Abstract
Interleukin (IL)-17-producing T helper cells (TH17) are a recently identified CD4+ T cell subset distinct from T helper type 1 (TH1) and T helper type 2 (TH2) cells1. TH17 cells can drive antigen specific autoimmune diseases and are considered the main population of pathogenic T cells driving experimental autoimmune encephalomyelitis (EAE)2, the mouse model for multiple sclerosis. The factors that are needed for the generation of TH17 cells have been well-characterized3–6. However, where and how the immune system controls TH17 cells in vivo remains unclear.
Here, by using a model of tolerance induced by CD3-specific antibody, a model of sepsis and influenza A viral infection (H1N1), we show that pro-inflammatory TH17 cells can be redirected to and controlled in the small intestine. TH17-specific IL-17A secretion induced expression of the chemokine CCL20 in the small intestine, facilitating the migration of these cells specifically to the small intestine via the CCR6/CCL20 axis. Moreover, we found that TH17 cells are controlled by two different mechanisms in the small intestine: first, they are eliminated via the intestinal lumen and simultaneously pro-inflammatory TH17 cells acquire a regulatory phenotype with in vitro and in vivo immune-suppressive properties (rTH17). These results identify mechanisms limiting TH17 cell pathogenicity and implicate the gastrointestinal tract as a site for control of TH17 cells.
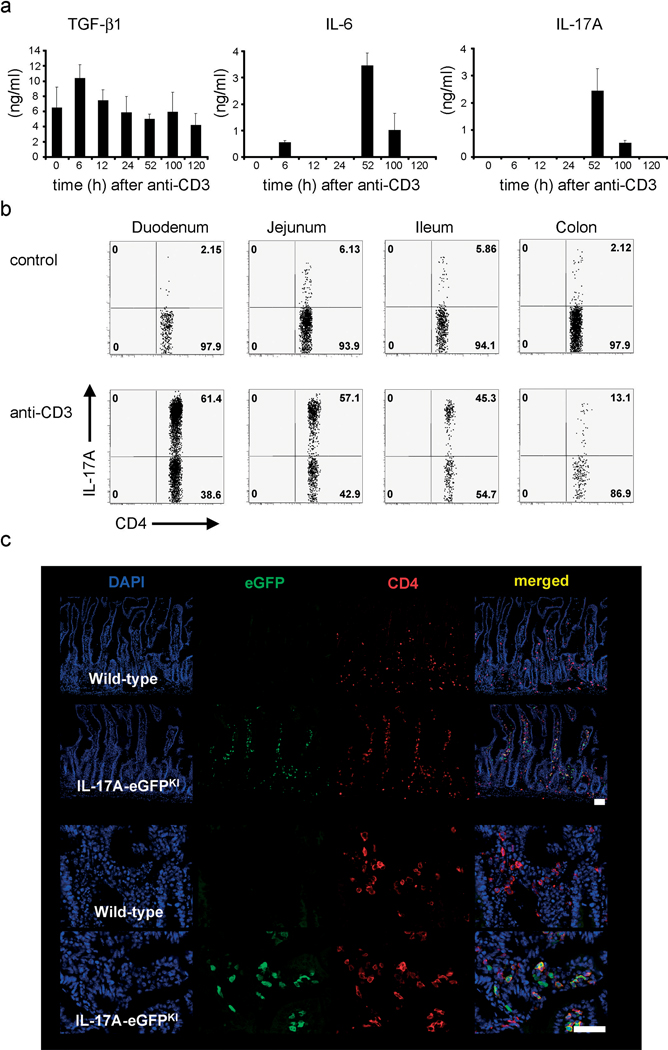
TH17 cells have been associated with the pathogenesis of several chronic inflammatory disorders including rheumatoid arthritis and multiple sclerosis2,7. To study the cellular and molecular mechanisms that control pathogenicity mediated by TH17 cells we first utilized the CD3-specific antibody treatment model. It is known that CD3-specific antibody treatment induces a ‘cytokine storm’ and local inflammation mainly in the small intestine8. Despite this it has been validated as an in vivo model of tolerization9 and is now under study in human clinical trials10. By mimicking antigen, CD3-specific antibody treatment leads to activation induced cell death (AICD) of T cells11,12 and consequently a systemic up-regulation of IL-69 and transforming growth factor-β (TGF-β1) induced by phagocyte engulfment of apoptotic T cells13. In line with these publications, we found that CD3-specific antibody treatment induced an immuno-regulatory environment marked by simultaneous expression of TGF-β1 and IL-6 (Fig.1a). The combination of these cytokines is important for the development of TH17 cells in vitro and in vivo as it has been previously clearly established3,4. Accordingly, we found elevated levels of IL-17A in plasma of CD3-specific antibody treated animals compared to controls (Fig.1a).
Figure 1. Accumulation of TH17 cells in the small intestine after CD3-specific antibody treatment.
Mice were injected with CD3-specific antibody. (a) Plasma levels of TGF-β1, IL-6 and IL-17A. (mean±s.e.m.; n=4). (b) Flow cytometric analysis of IL-17A-eGFP expression (gated on CD4+TCRβ+ events); numbers in quadrants indicate percent cells in each. (c) Immunofluorescence staining of frozen sections of the small intestine after CD3-specific antibody treatment (eGFP: green, CD4: red, and cell nuclei: DAPI). (Scale bar, 50µm). Data are representative of at least three independent experiments.
First, we aimed to investigate the source of IL-17A. It has been reported that a few hours after injection of CD3-specific antibody, there is a rapid disappearance of the majority of T cells from the circulation13,14. Surprisingly, in parallel with the disappearance of T cells from the periphery we found a concomitant increase in the percentage and the number of total T cells in the small intestine, in particular in the duodenum (Supplemental Fig.1a–c). In a newly generated IL-17A-eGFP knock-in mice (Supplemental Fig.2a–d and 3a–c) injected with CD3-specific antibody, 50–80% of the CD4+TCRβ+ T cells located in the duodenum were expressing IL-17A (Figs.1b and Supplemental Fig.1d,e). The percentage and number of TH17 cells in the intestine decreased from the duodenum to the colon in a gradient-like fashion (Fig.1b). Detection of CD4+eGFP+ T cells by immunofluorescence and two-photon-laser-scanning microscopy confirmed the high frequency of TH17 cells in the small intestine in situ (Fig.1c and Supplemental Fig.4a–c). Importantly, we also found TH17 cell infiltration in the duodenum when animals were injected with a therapeutic non-FcR-binding CD3-specific antibody15, although the frequency and numbers of the TH17 cells were lower compared to the FcR-binding antibody (Supplemental Fig.5a). Similar results were observed after antigen-specific stimulation when soluble myelin oligodendrocyte glycoprotein antigen (MOG) was administered to MOG-TCR transgenic mice (2D2 mice)16 (Supplemental Fig.5b). Taken together these data suggest that the generation and the accumulation of TH17 cells in the small intestine was not restricted to the CD3-specific antibody treatment but was a general mechanism following strong TCR stimulation.
