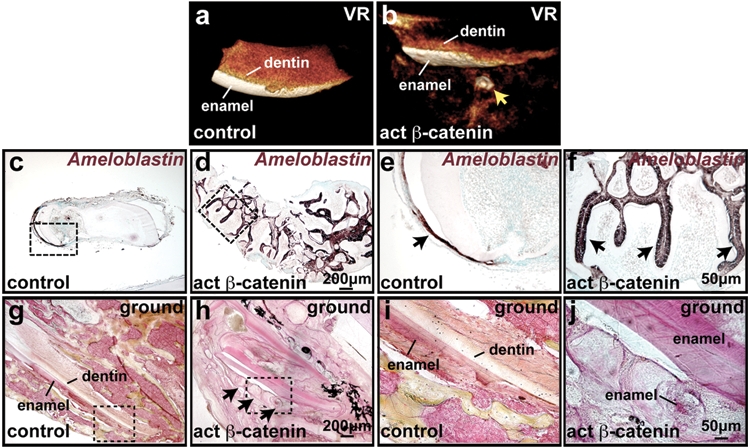
Implanted mutant incisors gave rise to well-mineralized dental structures. Incisors from adult mutant and control mice doxycycline treated for 10 days were implanted subcutaneously into nude mice and analyzed after 8 wks. (a,b) Analysis of the results of micro-CT imaging by three-dimensional volume rendering (VR) reveals formation of ectopic mineralized tissue in the mutant sample (arrow). (c-f) In situ hybridization of sectioned implant tissue for Ameloblastin (purple-brown) reveals multiple sites of ectopic dental differentiation in mutant samples. Boxed regions in (c,d) are magnified in (e,f). (g-j) Ground sections of implants stained with Villanueva Osteochrome. Boxed regions in (g,h) are magnified in (i,j). Ectopic formation of dentin and enamel is evident in the mutant.
Discussion
Deletion of the intracellular Wnt inhibitor Apc or β-catenin activation in embryogenesis or early post-natal life causes ectopic tooth formation from dental lamina and molar as well as incisor areas (Jarvinen et al., 2006; Kuraguchi et al., 2006; Liu et al., 2008; Wang et al., 2009). By contrast, we found that, in mature adult mutant mice, although β-catenin was activated in the diastema and both lingual and labial incisor epithelia, the formation of ectopic dental structures was most prominent in the labial incisor and was absent from the diastema and molar teeth 20 days after the induction of β-catenin mutation. This pattern of ectopic dental development is similar to that recently described for K14-CreER™-mediated inducible deletion of Apc in mature adult mice (Wang et al., 2009). Analysis of these data indicates that embryonic and adult oral epithelia differ in their sensitivity to the effects of β-catenin activation. Lack of adult molar phenotypes may also reflect the fact that dental lamina epithelial cells of molar teeth degenerate following eruption. Differential susceptibility within the incisor tooth may result from asymmetric distribution of key signaling molecules in labial and lingual mesenchyme (Wang et al., 2007), allowing the labial incisor to provide a particularly favorable environment for ectopic tooth development.
Although β-catenin was mutated only in epithelial cells in the mutants examined here, adjacent mesenchymal cells were induced to proliferate and differentiate into odontoblasts and papilla-like cells, presumably by secreted factors (such as FGFs) expressed in response to epithelial β-catenin activation. Several different populations of mesenchymal stem cells have been identified in teeth (Seo et al., 2004; Sonoyama et al., 2006; Song et al., 2009) and may contribute to these lineages in ectopic, induced tooth structures.
Analysis of our data indicates that adult rodent incisor epithelial cells can be forced to adopt an embryonic-like developmental program by activating β-catenin signaling. Further investigations will be needed to determine whether co-expression of additional factors could render other adult oral epithelial cell populations competent to participate in ectopic tooth formation. Since tight regulation of β-catenin signaling is necessary for the formation of normally shaped teeth from embryonic oral ectoderm (Jarvinen et al., 2006; Liu et al., 2008), potential future applications of our findings in tissue engineering approaches will likely require the development of methods for controlling and modulating spatial and temporal activation of the β-catenin pathway.
Supplementary Material
Acknowledgments
We thank Carolyn Gibson for helpful discussions; Boris Jerchow and Walter Birchmeier for Axin2lacZ mice; Adam Glick for K5-rtTA mice; and Leroy Ash for histology.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This study was funded by NIH R01-DE015342 and RC1-DE020337 (SEM).
