Abstract
Decreasing the temperature to 30°C is accompanied by significant enhancement of α2C-AR plasma membrane levels in several cell lines with fibroblast phenotype, as demonstrated by radioligand binding in intact cells or isolated membranes. No changes were observed on the effects of low-temperature after blocking receptor internalization in α2C-AR transfected HEK293T cells. In contrast, two pharmacological chaperones, dimethyl sulfoxide and glycerol, increased the cell surface receptor levels at 37°C, but not at 30°C. Further, at 37°C α2C-AR is co-localized with endoplasmic reticulum markers, but not with the lysosomal markers. Treatment with three distinct HSP90 inhibitors, radicicol, macbecin and 17-DMAG significantly enhanced α2C-AR cell surface levels at 37°C, but these inhibitors had no effect at 30°C. Similar results were obtained after decreasing the HSP90 cellular levels using specific siRNA. Co-immunoprecipitation experiments demonstrated that α2C-AR interacts with HSP90 and this interaction is decreased at 30°C. The contractile response to endogenous α2C-AR stimulation in rat tail artery was also enhanced at reduced temperature. Similar to HEK293T cells, HSP90 inhibition increased the α2C-AR contractile effects only at 37°C. Moreover, exposure to low-temperature of vascular smooth muscle cells from rat tail artery decreased the cellular levels of HSP90, but did not change HSP70 levels. These data demonstrate that exposure to low-temperature augments the α2C-AR transport to the plasma membrane by releasing the inhibitory activity of HSP90 on the receptor traffic, findings which may have clinical relevance for the diagnostic and treatment of Raynaud Phenomenon.
Keywords: α2C-AR, HSP90, heat shock proteins, intracellular traffic, molecular chaperones, low temperature, Raynaud Phenomenon
Research Highlights.
Highlights
at 37°C α2C-AR is localized mostly in the endoplasmic reticulum in HEK293T cells.
exposure to low-temperature stimulated the receptor transport to the cell surface.
inhibition of HSP90 activity has a similar effect on the α2C-AR subcellular localization as low-temperature.
in rat tail artery, HSP90 inhibitors are enhancing the contraction to α2C-AR stimulation.
these findings may have clinical relevance in Raynaud Phenomenon.
1. Introduction
The effects of circulating catecholamines are mediated by specific plasma membrane proteins, named adrenergic receptors. Adrenergic receptors are members of the G protein coupled receptors superfamily (GPCR) and are divided into β, α1 and α2-AR [1]. Three distinct genes have been identified that encode for separate subtypes of α2-AR [2,3]. Lacking specific ligands, the progress in understanding α2-AR pathophysiology was based on genetic models individually targeting each subtype [4,5]. These studies demonstrated distinct tissue distribution and functional roles for each α2-AR subtype. Specifically, α2C-AR is expressed in brain, atria, kidney, and hepatic cells, and in vascular smooth muscle cells (VSMC) from the peripheral vasculature [2–5]. Like other α2-AR subtypes, the cellular effects of α2C-AR are mediated by coupling to Gαi leading to inhibition of adenylate cyclase, inhibition of voltage Ca2+ channels, stimulation of phospholipase C, A2 and D and activation of MAP kinases [2–5]. A functional coupling to Gαs has also been reported for α2-AR, but it is apparent only at high agonist concentration or after inhibition of Gαi and its physiological significance remains unknown [6,7]. In the heterologous systems, α2C-AR is poorly transported to the plasma membrane [8,9]. In contrast, in the neuroendocrine cell lines the receptor is efficiently targeted to the plasma membrane, suggesting a cell specific α2C-AR intracellular trafficking [8,9]. Overall, α2C-AR remains the least characterized α2-AR subtype, and the mechanisms regulating the receptor intracellular trafficking are not fully understood.
However, a role of α2C-AR in the pathology of Raynaud Phenomenon has been suggested. This disease is characterized by enhanced vasoconstriction in response to cold, emotional stress or exposure to vibrations [10,11]. The involvement of an unknown α2-AR subtype was suggested by early publications of Flavahan and Freedman groups, based on the observation that the α2-AR stimulation modulates the vasoconstriction at reduced temperature, whereas α1-AR has no effect [12,13]. Subsequently, elegant work from Flavahan’s group demonstrated that the vascular tone at low-temperature is specifically modulated by the α2C-AR subtype, which is silent at 37°C but it is functional at lower temperatures [14,15].
During the last decade, significant progress was made in understanding the mechanisms controlling the intracellular protein traffic from the folding site represented by the endoplasmic reticulum to the functional destination [16,17]. It has been found that many newly synthesized proteins are transported along the biosynthetic pathway in an inefficient manner [17–19]. For example, within the GPCR class, only 50% of the newly synthesized δ-opioid receptors are transported to the plasma membrane [20]. The fate of the newly synthesized GPCR results from the interactions with several specialized proteins, generically named molecular chaperones [17–19]. These molecular chaperones are heterogeneous, with different subcellular localization (cytosol, Golgi, endoplasmic reticulum) and have different outcomes on the chaperoned protein, like improving the folding status and favoring the transport, or determining intracellular retention and proteasomal degradation. Thus, it is not surprising that interfering with the activity or expression of different molecular chaperones has been found to change the rate of intracellular transport for several proteins. Likewise, downregulation of the cellular levels of AHSA1, a HSP90 co-chaperone, enhanced the cell surface of CFTR Δ508 mutant [21]. In contrast, inhibition of HSP90 activity decreased the maturation rate of insulin receptor and nicotinic receptors [22,23]. Currently few specific pharamacological agents are available to modulate the activity of molecular chaperones. This deficit is partly compensated by several non-specific compounds, named pharmacological chaperones, which were shown to stabilize the misfolded proteins and allow their progression in the biosynthetic pathway [17,24,25]. The non-specific pharmacological chaperones are including osmolytes (dimethyl sulfoxide and glycerol), inhibitors of sarco(endoplasmic) reticulum Ca2+ ATP-ase and factors modifying the heat shock response. Interestingly, exposure to low-temperature has also been suggested to function in the same way as non-specific pharmacological chaperones, improving the subcellular transport of CFTR Δ508 mutant and potassium channels human ether-a-go-go-related gene channels [26–28].
Understanding the mechanisms regulating the intracellular trafficking of specific proteins can provide new therapeutic approaches to several diseases caused by accumulation of misfolded proteins. Therefore, in the present work we studied the subcellular localization of α2C-AR at 37°C and at low-temperature and we investigated the mechanisms underlying the particular receptor intracellular trafficking.
2 Material and Methods
2.1. Plasmids
Human α2C-AR wild-type receptor in pcDNA3.1+ vector was a gift from Dr. D. Bylund (University of Nebraska Medical Center). Human HA-tagged-α2C-AR was a gift from Drs. C. Hurt and T. Angelotti (Stanford University Medical School). Human α2C-322-325del-AR and not-tagged and 3xHA tagged α2B-AR in pcDNA 3.1+ vector were bought from Missouri S&T cDNA Resource Center. HSP90AB and GRP94 in pCMV5 vector were from Origene. DsRed-Rab7 was from Addgene (plasmid 12661). Human α2C-AR and α2B-AR tagged with GFP at their C-termini were generated by PCR after the stop codon was mutated, and the sequences restricted with HindIII/SalI in frame with GFP were ligated into the pEGFP-N1 vector (Clonetech).
2.2 Antibodies and chemicals
The sources of the antibodies used in the present study were as follows: anti-GFP, anti-hemagglutinin (anti-HA), Na+/K+ ATP-ase and β-actin were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); anti-HSP70 and anti-GM130 were from BD Biosciences and anti-HSP90 was from Enzo Life Sciences; rabbit polyclonal α2C-AR antibody corresponding to the aminoacids 309-324 from the receptor third intracellular loop was from Abcam; fluorescently labeled secondary antibodies (Alexa Fluor 594-labeled anti-mouse and anti-rabbit), and 4,6-diamidino-2-phenylindole were obtained from Invitrogen. Macbecin and 17-DMAG were from Enzo Life Sciences and radicicol was from Sigma Aldrich. Lactacystin and MG132 were from Tocris.
2.3 Cell culture and transient transfection
HEK293T cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum, 10 units/ml penicillin, and 100 μg/ml streptomycin. Transient transfection of the HEK293T cells was carried out using LipofectAMINE 2000 reagent (Invitrogen), following the manufacturer instructions. In brief, HEK293T cells were cultured on 10 cm2 dishes and transfected at ~80% confluency with 3 μg receptor construct in DMEM with no antibiotics and no FBS. Six hours later the cells were trypsinized and plated at a density of 106 cells/well in 6-well plates for western blot experiments, or 4×105 cells/well in 12-well plates for radioligand binding experiments and cAMP determination. For co-transfections experiments, the cells were cultured on 6-well plates and transfected with 0.5 μg α2C-AR and 2.5 μg pcDNA3.1 or GRP94 per well. After six hours the cells were trypinized and plated on 12-well plates as above. For siRNA studies, HEK293T cells in 10 cm2 dishes were first transfected with α2C-AR (3 μg/plate) and after 6 h were trypsinized and plated on 12-well plates together with siRNA complexes (3 nM/well) in Transfection Agent 1 following the manufacturer instructions (Applied Biosciences).