We next wanted to identify the molecular signals important for the generation of TH17 cells in vivo after CD3-specific antibody treatment. Since IL-6 is known to be important for TH17 cell generation, we evaluated the importance of this cytokine. Il6−/− and wild type mice were treated with CD3-specific antibody. In the Il6−/− mice, only a very small population of TH17 cells (about 2%) could be found by flow cytometry in the small intestine (Supplemental Fig. 6a) and IL-17A was undetectable in the plasma (data not shown). To study the cellular source of IL-6, we treated mice with clodronate-loaded liposomes, which eliminates most macrophages and a significant proportion of dendritic cells compared to PBS-loaded liposomes13 (Supplemental Fig.6c). IL-6 plasma levels were greatly reduced in mice treated with clodronate-loaded liposomes compared to control mice after CD3-specific antibody injection (Supplemental Fig.6d) and a profound reduction in TH17 cells was observed (Supplemental Fig.6b,c). Taken together, these data support the notion that IL-6 secreted by antigen-presenting cells (APCs) is critical for the generation of TH17 cells during CD3-specific antibody treatment.
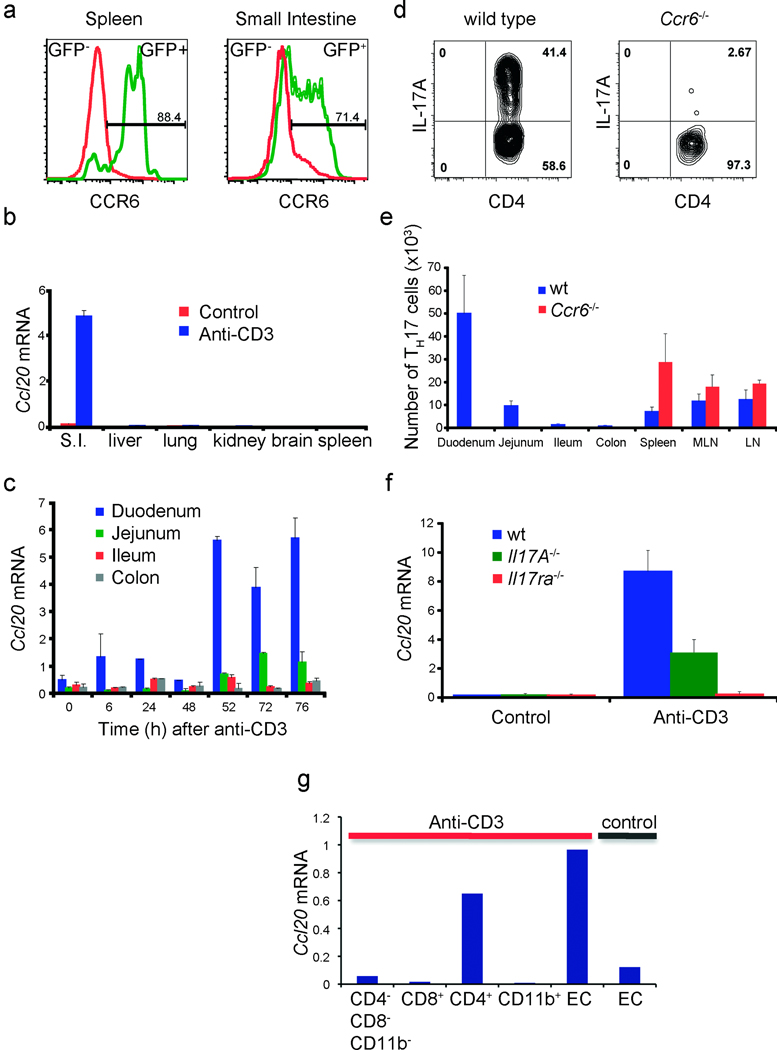
We next analyzed the mechanism leading to the specific accumulation of TH17 cells in the small intestine, predominantly in the duodenum. TH17 cells are known to express the chemokine receptor CCR617. While CCR6 is relevant in different autoimmune disease models7,18, the role of the CCR6-CCL20 axis in immune cell migration to the intestine during tolerance induction has not yet been evaluated. To study that, we analyzed the expression of CCR6 on CD4+ IL-17A-eGFP positive and negative cells (Fig.2a) and Ccl20 mRNA expression (Fig.2b) in the spleen and the gut. CCR6 was mainly expressed in the TH17 cells from the spleen and the gut 24 hours after CD3-specific antibody injection (Fig.2a). Strikingly, when we performed a time course to measure the mRNA levels of Ccl20 in different parts of the intestine during CD3-specific antibody treatment, we observed that Ccl20 was expressed at the highest level in the duodenum in steady state conditions and was selectively further up-regulated after CD3-specific antibody treatment (Fig.2b,c, Supplemental Fig.7). To test the importance of the CCR6/CCL20 axis for the migration of TH17 cells from the periphery to the duodenum, we treated Ccr6−/− and control mice with CD3-specific antibody. TH17 cell number (Fig.2e) and frequency (Fig.2d) were strongly reduced in the intestine of the Ccr6−/− compared to wild-type mice. In general, we did not observe signs of intestinal inflammation in the Ccr6−/− mice as we did in wild-type controls after CD3-specific antibody treatment (data not shown). Interestingly, we detected a higher number of TH17 cells in the spleen and lymph nodes of Ccr6−/− mice when compared to control animals (Fig.2e). This increase was accompanied by splenomegaly and enlargement of lymph nodes (data not shown), suggesting that CCR6 does not play a major role in the generation and expansion of TH17 cells. In conclusion, CCR6 seems to be essential for the migration of TH17 cells to the small intestine after CD3-specific antibody treatment, and the intestinal inflammation is dependent on this migration. Thus our data suggest that TH17 cell migrate to the small intestine leading to intestinal inflammation and damage. However we cannot exclude that a proliferation of gut resident TH17 cells also contributes to the observed phenomenon.
Figure 2. The axis CCR6/CCL20 is essential for the recruitment of TH17 cells to the Small Intestine.
(a) CCR6 expression 24h after anti-CD3 treatment. (b+c) Ccl20 mRNA expression (mean±s.e.m.; n=4). (d) IL-17A expression (gated on CD4+TCRβ+ events) as measured by intracellular cytokine staining. (e) TH17 cell numbers in different organs (mean±s.d.;n=5). (f) Ccl20 mRNA expression in duodenum of wild type, Il17a−/− and Il17ra−/− mice (mean±s.e.m.;n=4). (g) Ccl20 mRNA levels of epithelial and hematopoietic cells isolated from the small intestine. (b,d,e,f,g): 100 hours after the first anti-CD3 injection. Data are representative of at least three independent experiments.
To evaluate the contribution of IL-17A and IL-17F (TH17 signature cytokines) in the induction of CCL20 expression in the duodenum, we treated Il17a−/− or Il17ra−/− mice with CD3-specific antibody. We found decreased levels of CCL20 in the Il17a−/− and the Il17ra−/− mice versus the controls after CD3-specific antibody treatment (Fig.2f), suggesting that IL-17 signalling plays a major role in the induction of CCL20 in the duodenum. We next studied the cellular source of CCL20. Ccl20 mRNA was only detectable in the intestinal epithelial cells in untreated mice. Treatment with CD3-specific antibody led to a further up-regulation of Ccl20 mRNA by the epithelial cells. Additionally, the CD4+ T cells present in the small intestine after CD3-specific antibody treatment, most of which were TH17 cells, expressed high levels of Ccl20 mRNA (Figure2g). In conclusion, TH17 cells via IL-17A and IL-17F production directly up-regulate CCL20 production by the intestinal epithelial cells, which then leads to the subsequent recruitment of CCR6+ TH17 cells, which also produce CCL20.
