Abstract
The incidence of cardiovascular disease, including inflammatory heart diseases like myocarditis, is increased in men. Similarly, male BALB/c mice infected with coxsackievirus B3 (CVB3) develop more severe acute inflammation in the heart compared to females. To better understand the effect of male sex hormones on cardiac inflammation, we gonadectomized (Gdx) male BALB/c mice and examined acute CVB3-induced myocarditis compared to sham controls. Viral replication in the heart was not significantly altered between Gdx and sham mice. However, gonadectomy significantly reduced testosterone levels and inflammation in the heart. FACS analysis of cell populations isolated from the heart revealed that CD11b+ cells were significantly reduced in Gdx males. However, a GR1+F4/80+ subset of CD11b+ cells was significantly increased. Because this subset also expressed the interleukin (IL)-4R and IL-10, we refer to these cells as “alternatively activated” or M2 macrophages. A greater percentage of M2 macrophages in Gdx males expressed the inhibitory receptor Tim-3, while fewer expressed IL-1β and IL-10. Only M2 macrophages upregulated TLR4 and Tim-3, whereas GR1−IL-4Rlo macrophages did not. Additionally, IL-4+CD4+ Th2 cells, Foxp3+ regulatory T (Treg) cells and Tim-3+CD4+ T cells were significantly increased in the heart following Gdx. Thus, we report for the first time that the inhibitory receptor Tim-3 is expressed on M2 macrophages. Our findings show that sex hormones and/ or other mediators released from the testes inhibit anti-inflammatory populations in the heart including Tim-3+ M2, Tim-3+CD4+ T cells, Th2 and Treg resulting in more severe acute cardiac inflammation in males following CVB3 infection.
Keywords: Autoimmunity, Cytokines, Macrophages, Myocarditis, Sex differences, Th2, Tim-3, Tolerance, Treg, Virus
1. Introduction
The incidence and severity of heart disease, including myocarditis, is higher among men than women (Dec, 2003; Rosamond et al., 2008). Inflammation underlies the pathogenesis of many common cardiovascular diseases, such as myocardial infarction, atherosclerosis, myocarditis and congestive heart failure. Enteroviruses, like coxsackievirus B3 (CVB3), infect vascular tissues in culture and have been detected in atherosclerotic plaques from patients (Godeny et al., 1986; Kwon et al., 2004; Roivainen, 1999). CVB3 infection is commonly associated with myocarditis, an autoimmune disease that may progress to dilated cardiomyopathy (DCM) and congestive heart failure in susceptible individuals or mice (Fairweather and Rose, 2007a; 2007b; Feldman and McNamara, 2000; Huber, 2005; Woodruff, 1980). While most individuals recover from acute inflammation in the heart, some go on to develop chronic inflammation associated with autoantibody deposition, fibrosis, DCM and heart failure (Fairweather et al., 2004a; 2006). Although more men than women develop myocarditis, rates of infection with CVB3 are similar between the sexes worldwide (Dechkum et al., 1998; Khetsuriani et al., 2006; Schoub et al., 1985).
The lower incidence of heart disease in women has been attributed to the cardioprotective effects of estrogen (Rossouw et al., 2002). In CVB3-induced myocarditis male BALB/c mice develop significantly increased acute inflammation at day 8, 10 and 12 post infection (pi) compared to females (Fairweather and Rose, 2007b; Frisancho-Kiss et al., 2006a; 2007), yet there is no difference in viral replication in the heart indicating that the CVB3/ cardiac myosin inoculum amplifies inflammation by acting as an adjuvant (Fairweather et al., 2005a). Increased inflammation in males is associated with elevated levels of the proinflammatory cytokines interleukin (IL)-1β, IL-18 and interferon (IFN)-γ, while females have increased levels of IL-4 in the heart (Frisancho-Kiss et al., 2006a; Huber and Pfaeffle, 1994; Huber et al., 1999). IL-17 levels are not increased in male hearts during acute CVB3 myocarditis (Fairweather et al., 2008). We showed previously that more mast cells (MC) and macrophages from males express the proinflammatory receptor Toll-like receptor 4 (TLR4) during innate immunity and acute myocarditis (Frisancho-Kiss et al., 2007; Fairweather and Frisancho-Kiss, 2008). Male mice deficient in TLR4 signaling develop significantly reduced CVB3-induced myocarditis that correlates with lower levels of IL-1β and IL-18 in the heart (Fairweather et al., 2003). Thus, TLR4 signaling increases proinflammatory cytokines and acute inflammation in males.
Females respond to CVB3 infection by upregulating not only TLR4, but also the inhibitory receptor T cell immunoglobulin mucin (Tim)-3 on MCs, macrophages and T cells during innate immunity and acute myocarditis (Frisancho-Kiss et al., 2007; Fairweather and Frisancho-Kiss, 2008). The sex difference in the percentage of MCs and macrophages expressing TLR4 and Tim-3 is not observed for dendritic cells (DC) or B cells. The family of genes encoding Tim proteins has been linked to susceptibility to allergy and autoimmune diseases (Meyers et al., 2005). Tim-3 is expressed on T helper-type 1 (Th1) CD4+ T cells, Th17 cells, regulatory T (Treg) cell populations, monocyte/macrophages, natural killer cells, DCs and MCs (Frisancho-Kiss et al., 2006b; Monney et al., 2002; Su et al., 2008). Treatment of male mice with antibodies to block Tim-3 receptor signaling increases experimental autoimmune encephalomyelitis (EAE) and CVB3-induced myocarditis (Frisancho-Kiss et al., 2006b; Monney et al., 2002). We found that blocking Tim-3 signaling during the innate immune response to CVB3 infection increases the number of CD11b+ cells and reduces Tim-3+CD4+ and forkhead box p3 (Foxp3)+ Treg populations in the heart during acute myocarditis (Frisancho-Kiss et al., 2006b). Tim-3 expression may be elevated in females due to the location of Tim genes in the IL-4 gene complex (estrogen increases IL-4 in females) (Fairweather et al., 2008; Lang, 2004; Meyers et al., 2005). Estrogen can exert either pro- or anti-inflammatory affects on NF-κB transcription depending largely on the cell type being examined (De Bosscher et al., 2006; Straub, 2007). Estrogen, via estrogen receptor (ER)-α, has been shown to directly down-regulate NF-κB and Th1 responses in various human and murine cell types (Evans et al., 2001; Demyanets et al., 2006; Feldman et al., 2007; Wang et al., 2007). Estrogen is particularly potent at inhibiting lipopolysaccharide (LPS)/TLR4-induced proinflammatory pathways and is effective at inhibiting Th1 responses in both male and female mice (Demyanets et al., 2006; Paimela et al., 2007). We have shown that males have more CD11b+/ F4/80+ macrophages and CD117+ MCs in the heart than females during acute myocarditis (Frisancho-Kiss et al., 2007). Previous studies reported that estrogen decreases whereas testosterone increases MCs and macrophages (Cutolo et al., 1996; Fairweather et al., 2008; Lima et al., 2000; Mahoney et al., 2003; Mayo et al., 1997).
Thus, sex hormones alter expression of pro- and anti-inflammatory signaling pathways that determine the severity of acute inflammation. Yet, the effect of sex hormones on acute myocarditis is largely unknown. In this study, we examined the role of gonadectomy on the development of acute CVB3-induced myocarditis. Male BALB/c mice underwent gonadectomy, and then myocarditis and viral replication were compared to mice that received a sham operation, leaving the testes intact. Additionally, we characterized changes in the immunologic phenotype of infiltrating cells in the heart by FACS analysis and ELISA following gonadectomy. Findings from this study enhance our understanding of the role of sex hormones in regulating inflammation in the heart following infection.