2.4. Ligand binding in intact cells
The cells in 12-well plates were serum starved for 24 h to prevent differential proliferation at different temperatures and we found no differences in cell number in these conditions. Eighteen hours before the experimental procedure, half of the plates were transferred to a similar incubator at 30°C, whereas the other were incubated at 37°C and served as control. Two days after transfection the medium was aspired and the cells were incubated in DMEM containing 20 nM [3H]-RX821002 for four hours at 4°C. The binding was terminated by aspiration of the radioactivity and the cells were washed three times with DMEM, digested with 1 M NaOH, and the bound radioactivity was determined in a β-scintillation counter. The non-specific binding determined in presence of non-radioactive rauwolscine (10 μM) represented less than 10% of the total radioactivity and it was subtracted from the presented results. In preliminary experiments we found that performing the binding procedure at low-temperature prevents [3H]-RX821002 internalization. This was tested, by washing the cells three times with 50 mM glycine (pH=3) to remove plasma membrane bound radioactivity. Subsequently the cells were trypsinized and fractionated using Qproteome cell compartment kit (Qiagen) and the radioactivity was determined in each fraction. Most of the radioactivity (89 ± 3 %, n=3) was present in the initial acidic washouts, and the remaining was present in the membrane fraction (7 ± 2 %) and in the cytosolic fraction (5 ± 3%, n=3).
2.5. Flow cytometry
For measurement of total receptor expression, HEK293T cells were transiently transfected with 500 ng of GFP-tagged receptors for 48 h. The cells were collected, washed twice with PBS and resuspended at a density of 8×106 cells/mL. Total GFP fluorescence was then measured on a flow cytometer (BD Biosciences FASCalibur) as described previously [29,30].
2.6. Fluorescence microscopy
For fluorescence microscopic analysis of receptor subcellular localization, HEK293T cells were grown on coverslips pre-coated with poly-L-lysine in 6-well plates and transfected with 500 ng of GFP-tagged receptors. For colocalization of GFP-tagged receptors with the ER and lysosomal markers, HEK293T cells grown on coverslips were transfected with 500 ng of GFP-tagged receptors and 300 ng of pDsRed2-ER or pDsRed2-Rab7. The cells were fixed with 4% paraformaldehyde–4% sucrose mixture in PBS for 15 min and stained with 4, 6-diamidino-2-phenylindole for 5 min. For colocalization of GFP-tagged receptors with the cis-Golgi marker GM130 or with the plasma membrane marker Na+/K+ ATP-ase, HEK293T cells were permeabilized with PBS containing 0.2% Triton X-100 for 5 min, and blocked with 5% normal donkey serum for 1 h. The cells were then incubated with antibodies against GM130 (BD Biosciences) or Na+/K+ ATP-ase (Santa Cruz) at a dilution of 1:100 for 1 h. After washing with PBS (3 × 5 min), the cells were incubated with Alexa Fluor 594-labeled secondary antibody (1:1000 dilution) for 1 h at room temperature. The coverslips were mounted, and fluorescence was detected with a Leica DMRA2 epifluorescent microscope as described previously [30,31]. Images were deconvolved using SlideBook software and the nearest neighbor deconvolution algorithm (Intelligent Imaging Innovations).
2.7. Co-immunoprecipitation
Immuno-precipitation of the receptors was performed in similar manner as described [29]. In brief, HEK293T cells were cultured on 10 cm2 dishes and transfected with 3 μg of HA-tagged α2C-AR or α2B-AR for 48 h. The cells were washed twice with PBS and harvested. The cells were then lysed with 300 μl of lysis buffer containing 50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS and Complete Mini protease inhibitor cocktail. After gentle rotation for 1 h, samples were centrifuged for 15 min at 14,000 ×g and the supernatant was incubated with 50 μl of protein G Sepharose for 1 h at 4°C to remove non-specific bound proteins. Samples were then incubated with 5 μg of anti-GFP antibodies overnight at 4°C with gentle rotation followed by incubation with 50 μl of protein G sepharose beads for 5 h. Resin was collected by centrifugation and washed four times with 500 μl of lysis buffer. Immunoprecipitated receptors were eluted with 100 μl of 1xSDS-PAGE loading buffer, separated by 10% SDS-PAGE and visualized by immunoblotting using specific antibodies.
2.8. Western Blotting
Western blot analysis of protein expression was carried out as previously described [29–32]. Samples were separated by SDS-PAGE and transferred onto polyvinylidene difluoride membranes. The signal was detected using ECL Plus (PerkinElmer Life Sciences) and a Fuji Film luminescent image analyzer (LAS-1000 Plus) and quantitated using the Image Gauge program (Version 3.4).
2.9. Measurement of cAMP production
cAMP concentrations were measured by using cAMP enzymeimmunoassay system (Cayman Chemical Company) as described previously [32]. HEK293T cells on 10 cm2 plates were transfected with 3 μg α2C-AR and six hours later were split into 12-well plates. The cells were serum straved for 24 hours and then incubated at 37°C or at 30°C in absence or presence of macbecin (5×10−6 M) for the next 18 h. One hour before stimulation the medium was changed to PBS supplemented with isobutylmethylxanthine (100 μM). Then the cells were incubated with 10−8 M UK14304 for 5 min, followed by stimulation with forskolin (10 μM) for 15 min. The reactions were stopped by aspirating the medium and addition of 200 μl of acetic acid (4%). Twenty five microliters of cell lysate was then transferred microtitre plate and the cAMP levels were determined by ELISA according to the manufacturer protocol.
2.10. Contractile studies
Rat tail arteries were removed from male Wistar rats, and stored overnight in a cold (4°C), oxygenated Krebs bicarbonate solution of the following composition (mmol/L): NaCl 118, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2 PO4 1.2, NaHCO3 25 and glucose 8.3; pH 7.4. Artery segments were mounted in Mulvany myographs (J.P. Trading) with separated 6-mL organ baths containing Krebs bicarbonate solution, aerated with 95% O2 and 5% CO2, and maintained at 37°C. Tissue responses were measured as changes in isometric force, using a Harvard isometric transducer. Following a 30-min stabilization period, the optimal internal diameter was set to a tension equivalent to 0.9 times the estimated diameter at 100 mm Hg effective transmural pressure as described by Mulvany and Halpern [33]. To determine the maximum contractile response, the tissue was exposed to 100 mmol/L KCl. The segments were then allowed to equilibrate in fresh organ bath fluid in the presence of BRL44408 (α2A-AR receptor antagonist), L-NAME (NOS inhibitor), and macbecin for 30 minutes at 37°C. Subsequently concentration-response curves were constructed with the α2-AR receptor agonist UK14304. Then, the protocol was repeated at 30°C, after washing and one hour re-equilibration at this temperature. This washing period was sufficient to fully restore the response to UK14304 when the experiment was repeated at 37°C.
2.11. Isolation of vascular smooth muscle cells from rat tail artery
All procedures were reviewed and approved by the health sciences animal and welfare committee of the LSU Health Sciences Center. Central tail arteries from male Wistar rats were dissected, immersed in cold PBS without Ca2+ and Mg2+, and cleaned by the connective tissue. The arteries were cut in small pieces (~ 0.5 cm each) and incubated with collagenase (type, 1 mg/ml) elastase (0.5 mg/ml), trypsin inhibitor (2 mg/ml) and bovine serum albumin (2 mg/ml) for three hours at 37°C with gentle rotation. The cells were collected by centrifugation (200 g for 5 min) and plated at a density of ~106 cells in 10 cm2 dishes containing DMEM supplemented with 10 % FBS and 10 units/ml penicillin, and 100 μg/ml streptomycin. The medium was changed each 2–3 days and the cells were trypsinized near confluency. The vascular smooth muscle phenotype was confirmed by anti-caldesmon antibodies which demonstrated that over 95% of the cells were smooth muscle myocytes. All experiments were performed in the second passage on cells plated on 6-well plates at a density of ~5×105 cells/well. The cells were serum starved for 48 h and then expose to 30°C for 18 h in similar manner as described for HEK293T cells.
2.12. Statistical Analysis
Each experiment was repeated at least in two independent transfections and the data are shown as mean ± SD. The statistical differences were tested using using one-way ANOVA followed by Bonferonni test, p < 0.05 being considered significantly different. The Kd values for α2C-AR and α2B-AR at 37°C and at 30°C were calculated using Graph Pad Software (version 5.01) and nonlinear regression for best fit to a one-site binding model.