Of note the intestinal inflammation after CD3-specific antibody treatment was transient and 100% of the mice recovered. To better understand the mechanisms underlying this process, we first assessed apoptosis of TH17 cells in the small intestine but we did not detect a significant number of apoptotic cells (data not shown). When we studied the in vivo proliferation capacity of CD4+TCRβ+ T cells from the CD3-specific antibody treated animals, we found that TH17 cells from the duodenum were actively proliferating (Supplemental Figs.8 and 9a,b). Using IL17A-eGFPxFoxP3-mRFP mice we determined that CD4+IL-17A+ T cells were proliferating at a higher rate than CD4+IL-17A− T cells in the duodenum (Supplemental Figs. 8 and 9). Using two-photon-laser-scanning microscopy, we found that the TH17 cells in the duodenum did not show the typical behaviour of an apoptotic T cell, conversely, they behaved like activated T cells in terms of their pattern of speed and direction of migration (Supplemental-video). Taken together these data indicate that TH17 cells do not die in the small intestine, but are rather actively proliferating.
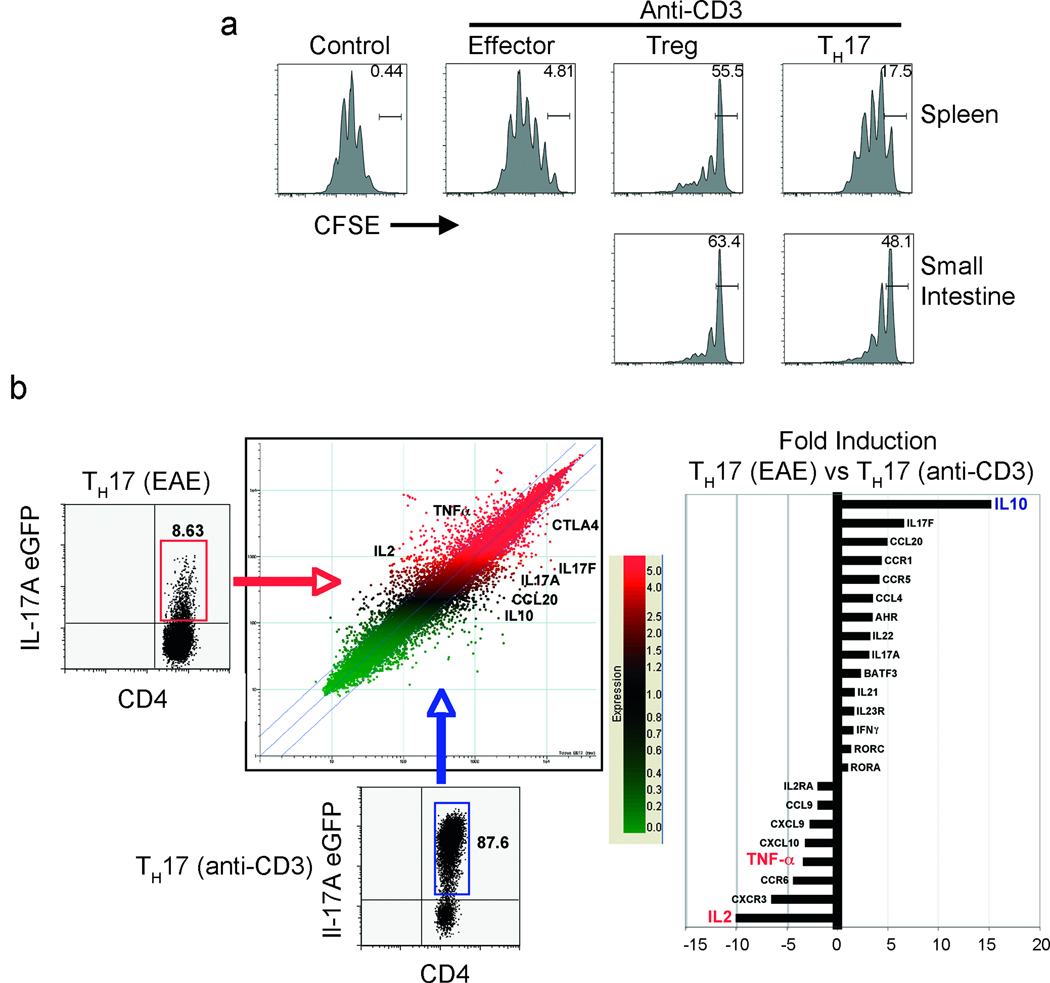
In line with previous publications8 we found that CD3-specific antibody treatment caused diarrhoea, edema, inflammation and tissue destruction in the small intestine (Supplemental Fig.10a,b), which correlated with the recruitment of TH17 cells. However the intestinal pathology was only transient and mice fully recovered. We therefore began to investigate the fate of TH17 cells in the small intestine. Interestingly we found a fraction of TH17 cells in the intestinal lumen of the CD3-specific antibody treated mice (Supplemental Fig.10c,d). Given the sever inflammation, diarrhoea and tissue damage (Supplemental Fig.10a,b), it is most likely that these cells were passively washed out, although an active mechanism cannot be excluded. Considering that the remaining TH17 cells in the duodenum were actively proliferating, but the intestinal pathology was only transient, we were curious about the functional capabilities of these cells. Surprisingly, we found that the remaining TH17 cells in the duodenum were able to suppress proliferation of responder T cells in vitro (Fig.3a). To study the molecular properties of these suppressive TH17 cells (which we refer from now on as to rTH17 cells), we performed a genome-wide transcriptional profiling assay (Fig. 3b). We compared the gene expression pattern of rTH17 from CD3-specific antibody treated mice and genes expressed by pro-inflammatory TH17 cells that were harvested from the CNS of EAE induced mice. The signature genes of TH17 cells, like Rorc, Rora, Il17a, Il22, or Il23r, were similarly expressed between both types of TH17 cells. Also the activation status of these cells appeared to be similar, since activation markers like CD69, CD25 and CD44 were equally expressed. However, we found that the rTH17 cells from the CD3-specific antibody treated mice showed a non-inflammatory gene expression profile compared to pro-inflammatory TH17 cells isolated from the central nervous system (CNS) of EAE-induced mice. Notably, the expression levels of Tnf-α and Il-2, two cytokines with clear pro-inflammatory roles19,20, were greatly reduced in the rTH17 cells from the small intestine. In contrast, these cells expressed high levels of IL-10, a cytokine with potent anti-inflammatory activities21 (Supplemental Fig.11b + Fig. 3b). These data are supported by a previous report showing that in vitro generated non-pathogenic TH17 cells are able to express IL-1022. To evaluate the molecular mechanisms involved in the suppressive function of the rTH17 cells different molecules were blocked in an in vitro suppression assay using monoclonal antibodies (Supplemental Fig.11a). The suppressive capacity of the rTH17 cells was partially dependent on IL-10, CTLA-4 and TGF-β. Blocking all three pathways resulted in a lack of suppression by the rTH17 cells. TH17 cells isolated from the spleen showed an intermediate phenotype. They exhibited a limited capacity to suppress the proliferation of T cells in vitro (Fig.3a), and also produced more TNF-α and IL-2, but less IL-10 compared to TH17 cells isolated from the small intestine (Supplemental Fig.11b). However since some of the TH17 cells in the small intestine down-regulated CCR6 (Fig.2a) it is possible that some rTH17 might have migrated back from the small intestine to the spleen. If development of the suppressive capability occurred in the small intestine, then preventing the migration of the TH17 cells to that site should prevent the development of these tolerogenic cells. To test this hypothesis, we analyzed TH17 cells isolated from the spleen of Ccr6−/− mice, since we showed already that these TH17 cells are unable to migrate to the small intestine. Consistent with the hypothesis, Ccr6−/− TH17 cells in the spleen showed high TNF-α production, failed to suppress T cell proliferation in vitro, and were even proinflammatory, causing IBD in vivo upon transfer into a lymphopenic host (Supplemental Fig.12 a–d). These data argue that proinflammatory TH17 cells do indeed acquire their suppressive phenotype in the small intestine.