2. Materials and methods
2.1 Mice
Male BALB/cJ (BALB/c) mice (6 weeks of age) were obtained from the Jackson Laboratory (Bar Harbor, ME). Mice were maintained under pathogen-free conditions in the animal facility at the Johns Hopkins School of Medicine, and approval was obtained from the Animal Care and Use Committee of the Johns Hopkins University for all procedures.
2.2 Gonadectomy
At 6 to 7 weeks of age, male BALB/c mice were bilaterally gonadectomized (Gdx) or received a sham operation under a Ketamine (50mg/ kg)/ xylazine (5mg/ kg) anesthesia (Phoenex Pharmaceutical, St. Joseph, MO), as previously (Cernetich et al., 2006; Hannah et al., 2008). Mice were allowed two weeks to recover from the operations before they were infected with CVB3 at around 8 weeks of age. Individual experiments were repeated at least three times, providing consistent findings.
2.3 Myocarditis
After recovering from the gonadectomy or sham operations, 7 to 10 mice/ group were inoculated ip with a heart-passaged stock of CVB3 (containing CVB3 and cardiac myosin) to assess myocarditis. CVB3 (Nancy strain) was originally obtained from the American Type Culture Collection (ATCC, Manassas, VA) and grown in Vero cells (ATCC, Manassas, VA) according to the method in Fairweather and Rose (2007b). CVB3 was diluted in sterile saline and 103 PFU injected ip on day 0 and tissues and sera collected on day 12 pi (acute myocarditis). Mice inoculated ip with PBS or uninfected heart homogenate did not develop myocarditis (data not shown). Hearts were cut longitudinally and fixed in 10% phosphate-buffered formalin and embedded in paraffin for histological analysis. Sections 5μm thick were cut at various depths in the tissue section and stained with H&E to determine the level of inflammation or toluidine blue to detect MC granules. The number of MCs in 50 fields of view was counted with the aid of a microscope eyepiece grid (Fairweather et al., 2004a; 2004b). Sections were examined by two independent investigators and myocarditis assessed as the percentage of the heart section with inflammation compared to the overall size of the heart section, with the aid of a microscope eyepiece grid. Individual experiments using 7 to 10 mice/ group were conducted 3–4×, providing consistent findings.
2.4 Cytokine and Testosterone Measurement
Blood was collected at day 12 pi by heart puncture, allowed to clot at room temperature, centrifuged and sera collected and frozen at −80°C within 30 minutes. Hearts were collected at day 12 pi, frozen in dry ice immediately and stored at −80°C until homogenized. Tissues were homogenized at 10% weight/ volume in 2% MEM and supernatants were stored at −80°C until used in ELISA (or plaque assay). Cytokines and testosterone were measured in heart supernatants and/ or sera using Quantikine cytokine ELISA kits (R&D Systems, Minneapolis, MN) according to manufacturer's instructions, as previously (Fairweather et al., 2003). Cytokines were below detectable levels in the 2% MEM used to homogenize samples (data not shown). Cytokines/ hormones were expressed as pg/ mL for sera or pg/ g for heart tissue ±SEM.
2.5 Plaque Assay
The level of infectious virus was determined in heart homogenates (see Section 2.4) at day 12 pi by plaque assay, according to standard procedures (Fairweather et al., 2003; Fairweather and Rose, 2007b). Dilutions of tissue supernatants were incubated on Vero cell monolayers (ATCC, Manassas, VA) for 1h at 37°C and 5% CO2 to allow viral attachment and then incubated for three days to allow plaque formation. Virus titers were expressed as the mean PFU/ g tissue ±SEM and the limit of detection was 10 PFU/ g of tissue.
2.6 Heart Digestion and FACS Analysis
Male BALB/c mice (15 mice/ group) were infected with 103 PFU CVB3 ip after recovering from gonadectomy or sham operations and leukocytes isolated from the heart on day 10 pi during acute myocarditis. The heart was perfused at a constant flow of 14 mL/ min with cold PBS (Biofluids, Rockville, MD) for 2 min, as previously (Fairweather et al., 2005b; Lenzo et al., 2002). Briefly, immune cells were released from the myocardium by digestion with collagenase II (1 mg/ mL, Sigma-Aldrich, St. Louis, MO) and protease XIV (0.5 mg/ mL, Sigma-Aldrich, St. Louis, MO) in PBS for 7 min at 37°C, and single cell separation completed using razor blades to dislodge immune cells from the tissue. The total number of cells isolated after collagenase digestion of the heart was not significantly different between Gdx and sham mice. Individual cell suspensions from 3 mice were pooled to form 5 samples per group. A sample of cells from the digested heart was used to determine the percent CD45+ cells from total live heart cells. Immune cells were then separated from heart cells using a magnetic column and mouse panleukocyte CD45 MicroBeads (30F11.1; Miltenyi Biotec, Auburn, CA), as previously (Fairweather et al., 2005b; Frisancho-Kiss et al., 2006b; 2007).
Immune cells were obtained from the heart and stained with the following fluorochrome conjugated mAbs and corresponding isotype controls (BD Biosciences, San Diego, CA; R&D Systems, Minneapolis, MN) diluted in 1% FBS in PBS (Invitrogen Life Technologies, Carlesbad, CA): CD45 (30F11.1), CD11b (M1/70), F4/80 (BM8), GR-1 (RB6-8C5), B220 (RA3-6B2), CD3e (145-2C11), CD4 (GK1.5), CD8 (53-6.7), CD117 (ACK2), TLR4 (MTS5510), Tim-3 (8B.2C12), IL-4R (mIL-4R-M1), CTLA-4 (CD152/FAB434F), Foxp3 (FJK-165), IL-4 (11B11), IFN-γ (XMG1.2), IL-17A (eBio TC11), IL-1β (polyclonal) and IL-10 (JES5-16E3). For intracellular staining, cells were fixed and permeabilized using BD Cytofix/ Cytoperm or an anti-mouse Foxp3 staining kit (BD Biosciences). To measure intracellular cytokines, cells were stimulated for 4 hours in the presence of PMA/ Ionomycin/ Brefeldin A (Leukocyte Activation Cocktail, BD Biosciences, San Diego, CA) at 37°C in a CO2 incubator. Cells were washed and stained for cell surface markers, then fixed and permeabilized, as above, and stained with antibodies against cytokines. Cell fluorescence was measured using a LSR flow cytometer (BD Biosciences, San Diego, CA) and data analyzed using FlowJo (Tree Star Inc., Ashland, OR). Approximately 5,000 events/ cells were counted for each sample.