3. Results
3.1. The effects of low-temperature on the α2C-AR plasma membrane levels
Previously it has been shown that the functional responses to α2C-AR stimulation are enhanced at low-temperature and that the receptor accumulates intracellularly at 37°C [8,9,14,15]. However, the mechanisms underlying the particular receptor trafficking remain poorly characterized. To fill this gap, in the present study the plasma membrane α2C-AR levels in transfected cell lines were determined by radioligand binding in intact cells. As different α2C-AR localization were noted on in fibroblasts and neuro-endocrine cells [8,9], the effects of low-temperature were evaluated in a variety of cell lines (Fig 1A). Exposure to 30°C significantly enhanced the α2C-AR plasma membrane levels in all cell lines with fibroblast phenotype in time-dependent manner (Fig 1A). In six such cell lines, a significant increase in cell surface receptor levels was observed after 6 hours, but the maximal effect was observed after 18 h exposure at 30°C (Fig 1A). In contrast, exposure to low-temperature had no effect on the receptor levels in the neuro-endocrine cell line, PC12 (Fig 1A). The largest increase of α2C-AR plasma membrane levels at 30°C was found in HEK293T cells (Fig 1A), and this cell line was selected to further study the mechanisms involved in the regulation of receptor trafficking by low-temperature.
Figure 1. The effects of low-temperature on the plasma membrane expression of human α2C-AR.
A. The six cell lines indicated were transfected with α2C-AR (3μg/10cm2 plate) and after 6 hours were trypsinized and plated on 12-well plates at ~80% confluence. Twelve hours later the medium was changed to DMEM without FBS and after another 12 h the cells were exposed to 30°C for the time periods indicated on the x-axis. The α2C-AR cell surface levels were determined by [3H]-RX821002 binding in intact cells as described in Material and Methods; mean ± SD, n=4–12 for each point B. Time-dependent increase in α2C-AR plasma membrane levels in transfected HEK293T exposed at 30°C. The cells were transfected as in A, exposed for 18 h at the temperatures indicated on the x-axis and the cell surface levels were determined in intact cells; mean ± SD, n=4–12 in each case. C. The plasma membrane levels of α2C-AR at 37°C (black symbols) and at 30°C (white symbols) determined in intact HEK293T cells. The cells were incubated at 4°C for 4 h with the indicated concentarations of [3H]-RX821002 and the bound radioactivity was determined by liquid scintillation spectrometry; n=4–12 for each point, from four different transfections. D. Same as in E, but for α2B-AR transfected HEK293T cells; n=4–12 for each point. E. Total levels of α2C-AR (left columns) and α2B-AR (right columns) at 37°C (black columns) and at 30°C (white columns) were determined by flow cytometry measuring GFP fluorescence; data are presented as mean ± SD from the values obtained at 37°C, n=3 from three independent transfections. F. The plasma membrane levels of α2C-AR wild type (wt) and α2C322-325del-AR isoform (GAGP) at 37°C (black bars) and at 30°C (white bars) determined in intact HEK293T cells; mean ± SD, n=12 in each case from four different transfections. G. The plasma membrane levels of untagged, GFP-tagged and HA-tagged α2C-AR in HEK293T cells at 37°C (black bars) and at 30°C (white bars); mean ± SD, n=4–12 in each case
Next, the temperature ranges stimulating the α2C-AR trafficking to the plasma membrane were determined. Because long-term exposure at temperatures lower than 25°C induces irreversible changes in the cytoskeletal structures [25,28], the present study was limited to study the effects of temperatures above 28°C. The maximal increase in the cell surface receptor levels was found at 30°C (Fig 1B). As exposure to low-temperatures in the range of 28–32°C is often used to enhance the plasma membrane expression of misfolded proteins [17,25,28], the effects of low-temperature were also assessed on the closest α2C-AR homologue, α2B-AR. Although these two receptors share more than 80% homology, exposure to low-temperature had no effect on the α2B-AR plasma membrane levels, (Fig 1D). In contrast, significant augmentation of the α2C-AR cell surface levels was found in cells exposed to 30°C (Fig 1C). Similar results were obtained in the purified isolated plasma membrane fraction (Supplementary Figure 1). These increases cannot be explained by changes of the affinity of the ligand for the receptor, because similar Kd values were calculated at 37°C and 30°C by the two different methods (Table 1). To further eliminate the possibility that the observed enhancement in the plasma membrane receptor number is the result of enhanced total receptor levels due to increased synthesis or diminishment in the protein degradation at low-temperature, the total cellular levels of α2C-AR and α2B-AR were determined by flow-cytometry. No significant differences in the total number of receptors were found at 37°C or at 30°C for any α2-AR subtype (Fig 1E).
Table 1.
Ligand binding properties of α2C-AR at 37°C and 30°C in isolated membranes and in intact cells.
| Receptor | Isolated Membranes | Intact Cells | ||
|---|---|---|---|---|
| 37°C | 30°C | 37°C | 30°C | |
| α2C-AR | 5.72 ± 1.4 nM | 6.36 ± 0.98 nM | 1.87 ± 0.33 nM | 2.08 ± 0.32 nM |
The receptor levels in transfected HEK293T cells were determined by [3H]-RX821002 binding in isolated membranes or in intact cells as described in Material and Methods. The Kd values in each case were determined by nonlinear regression for best fit to a one-site binding model.
An α2C-AR splicing variant missing four amino acids in the positions 322GAGP325 (α2C322-325del-AR) in the third intracellular loop has been identified and it has been proposed to contribute to the ethnic differences to cardiovascular stress responses [1,34,35]. However, when transfected in HEK293T cells, both α2C-AR isoforms showed similar augmentations in the plasma membrane levels at low-temperature (Fig 1F). For many biochemical approaches, receptor tagging is a common method enabling visualization and receptor pull-down and for this study GFP- and HA-α2C-AR were generated. These tagged receptors displayed the same temperature-dependent upregulation in the cell surface receptor levels as parent construct (Fig 1G).
3.2. Subcellular distribution of α2C-AR at physiological temperature
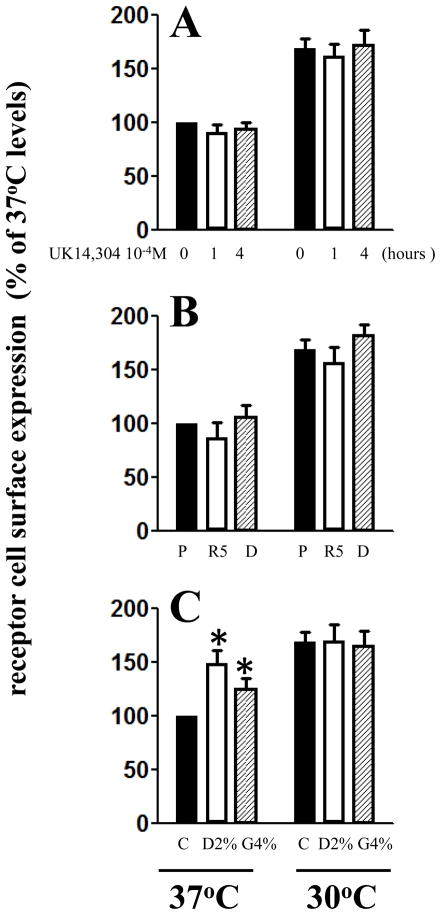
The receptor number present at the plasma membrane is the result of the fine equilibrium between receptor internalization and receptor export. To assess if the effects of low-temperature on the α2C-AR are caused by inhibition of receptor internalization, first the effects of typical α2 agonist, UK14304 were tested on the receptor cell surface levels at 37°C and at 30°C. As shown in Fig 2A, even incubations up to four hours with the agonist did not change the effects of low-temperature on the upregulation of α2C-AR plasma membrane. To further test the involvement of receptor internalization, the effects of two well characterized proteins interfering with GPCR internalization, Rab5 and Dynamin I, were investigated. Co-transfection with dominant negative isoforms Rab5N135I and Dynamin I K44A did not change the α2C-AR plasma membrane levels at 37°C or at 30°C (Fig 2B). In contrast, the treatment with the non-specific chemical chaperones, dimethyl sulfoxide and glycerol significantly enhanced the receptor plasma membrane levels at 37°C, but they were ineffective at 30°C (Fig 2C). The main mechanism involved in the actions of chemical chaperones is stabilization of the mildly misfolded proteins allowing their inclusion in the biosynthetic pathway. Thus, these results indicate that defects in the receptor export, but not in the receptor internalization are the explanation for α2C-AR intracellular localization at 37°C.
Figure 2. The roles of receptor internalization and chemical chaperones in the temperature-dependent α2C-AR trafficking.
A. The cells were processed as above and α2C-AR transfected HEK293T cells maintained at 37°C or at 30°C for 18 h prior determinations were incubated with UK14304 (10−4M) for 1 (white bars) and 4 h (hatched bars) at each temperature and the receptor plasma membrane levels were determined in intact cells by [3H]-RX821002 binding. mean ± SD, n=6–10 in each case. B. Same as in A, but HEK293T cells were co-transfected with α2C-AR (0.5 μg) and 3 μg of pcDNA 3.1+ (P, black bars), Rab5N135I (R5, white bars ) or Dynamin I K44A (D, hatched bars) and the receptor cell surface levels were determined in similar manner, mean ± SD, n=4–8 from 2 different transfections. C. The effects of DMSO (2%, white bars) and glycerol (4% hatched bars) on the α2C-AR plasma membrane levels at 37°C and at 30°C; n=8–12 in each case from four different transfections, * - p < 0.05.