References
- Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG. (2006). Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone 38:758-768 [DOI] [PubMed] [Google Scholar]
- Andl T, Reddy ST, Gaddapara T, Millar SE. (2002). WNT signals are required for the initiation of hair follicle development. Dev Cell 2:643-653 [DOI] [PubMed] [Google Scholar]
- Cobourne MT, Miletich I, Sharpe PT. (2004). Restriction of sonic hedgehog signalling during early tooth development. Development 131:2875-2885 [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. (1999). Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126:4557-4568 [DOI] [PubMed] [Google Scholar]
- Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. (2000). Sonic hedgehog regulates growth and morphogenesis of the tooth. Development 127:4775-4785 [DOI] [PubMed] [Google Scholar]
- Diamond I, Owolabi T, Marco M, Lam C, Glick A. (2000). Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol 115:788-794 [DOI] [PubMed] [Google Scholar]
- Feldkamp LA, Davis LC, Kress JW. (1984). Practical cone-beam algorithm. Jrnl Optic Soc Amer A1:612-619 [Google Scholar]
- Gordon MD, Nusse R. (2006). Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem 281:22429-22433 [DOI] [PubMed] [Google Scholar]
- Gritli-Linde A, Bei M, Maas R, Zhang XM, Linde A, McMahon AP. (2002). Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization. Development 129:5323-5337 [DOI] [PubMed] [Google Scholar]
- Harada H, Toyono T, Toyoshima K, Yamasaki M, Itoh N, Kato S, et al. (2002). FGF10 maintains stem cell compartment in developing mouse incisors. Development 129:1533-1541 [DOI] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, et al. (1999). Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J 18:5931-5942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvinen E, Salazar-Ciudad I, Birchmeier W, Taketo MM, Jernvall J, Thesleff I. (2006). Continuous tooth generation in mouse is induced by activated epithelial Wnt/beta-catenin signaling. Proc Natl Acad Sci USA 103:18627-18632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. (2002). Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol 22:1172-1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraguchi M, Wang XP, Bronson RT, Rothenberg R, Ohene-Baah NY, Lund JJ, et al. (2006). Adenomatous polyposis coli (APC) is required for normal development of skin and thymus. PLoS Genet 2:e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Thirumangalathu S, Gallant NM, Yang SH, Stoick-Cooper CL, Reddy ST, et al. (2007). Wnt-beta-catenin signaling initiates taste papilla development. Nat Genet 39:106-112 [DOI] [PubMed] [Google Scholar]
- Liu F, Chu EY, Watt B, Zhang Y, Gallant NM, Andl T, et al. (2008). Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev Biol 313:210-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, et al. (2003). Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA 100:3299-3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, et al. (2003). beta-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem 278:40231-40238 [DOI] [PubMed] [Google Scholar]
- Neubuser A, Peters H, Balling R, Martin GR. (1997). Antagonistic interactions between FGF and BMP signaling pathways: a mechanism for positioning the sites of tooth formation. Cell 90:247-255 [DOI] [PubMed] [Google Scholar]
- Ritman EL. (2002). Molecular imaging in small animals—roles for micro-CT. J Cell Biochem Suppl 39:116-124 [DOI] [PubMed] [Google Scholar]
- Ritman EL. (2004). Micro-computed tomography—current status and developments. Annu Rev Biomed Eng 6:185-208 [DOI] [PubMed] [Google Scholar]
- Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. (2004). Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364:149-155 [DOI] [PubMed] [Google Scholar]
- Song J-S, Stefanik D, Damek-Poprawa M, Alawi F, Akintoye SO. (2009). Differentiation and regenerative capacities of human odontoma-derived mesenchymal cells. Differentiation 77:29-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, et al. (2006). Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 1:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Amand TR, Zhang Y, Semina EV, Zhao X, Hu Y, Nguyen L, et al. (2000). Antagonistic signals between BMP4 and FGF8 define the expression of Pitx1 and Pitx2 in mouse tooth-forming anlage. Dev Biol 217:323-332 [DOI] [PubMed] [Google Scholar]
- Wang XP, Suomalainen M, Felszeghy S, Zelarayan LC, Alonso MT, Plikus MV, et al. (2007). An integrated gene regulatory network controls stem cell proliferation in teeth. PLoS Biol 5:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XP, O’Connell DJ, Lund JJ, Saadi I, Kuraguchi M, Turbe-Doan A, et al. (2009). Apc inhibition of Wnt signaling regulates supernumerary tooth formation during embryogenesis and throughout adulthood. Development 136:1939-1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HM, Jerchow B, Sheu TJ, Liu B, Costantini F, Puzas JE, et al. (2005). The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development 132:1995-2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Andl T, Yang SH, Teta M, Liu F, Seykora JT, et al. (2008). Activation of beta-catenin signaling programs embryonic epidermis to hair follicle fate. Development 135:2161-2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