2.7 Statistical Analysis
Normally distributed data were analyzed by the Student's t test. The Mann-Whitney U test was used to evaluate nonparametric data. Significant differences were obtained by comparing CVB3-infected Gdx mice to infected sham controls. Test values with a p < 0.05 were considered significantly different from control values. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
3. Results
3.1 Gonadectomy decreases testosterone levels in the sera and hearts of male mice
The heart is a target organ for androgenic anabolic steroids and androgen receptors have been detected in the heart of rats and other species (Melchert et al., 2001). Interestingly, hearts from rats were found to accumulate testosterone to levels greater than other target tissues like skeletal muscle or prostate tissue (Krieg et al., 1978). In order to assess whether gonadectomy influenced systemic and local (i.e. heart) levels of testosterone, samples were obtained during acute myocarditis (day 12 pi) and testosterone levels measured in the sera and heart homogenates of individual mice by ELISA. Gonadectomy significantly reduced the level of testosterone detected in the sera (p = 0.03; Fig. 1A) and heart (p = 0.04; Fig. 1B) during acute CVB3-induced myocarditis. Yet even after gonadectomy, testosterone levels remained relatively high in the heart compared to systemic levels by this detection method (Fig. 1).
Fig. 1.
Gonadectomy decreases testosterone levels during acute CVB3-induced myocarditis. Male BALB/c mice were infected with 103 PFU CVB3 ip on day 0, after recovery from gonadectomy (Gdx) or sham operation, and testosterone levels in the sera (A) or heart (B) were measured by ELISA at day 12 pi. Similar results were obtained in 3 separate experiments. Data show the mean ±SEM of 7 to 10 mice/ group. *, p < 0.05.
3.2 Gonadectomy reduces inflammation in the heart
Previously we found that male BALB/c mice develop more severe CVB3-induced myocarditis at day 8, 10 or 12 pi compared to female mice (Frisancho-Kiss et al., 2006a; 2007), suggesting that sex hormones influence inflammation in the heart of males. Following gonadectomy of male mice, the percent acute CVB3-induced myocarditis at day 12 pi was significantly decreased (n = 31/ group, p = 0.0003; Fig. 2). Thus, gonadectomy of male mice resulted in low levels of cardiac inflammation of around 15% (Fig. 2A), levels similar to those observed in intact female mice at day 12 pi in our previous studies.
Fig. 2.
Gonadectomy decreases acute inflammation in the heart. Male BALB/c mice were infected with 103 PFU CVB3 ip on day 0, after recovery from gonadectomy (Gdx) or sham operation, and hearts collected on day 12 pi during acute myocarditis. Myocardial sections were stained with H&E to detect inflammation and assessed as the percentage of the heart section with inflammation compared to the overall size of the heart section (A). Representative histology sections are shown for sham (B) and Gdx (C) males (original magnification ×64). Data show the mean ±SEM of 31 mice/ group combining 4 separate experiments. Myocarditis was decreased in all 4 independent experiments, using 7 to 10 mice/ group. *** p <0.001.
3.3 Viral replication is not decreased following gonadectomy
Since reduced inflammation in the heart of Gdx males could be due to reduced viral replication, we examined the level of viral replication in the heart of Gdx and sham mice at day 12 pi. We found that viral replication was not significantly decreased in any of three separate experiments (one representative experiment shown in Fig. 3, p = 0.57). Thus, changes in viral replication are not responsible for the decreased inflammation observed in Gdx males.
Fig. 3.
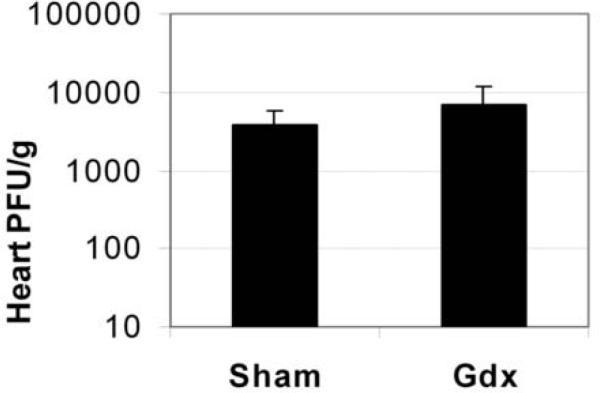
Viral replication is not decreased by gonadectomy. Sham or gonadectomized (Gdx) males were infected with 103 PFU CVB3 ip on day 0 and compared for the level of viral replication in the heart at day 12 pi, during acute myocarditis. Data are presented as the mean PFU/ g heart ±SEM of 7 to 10 mice/ group. One representative example of three separate experiments is shown, p = 0.57.
3.4 FACS analysis of cell populations in the heart: CD45+ and CD11b+ cells decrease
We previously showed that CD11b+ cells, which represent macrophages, neutrophils, MCs and DCs, are significantly increased in the hearts of males during acute CVB3-induced myocarditis, whereas B cells, Tim-3+CD4+ T cells and Foxp3+ Treg are increased in females (Frisancho-Kiss et al., 2007). To better understand the mechanisms involved in the decrease in inflammation observed in Gdx males compared to sham controls, we examined specific cell populations in the heart by FACS. Because more cells can be isolated from the heart at day 10 pi, and some cells are lost in the isolation procedure, we isolated CD45+ leukocytes from the heart of Gdx or sham CVB3-infected males at day 10 pi. Confirming the histological findings at day 12 pi (Fig. 2), the percentage of CD45+ leukocytes compared to total heart cells was significantly decreased in Gdx males at day 10 pi (p = 0.005; Fig. 4A and B). Comparing the relative proportion of CD11b+ cells, B cells (B220+), T cells (CD4+ or CD8+) and MCs (CD117+) in the CD45+ fraction, we found that only cells expressing CD11b (Fig. 4C) were significantly decreased in Gdx males (n = 10/ group, p = 0.007, Fig. 4D). In the CD11b+ population, F4/80 expression was not altered by gonadectomy (sham 41.3% vs. Gdx 40.5%). Although CD11b can be expressed on MCs, the CD117-expressing MCs from the CD45+ population were not significantly different in Gdx compared to sham males by FACS analysis (Fig. 4D) or histology of toluidine blue-stained sections (total MC number: Expt. 1 Sham 41.1 ±5.1 vs. Gdx 37.4 ±4.0, p = 0.58; Expt. 2 Sham 23.5 ±2.4 vs. Gdx 28.5 ±2.5, p = 0.16; Expt. 3 Sham 22.6 ±4.1 vs. Gdx 29.1 ±3.8, p = 0.26). We did not examine CD11b+CD11c+ DCs in this study because we showed previously that there were no sex differences in TLR4 or Tim-3 expression on DCs (Frisancho-Kiss et al., 2007). We also did not examine CD11b+GR1+F4/80− neutrophils in this study. Thus, gonadectomy significantly decreases inflammation in the heart, specifically affecting immune cells expressing CD11b.
Fig. 4.
Males develop significantly reduced CD45+ and CD11b+ inflammation in the heart following gonadectomy. Sham or gonadectomized (Gdx) males were infected with 103 PFU CVB3 ip on day 0 and compared by FACS analysis on day 10 pi during acute myocarditis for the percentage of CD45+ leukocytes compared to total heart cells (A and B) or the relative proportion of individual immune cells (C and D). The following cell types were evaluated by FACS (D): macrophages/ neutrophils/ MCs/ DCs (CD11b), B cells (B220), CD4+ T cells (CD4), CD8+ T cells (CD8), and MCs (CD117). One representative sham (light line/ red) and Gdx (heavy line/ blue) are compared by FACS for CD45 (B) or CD11b (C) expression within the gated region. Isotype controls are depicted as shaded grey (B and C). Similar results were obtained in 2 separate experiments. Data show the mean ±SEM of 10 pooled samples/ group. Each pooled sample consists of cells from 3 mice for a total of 30 mice/ group. **, p < 0.01.