To further confirm this hypothesis, deconvolution microscopy was used to determine GFP-α2C-AR subcellular localization at 37°C and at 30°C. As expected from radio-ligand binding experiments, at 37°C most of the receptor was found to accumulate intracellularly in the perinuclear regions, overlapping with the endoplasmic reticulum marker pDsRed2-ER (Fig 3A). In contrast, at 30°C, most of the GFP-α2C-AR was present at the plasma membrane (Fig 3B). In agreement with previous reports, occasionally at 37°C the receptor was found to be co-localized with the cis-Golgi marker, GM130 (Fig 3C). However, either at 37°C or at 30°C, the receptor did not co-localize with the lysosomal marker, Rab7 (Fig 3D). These findings indicate again that defects in the receptor export, but not in the receptor internalization, are responsible for α2C-AR intracellular accumulation at the physiological temperature.
Figure 3. The subcellular localization of GFP-tagged α2C-AR at 37°C (left column) and at 30°C (right column).
HEK293T were transfected with 1 μg per 10 cm2 plate with GFP-tagged receptors and processed in the same way as for radio-ligand binding. The individual organelles were identified as follows: A. The endoplasmic reticulum was visualized by co-transfection with the specific marker pDsRed2-ER. B. The plasma membrane was stained using Na+/K+ ATP-ase antibody as described in Material and Methods. C. Cis-Golgi was labeled after incubation with GM130 antibody. D. The lysosomal compartment was visualized by co-transfection with DsRed2-Rab7. The cells were fixed with 4% paraformaldehyde–4% sucrose mixture and the distribution of α2C-AR was analyzed by fluorescence microscopy. Blue: DNA staining by 4,6-diamidino-2-phenylindole (nuclear), green: GFP-α2C-AR, red: specific organelle indicated in the figure, yellow: co-localization of the receptor with the respective organelle marker. The images are representative from at least six different coverslips, obtained from three independent transfections..
3.3. The effects of HSP90 inhibition on the α2C-AR intracellular traffic in HEK293T cells
Recently it has been shown that alterations in the HSP90 activity may change the intracellular trafficking of different proteins like CFTR, AchR and the insulin receptor [21–23]. To test if this is the case for α2C-AR, the effects of three distinct HSP90 inhibitors were tested on the receptor cell surface levels at 37°C and at 30°C. At 37°C, macbecin, 17-DMAG and radicicol significantly enhanced the number of α2C-AR plasma membrane binding sites to similar levels as observed at 30°C (Fig 4A). In contrast, these compounds were ineffective at 30°C. Macbecin pretreatment did not change the Kd values of [3H]-RX821002 binding to α2C-AR at 37°C or at 30°C (Supplementary Table 1), indicating that these effects are not due to changes in the ability of the receptor to bind the ligand. Further, although HSP90 inhibitors also slightly increase the α2B-AR plasma membrane levels, this effect is significantly smaller than the increase observed on the α2C-AR (Fig 4A). The effects were dose-dependent and similar between the α2C-AR wild-type and α2C322-325del-AR splicing variant (Fig 4B). To exclude the possibility that these inhibitors may modulate receptor traffic independent of HSP90, the relation between endogenous levels of HSP90 and α2C-AR cell surface expression was examined. Using HSP90 siRNA in α2C-AR transfected HEK293T cells a reduction of about 50% in the protein levels was obtained (Fig 4C). This reduction was enough to enhance the plasma membrane receptor levels at 37°C to the same levels as found by using HSP90 inhibitors (Fig 4D). Again, the diminishment in HSP90 levels had no effect on the receptor cell surface levels at 30°C, strongly suggesting that low-temperature stimulate receptor traffic to the cell surface by interfering with HSP90 activity. Co-immunoprecipitation experiments demonstrated interactions between α2C-AR and the cytosolic HSP90 (Fig 5A). Interestingly, these interactions were temperature-dependent, as exposure to 30°C for 18 h reduced the interactions between the two proteins with about ~80% (Fig 5B). A similar inhibition in the interactions between α2C-AR and HSP90 was found in the cells pretreated with macbecin at 37°C (Fig. 5B). In contrast, the weak interactions observed between HSP90 and α2B-AR were not temperature-sensitive and not significantly affected by macbecin (Fig 5A and B).
Figure 4. The effects of manipulations in HSP90 activity on the temperature-dependent α2C-AR plasma membrane expression.
A. HEK293T cells were transfected with α2C-AR or with α2B-AR (right columns, 3 μg/10cm2 plate left columns) and subsequently plated on 12-well plates as described in Figure 1. Six hours after FBS was withdrawn the cells were incubated for 1 h with either vehicle (V), macbecin (M, 5 μM), 17-DMAG (17, 0.5 μM) or radicicol (R, 10 μM) and subsequently exposed to 37°C or to 30°C for the next 18h in the presence of the HSP90 inhibitors. The receptor plasma membrane levels were determined in intact cells as described in Material and Methods. Mean ± SD from 4–6 independent transfections. * - indicate p<0.05 compared to the vehicle. B. Dose-dependent effects of 17-DMAG on the wild-type α2C-AR (triangles) and α2C322-325del-AR (squares) at 37°C (black symbols) or at 30°C (white symbols). HEK293T were transfected as in A, and exposed to increased concentrations of 17-DMAG indicated on the x-axis; mean ± SD with n=4–8 for each point from four different transfections. C. Left panel demonstrate the reduction of HSP90 expression levels in HEK293T cells by siRNA targeting. HEK293T cells were first transiently transfected with α2C-AR (3 μg/10cm2 plate). Six hours later the cells were trypsinized and replated on 12-well plates and transfected with siRNA following the manufacturer instructions (Ambion). After 12 hours the medium was changed to DMEM without FBS and then processed as for radio-ligand binding experiments. The result is representative from three independent transfections. Right panel: quantitative data expressed as percentage of the HSP90 levels in control cells transfected with control siRNA at 37°C. mean ± SD from three independent transfections. D. The effects of HSP90 downregulation using siRNA on the α2C-AR plasma membrane levels at 37°C and at 30°C determined by [3H]RX821002 binding in intact cells in the same transfections as used for the western blot experiments presented in C; mean ± SD, n=9 from three independent transfection. * - indicate p<0.05 compared to the levels in control siRNA transfected cells at 37°C.
Figure 5. Interactions between α2C-AR and HSP90 in HEK293T cells.
A. HEK293T cells were transfected with empty vector pcDNA 3.1, or HA-tagged α2C-AR and 3xHA tagged α2B-AR (3 μg/10cm2 plate each) and 6 hours later the medium was changed to DMEM without FBS, followed by incubation at 30°C or at 37°C for the subsequent 18 h. Some plates were treated with macbecin (5 μM). Subsequently, the cells were solubilized and immunoprecipitated with HA antibody as described under Material and Methods. The HA immunoprecipitates (20 μg/lane) were separated by 10 % SDS-Page and the HSP90 levels were revealed by Western-blotting. The experiment shown is representative from three independent transfections. Similar results were obtained in case of α2C-AR using GFP-tagged receptor and GFP antibody. B. Quantification of the data presented in A. mean ± SD, n=3, *-indicate statistical significant differences compared with control cells (p < 0.05).
HSP90 chaperone class comprises from cytosolic (HSP90α and β), endoplasmic reticulum (GRP94) and mitochondrial (TRAP-1) isoforms [36–38]. The mitochondrial isoform is not involved in the regulation of protein trafficking from the endoplasmic reticulum to the plasma membrane, but to distinguish between the other isoforms, the endoplasmic reticulum isoform GRP94 was overexpressed in HEK293T cells. No differences in the effects of low-temperature on the α2C-AR plasma membrane levels were found between control and GRP94 overexpressing cells (Fig 6A), supporting that the cytosolic HSP90 isoforms are modulating receptor traffic. These cytosolic isoforms were proposed to downregulate the cellular levels of some of its client proteins through proteasomal degradation. However, this appear to be not the case for α2C-AR, because in HEK293T cells two specific proteasomal inhibitors, MG132 and lactacystin, failed to modify the effects of low-temperature on the receptor cell surface expression (Fig 6B).
Figure 6. The effects of GRP94 overexpression and proteasomal inhibition on the α2C-AR temperature-sensitive trafficking.
A. HEK293T cells were co-transfected with α2C-AR and pcDNA 3.1 (left columns) or GRP94 (right columns). The cell surface α2C-AR levels in cells maintained at 37°C (black columns) or at 30°C for 18 h (white columns) were determined in intact cells as above. mean ± SD, n=9 from three independent transfections. B. α2C-AR tranfected HEK293T cells were incubated with DMSO (vehicle, 0.1%), MG132 (10 μM) or lactacystin (5μM) and subsequently exposed to 30°C for 18h or maintained at 37°C. The α2C-AR plasma membrane levels were determined in intact cells. n=9 from three independent transfections.