Figure 3. Functional and molecular characterization of rTH17 cells.
(a) Suppression assay was performed using eGFP−mRFP−CD4+ (Effector), eGFP−mRFP+CD4+ (Tregs) or eGFP+mRFP−CD4+ (TH17) cells sorted from spleen or small intestine. (Bar represents undivided CFSE labelled CD4+CD25− responder T cells). Data are representative of six independent experiments. (b) Gene expression analysis comparing TH17 cells (eGFP+mRFP−CD4+) from central nervous system at day 17 after EAE induction versus TH17 cells isolated from the small intestine of anti-CD3 treated IL-17A-eGFP × Foxp3-mRFP double reporter mice mice.
To confirm our findings in an animal disease model, EAE-induced mice were treated with CD3-specific antibody. In line with a previous publication23 we observed a protective effect when the treatment was administered during the course of the disease (Supplemental Fig.13a). More importantly, we demonstrated that TH17 cells were recruited to the duodenum of the CD3-specific antibody treated animals and these mice had strongly reduced numbers of TH17 cells in the central nervous system (data not shown). Using a MOG specific tetramer, we determined that a significant percentage of TH17 cells in the duodenum were antigen specific (Supplemental Fig.13b), demonstrating that MOG specific TH17 cells were recruited to the duodenum following CD3-specific antibody treatment. In contrast the frequency of MOG-tetramer positive TH17 cells was much lower in other organs of EAE induced mice, which had not been treated with CD3-specific antibody (data not shown). This argues against a general increase in MOG specific TH17. Therefore our results show that antigen specific TH17 cells, with proinflammatory properties, generated in the periphery can be redirected to the small intestine. In order to confirm that rTH17 isolated from the small intestine of CD3-specific antibody treated mice are indeed in vivo immune-suppressive we tested their suppressive capacity in an EAE transfer model. We co-transferred MOG-specific in vitro differentiated TH17 either alone or together with MOG-specific rTH17 isolated from the small intestine of CD3-specific antibody treated 2D2 transgenic mice. Strikingly we found that rTH17 cells were able to completely suppress the development of EAE in these transfer experiments (Supplemental Fig.13c,d). Suggesting that the rTH17 cells are indeed stable in terms of their immune suppressive function.
As mentioned above CD3-specific antibody treatment is already used in clinical trials9,10, and we therefore aimed to confirm our results using Teplizumab (hOKT3γ1(Ala-Ala)), one CD3-specific antibody used in these trials. To that end we used a humanized mouse system: we reconstituted Balb/c Rag-1−/−γc−/− mice with human PBMC. Two weeks after the transfer we treated these mice with either OKT-3, a FcR-binding CD3-specific antibody used in the first human studies, or Teplizumab, a FcR non-binding CD3-specific antibody. Strikingly we found human T cells in the small intestine after treatment with both of these CD3-specific antibodies (Supplemental Fig.14a,b). The presence of human IL-17A+, IL-10+ and CCL20+ producing cells in the small intestine in OKT-3 and Teplizumab treated mice was confirmed by real-time PCR (Supplemental Fig.14c).
Taken together our results obtained in the CD3-specific antibody model suggest that TH17 cells, by up-regulating CCL20 expression in the duodenum via IL-17 signalling, have developed an elegant mechanism to limit the pathogenicity in order to avoid a life-threatening immune response. This predicted in turn that this mechanism should be general to most strong immune responses that result in TH17 cells.
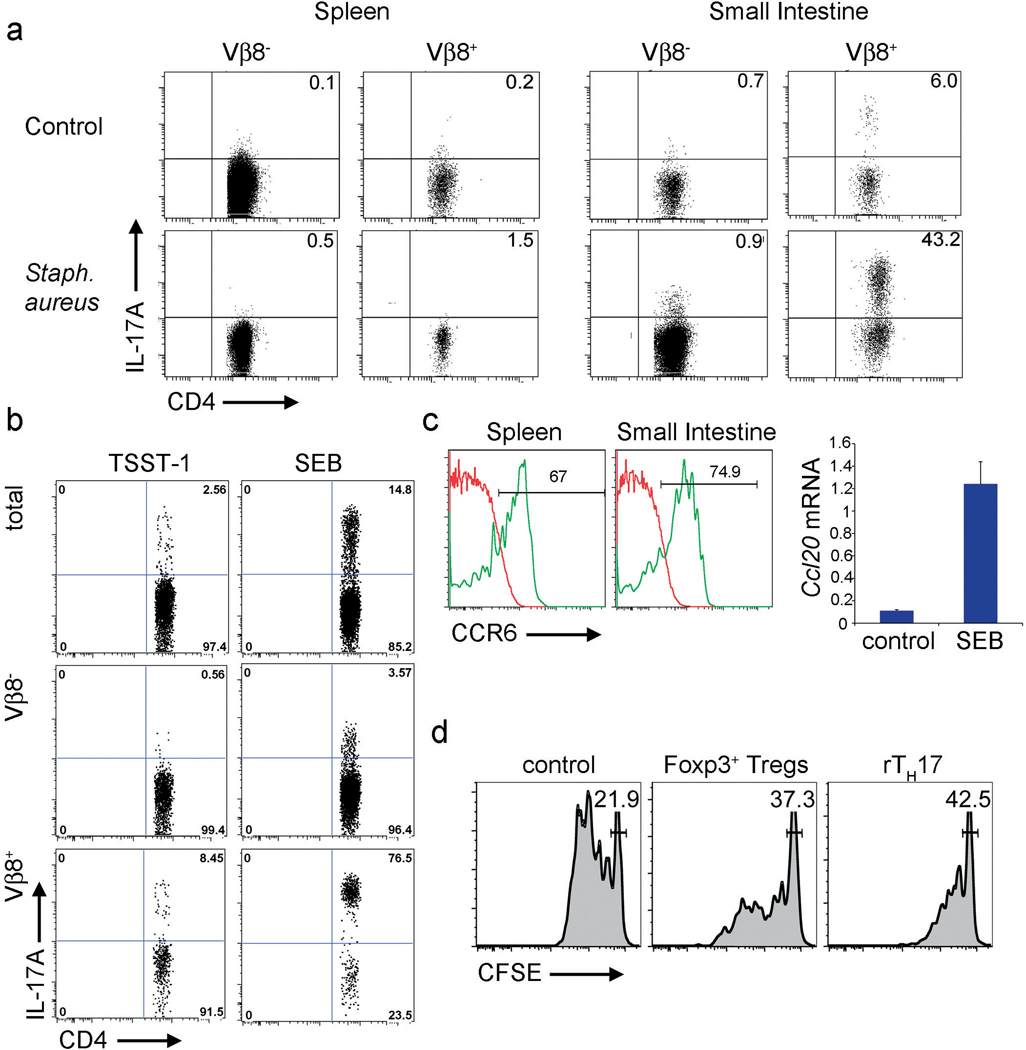
TH17 cells play a crucial role in controlling different microorganisms in vivo24. We next investigated, whether this mechanism of TH17 cell control also functions during a strong immune response elicited by pathogenic microorganisms. We first used a murine model of sepsis. We injected Staphylococcus aureus, which is one of most frequent organisms responsible for sepsis in humans25,26, intravenously into IL-17A eGFP reporter mice. Mice were sacrificed three days after the injection, at a time when they displayed severe clinical symptoms of sepsis (weight loss, dehydration, lethargy). Strikingly we found the highest frequency and number of TH17 in the small intestine (Fig.4a). Interestingly, most TH17 appeared to be TCR Vβ8+. The injection of the superantigen SEB (Staphylococcus aureus Enterotoxin B), which is produced by the bacteria used in these experiments and binds to Vβ8+ T cells, was sufficient to induce the accumulation of TH17 in the small intestine just as in the anti-CD3 