3.5 FACS analysis of CD4+ T cell populations in the heart: Tim-3 and Treg increase
Gonadectomy of male BALB/c mice significantly increased CD4+ T cells expressing Tim-3 (p = 0.04, Fig. 5A) or CTLA-4 (p = 0.03, Fig. 5A) and significantly increased the percentage of Foxp3+ Treg (p = 0.003, Fig. 5A) in the heart during acute myocarditis at day 10 pi. Thus, gonadectomy of males results in a regulatory CD4+ T cell phenotype resembling that of intact females (Frisancho-Kiss et al., 2007; Huber, 2008).
Fig. 5.
Gonadectomy increases Th2, Tim-3+ and CTLA-4+ T cells in the heart of males. Mice received 103 PFU CVB3 ip on day 0 after recovery from operations and CD45+ immune cells were isolated from the heart on day 10 pi, during acute myocarditis. FACS analysis depicts expression of Tim-3, CTLA-4 (intracellular) or Foxp3 (intracellular) on/ in CD4+ T cells (A). Intracellular levels of IL-4 (Th2), IFN-γ (Th1) or IL-17 (Th17) were assessed in CD4+ T cells after stimulation for 4 hours with a leukocyte activation cocktail of PMA, Ionomycin and Brefeldin A (B). Similar results were obtained in 2 separate experiments. Data show the mean ±SEM of 5 pooled samples/ group. Each pooled sample consists of cells from 3 mice for a total of 15 mice/ group. *, p < 0.05; **, p ≤ 0.01.
3.6 FACS analysis of Th cell populations in the heart: Th2 cells increase
In this study, we examined the effect of gonadectomy on Th populations in the heart by FACS analysis of intracellular cytokine levels at day 10 pi. We found that gonadectomy of males significantly increased the percentage of CD4+ T cells expressing IL-4 (Th2 cells) (p = 0.01, Fig. 5B), but did not alter CD4+ T cells expressing IFN-γ(Th1 cells) or IL-17 (Th17 cells) (Fig. 5B). Tim-3 has been reported to be expressed only on Th1 or Th17 cells, not Th2 cells (Nakae et al., 2008; Su et al., 2008). It is interesting to note that significantly more CD4+ T cells expressed Tim-3 in Gdx males (Fig. 5A), yet the percentage of Th1 or Th17 cells was unchanged (Fig. 5B).
3.7 Cytokine profile in the heart following gonadectomy
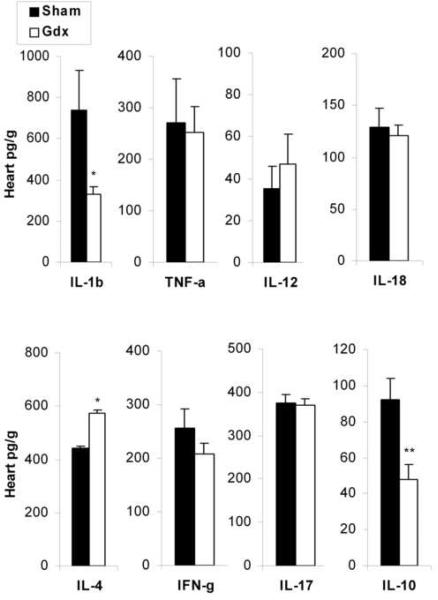
Cytokine profiles detected in the heart homogenate reflect the influence of the inflammatory infiltrate (i.e. increased inflammation in males directly correlates to IL-1β and IL-18 levels in the heart). To better understand the mechanisms responsible for decreased inflammation in Gdx males, we compared the cytokine profile of male mice that underwent gonadectomy to sham controls by ELISA at day 12 pi. We found that Gdx males produced significantly less IL-1β(p = 0.04, Fig. 6) and IL-10 (p = 0.004, Fig. 6) in the heart and significantly more IL-4 (p = 0.03, Fig. 6). The increased level of IL-4 by ELISA (Fig. 6) was consistent with the significant increase in Th2 cells that we observed in the hearts of Gdx males (Fig. 5B).
Fig. 6.
Gonadectomy reduces IL-1β and IL-10 levels in the heart while increasing IL-4 during acute myocarditis. Sham or gonadectomized (Gdx) males were infected with 103 PFU CVB3 ip on day 0 and compared for cytokines levels in the heart at day 12 pi by ELISA. Data are presented as the mean pg cytokines/ g heart ±SEM of 7 to 10 mice/ group. One representative example of four separate experiments is shown. *, p < 0.05; **, p < 0.01.
3.8 Gonadectomy shifts macrophages to a GR1+F4/80+ M2 phenotype
Regulatory macrophages have been broadly characterized into two groups 1) as CD11b+GR1+F4/80+ immature myeloid or myeloid-derived suppressor cells and 2) as IL-4R+IL-10+F4/80+ macrophages termed alternatively activated or M2 macrophages because of the requirement for IL-4 (instead of IFN-γ required by classically activated or GR1− M1 macrophages) (Anthony et al., 2007; van Ginderachter et al., 2006; Martinez et al., 2008; Sica and Bronte, 2007; Terrazas et al., 2001). We are calling the CD11b+GR1+F4/80+ cells found in this study alternatively activated or M2 macrophages because they express markers for immature myeloid suppressor cells i.e. CD11b and GR1 (Fig. 7) and IL-4R, IL-1β and IL-10 found on/ in M2 cells (Fig. 8).
Fig. 7.
Gonadectomy decreases GR1−F4/80+ M1 macrophages (A) and increases GR1+F4/80+ M2 macrophages (B and C) in the heart of males. Mice received 103 PFU CVB3 ip on day 0 and CD45+ immune cells were isolated from the heart on day 10 pi, during acute myocarditis. An example of the shift in M2 population in sham (middle) vs. Gdx (right) and isotype control (left) is shown in (C). Similar results were obtained in 2 separate experiments. Data show the mean ±SEM of 5 pooled samples/ group. Each pooled sample consists of cells from 3 mice for a total of 15 mice/ group. *, p < 0.05.
Fig. 8.
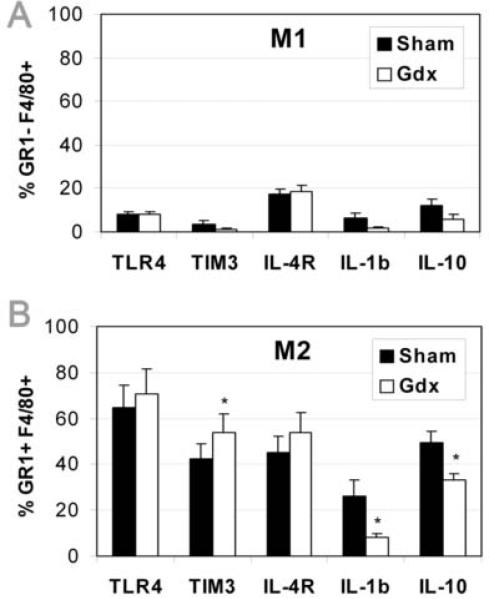
Gonadectomy increases Tim-3 expression and decreases IL-1β/ IL-10 expression on/ in GR1+F4/80+ M2 macrophages during acute myocarditis. Mice received 103 PFU CVB3 ip on day 0 and CD45+ immune cells were isolated from the heart on day 10 pi, during acute myocarditis. Expression levels of TLR4, Tim-3, IL-4R, IL-1β and IL-10 are shown for GR1−F4/80+ M1 (A) and GR1+F4/80+ M2 (B) macrophages. Similar results were obtained in 2 separate experiments. Data show the mean ±SEM of 5 pooled samples/ group. Each pooled sample consists of cells from 3 mice for a total of 15 mice/group. *, p < 0.05.