3.4. The effects of low-temperature and HSP90 inhibition on the α2C-AR functional responses
To test if low-temperature and HSP90 are also modulating the functional responses to α2C-AR stimulation, the cAMP levels were determined in HEK293T cells. The α2-AR agonist UK14304 (10−8M) itself had no effect on the basal cAMP levels in HEK293T cells at 37°C or at 30°C. Also, at 37°C, UK14304 had minimal effects on the forskolin-stimulated increase in cAMP levels (Fig 7A). Exposure to low-temperature up to 18 h at 30°C did not change the ability of forskolin to enhance the cAMP levels (Fig 7A). However, inhibition of cAMP formation by UK14304 was enhanced by exposure to low-temperature in time-dependent manner (Fig 7A), with a maximal effects after 18 h, similar to the effects observed on the plasma membrane receptor levels (Fig 1C). Further, pre-treatment with the HSP90 inhibitors macbecin significantly enhanced the effects of receptor stimulation on this parameter (Fig 7B). Noteworthy, the UK14304 effects at 37°C in presence of macbecin were not statistically different from the effects of the agonist alone at 30°C. Lastly, at 30°C macbecin did not change the effects of α2C-AR stimulation on the cAMP levels, demonstrating that the inhibitors of HSP90 are increasing the receptor activity only at 37°C (Fig 7B).
Figure 7. The effects of low-temperature and macbecin on α2C-AR mediated cAMP inhibition in HEK293T cells.
A. α2C-AR transfected HEK293T cells (3 μg/10 cm2 dish) were plated in 24 well plates and serum-starved for 24 h. Subsequently, the cells were incubated at 30°C for the periods indicated under the graph. At the end of the incubation period the medium was changed to PBS containing 100 μM IBMX for one hour. Subsequently the cells were pretreated with UK14304 (10 nM) for 5 min, followed by stimulation with forskolin (10 μM) for 15 min. The reactions were stopped by medium aspiration and addition of 200 μl thhricloracetic acid and cAMP levels were determined using cAMP Elisa Kit (Cayman Biochemicals) as described in Material and Methods. B. Same as in A., but the cells were incubated at 37°C or at 30°C in absence or presence of macbecin (5 μM) for 18 h. n=12–15 in each case from three independent transfections. * - shows statistical significant differences between the effects of UK14304 at 37°C and all other series with p < 0.05.
Cold-induced α2C-AR translocation to the plasma membrane has been proposed to play a role in Raynaud Phenomenon. Therefore, this study was extended to a more appropriate model for this disease, namely contraction of the rat tail artery. In this preparation, rauwolscine (1 μM), an α2-AR inhibitor, reduced the contractile effects of UK14304 with more than 80% (n=4, data not shown). The remaining effect could be possibly attributed to activation of α1-AR [12], but these effects were previously shown to be temperature-indpendent [12,14]. Both α2A-AR and α2C-AR subtypes are expressed in this tissue [14], and for this reason these experiments were conducted in presence of the α2A-AR inhibitor BRL44408 and L-NAME to prevent the contribution of endothelial vasoactive factors. Therefore, in these experimental conditions, the contraction to UK14304 can be largely attributed to activation of vascular α2C-AR. In agreement with the results in HEK293T cells, the contractile effects in response to α2C-AR stimulation were enhanced after one hour exposure at 30°C (Fig 8A and B). Again, pre-treatment with macbecin significantly increased the contractile effects of α2C-AR at 37°C, but it was without effect at 30°C (Fig 8A and B). Importantly, the log EC50 values of the UK14304 contractile effects were not statistically different in these conditions (−6.76 ± 0.13 and −6.537 ± 0.06 at 37°C, vs. −6.90 ± 0.11 and −6.753 ± 0.19 at 30°C, in absence or presence of macbecin, respectively), indicating that macbecin is not affecting the affinity of the agonist for α2C-AR. Together, these results indicate that low-temperature may reduce HSP90 activity and thus preventing the inhibitory action on the receptor trafficking. To test if this is the case, the HSP90 levels were determined in VSMC from rat tail artery. The expression of α2C-AR was confirmed in these cells by western-blot. The predicted molecular weight of the α2C-AR is 49.5 Kda, but we detected multiple receptor species, with the major band around 65 kDa in both VSMC and HEK293T cells (Fig 9A), in agreement with previous reports [14,15], reflecting post-translational modifications of the receptor. From these experiments it can be estimated that the endogenous receptor levels in VSMC are about ~ 11 times less than in transfected HEK293T cells. However, as expected from the flow cytometry results (Fig 1H), no differences were observed in total levels of the receptors in cells maintained at 37°C or exposed to 30°C for 18 h in both cell types (Fig 9A). In contrast, exposure to 30°C of VSMC from rat tail artery significantly reduced the HSP90 cellular levels (Fig 9B and C). This effect is specific for HSP90, as no changes in the levels of a α2C-AR, β-actin or of HSP70 were found in these conditions (Fig 9B and C).
Figure 8. The effects of low-temperature and HSP90 inhibition on the contractile response to α2C-AR stimulation in rat tail artery.
A. Cumulative dose-effect contractile response of rat tail artery to UK14304 in presence of L-NAME (100 μM) and α2A-AR antagonist BRL44408 (1 μM). The responses obtained at 37°C (black symbols) and after one hour exposure to 30°C (white symbols) in absence (squares) or after pretreatment with 10 μM macbecin (triangles) are expressed as percentage of the control contraction induced by 100 mM KCl. mean ± SD from four different preparations obtained from four different animals. One-way ANOVA followed by Bonferroni’s Multiple Comparison Test revealed that the contractile effects at 37°C in control arteries are significantly lower compared to the other three conditions. B. Same data as in A presented as maximal contractile response to 100 μM UK14304 at 37°C (left columns) and at 30°C (right columns) in absence (black columns) or presence of 10 μM macbecin (white columns). * - indicate statistical significant differences with p < 0.05.
Figure 9. The influence of temperature on the HSP90 and HSP70 levels in the vascular smooth muscle cells from rat tail artery.

A. α2C-AR levels in VSMC from rat tail artery and HEK293T cells. 20 μg of cell lysates obtained from VSMC from rat tail artery (P2, left lanes) and α2C-AR transfected HEK293T (right lanes) maintained for 18 h at 37°C or at 30°C were separated by 10% SDS PAGE. The α2C-AR levels were determined by western blotting using a specific antibody. Similar results were obtained in three other experiments. B. HSP90 and HSP70 levels in VSMC from rat tail artery. VSMC from rat tail artery (P2) were serum starved for 48 h and subsequently maintained at 37°C or exposed to 30°C for 18 h. The cell lysates (20 μg/lane) were separated by 10 % SDS-Page and subject to western-blotting with specific antibodies. A representative blot out of three independent cell isolations is shown. C.. The levels of HSP90 (black column) and HSP70 (white column) at 30°C in VSMC from rat tail artery quantified from the experiments shown in B. Data are expressed as % of the levels of each HSP at 37°C. mean ± SD, from three independent experiments. * - indicate statistical significant differences with p < 0.05.
Discussion
Within the GPCR class, α2C-AR has particular characteristics, being poorly transported to the cell surface in fibroblasts and in vascular smooth muscle cells [8,9,12–15]. The present study was undertaken to clarify the mechanisms controlling α2C-AR trafficking in fibroblasts and VSMC. Two major findings resulted from these experiments, identification of the endoplasmic reticulum as the major site for the receptor intracellular accumulation and the role of HSP90 in the α2C-AR trafficking. Also, it has been found that the effects of low-temperature are specific for this receptor, because neither its closest homologue α2B-AR (Fig 1D,F), nor β2-AR or β1-AR (Filipeanu, unpublished results) cell surface levels are modified after exposure to low-temperature.