studies. As a control we injected mice with TSST-1 (Toxic Shock Syndrome Toxin 1), a superantigen that does not bind to Vβ8 and is not produced by the bacteria we used. Of note, we observed that the administration of TSST-1 was less effective at inducing the accumulation of TH17 cells in the small intestine (Fig.4b). Finally we could confirm that the TH17 cells, induced by SEB treatment, expressed CCR6 and that CCL20 is specifically up-regulated in the small intestine following SEB treatment (Fig.4c). Furthermore, while a subpopulation of the TH17 cells was found in the intestinal lumen (supplemental Fig.15), the remaining TH17 cells demonstrated an immune-suppressive phenotype (Fig.4d + Supplemental Fig.16), again comparable to our results obtained in the CD3-specific antibody treatment. Interestingly it is known that SEB can induce tolerance27, which is in line with our results that SEB leads to the generation of rTH17 cells. Accordingly we found that SEB and to a lesser extent TSST-1 treatment of EAE-induced mice led to the amelioration of disease (data not shown), which is in line with one previous publication28.
Figure 4. TH17 cells are recruited to the small intestine during sepsis.
(a + b) IL-17A eGFP expression is shown (gated on CD4+TCRβ+ events). Mice were injected with Staphylococcus aureus (a) or SEB and TSST-1 (b). (c) CCR6 expression 24 hours after the first SEB injection (left). Ccl20 mRNA levels in the small intestine 100 hours after the first injection (right). (d) In vitro suppression assay using CD4+IL-17A eGFP+ cells from the small intestine or CD4+Foxp3 mRFP+ cells from the spleen of SEB treated mice as suppressor cells. Results are representative of at least two independent experiments.
In addition to anti-bacterial immunity, viruses are the next key class of pathogens to which we must respond, yet contain excessive immunopathology which is commonly the cause of morbidity and mortality29. To address such an immune response, we analysed influenza, a viral infection that has devastated human populations. Notably, we again found increased TH17 cell frequencies in the small intestine in mice infected with Influenza A (H1N1) (Supplemental Fig. 17).
In conclusion, we propose a general mechanism that could explain how a pro-inflammatory TH17 immune response, which is beneficial in clearing infection, but immunopathogenic in excess, can be controlled by the mechanism we describe here: namely by acquisition of an immune-suppressive phenotype or elimination into the intestinal lumen (Supplemental Fig. 18). These findings and further studies aiming to identify the underlying mechanism of the conversion of pro-inflammatory TH17 cells into rTH17 cells may help in designing new strategies to control auto-reactive TH17 cells in autoimmune diseases like multiple sclerosis.
Method summary
Anti-CD3, SEB, TSST-1 treatment and Staphyloccocus aureus infection
Mice were injected i.p. three times with either CD3-specific antibody (clone 2C11, 20 µg/mouse) SEB (50 µg/mouse) or TSST-1 (50 µg/mouse) at 0, 48 and 96 hours. Mice were analyzed 100 hours after the first injection, if not otherwise specified. Staphylococcus aureus was injected i.v. (1×108 cfu/mouse) in order to induce sepsis. Mice were sacrificed three days after the injection.
Flow cytometric analysis
Cells were isolated from the organ as indicated. IL-17A eGFP and CCR6 expression was assessed directly after isolation. When indicated cells were restimulated and intracellular cytokine staining for IL-17A was performed. Numbers in dot plot quadrants indicate percent cells in each. Cells were gated on CD4+TCRβ+ events.
Real-time PCR
Ccl20 mRNA expression was measured in different tissues as indicated using RT-PCR.
In vitro suppression assay
Differrent suppressor cells were co-cultured with CFSE labelled CD4+CD25− responder T cells, which were isolated from the spleen of CD45.1 congenic mice. Bar represents undivided CFSE labelled responder T cells.
Methods
Mice
BALB/c mice (blastocyst donors), CD1 mice (foster mothers), Tet-Cre transgenic mice (“deletor” mice, C57BL/6 background), C57BL/6 mice (B6), C57BL/6.Ly5.1 mice (CD45.1+), IL-6−/− mice and CCR6−/− mice were purchased from The Jackson Laboratories. MOG-transgenic mice (2D2 mice, C57BL/6 background)1 and Foxp3 reporter mice (FIR mice, C57BL/6 background)2 were intercrossed with the IL-17A-eGFP reporter mice. We also used Il-17a−/−, Il-17ra−/− and IL-10-eGFP mice (Tiger mice)3–5. All the mice were kept under specific pathogen-free conditions in the animal care facility at Yale University. The mice were studied at 6–12 week of age. All the experiments were approved by the Institutional Animal Care and Use Committee of Yale University.