Previously we showed that CD11b+, F4/80+ and GR1+ cell populations are increased in males compared to females with acute CVB3-induced myocarditis (Frisancho-Kiss et al., 2007). In this study, we observed a shift in the F4/80+ macrophage population from significantly more GR1− (M1) macrophages in sham males (p = 0.02, Fig. 7A) to significantly more GR1+ (M2) macrophages in Gdx males (p = 0.02, Fig. 7B and C). Note that the F4/80+ macrophages within the CD11b+ population were not altered by gonadectomy (sham 41.3% vs. Gdx 40.5%). Thus, gonadectomy decreases GR1− macrophages while increasing GR1+ macrophages in the heart during acute CVB3-induced myocarditis.
3.9 Gonadectomy increases Tim-3+ M2 macrophages
Not only do females have significantly more Tim-3+CD4+ T cells and Foxp3+ Treg cells in their hearts during acute CVB3-induced myocarditis, but they also have significantly more macrophages and MCs expressing Tim-3 (Frisancho-Kiss et al., 2007). When we compared receptor and cytokine expression between GR1− (M1) and GR1+ (M2) macrophage populations by FACS at day 10 pi, we found that TLR4, Tim-3, IL-4R, IL-1β and IL-10 levels were expressed at relatively low levels on/ in GR1−F4/80+ M1 macrophages in both sham and Gdx males (Fig. 8A). In contrast, these receptors and cytokines were expressed at relatively high levels on GR1+F4/80+ M2 (Fig. 8B) compared to M1 macrophages (Fig. 8A), similar to the expression levels we had observed on macrophages in previous studies (Fairweather and Frisancho-Kiss, 2008; Frisancho-Kiss et al., 2007). Importantly, we found that gonadectomy altered the phenotype of M2 macrophages in males during acute myocarditis. Gdx males had significantly more GR1+ M2 macrophages expressing Tim-3 (p = 0.03, Fig. 8B), and significantly fewer M2 expressing IL-1β (p = 0.04, Fig. 8B) or IL-10 (p = 0.04, Fig. 8B). These data indicate that the primary macrophage population expressing TLR4 and Tim-3 in the heart of BALB/c sham and Gdx males is alternatively activated M2 macrophages. Additionally, these findings suggest that male sex hormones or other mediators released from the testes induce more M2 macrophages to express IL-1β and IL-10 than Tim-3. The TLR4+IL-1β+ M2 macrophages (possibly the M2b population suggested by Martinez et al., 2008) may function in a more proinflammatory/ profibrotic manner than those expressing Tim-3 (possibly the M2a population suggested by Martinez et al., 2008).
4. Discussion
In this study we show that sex hormones or other mediators released from the testes increase inflammation in the heart following CVB3 infection by inhibiting regulatory macrophages and T cells (i.e. Tim-3, M2, Th2 and Treg) and by increasing proinflammatory/ profibrotic macrophages (i.e. M1 and TLR4+IL-1β+ M2) (Fig. 9). Most of the macrophages in the heart of sham males were M1 (70% M1 to 30% M2), which shifted to roughly 50% M1 and 50% M2 following gonadectomy (Fig. 7). These data suggest that a primary immunologic effect of male sex hormones released from the testes is to increase proinflammatory M1 and TLR4+IL-1β+IL-10+ alternatively activated M2 macrophages in the heart following CVB3 infection (Fig. 9). Although IL-10 released from M2 in males reduces acute inflammation in the heart, IL-1β in the context of IL-4 (i.e. IL-4 is necessary for the development of M2) is profibrotic further contributing to cardiac pathology (Fairweather et al., 2004a; 2004b).
Fig. 9.
Sex hormones or other mediators released from the testes (male gonads) increase inflammation in the heart following CVB3 infection by increasing proinflammatory GR1−F4/80+ M1 and IL-1β+IL-10+ M2 macrophage populations, and by inhibiting regulatory populations like Tim-3+ M2 macrophages, Tim-3+ T cells, Th2 cells and Treg during acute CVB3-induced myocarditis.
Regulatory CD11b+GR1+F4/80+ macrophages have been referred to by various names in different disease models such as immature myeloid cells, alternatively activated M2 macrophages (expressing IL-4R, IL-10, mannose receptor and/ or arginase), myeloid-derived suppressor cells (MDSC) and tumor-associated macrophages (TAM) (Anthony et al., 2007; van Ginderachter et al., 2006; Martinez et al., 2008; Sica and Bronte, 2007; Terrazas et al., 2001). Although M1 macrophages are characterized as expressing high IL-12 and nitric oxide and low/ no IL-10, it is worth noting that LPS has been reported to activate both arginase (associated with M2) and iNOS (associated with M1) (van Ginderachter et al., 2006). LPS has also been shown to induce an M2-type cell in culture (M2b) that expresses both IL-1β and IL-10 (Martinez et al., 2008). IL-4 and IL-13 are critical for the induction of M2, and M2 cells are characterized by expression of the IL-4R (Cihakova et al., 2008; Martinez et al., 2008; Sica and Bronte, 2007). The abundance of MCs and IL-4 in the peritoneum, spleen and heart of male BALB/c mice may be responsible for promoting the development of M2 following CVB3 infection (Fairweather et al., 2004b; Frisancho-Kiss et al., 2006b).
An important finding from this study is that alternatively activated or M2 macrophages express TLR4 and Tim-3, and are a primary source of IL-1β and IL-10 during acute myocarditis (compare Fig. 6 and 8B). Our data suggest that the population of M2 expressing Tim-3+ is regulatory (Frisancho-Kiss et al., 2006b; 2007). Although Zhu et al. (2007) describe a regulatory CD11b+GR1+F4/80+ population that decreases EAE, they do not identify whether the monocytes express Tim-3. Additionally, GR1+F4/80+ M2 monocytes are known to play an important role in apoptosis, clearing damaged tissue and wound healing, particularly in the heart (Martinez et al., 2008; Nahrendorf et al., 2007; Sica and Bronte, 2007; van Ginderachter et al., 2006). A similar role has been proposed for Tim-3 (Su et al., 2008). Thus, in this study we show that male sex hormones or other mediators from the testes decrease Tim-3 expression on M2 and T cells resulting in increased acute cardiac inflammation. These findings agree with our previous reports that Tim-3 regulates CD11b+ monocytes/ macrophages during acute CVB3-induced myocarditis in males (Frisancho-Kiss et al., 2006b; 2007).
Macrophages and MCs are known to express estrogen and androgen receptors (Cutolo et al., 1996; Mayo et al., 1997; Zhao et al., 2001). Testosterone has been shown to increase the number of MCs and macrophages, histamine and cytokine release (Cutolo et al., 1996; Frisancho-Kiss et al., 2007; Lima et al., 2000; Mayo et al., 1997; Zhao et al., 2001). Although males have increased numbers of CD11b+ monocytes/ macrophages and MCs during acute CVB3-induced myocarditis compared to females (Frisancho-Kiss et al., 2007), only CD11b+F4/80+GR1− M1 macrophages were decreased by gonadectomy in males while MCs were not altered. Thus, findings from this study suggest that sex hormones released from the testes increase macrophages in the heart, but not MCs. This may be due to the fact that monocytes/ macrophages are recruited to the heart during the inflammatory response whereas MCs reside in the heart (Fairweather and Frisancho-Kiss, 2008; Galli et al., 2005; Sica and Bronte, 2007) and although testosterone was significantly reduced following Gdx, testosterone levels remained relatively high in the heart (Fig. 1B). Thus, male sex hormones may increase inflammation by amplifying the number of macrophages (i.e. M1 and TLR4+IL-1β+ M2) and/ or by transcriptionally upregulating the proinflammatory effects of TLR4 signaling by macrophages. We are currently replacing testosterone levels in Gdx males to determine whether the changes in the immune response following gonadectomy are due to testosterone.