Previously, based on the effects of α2-AR antagonists, the receptor localization in the peripheral vasculature, and specific upregulation of the plasma membrane levels at reduced temperature, α2C-AR has been proposed to play a major role in the pathology of Raynaud Phenomenon [12–15]. Although Raynaud Phenomenon is often perceived as a rare disease, its world-wide incidence ranges from 4 to 20% of the general population, the prevalence being higher in cold climates [10,11]. Even if other factors like emotional stress and vibrations can precipitate the symptoms, cold-exposure remains the major triggering factor for this disease [10,11]. In the last decade many cellular biology studies established that exposure to decreased temperatures (28–32°C) efficiently enhanced plasma membrane targeting of misfolded proteins [24,28]. The mechanisms involved in this effect appear to be similar to the actions of the molecular chaperones [28]. The results from the present work are in full agreement with this hypothesis, as the stimulatory effects of DMSO and glycerol on the α2C-AR plasma membrane levels were clearly visible at 37°C, but absent in the cells incubated at 30°C (Fig 2C). In addition, interfering with receptor internalization did not change the effects of low-temperature on the receptor trafficking, indicating that α2C-AR poor plasma membrane targeting is due to defects in the receptor export (Fig 2A, B). This idea is also supported by the co-localization experiments showing that the endoplasmic reticulum is the major site for the receptor intracellular accumulation at 37°C (Fig 3). Interestingly, the polymorphic variant α2C322-325del-AR displayed similar increases in the cell surface levels at low-temperature as α2C-AR wild-type (Fig 1H), indicating that the 322GAGP325 fragment from the third intracellular is not essential for the temperature-dependent trafficking. The subcellular α2C-AR localization findings from this study are in full agreement with earlier work from Kobilka’s group demonstrating that this receptor accumulates in the endoplasmic reticulum and cis-Golgi at physiological temperature in cell lines with fibroblast phenotype [8,9]. However, other studies reported a trans-Golgi localization of the receptor in α2C-AR transfected HEK293T cells [14,15,39]. The reasons for this discrepancy are unclear, but it could be related to the differences in the transfection procedure and/or in the organelle markers used. Very recently, Angelotti et al, also found that in physiological conditions α2C-AR is targeted to the endoplasmic reticulum, possibly by a hydrophobic motif located in the receptor N-terminus [40]. In addition, our study is first to directly quantify the amount of the receptor translocated from intracellular organelles to the plasma membrane at low-temperature by radioligand binding. We found similar results using untagged and tagged α2C-AR (Fig 1G), indicating that this receptor has an intrinsic folding defect and exposure to low-temperature facilitates the receptor stabilization and allows its inclusion in the export trafficking pathways.
Our data demonstrate for the first time the role of HSP90 in the α2C-AR intracellular traffic regulation. The folding of the newly synthesized proteins and the subcellular transport is assisted by many specialized proteins, broadly named molecular chaperones [16,17]. These molecular chaperones belong to different classes and intervene at different steps during protein maturation or trafficking, modulating the transport rate and the subcellular localization [16–19]. In the case of misfolded proteins it has been repeatedly demonstrated that several molecular chaperones, actively prevent formation of aggregates by triggering the unfolded protein response [17, 25]. In particular, HSP90 has been shown to modulate the folding, stabilization, activation, and assembly of a wide range of proteins [36–38]. Still, in contrast with other molecular chaperones, HSP90 has a distinct repertoire of specific ‘client’ proteins with which it interacts, playing the role of scaffold and regulating the maturation and signaling of these molecules [36–38]. Alterations in the HSP90 activity have been demonstrated to modify the intracellular trafficking and plasma membrane targeting of different mutants of CFTR, insulin receptor and nicotinic receptor [21–23]. Thus far, just one another GPCR member, the cannabinoid CB2 receptor has been reported to interact with HSP90 and this interaction is required for the receptor mediated cell migration through the Gαi-Rac1 pathway [41]. However, no attempt to quantify the HSP90 effects on the receptor subcellular localization and plasma membrane expression was performed in the respective study.
The inhibitory role of HSP90 on the α2C-AR traffic to the plasma membrane was demonstrated in the present study by two separate and complimentary means, inhibition of its activity using specific inhibitors and decreasing the cellular levels using specific siRNA (Fig 4). Similar results were obtained with both approaches, demonstrating that HSP90 activity is essential for the receptor accumulation at the physiological temperature. Again, wild-type α2C-AR and α2C322-325del-AR polymorphic variant have similar sensitivity (Fig 4B), clearly showing that both isoforms have similar trafficking properties at least in respect to the effects of low-temperature and HSP90 modulation. Because no changes were observed in the total receptor levels at the two temperatures (Fig 1E and Fig 9A), and the specific proteasomal inhibitors MG132 and lactacystin have no effects on the α2C-AR trafficking (Fig 6B), it can be concluded that low-temperature acts by releasing the inhibitory mechanisms preventing the receptor transport at physiological temperature. Based on the absence of HSP90 inhibitors at 30°C, it can be assumed that these mechanisms are at least in part mediated by HSP90.
HSP90 has multiple isoforms with different subcellular localization and different functions [36–38]. The current HSP90 inhibitors are a little more effective against the cytosolic isoforms [42]. Indeed, overexpression of GRP94, the endoplasmic reticulum HSP90 isoform, had no effect on the α2C-AR trafficking. This finding is not surprising, considering that in contrast to other endoplasmic reticulum resident molecular chaperones, GRP94 has been suggested to have a limited number of interacting partners [43]. The correlation between the data obtained with three distinct HSP90 inhibitors and specific down-regulation of cytosolic HSP90 levels using siRNA, demonstrate that only these isoforms are modulating α2C-AR temperature-dependent trafficking. The two HSP90 cytosolic isoforms are designed α and β and are closely related (86% identitity and 93 % similarity), with the most important sequence difference in the N-terminus. [37]. Although both isoforms are present under basal conditions, HSP90α usually shows a larger increase after heat shock and therefore is credited to be the inducible isoform, whereas HSP90β which has lesser variations is considered the constitutive isoform [36–38]. However, each isoform may substitute the other in the cellular functions. Also, the experimental tools to differentiate between the HSP90 isoforms are limited, as the two cytosolic isoforms have similar sensitivity to HSP90 inhibitors, share the same co-chaperones, form heterodimers and the antibodies cross-react. Based on these reasons, no attempt was made in the present study to differentiate which isoform is crucial for the temperature-sensitive α2C-AR trafficking.
The enhanced α2C-AR plasma membrane expression at low-temperature and/or after HSP90 inhibition is reflected by increased functional responses after receptor stimulation in these conditions. The classical physiological view attributes all the GPCR function to the receptors present at the cell surface, freely accessible to the extracellular ligands. However, this paradigm was challenged in the last decade, activation of cellular signaling by receptors with intracellular localization being demonstrated in several situations [44–46]. However, the large pool of α2C-AR localized in the endoplasmic reticulum at physiological temperature appears unable to contribute to cellular responses. In fact, the effects on cAMP and vascular tone observed at 37°C are exclusively due to activation of the receptor fraction with plasma membrane localization, as they are eliminated by addition of the extracellular α2-AR antagonist, rauwolscine (data not shown). The inability of intracellular α2C-AR to trigger cellular signaling may be related to the absence of molecules required to trigger signaling (i.e. G protein subunits) at this level. However, recent data indicate that GPCR are associated in signaling complexes with the corresponding molecules early in the biosynthetic pathway [47]. More probably, appropriate receptor activators are unable to reach the intracellular α2C-AR. Still, our results cannot exclude the possibility that intracellular α2C-AR activates other unknown yet signaling mechanisms. In contrast, when the receptor expression at the cell surface is increased by low-temperature and/or HSP90 inhibition, the inhibition of cAMP levels and contractile effects in response to the α2-agonist are markedly enhanced. The similarity of the effects of low-temperature and HSP90 inhibition on α2C-AR functional responses in HEK293T cells and rat tail artery demonstrate that the temperature-sensitive receptor trafficking is not limited to heterologous transfection systems. The effects of low-temperature were absent only in PC12, a neuro-endocrine cell line, in agreement with previous findings [9]. Different expression of HSP90 isoforms (or specific co-chaperones) in neurons and in smooth muscle cells have been reported [48] and this fact may explain the cell-specific receptor trafficking.
The current study reveals a novel aspect of HSP90 inhibitors, specifically modulation of vascular tone. Previously, impairment of the endothelium-dependent relaxation by these agents was observed in the porcine coronary arteries and rat thoracic aorta [49,50], but a direct effect on vascular smooth muscle, as in the present study, has not been reported. Various HSP90 inhibitors are currently in clinical trials for treatment of different types of cancer [51,52]. When the data and findings from these trials is reported, it will be interesting to determine if there is an association between the use of HSP90 inhibitors and clinical manifestations of Raynaud Phenomenon and it will clarify if the endogenous HSP90 levels may be used as biomarker for the susceptibility to the disease.
In correlation with the findings on the receptor cell surface levels, the effects of low-temperature and HSP90 inhibitors on the α2C-AR functional effects in HEK293T cells and rat tail artery were not additive, indicating that a common mechanism may underlie these effects. This conclusion is supported by the co-immunoprecipitation experiments which demonstrated strong interaction between these two proteins at 37°C (Fig 5A). Based on these data, α2C-AR should be added to the growing list of HSP90-interacting proteins. The interactions between α2C-AR and HSP90 were decreased at 30°C, supporting the idea that low-temperature may release the inhibitory action of HSP90 on the receptor traffic. This temperature-dependent interaction was specific for α2C-AR, as it was not observed in the case of α2B-AR (Fig 5). HEK293T cells express large amounts of endogenous HSP90 compared to VSMC from rat tail artery (Supplementary Fig 2), and this fact may explain the long time interval required to observe the maximal effect of low-temperature on the α2C-AR plasma membrane levels (~18 h, Fig 1A), which is in contrast with rapid onset of the Raynaud Phenomenon [10,11]. Endogenous HSP90 levels are well known to be higher in cancer or immortalized cell lines compared to normal cells [51,52]. Thus, the high endogenous HSP90 levels in HEK293T may mask the contribution of other mechanisms like Rho kinase, Rap GTP-ase and JNK [14,15,39,53,54] to the temperature-dependent α2C-AR intracellular trafficking. However, a clear and specific reduction of about 50% in HSP90 levels was found in VSMC from rat tail artery maintained at 30°C for 18h (Fig 9). Although mild heat shock is the hallmark of heat shock protein upregulation, currently little is known about to the effects of low-temperature on the HSP levels. Recently it has been proposed that cold-exposure may destabilize HSP90 in cell free environment leading to its rapid degradation [55]. Still, considering that the largest effect at 30°C on the α2C-AR trafficking was observed in HEK293T cells, additional mechanisms may regulate the interactions between α2C-AR and HSP90 at low temperature, including translocation of HSP90 into cellular compartments in which is not able to bind to receptor. Interestingly, stimulation of estrogen receptors through activation of Rap GTP-ase have been also proposed to modulate the effects of low-temperature on the α2C-AR [53,54]. On the other hand, HSP90 inhibition has been shown to block the non-genomic estrogen signaling [49] and to prevent GPCR activation of small GTP-ases [41]. Thus, HSP90 may integrate different subcellular mechanisms to regulate temperature-sensitive α2C-AR trafficking.