Generation of IL-17A-IRES-eGFP reporter mice
A BAC clone consisting of Il-17a genomic DNA derived from C57BL/6 mice was purchased from BacPac (Oakland, CA). An 8-kb BamHI-MluI fragment comprising exons 1, 2 and 3 for Il-17a gene was cloned into pEasy-Flox vector adjacent to the thymidine kinase selection marker. The IRES-eGFP cassette was linked to a LoxP-flanked neomycin (Neo) selection marker to obtain the IRES-eGFP-Neo cassette. The targeting construct was generated by cloning the IRES-eGFP-Neo cassette into a SacII site between the translation stop codon (UGA) and the polyadenylation signal (A2UA3) of the Il-17a gene. The targeting construct was linearized by ClaI cleavage and subsequently electroporated into Bruce4 C57BL/6 ES cells. Transfected ES cells were selected in the presence of 300 µg/ml G418 and 1 µM ganciclovir. Drug resistant ES cell clones were screened for homologous recombination by PCR. To obtain chimeric mice, correctly targeted ES clones were injected into BALB/c blastocysts, which were then implanted into CD1 pseudopregnant foster mothers. Male chimeras were bred with C57BL/6 to screen for germ-line transmitted offspring. Germ-line transmitted mice were bred with germline Cre transgenic mice (Tet-Cre mice) to remove the neomycin gene. Mice bearing the targeted Il-17a allele were screened by PCR (IL17AKI sense: CACCAGCGCTGTGTCAAT, IL17AKI anti-sense: ACAAACACGAAGCAGTTTGG and IL17A IRES: ACCGGCCTTATTCCAAGC).
Antibodies, Tetramers and intracellular cytokine staining
Anti-CD4 (L3T4), anti-CD62L (MEL-14), anti-CD44 (IM7), anti-CD45.1 (A20) and anti-CD45.2 (104), anti-TCRβ, anti-IL-2, anti-IL-17A, anti-TNFα, anti-Ki-67 and anti-BrdU were purchased from Becton Dickinson Pharmingen. For intracellular cytokine staining, the cells were restimulated with PMA (Sigma, 20 ng/ml) and ionomycin (Sigma, 0.5 µg/ml) for 4 hr. Golgistop (BD Bioscience) was added during the last 3 hours of restimulation. After restimulation, the cells were washed and a ficoll gradient was performed. The cells were fixed with 1% parafomaldehyde (Electron microscopy grade) for 10 minutes on ice. After two washes, cells were incubated with anti-GFP-FITC antibody (Rockland) and anti-IL-17A-PE (BD Bioscience) in wash/perm solution (BD Bioscience) for 30 minutes on ice. Cells were washed two times and resuspended in PBS. Acquisitions were made with a LSRII cytometer (BD Bioscience).
For ex vivo-staining with MOG38–49/I-A(b)-tetramer-APC labeled (mouse myelin oligodendrocyte glycoprotein 38–49, “GWYRSPFSRWH”, NIH Tetramer Facility), single cell suspensions were incubated at a density of 107 cells/ml with neuraminidase (0.7 µU/ml, neuraminidase type X from Clostridium perfringens, Sigma) in serum free DMEM at 37 °C/10% CO2 for 25 min before incubation with the I-A(b) multimers (30 µg/ml) in DMEM supplemented with 2% FCS (pH 8.0) at RT for 4 h. After washing, cells were stained for 7-AAD (Molecular Probes), CD4 (RM4-5) and TCRβ. hCLIP/I-A(b)-tetramer-APC labeled was used as a control (“PVSKMRMATPLLMQA”, NIH Tetramer Facility). The percentage of tetramer cells was determined in the CD4/TCRβ gate of live (7-AAD− cells. Stained cells were analyzed on LSRII cytometer (BD Bioscience) and data were analyzed with FlowJo software (Treestar).
Flow Cytometry and FACS sorting
Harvested lymphocytes were treated with ammonium-chloride lysis buffer (BioSource International, Caramillo, CA, no. P304) to remove red blood cells and washed with RPMI containing 10% FBS (Gemini Biological Products, Calabasas, CA). Cells were then stained with 1:400 dilution of the indicated antibodies together with 10µg/ml anti-Fc-Receptor blocking antibody (2.4G2, American Type Culture Collection) in PBS containing 2% FBS and then washed twice with PBS. For isolating T cells, CD4+ T cells were first enriched by magnetic-activated cell sorting beads (Miltenyi Biotec, Auburn, CA) and then stained with the indicated antibodies. The Becton Dickinson FACSVantage system and MoFlo sorter (DAKO Cytomation) were used for fluorescence detection and cell sorting.
TH17 Differentiation in vitro
Splenocytes from IL-17A-IRES-eGFP mice and C57BL/6 mice were incubated with CD4-microbeads and then positively selected through LS columns (Miltenyi Biotec). After enrichment, naive cells (CD4+ CD25−CD62Lhi CD44low) were FACS sorted as mentioned above. CD4+ naive T cells were cultured for 5 days at 106 cells/ml with plate bound anti-CD3 (5µg/ml) and soluble anti-CD28 (2µg/ml) in medium (Bruff’s media supplemented with 10% FCS, L-glutamine, penicillin, and streptomycin) under TH17 conditions (TGF-β, IL-6, IL-23, anti-IFN-γ, anti-IL4). IL-17A (eGFP) expression was determined by flow cytometry.
Multiphoton Imaging
The small intestine (duodenum) from an IL-17A-eGFP × Foxp3-mRFP double reporter mouse treated with CD3-specific antibody was mounted on a glass slide in a chamber consisting of a silicone isolator (20mm diameter × 0.5mm, Electron Microscopy Sciences). The tissue was immersed in PBS and covered by a glass coverslip. An Olympus BX50WI microscope equipped with a 20×, 0.95NA Olympus objective and a LaVision TriMScope Multiphoton System controlled by Imspector Software (LaVision Biotec) was used to collect images. For excitation, a Coherent Chameleon Ti:Sapphire laser was tuned to 960nm. Images of 300×300mm size were recorded at a resolution of 1024×1024 pixels with 1 mm z-spacing. Emitted light was collected with nondescanned detectors after having passed 435/90, 525/50 and 615/100 bandpass filters. Volocity software (Improvision) was used to create 3-dimensional image stacks, and Quicktime Pro was used to generate image sequences.
In Vivo T Cell Stimulation and Intestinal Lymphocyte Isolation
Different mice in C57BL/6 background were injected with anti-CD3 (20 µg, 145 2C11)5–6 i.p. 1–3 times at an interval of 2 days between injections and sacrificed 4 hr after the final injection. For the controls, isotype control or PBS was injected. The IEL and LPL were collected as described with some modifications5. In brief, small or large intestines were removed and Peyer’s patches were dissected. The first 2 cm of the small intestine were considered as Duodenum. Intestines were opened longitudinally and then were cut into strips 1 cm in length. Tissues were washed with Hank’s Buffered Saline and incubated in the presence of 5 mM of EDTA at 37°C for 30 min. The released cells were loaded onto a Percoll gradient and centrifuged. The cells between 40% and 100% Percoll were collected and used as intestinal epithelial lymphocytes. LPL were collected by digesting gut tissue, which was removed for IEL isolation as described above. The tissue was digested with collagenase IV (100 U, Sigma) at 37°C for 1 hr and loaded onto a Percoll gradient and centrifuged. The cells between 40% and 100% Percoll were collected and used as LPL.