Interestingly, several aspects of the proinflammatory response were not affected by gonadectomy in males. IFN-β and IL-17, cytokines associated with proinflammatory responses in experimental models of autoimmune disease (Cihakova et al., 2008; Dardalhon et al., 2008), were not altered by gonadectomy in males as assessed in whole heart homogenates (Fig. 6) or Th1/ Th17 cells (Fig. 5B). We have shown previously that IL-17 levels are not increased in the heart of males compared to females (Fairweather et al., 2008). This contrasts with results from experimental autoimmune myocarditis (EAM), where the Th17 response has been reported to be involved in the development of disease in mice (Chang et al., 2008; Rangachari et al., 2006; Sonderegger et al., 2006; Valaperti et al., 2008). Although CVB3 infection leads to a predominant Th1 response due to TLR4-induced IL-18 (Frisancho-Kiss et al., 2006a), autoimmune disease models using complete Freund's adjuvant (CFA) most likely generate a dominant Th17 response due to the Mycobacterium component of the adjuvant (Fairweather et al., 2008; Steinman, 2008; Stockinger et al., 2007). CFA is used to induce autoimmunity in several models including EAE, collagen-induced arthritis, experimental autoimmune uveitis and EAM (Cihakova et al., 2004; 2008; Kaya et al., 2001; Steinman, 2008). However, Tim-3 signaling and CD11b+GR1+ monocytes have been shown to decrease Th1 and Th17 responses (Ehirchiou et al., 2007; Martinez et al., 2008; Su et al., 2008; Valperti et al., 2008).
We showed previously that significantly more macrophages from males express TLR4 compared to females following CVB3 infection (Frisancho-Kiss et al., 2007). Here we found that gonadectomy did not alter the percentage of macrophages expressing TLR4 in the heart (Fig. 8). Surprisingly, the primary macrophage subtype expressing TLR4 during acute myocarditis was not M1 macrophages but IL-4R+IL-1β+IL-10+ M2 macrophages that expressed GR1 (Fig. 8). Both sham and Gdx males expressed TLR4 and Tim-3 receptors on M2 cells at levels comparable to those we reported previously (Fig. 8B) (Frisancho-Kiss et al., 2007), suggesting that the inflammatory cells expressing TLR4 and IL-1β in males are M2 rather than M1 macrophages and that cross-regulation of TLR4 and Tim-3 signaling occurs on M2. Findings from this study provide a fresh understanding of why men and male mice respond to CVB3 infection with increased cardiac inflammation compared to females.
Acknowledgments
We thank P.G. Fallon for technical assistance. This research was supported by National Institutes of Health Grants R01 HL087033 (D.F.), R01 HL67290 (N.R.R.), R01 AI054995 (S.L.K.) and P30 ES03819.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicting financial interests.
References
- Anthony RM, Rutitzky L, Urban JF, Jr., Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nature Rev. Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernetich A, Garver LS, Jedlicka AE, Klein PW, Kumar N, Scott AL, Klein SL. Involvement of gonadal steroids and gamma interferon in sex differences in response to blood-stage malaria infection. Infect. Immun. 2006;74:3190–3203. doi: 10.1128/IAI.00008-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Hanawa H, Yoshida T, Hayashi M, Liu H, Ding L, Otaki K, Hao K, Yoshida K, Kato K, Toba K, Kodama M, Maruyama H, Miyazaki J, Aizawa Y. Alteration of IL-17 related protein expressions in experimental autoimmune myocarditis and inhibition of IL-17 by IL-10-Ig fusion gene transfer. Circ. J. 2008;72:813–819. doi: 10.1253/circj.72.813. [DOI] [PubMed] [Google Scholar]
- Cihakova D, Sharma R, Fairweather D, Afanasyeva M, Rose NR. Animal models for autoimmune myocarditis and autoimmune thyroiditis. In: Perl A, editor. Methods Mol. Med. Autoimmunity: Methods and Protocols. Vol. 102. 2004. pp. 175–194. [DOI] [PubMed] [Google Scholar]
- Cihakova D, Barin JG, Afanasyeva M, Kimura M, Fairweather D, Berg M, Talor MV, Baldeviano GC, Frisancho-Kiss S, Gabrielson K, Bedja D, Rose NR. Interleukin-13 protects against experimental autoimmune myocarditis by regulating macrophage differentiation. Am. J. Pathol. 2008;172:1195–1208. doi: 10.2353/ajpath.2008.070207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo M, Accardo S, Villaggio B, Barone A, Coviello DA, Carabbio C, Felli L, Miceli D, Farruggio R, Carruba G, Castagnetta L. Androgen and estrogen receptors are present in primary cultures of human synovial macrophages. J. Clin. Endocrinol. Metab. 1996;81:820–827. doi: 10.1210/jcem.81.2.8636310. [DOI] [PubMed] [Google Scholar]
- Dardalhon V, Korn T, Kuchroo VJ, Anderson AC. Role of Th1 and Th17 cells in organ-specific autoimmunity. J. Autoimm. 2008 doi: 10.1016/j.jaut.2008.04.017. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bosscher K, Vanden Berghe W, Haegeman G. Cross-talk between nuclear receptors and nuclear factor κB. Oncogene. 2006;25:6868–6886. doi: 10.1038/sj.onc.1209935. [DOI] [PubMed] [Google Scholar]
- Dec GW. Introduction to clinical myocarditis. In: Cooper LT Jr., editor. Myocarditis: From Bench to Bedside. Humana Press; Totowa, New Jersey: 2003. pp. 157–281. [Google Scholar]
- Dechkum N, Pangsawan Y, Jayavasu C, Saguanwongse S. Coxsackie B virus infection and myopericarditis in Thailand, 1987–1989. Southeast Asian J. Trop. Med. Public Health. 1998;29:273–276. [PubMed] [Google Scholar]
- Demyanets S, Pfaffenberger S, Kaun C, Rega G, Speidl WS, Kastl SP, Weiss TW, Hohensinner PJ, Dietrich W, Tschugguel W, Bochkov VN, Awad EM, Maurer G, Huber K, Wojta J. The estrogen metabolite 17b-dihydroequilenin counteracts interleukin-1α induced expression of inflammatory mediators in human endothelial cells in vitro via NF-κB pathway. Thromb. Haemost. 2006;95:107–116. [PubMed] [Google Scholar]
- Ehirchiou D, Xiong Y, Xu G, Chen W, Shi Y, Zhang L. CD11b facilitates the development of peripheral tolerance by suppressing Th17 differentiation. J. Exp. Med. 2007;204:1519–1524. doi: 10.1084/jem.20062292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Eckert A, Lai K, Adelman SJ, Harnish DC. Reciprocal antagonism between estrogen receptor and NF-κB activity in vivo. Circ. Res. 2001;89:823–830. doi: 10.1161/hh2101.098543. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Frisancho-Kiss S. Mast cells and inflammatory heart disease: potential drug targets. Cardiovasc. Hematol. Disord. Drug Targets. 2008;8:80–90. doi: 10.2174/187152908783884957. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Rose NR. Immunopathogenesis of autoimmune disease. In: Luebke R, House R, Kimber I, editors. Immunotoxicology and Immunopharmacology. 3rd Ed. CRC Press; Boca Raton: 2007a. pp. 423–436. [Google Scholar]