In conclusion, two new important features of α2C-AR intracellular trafficking were characterized in the present investigation, identification of the endoplasmic reticulum as the major site of the receptor intracellular accumulation at 37°C and demonstration that low-temperature acts by weakening the α2C-AR interactions with cytosolic HSP90 to promote the receptor transport to the cell surface.
Supplementary Material
Acknowledgments
We are indebted to Drs. David Bylund, Carl Hurt and Tim Angelotti for sharing plasmids. We thank to Drs Stephania Cormier, Patrice Delafontaine, Kurt Varner, Peter Winsauer and Guangyu Wu for many helpful suggestions. The work presented in this paper was supported by NIH Grant P20-RR-018766 (CMF and DRK) and TI Pharma grant T2-301 (AHD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Small KM, McGraw DW, Liggett SB. Pharmacology and physiology of human adrenergic receptor polymorphisms. Annu Rev Pharmacol Toxicol. 2003;43:381–411. doi: 10.1146/annurev.pharmtox.43.100901.135823. [DOI] [PubMed] [Google Scholar]
- 2.Saunders C, Limbird LE. Localization and trafficking of alpha2-adrenergic receptor subtypes in cells and tissues. Pharmacol Ther. 1999;84:193–205. doi: 10.1016/s0163-7258(99)00032-7. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald E, Kobilka BK, Scheinin M. Gene targeting--homing in on alpha 2-adrenoceptor subtype function. Trends Pharmacol Sci. 1997;18:211–219. doi: 10.1016/s0165-6147(97)01063-8. [DOI] [PubMed] [Google Scholar]
- 4.Brede M, Philipp M, Knaus A, Muthig V, Hein L. Alpha2-adrenergic receptor subtypes – novel functions uncovered in gene-targeted mouse models. Biol Cell. 2004;96:343–348. doi: 10.1016/j.biolcel.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Knaus AE, Muthig V, Schickinger S, Moura E, Beetz N, Gilsbach R, Hein L. Alpha2-adrenoceptor subtypes--unexpected functions for receptors and ligands derived from gene-targeted mouse models. Neurochem Int. 2007;51:277–81. doi: 10.1016/j.neuint.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 6.Eason MG, Liggett SB. Identification of a Gs coupling domain in the amino terminus of the third intracellular loop of the alpha 2A-adrenergic receptor. Evidence for distinct structural determinants that confer Gs versus Gi coupling. J Biol Chem. 1995;270:24753–24760. doi: 10.1074/jbc.270.42.24753. [DOI] [PubMed] [Google Scholar]
- 7.Wade SM, Lim WK, Lan KL, Chung DA, Nanamori M, Neubig RR. G(i) activator region of alpha(2A)-adrenergic receptors: distinct basic residues mediate G(i) versus G(s) activation. Mol Pharmacol. 1999;56:1005–1013. doi: 10.1124/mol.56.5.1005. [DOI] [PubMed] [Google Scholar]
- 8.Daunt DA, Hurt C, Hein L, Kallio J, Feng F, Kobilka BK. Subtype-specific intracellular trafficking of alpha2-adrenergic receptors. Mol Pharmacol. 1997;51:711–20. doi: 10.1124/mol.51.5.711. [DOI] [PubMed] [Google Scholar]
- 9.Hurt CM, Feng FY, Kobilka B. Cell-type specific targeting of the alpha 2c-adrenoceptor. Evidence for the organization of receptor microdomains during neuronal differentiation of PC12 cells. J Biol Chem. 2000;275:35424–35431. doi: 10.1074/jbc.M006241200. [DOI] [PubMed] [Google Scholar]
- 10.Wigley FM. Raynaud’s Phenomenon. N Engl J Med. 2002;347:1001–1008. doi: 10.1056/NEJMcp013013. [DOI] [PubMed] [Google Scholar]
- 11.Flavahan NA. Regulation of vascular reactivity in scleroderma: new insights into Raynaud’s phenomenon. Rheum Dis Clin North Am. 2008;34:81–87. doi: 10.1016/j.rdc.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flavahan NA, Lindblad LE, Verbeuren TJ, Shepherd JT, Vanhoutte PM. Cooling and alpha 1- and alpha 2-adrenergic responses in cutaneous veins: role of receptor reserve. Am J Physiol. 1985;249:H950–H955. doi: 10.1152/ajpheart.1985.249.5.H950. [DOI] [PubMed] [Google Scholar]
- 13.Freedman RR, Baer RP, Mayes MD. Blockade of vasospastic attacks by alpha 2-adrenergic but not alpha 1-adrenergic antagonists in idiopathic Raynaud’s disease. Circulation. 1995;92:1448–1451. doi: 10.1161/01.cir.92.6.1448. [DOI] [PubMed] [Google Scholar]
- 14.Chotani MA, Flavahan S, Mitra S, Daunt D, Flavahan NA. Silent alpha(2C)-adrenergic receptors enable cold-induced vasoconstriction in cutaneous arteries. Am J Physiol Heart Circ Physiol. 2000;278:H1075–H1083. doi: 10.1152/ajpheart.2000.278.4.H1075. [DOI] [PubMed] [Google Scholar]
- 15.Bailey SR, Eid AH, Mitra S, Flavahan S, Flavahan NA. Rho kinase mediates cold-induced constriction of cutaneous arteries: role of alpha2C-adrenoceptor translocation. Circ Res. 2004;94:1367–1374. doi: 10.1161/01.RES.0000128407.45014.58. [DOI] [PubMed] [Google Scholar]
- 16.Duvernay MT, Filipeanu CM, Wu G. The regulatory mechanisms of export trafficking of G protein-coupled receptors. Cell Signal. 2005;17:1457–1465. doi: 10.1016/j.cellsig.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 17.Dong C, Filipeanu CM, Duvernay MT, Wu G. Regulation of G protein-coupled receptor export trafficking. Biochim Biophys Acta. 2007;1768:853–870. doi: 10.1016/j.bbamem.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conn PM, Janovick JA, Brothers SP, Knollman PE. ‘Effective inefficiency’: cellular control of protein trafficking as a mechanism of post-translational regulation. J Endocrinol. 2006;190:13–16. doi: 10.1677/joe.1.06771. [DOI] [PubMed] [Google Scholar]
- 19.Ritter SL, Hall RA. Fine-tuning of GPCR activity by receptor-interacting proteins. Nat Rev Mol Cell Biol. 2009;10:819–30. doi: 10.1038/nrm2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petaja-Repo UE, Hogue M, Laperriere A, Walker P, Bouvier M. Export from the endoplasmic reticulum represents the limiting step in the maturation and cell surface expression of the human delta opioid receptor. J Biol Chem. 2000;275:13727–13736. doi: 10.1074/jbc.275.18.13727. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Venable J, LaPointe P, Hutt DM, Koulov AV, Coppinger J, Gurkan C, Kellner W, Matteson J, Plutner H, Riordan JR, Kelly JW, Yates JR, 3rd, Balch WE. Hsp90 cochaperone Aha1 downregulation rescues misfolding of CFTR in cystic fibrosis. Cell. 2006;127:803–815. doi: 10.1016/j.cell.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 22.Ramos RR, Swanson AJ, Bass J. Calreticulin and Hsp90 stabilize the human insulin receptor and promote its mobility in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2007;104:10470–10475. doi: 10.1073/pnas.0701114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo S, Zhang B, Dong XP, Tao Y, Ting A, Zhou Z, Meixiong J, Luo J, Chiu FC, Xiong WCL. Mei, HSP90 beta regulates rapsyn turnover and subsequent AChR cluster formation and maintenance. Neuron. 2008;60:97–110. doi: 10.1016/j.neuron.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robben JH, Deen PM. Pharmacological chaperones in nephrogenic diabetes insipidus: possibilities for clinical application. BioDrugs. 2007;21:157–66. doi: 10.2165/00063030-200721030-00003. [DOI] [PubMed] [Google Scholar]