For the lumen content isolation and analysis, mice were anesthetized using Isoflurane. One ligation was made after the pylorus and a second one about 4–5 cm distal from the first ligation. A small incision without breaching the vessel proximal to the second ligation was made and 10 ml of pre-warmed PBS (2ml/minute) was injected using a syringe and a 27G1/2 needle. The fluids were collected in a Petri dish placed under the incision proximal to the second ligation. The collected fluids were incubated for 15 min. in HBSS/ EDTA and then filtered through a 70 µm cell strainer before FACS analysis.
Adoptive Transfer of CD4+ T Cells
CD4+FoxP3+ and the CD4+FoxP3− T cells from the thymus or from the periphery of IL17A-eGFP × Foxp3-mRFP double reporter mice were collected and purified by MACS (Miltenyi Biotec). After MACS enrichment, total CD4+Foxp3+ and CD4+ foxp3− T cells were FACS sorted and 4×106 T cells were adoptively transferred (i.v.) into sub-lethally irradiated, sex-matched wild-type CD45.1+ recipient mice. Four weeks after transfer, animals were injected with CD3-specific antibody (20 µg) and the small intestines were recovered and examined for eGFP and mRFP by FACS.
Immunofluorescence microscopy
Small intestines were removed from IL-17A-eGFP reporter mice and wild-type littermates after CD3-specific antibody treatment in vivo. Small intestines were fixed in 4% paraformaldehyde for 16 hours. After two washes with PBS 20% sucrose solution was added. 16 hours later the 20% sucrose solution was replaced with 30% sucrose solution. On the next day, the samples were washed and then snap frozen in OCT and stored in −80°C. Cryosections were cut at 12 microns on a Leica model CM1850 freezing microtome, applied to Superfrost Plus Gold slides (Fisher Scientific), air dried, and PAP pen applied (Zymed Laboratories). Sections were blocked for 30 min at ambient temperature with serum-free protein block (DakoCytomation) and were stained with anti-CD4-PE (BD) and anti-GFP-Alexa488 (Invitrogen) overnight at 4°C. Samples were washed 3 times by immersing in PBS for 5 minutes and then mounted with Prolong gold mounting media with DAPI (Invitrogen). Sections were observed under darkfield in independent fluorescence channels using an automated Olympus BX-61 microscope.
Experimental Autoimmune Encephalomyelitis
Mice were immunized subcutaneously with 250 µg of MOG35–55 (Yale Keck facility) emulsified in CFA (BD Difco). Mice received 400 ng pertussis toxin (PTx, List Biological Laboratories, INC.) intraperitoneally at the time of immunization and 48 h later. Mice were checked for clinical symptoms daily, and signs were translated into clinical score as follows: 0, no detectable signs of EAE; 0.5, tail weakness; 1, complete tail paralysis; 2, partial hind limb paralysis; 2.5, unilateral complete hind limb paralysis; 3, complete bilateral hind limb paralysis; 3.5, complete hind limb paralysis and partial forelimb paralysis; 4, total paralysis of forelimbs and hind limbs (mice with a score above 3.5 to be killed); 5, death. All animal experiments were conducted according to the IACUC policies.
Cytokine assays
Cytokines were quantified in plasma by ELISA (TGF-β1, Promega) or by Cytometric Bead Array (IL-6 and IL-17A, BD Bioscience) following manufacturer’s instructions. The plasma was obtained by centrifugation of blood collected on EDTA-coated tubes after cardiac puncture.
Gene Expression Analysis
100 ng of total RNA extracted (RNAsy, Qiagen) from intestinal rTH17 cells (from CD3-specific antibody treated animals) or from pro-inflammatory TH17 cells (from EAE induced mice) were used to perform a genome-wide transcriptional profiling assay (GeneChip Mouse 1.0 ST Array, Affymetrix). Data was analyzed with GeneSpring GX 10 (Agilent Technologies).
Relative gene expression analysis
RNA from cells/tissues was isolated with the RNEasy/Qiashredder purification system (Qiagen) in accordance with the manufacturer’s protocol. RNA was subjected to reverse transcriptase with Superscript II (Invitrogen) with oligo(dT) primer in accordance with the manufacturer’s protocol. cDNA was semi-quantified using commercially available primer/probe sets (Applied Biosystems) and analysed with the ΔΔCt (change in cycle threshold) method. All results were normalized to Hprt quantified in parallel amplification reactions during each PCR quantification.
Suppression Assays
CFSE (2µM) labeled CD4+CD25− T cells (responder cells) were cultured in 96-well round bottom plates at 2×104 cells/well with 105 irradiated APCs (spleenocytes MACS depleted for CD4+ and CD8+ T cells) as feeder cells in the presence of 2×104 cells/well of FACS sorted CD4+IL-17A+Foxp3− or CD4+ IL17A−Foxp3+ T cells. Cell cultures were stimulated with 2 µg/ml of anti-CD3 antibody (2C11) in the presence or not of anti-TGFβ (1D11), anti-CTLA-4 (9H10) and anti-IL10R. After 4 days, cells were harvested, stained and CFSE signal was analyzed by flow cytometry.
Sepsis induced by infection and superantigen treatment
Staphylococcus aureus (ATCC 14458, SEB+ TSST-1−) was injected intravenously into IL-17A eGFP reporter mice (108 cfu/mouse). Mice were sacrificed three days after the injection, at a time when they displayed severe clinical symptoms of sepsis (weight loss, dehydration, lethargy) and the presence of CD4+IL17A+ T cells was tested in different organs (Spleen, Lymph node, Small intestine) using FACS analysis. Similar experiments were done injecting the superantigens SEB and TSST-1. All of them were purchased from Toxin Technology Inc.. All superantigens were administered 3 times (0, 48, 96 hours) intraperitoneally at 50µg/mouse.
For the influenza A infection, mice were infected with 1 × 104 PFU of influenza A/PR8 (H1N1) virus via the intranasal route. Infection was performed by the intranasal application of 50 µL virus stock diluted in PBS (or an equal volume of PBS as a control) to mice that had been deeply anesthetized with anafane (Ivesco). Lungs and Small intestines were harvested 3 and 5 days after infection for flow cytometry analysis.
PBMC isolation and administration
Human leukocytes were collected by leukapheresis of adult volunteer donors under a protocol approved by the Yale Human Investigations Committee. The PBMC were isolated using Lymphocyte Separation Medium (Cappel) according to the manufacturer’s instructions. The cells were stored in 10% DMSO/90% FBS at −196°C and were thawed and washed before use. Rag1−/− × γc−/− double knockout mice were reconstituted with 5 × 107 human PBMC by i.p. inoculation 2 wk before anti-CD3 specific antibody treatment. The number of human T cells (hCD45+hCD4+) in the small intestine was evaluated by flow cytometry. Animals demonstrated no signs of graft-vs-host disease. Rare animals that failed to reconstitute with human T cells were, by prior design, excluded from analysis.