- Fairweather D, Rose NR. Coxsackievirus-induced myocarditis in mice: a model of autoimmune disease for studying immunotoxicity. Methods. 2007b;41:118–122. doi: 10.1016/j.ymeth.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather D, Yusung S, Frisancho(-Kiss) S, Barrett M, Gatewood S, Steele R, Rose NR. IL-12Rβ1 and TLR4 increase IL-1βand IL-18-associated myocarditis and coxsackievirus replication. J. Immunol. 2003;170:4731–4737. doi: 10.4049/jimmunol.170.9.4731. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Frisancho-Kiss S, Yusung SA, Barrett MA, Gatewood SJL, Davis SE, Njoku DB, Rose NR. IFN-γ protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines TGF-β1, IL-1β, and IL-4 in the heart. Am. J. Pathol. 2004a;165:1883–1894. doi: 10.1016/s0002-9440(10)63241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather D, Frisancho-Kiss S, Gatewood S, Njoku D, Steele R, Barrett M, Rose NR. Mast cells and innate cytokines are associated with susceptibility to autoimmune heart disease following coxsackievirus B3 infection. Autoimmunity. 2004b;37:131–145. doi: 10.1080/0891693042000196200. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Frisancho-Kiss S, Rose NR. Viruses as adjuvants for autoimmunity: evidence from coxsackievirus-induced myocarditis. Rev. Med. Virol. 2005a;15:17–27. doi: 10.1002/rmv.445. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Frisancho-Kiss S, Yusung SA, Barrett MA, Davis SE, Steele RA, Gatewood SJ, Rose NR. IL-12 protects against coxsackievirus B3-induced myocarditis by increasing IFN-γ and macrophage and neutrophil populations in the heart. J. Immunol. 2005b;174:261–269. doi: 10.4049/jimmunol.174.1.261. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Frisancho-Kiss S, Njoku DB, Nyland JF, Kaya Z, Yusung SA, Davis SE, Frisancho JA, Barrett MA, Rose NR. Complement receptor 1 and 2 deficiency increases coxsackievirus B3-induced myocarditis and heart failure by increasing macrophages, IL-1β and immune complex deposition in the heart. J. Immunol. 2006;176:3516–3524. doi: 10.4049/jimmunol.176.6.3516. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Frisancho-Kiss S, Rose NR. Sex differences in autoimmune disease from a pathologic perspective. Am. J. Pathol. 2008;173:600–609. doi: 10.2353/ajpath.2008.071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman AM, McNamara D. Myocarditis. N. Engl. J. Med. 2000;343:1388–1398. doi: 10.1056/NEJM200011093431908. [DOI] [PubMed] [Google Scholar]
- Feldman I, Feldman GM, Mobarak C, Dunkelberg JC, Leslie KK. Identification of proteins within the nuclear factor-kappa B transcriptional complex including estrogen receptor-alpha. Am. J. Obstet. Gynecol. 2007;196:394.e1–394.e11. doi: 10.1016/j.ajog.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisancho-Kiss S, Nyland JF, Davis SE, Frisancho JA, Barrett MA, Rose NR, Fairweather D. Sex differences in coxsackievirus B3-induced myocarditis: IL-12Rβ1 signaling and IFN-γ increase inflammation in males independent from STAT4. Brain Res. 2006a;1126:139–147. doi: 10.1016/j.brainres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Frisancho-Kiss S, Nyland JF, Davis SE, Barrett MA, Gatewood SJL, Njoku DB, Cihakova D, Silbergeld EK, Rose NR, Fairweather D. Cutting Edge: T cell Ig mucin-3 reduces inflammatory heart disease by increasing CTLA-4 during innate immunity. J. Immunol. 2006b;176:6411–6415. doi: 10.4049/jimmunol.176.11.6411. [DOI] [PubMed] [Google Scholar]
- Frisancho-Kiss S, Davis SE, Nyland JF, Frisancho JA, Cihakova D, Rose NR, Fairweather D. Cutting Edge: Cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J. Immunol. 2007;178:6710–6714. doi: 10.4049/jimmunol.178.11.6710. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nature Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- Godeny EK, Sprague EA, Schwartz CJ, Gauntt CJ. Coxsackievirus group B replication in cultured fetal baboon aortic smooth muscle cells. J. Med. Virol. 1986;20:135–149. doi: 10.1002/jmv.1890200206. [DOI] [PubMed] [Google Scholar]
- Hannah MF, Bajic VB, Klein SL. Sex differences in the recognition of and innate antiviral responses to Seoul virus in Norway rats. Brain Behav. Immun. 2008;22:503–516. doi: 10.1016/j.bbi.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SA. Increased susceptibility of male BALB/c mice to coxsackievirus B3-induced myocarditis: role for CD1d. Med. Microbiol. Immunol. 2005;194:121–127. doi: 10.1007/s00430-004-0221-6. [DOI] [PubMed] [Google Scholar]
- Huber SA. Coxsackievirus B3-induced myocarditis: Infection of females during the estrus phase of the ovarian cycle leads to activation of T regulatory cells. Virology. 2008;378:292–298. doi: 10.1016/j.virol.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SA, Pfaeffle B. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J. Virol. 1994;68:5126–5132. doi: 10.1128/jvi.68.8.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SA, Kupperman J, Newell MK. Estradiol prevents and testosterone promotes Fas-dependent apoptosis in CD4+ Th2 cells by altering Bcl 2 expression. Lupus. 1999;8:384–387. doi: 10.1177/096120339900800511. [DOI] [PubMed] [Google Scholar]
- Kaya Z, Afanasyeva M, Wang Y, Dohmen KM, Schlichting J, Tretter T, Fairweather D, Holers VM, Rose NR. Contribution of the innate immune system to autoimmune myocarditis: a role for complement. Nature Immunol. 2001;2:739–745. doi: 10.1038/90686. [DOI] [PubMed] [Google Scholar]
- Kher A, Wang M, Tsai BM, Pitcher JM, Greenbaum ES, Nagy RD, Patel KM, Wairiuko GM, Markel TA, Meldrum DR. Sex differences in the myocardial inflammatory response to acute injury. Shock. 2005;23:1–10. doi: 10.1097/01.shk.0000148055.12387.15. [DOI] [PubMed] [Google Scholar]
- Khetsuriani N, LaMonte-Fowlkes A, Oberste MS, Pallansch MA. Enterovirus surveillance- United States, 1970–2005. MMWR Surveill. Summ. 2006;55(SS08):1–20. [PubMed] [Google Scholar]
- Krieg M, Smith K, Bartsch W. Demonstration of a specific androgen receptor in rat heart muscle: relationship between binding, metabolism, and tissue levels of androgens. Endocrinology. 1978;103:1686–1694. doi: 10.1210/endo-103-5-1686. [DOI] [PubMed] [Google Scholar]
- Kwon TW, do Kim K, Ye JS, Lee WJ, Moon MS, Joo CH, Lee H, Kim YK. Detection of enterovirus, cytomegalovirus, and Chlamydia pneumoniae in atheromas. J. Microbiol. 2004;42:299–304. [PubMed] [Google Scholar]