- 25.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 26.Hegedus T, Aleksandrov A, Cui L, Gentzsch M, Chang XB, Riordan JR. F508del CFTR with two altered RXR motifs escapes from ER quality control but its channel activity is thermally sensitive. Biochim Biophys Acta. 2006;1758:565–572. doi: 10.1016/j.bbamem.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Dennis AT, Trieu P, Charron F, Ethier N, Hebert TE, Wan X, Ficker E. Intracellular potassium stabilizes human ether-à-go-go-related gene channels for export from endoplasmic reticulum. Mol Pharmacol. 2009;75:927–937. doi: 10.1124/mol.108.053793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Fageeh MB, Smales CM. Control and regulation of the cellular responses to cold shock: the responses in yeast and mammalian systems. Biochem J. 2006;397:247–259. doi: 10.1042/BJ20060166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou F, Filipeanu CM, Duvernay MT, Wu G. Cell-surface targeting of alpha2-adrenergic receptors -- inhibition by a transport deficient mutant through dimerization. Cell Signal. 2006;18:318–327. doi: 10.1016/j.cellsig.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Filipeanu CM, Zhou F, Claycomb WC, Wu G. Regulation of the cell surface expression and function of angiotensin II type 1 receptor by Rab1-mediated endoplasmic reticulum-to-Golgi transport in cardiac myocytes. J Biol Chem. 2004;279:41077–41084. doi: 10.1074/jbc.M405988200. [DOI] [PubMed] [Google Scholar]
- 31.Filipeanu CM, Zhou F, Fugetta EK, Wu G. Differential regulation of the cell-surface targeting and function of beta- and alpha1-adrenergic receptors by Rab1 GTPase in cardiac myocytes. Mol Pharmacol. 2006;69:1571–1578. doi: 10.1124/mol.105.019984. [DOI] [PubMed] [Google Scholar]
- 32.Filipeanu CM, Zhou F, Lam ML, Kerut KE, Claycomb WC, Wu G. Enhancement of the recycling and activation of beta-adrenergic receptor by Rab4 GTPase in cardiac myocytes. J Biol Chem. 2006;281:11097–11103. doi: 10.1074/jbc.M511460200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- 34.Small KM, Forbes SL, Rahman FF, Bridges KM, Liggett SB. A four amino acid deletion polymorphism in the third intracellular loop of the human alpha 2C-adrenergic receptor confers impaired coupling to multiple effectors. J Biol Chem. 2000;275:23059–23064. doi: 10.1074/jbc.M000796200. [DOI] [PubMed] [Google Scholar]
- 35.Kurnik D, Friedman EA, Muszkat M, Sofowora GG, Xie HG, Dupont WD, Wood AJ, Stein CM. Genetic variants in the alpha2C-adrenoceptor and G-protein contribute to ethnic differences in cardiovascular stress responses. Pharmacogenet Genomics. 2008;18:743–750. doi: 10.1097/FPC.0b013e3282fee5a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Picard D. Heat-shock protein 90, a chaperone for folding and regulation. Cell Mol Life Sci. 2002;59:1640–1648. doi: 10.1007/PL00012491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taherian A, Krone PH, Ovsenek N. A comparison of Hsp90alpha and Hsp90beta interactions with cochaperones and substrates. Biochem Cell Biol. 2008;86:37–45. doi: 10.1139/o07-154. [DOI] [PubMed] [Google Scholar]
- 38.Wandinger SK, Richter K, Buchner J. The Hsp90 chaperone machinery. J Biol Chem. 2008;283:18473–18477. doi: 10.1074/jbc.R800007200. [DOI] [PubMed] [Google Scholar]
- 39.Jeyaraj SC, Chotani MA, Mitra S, Gregg HE, Flavahan NA, Morrison KJ. Cooling evokes redistribution of alpha2C-adrenoceptors from Golgi to plasma membrane in transfected human embryonic kidney 293 cells. Mol Pharmacol. 2001;60:1195–1200. doi: 10.1124/mol.60.6.1195. [DOI] [PubMed] [Google Scholar]
- 40.Angelotti T, Daunt D, Shcherbakova OG, Kobilka B, Hurt CM. Regulation of G-protein coupled receptor traffic by an evolutionary conserved hydrophobic signal. Traffic. 2010;11:560–578. doi: 10.1111/j.1600-0854.2010.01033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He F, Qiao ZH, Cai J, Pierce W, He DC, Song ZH. Involvement of the 90-kDa heat shock protein (Hsp-90) in CB2 cannabinoid receptor-mediated cell migration: a new role of Hsp-90 in migration signaling of a G protein-coupled receptor. Mol Pharmacol. 2007;72:1289–1300. doi: 10.1124/mol.107.036566. [DOI] [PubMed] [Google Scholar]
- 42.Xu W, Mimnaugh E, Rosser MF, Nicchitta C, Marcu M, Yarden Y, Neckers L. Sensitivity of mature Erbb2 to geldanamycin is conferred by its kinase domain and is mediated by the chaperone protein Hsp90. J Biol Chem. 2001;276:3702–3708. doi: 10.1074/jbc.M006864200. [DOI] [PubMed] [Google Scholar]
- 43.Argon Y, Simen BB. GRP94, an ER chaperone with protein and peptide binding properties. Semin Cell Dev Biol. 1999;10:495–505. doi: 10.1006/scdb.1999.0320. [DOI] [PubMed] [Google Scholar]
- 44.Filipeanu CM, Henning RH, Nelemans SA, de Zeeuw D. Intracellular angiotensin II: from myth to reality? J Renin Angiotensin Aldosterone Syst. 2001;2:219–226. doi: 10.3317/jraas.2001.035. [DOI] [PubMed] [Google Scholar]
- 45.O’Malley KL, Jong YJ, Gonchar Y, Burkhalter A, Romano C. Activation of metabotropic glutamate receptor mGlu5 on nuclear membranes mediates intranuclear Ca2+ changes in heterologous cell types and neurons. J Biol Chem. 2003;278:28210–28219. doi: 10.1074/jbc.M300792200. [DOI] [PubMed] [Google Scholar]
- 46.Rozenfeld R, Devi LA. Regulation of CB1 cannabinoid receptor trafficking by the adaptor protein AP-3. FASEB J. 2008;22:2311–2322. doi: 10.1096/fj.07-102731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dupré DJ, Robitaille M, Ethier N, Villeneuve LR, Mamarbachi AM, Hébert TE. Seven transmembrane receptor core signaling complexes are assembled prior to plasma membrane trafficking. J Biol Chem. 2006;281:34561–34573. doi: 10.1074/jbc.M605012200. [DOI] [PubMed] [Google Scholar]
- 48.Quraishi H, Brown IR. Expression of heat shock protein 90 (hsp90) in neural and nonneural tissues of the control and hyperthermic rabbit. Exp Cell Res. 1995;219:358–363. doi: 10.1006/excr.1995.1239. [DOI] [PubMed] [Google Scholar]
- 49.Yetik-Anacak G, Xia T, Dimitropoulou C, Venema RC, Catravas JD. Effects of hsp90 binding inhibitors on sGC-mediated vascular relaxation. Am J Physiol Heart Circ Physiol. 2006;(291):H260–H268. doi: 10.1152/ajpheart.01027.2005. [DOI] [PubMed] [Google Scholar]
- 50.Han G, Ma H, Chintala R, Fulton DJ, Barman SA, White RE. Essential role of the 90-kilodalton heat shock protein in mediating nongenomic estrogen signaling in coronary artery smooth muscle. J Pharmacol Exp Ther. 2009;329:850–855. doi: 10.1124/jpet.108.149112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koga F, Kihara K, Necker L. Inhibition of cancer invasion and metastasis by targeting the molecular chaperone heat-shock protein 90. Anticancer Res. 2009;29:797–807. [PubMed] [Google Scholar]
- 52.Taldone T, Gozman A, Maharaj R, Chiosis G. Targeting Hsp90: small-molecule inhibitors and their clinical development. Curr Opin Pharmacol. 2008;8:370–374. doi: 10.1016/j.coph.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eid AH, Maiti K, Mitra S, Chotani MA, Flavahan S, Bailey SR, Thompson-Torgerson CS, Flavahan NA. Estrogen increases smooth muscle expression of alpha2C-adrenoceptors and cold-induced constriction of cutaneous arteries. Am J Physiol Heart Circ Physiol. 2007;293:H1955–H1961. doi: 10.1152/ajpheart.00306.2007. [DOI] [PubMed] [Google Scholar]
- 54.Eid AH, Chotani MA, Mitra S, Miller TJ, Flavahan NA. Cyclic AMP acts through Rap1 and JNK signaling to increase expression of cutaneous smooth muscle alpha2C-adrenoceptors. Am J Physiol Heart Circ Physiol. 2008;295:H266–H272. doi: 10.1152/ajpheart.00084.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tantos A, Friedrich P, Tompa P. Cold stability of intrinsically disordered proteins. FEBS Lett. 2009;583:465–469. doi: 10.1016/j.febslet.2008.12.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.