Endoscopic procedure
Colonoscopy was performed in a blinded fashion for colitis scoring using the Coloview system (Karl Storz, Germany). Briefly: Colitis scoring was based on granularity of mucosal surface, stool consistence, vascular pattern, translucency of the colon and fibrin visible (0–3 points for each).
Statistical analysis
Where indicated, the Student t test for non-paired data and the Mann-Whitney U test were used to calculate statistical significance for differences in a particular measurement between different groups. A P-value of less than 0.05 was considered significant.
Supplementary Material
Acknowledgements
The authors would like to thank F. Manzo for expert administrative assistance, L. Evangelisti and C. Hughes for generating ES cells and chimeric mice, respectively, J. Stein for initial screening of knock-in mice, T. Ferrandino for assistance with the mouse colony and E. Eynon and J. Alderman for managing the mouse program. A. Lin for assistance with Gene Array analysis. We also thank T. Taylor and G. Tokmoulina for expert help with the FACS sorting and D. Gonzalez for help with the multiphoton microscopy. We would like to thank Dr. J. P. Allison for providing the anti-CTLA-4 antibody, Dr. F. Waldron-Lynch, Dr. J. S. Pober for providing PBMCs, and the NIH Tetramer core facility for providing the tetramers. W.O. was supported by a fellowship from the National Multiple Sclerosis Society. S.H. was supported by the DFG (HU 1714/1-1) and by a James Hudson Brown – Alexander B. Coxe Fellowship. E.E. was supported by the Spanish Ministry of Science postdoctoral fellowship and by a James Hudson Brown – Alexander B. Coxe Fellowship. R.A.F. is an Investigator of the Howard Hughes Medical Institute. The generation of mice for this work was supported by the Transgenic Core of the Yale DERC DK45735 and some of the work supported by a Pilot project from DK45735.
Footnotes
Author Contributions
E.E., S.H. and R.A.F. designed the study and wrote the manuscript; N.G. did the in vitro suppression assays and the flow analysis for IL-10 expression; A.E.H. and A.M.H did the two-photon-laser-scanning microscopy experiments; T.T. did the immunohistochemistry analysis; W.O. supported the work with key suggestions and by editing the manuscript; E.E. and S.H. did all other in vitro and in vivo experimental work; Y.Y.W. provided Foxp3-mRFP mice; A.R. did the viral infection experiments; N.V. provided clodronate-loaded liposomes, V.K.K provided 2D2 mice and feedback on the manuscript; Y.I. provided Il17a−/− mice and feedback on the manuscript; J.K.K. provided the Il17ra−/− mice and feedback on the manuscript and J.A.B. provided CD3-specific antibodies and feedback on the manuscript; K.H. provided Teplizumab and key suggestions.
References
- 1.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 2.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 4.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441(7090):231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 5.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9(6):641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang L, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454(7202):350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirota K, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204(12):2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merger M, et al. Defining the roles of perforin, Fas/FasL, and tumour necrosis factor alpha in T cell induced mucosal damage in the mouse intestine. Gut. 2002;51(2):155–163. doi: 10.1136/gut.51.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol. 2007;7(8):622–632. doi: 10.1038/nri2134. [DOI] [PubMed] [Google Scholar]
- 10.Herold KC, et al. Treatment of patients with new onset Type 1 diabetes with a single course of anti-CD3 mAb Teplizumab preserves insulin production for up to 5 years. Clin Immunol. 2009;132(2):166–173. doi: 10.1016/j.clim.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carpenter PA, et al. Non-Fc receptor-binding humanized anti-CD3 antibodies induce apoptosis of activated human T cells. J Immunol. 2000;165(11):6205–6213. doi: 10.4049/jimmunol.165.11.6205. [DOI] [PubMed] [Google Scholar]
- 12.Smith CA, Williams GT, Kingston R, Jenkinson EJ, Owen JJ. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989;337(6203):181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- 13.Perruche S, et al. CD3-specific antibody-induced immune tolerance involves transforming growth factor-beta from phagocytes digesting apoptotic T cells. Nat Med. 2008;14(5):528–535. doi: 10.1038/nm1749. [DOI] [PubMed] [Google Scholar]
- 14.Chatenoud L, Primo J, Bach JF. CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J Immunol. 1997;158(6):2947–2954. [PubMed] [Google Scholar]
- 15.Alegre ML, et al. An anti-murine CD3 monoclonal antibody with a low affinity for Fc gamma receptors suppresses transplantation responses while minimizing acute toxicity and immunogenicity. J Immunol. 1995;155(3):1544–1555. [PubMed] [Google Scholar]
- 16.Bettelli E, et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197(9):1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Annunziato F, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204(8):1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reboldi A, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10(5):514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 19.Brown SJ, Mayer L. The immune response in inflammatory bowel disease. Am J Gastroenterol. 2007;102(9):2058–2069. doi: 10.1111/j.1572-0241.2007.01343.x. [DOI] [PubMed] [Google Scholar]
- 20.Petitto JM, Streit WJ, Huang Z, Butfiloski E, Schiffenbauer J. Interleukin-2 gene deletion produces a robust reduction in susceptibility to experimental autoimmune encephalomyelitis in C57BL/6 mice. Neurosci Lett. 2000;285(1):66–70. doi: 10.1016/s0304-3940(00)00996-4. [DOI] [PubMed] [Google Scholar]
- 21.Pestka S, et al. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 22.McGeachy MJ, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8(12):1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 23.Kohm AP, et al. Treatment with nonmitogenic anti-CD3 monoclonal antibody induces CD4+ T cell unresponsiveness and functional reversal of established experimental autoimmune encephalomyelitis. J Immunol. 2005;174(8):4525–4534. doi: 10.4049/jimmunol.174.8.4525. [DOI] [PubMed] [Google Scholar]
- 24.Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009;2(5):403–411. doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Creixems M, et al. Bloodstream infections: evolution and trends in the microbiology workload, incidence, and etiology, 1985–2006. Medicine (Baltimore) 2008;87(4):234–249. doi: 10.1097/MD.0b013e318182119b. [DOI] [PubMed] [Google Scholar]
- 26.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 27.Ochi A, Yuh K, Migita K. Not every superantigen induces tolerance in vivo. Semin Immunol. 1993;5(1):57–63. doi: 10.1006/smim.1993.1008. [DOI] [PubMed] [Google Scholar]
- 28.Soos JM, Schiffenbauer J, Johnson HM. Treatment of PL/J mice with the superantigen, staphylococcal enterotoxin B, prevents development of experimental allergic encephalomyelitis. J Neuroimmunol. 1993;43(1–2):39–43. doi: 10.1016/0165-5728(93)90073-8. [DOI] [PubMed] [Google Scholar]
- 29.Chowell G, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med. 2009;361(7):674–679. doi: 10.1056/NEJMoa0904023. [DOI] [PubMed] [Google Scholar]
References
- 1.Bettelli E, et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci U S A. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakae S, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 4.Ye P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamanaka M, et al. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941–952. doi: 10.1016/j.immuni.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Alegre ML, et al. An anti-murine CD3 monoclonal antibody with a low affinity for Fc gamma receptors suppresses transplantation responses while minimizing acute toxicity and immunogenicity. J Immunol. 1995;155:1544–1555. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.