- Lang TJ. Estrogen as an immunomodulator. Clin. Immunol. 2004;113:224–230. doi: 10.1016/j.clim.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Lenzo JC, Fairweather D, Cull V, Shellam GR, Lawson CM. Characterisation of murine cytomegalovirus myocarditis: cellular infiltration of the heart and virus persistence. J. Mol. Cell. Cardiol. 2002;34:629–640. doi: 10.1006/jmcc.2002.2003. [DOI] [PubMed] [Google Scholar]
- Lima AP, Lunardi LO, Rosa e Silva AAM. Effects of castration and testosterone replacement on peritoneal histamine concentration and lung histamine concentration in pubertal male rats. J. Endocrin. 2000;167:71–75. doi: 10.1677/joe.0.1670071. [DOI] [PubMed] [Google Scholar]
- Mahoney PM, Hurst PR, McLeod BJ, McConnell MA, Thompson EG. Effect of estradiol treatment on mast cell populations and microflora in the vaginal cul-de-sac of seasonally anestrous brushtail possums (Trichosurus vulpecula) Reprod. 2003;125:733–741. doi: 10.1530/rep.0.1250733. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front. Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- Mayo JC, Sainz RM, Antolin I, Uria H, Menendez-Pelaez A, Rodriguez C. Androgen-dependent mast cell degranulation in the Harderian gland of female Syrian hamsters: in vivo and organ culture evidence. Anat. Embryol. 1997;196:133–140. doi: 10.1007/s004290050086. [DOI] [PubMed] [Google Scholar]
- Melchert RB, Kennedy RH, Acosta D., Jr. Cardiovascular effects of steroidal agents. In: Acosta D Jr., editor. Cardiovascular Toxicology. Taylor & Francis; London: 2001. pp. 425–475. [Google Scholar]
- Meyers JH, Sabatos CA, Chakravarti S, Kuchroo VK. The TIM gene family regulates autoimmune and allergic diseases. Trends Mol. Med. 2005;11:362–369. doi: 10.1016/j.molmed.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, Freeman GJ, Kuchroo VJ. Th1-specific cell surface protein Tim-3 regulates macrophage activation and the severity of autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo J-L, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilized two monocyte subsets with divergent and complementary functions. J. Exp. Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae S, Iikura M, Suto H, Akiba H, Umetsu DT, Dekruyff RH, Saito H, Galli SJ. TIM-1 and TIM-3 enhancement of Th2 cytokine production by mast cells. Blood. 2007;110:2565–2568. doi: 10.1182/blood-2006-11-058800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paimela T, Ryhanen T, Mannermaa E, Ojala J, Kalesnykas G, Salminen A, Kaarniranta K. The effect of 17beta-estradiol on IL-6 secretion and NF-kappaB DNA-binding activity in human retinal pigment epithelial cells. Immunol. Lett. 2007;110:139–144. doi: 10.1016/j.imlet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Rangachari M, Mauermann N, Marty RR, Dirnhofer S, Kurrer MO, Komnenovic V, Penninger JM, Eriksson U. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J. Exp. Med. 2006;203:2009–2019. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roivainen M. Enteroviruses and myocardial infarction. Am. Heart J. 1999;138:S479–S483. doi: 10.1016/s0002-8703(99)70280-2. [DOI] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics- 2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J, Writing Group for the Women's Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. J. Am. Med. Assoc. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Schoub BD, Johnson S, McAnerney JM, Dos Santos IL, Klaassen KI. Epidemic Coxsackie B virus infection in Johannesburg, South Africa. J. Hyg. (Lond) 1985;95:447–455. doi: 10.1017/s0022172400062872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonderegger I, Rohn TA, Kurrer MO, Iezzi G, Zou Y, Kastelein RA, Bachmann MF, Kopf M. Neutralization of IL-17 by active vaccination inhibits IL-23-dependent autoimmune myocarditis. Eur. J. Immunol. 2006;36:2849–2856. doi: 10.1002/eji.200636484. [DOI] [PubMed] [Google Scholar]
- Steinman L. A rush to judgment on Th17. J. Exp. Med. 2008;205:1517–1522. doi: 10.1084/jem.20072066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B, Veldhoen M, Martin B. Th17 T cells: linking innate and adaptive immunity. Semin. Immunol. 2007;19:353–361. doi: 10.1016/j.smim.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Straub RH. The complex role of estrogens in inflammation. Endocrine Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- Styrt B, Sugarman B. Estrogens and infection. Rev. Infect. Dis. 1991;13:1139–1150. doi: 10.1093/clinids/13.6.1139. [DOI] [PubMed] [Google Scholar]
- Su EW, Lin JY, Kane LP. TIM-1 and TIM-3 proteins in immune regulation. Cytokine. 2008;44:9–13. doi: 10.1016/j.cyto.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrazas LI, Walsh KL, Piskorska D, McGuire E, Harn DA., Jr. The schistosome oligosaccharide lacto-N-neotetraose expands Gr1+ cells that secret anti-inflammatory cytokines and inhibit proliferation of naive CD4+ cells: A potential mechanism for immune polarization in helminth infections. J. Immunol. 2001;167:5294–5303. doi: 10.4049/jimmunol.167.9.5294. [DOI] [PubMed] [Google Scholar]
- Valaperti A, Marty RR, Kania G, Germano D, Mauermann N, Dirnhofer S, Leimenstoll B, Blyszczuk P, Dong C, Mueller C, Hunziker L, Eriksson U. CD11b+ monocytes abrogate Th17 CD4+ T cell-mediated experimental autoimmune myocarditis. J. Immunol. 2008;180:2686–2695. doi: 10.4049/jimmunol.180.4.2686. [DOI] [PubMed] [Google Scholar]
- Van Ginderachter JA, Movahedi K, Hassanzadeh G, Meerschaut S, Beschin A, Raes G, de Baetselier P. Classical and alternative activation of mononuclear phagocytes: Picking the best of both worlds for tumor promotion. Immunobiology. 2006;211:487–501. doi: 10.1016/j.imbio.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Wang X, Belguise K, Kersual N, Kirsch KH, Mineva ND, Galtier F, Chalbos D, Sonenshein GE. Oestrogen signaling inhibits invasive phenotype by repressing RelB and its target BCL2. Nature Cell Biol. 2007;9:470–478. doi: 10.1038/ncb1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff JF. Viral myocarditis: a review. Am. J. Pathol. 1980;101:425–484. [PMC free article] [PubMed] [Google Scholar]
- Zhao XJ, McKerr G, Dong Z, Higgins CA, Carson J, Yang ZQ, Hannigan BM. Expression of estrogen and progesterone receptors by mast cells alone, but not lymphocytes, macrophages or other immune cells in human upper airways. Thorax. 2001;56:205–211. doi: 10.1136/thorax.56.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Bando Y, Xiao S, Yang K, Anderson AC, Kuchroo VK, Khoury SJ. CD11b+Ly-6Chi suppressive monocytes in experimental autoimmune encephalomyelitis. J. Immunol. 2007;179:5228–5237. doi: 10.4049/jimmunol.179.8.5228. [DOI] [PubMed] [Google Scholar]