Abstract
Levels of urinary dialkylphosphates (DAPs) are currently used as a biomarker of human exposure to organophosphorus insecticides (OPs). It is known that OPs degrade on food commodities to DAPs at levels that approach or exceed those of the parent OP. However, little has been reported on the extent of DAP absorption, distribution, metabolism and excretion.
The metabolic stability of O,O-dimethylphosphate (DMP) was assessed using pooled human and rat hepatic microsomes. Time-course samples were collected over two hours and analyzed by LC-MS/MS. It was found that DMP was not metabolized by rat or pooled human hepatic microsomes.
Male Sprague-Dawley rats were administered DMP at 20 mg kg−1 via oral gavage and I.V. injection. Time-course plasma and urine samples were collected and analyzed by LC-MS/MS. DMP oral bioavailability was found to be 107 ± 39 % and the amount of orally administered dose recovered in the urine was 30 ± 9.9 % by 48 hrs.
The in vitro metabolic stability, high bioavailability and extent of DMP urinary excretion following oral exposure in a rat model suggests that measurement of DMP as a biomarker of OP insecticide exposure may lead to overestimation of human exposure.
Keywords: dialkylphosphate, dimethylphosphate, biomonitoring, biomarker, exposure assessment, pharmacokinetics, microsomes
Introduction
Organophosphorus insecticides (OPs) are among the most widely used insecticides in US and global agriculture. An estimated 60 million pounds of the roughly 40 currently registered OPs are applied annually to US agriculture (Barr et al., 2004; Zhang et al., 2008). They share a common mode of toxicity through the non-species specific inhibition of acetylcholinesterase (AChE) (Casida and Quistad, 2004; Mileson et al., 1998). The association between low-level lead exposure in children and the development of subtle neurologic effects in children led scientists to develop and investigate parallel hypotheses for OPs (Whitney et al., 1995; Fenske et al., 2000; Chanda and Pope, 1996). Recognition of these ideas aided in the passage of the 1996 Food Quality Protection Act that charged the US Environmental Protection Agency with assessing the aggregate and cumulative exposure of OPs to humans with careful attention being paid to childhood exposure (Loewenherz et al., 1997).
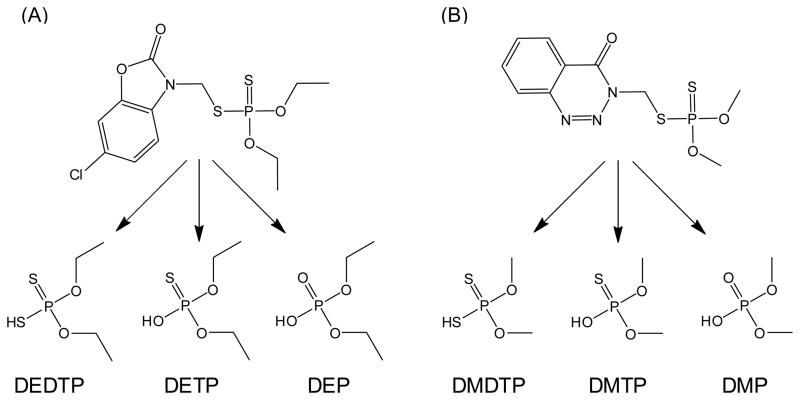
The majority of OP insecticides registered with the US Environmental Protection Agency are metabolized to at least one of six dialkylphosphate (DAP) metabolites (Fig. 1) (Wessels et al., 2003). Following human OP exposure and metabolism, DAPs are eliminated from the body in the urine (Shafik et al., 1971; Shafik et al., 1973; Bradway and Shafik, 1977; Fenske et al., 2000; Bicker et al., 2005). Monitoring the levels of these six common dialkylphosphate urinary metabolites, rather than the individual parent OPs, has allowed researchers to develop a single analytical method with which to monitor human spatial and temporal exposure to OPs (Aprea et al., 1996; Aprea et al., 2000; Barr et al., 2004; Loewenherz et al., 1997; Fenske et al., 2000; Lu et al., 2001; Bradman et al., 2005). Additionally, it is becoming more and more common for researchers to employ the biomonitoring of DAPs to investigate associations between human OP exposure and the risk of developing adverse health effects (Eskenazi et al., 2004; Young et al., 2005; Castorina et al., 2003; Eskenazi et al., 2007). Though the measurement of DAPs offers several analytical and cost-effective advantages, their use as human biomarkers of OP exposure has limitations.
Fig. 1.
DAPs commonly used in biomonitoring studies and specific examples of DAPs found in urine following exposure to the OPs phosalone (A) and azinphos-methyl (B). Abbreviations: DEDTP, O,O-diethyldithiophosphate; DETP, O,O-diethylthiophosphate; DEP, O,O-diethylphosphate; DMDTP, O,O-dimethyldithiophosphate; DMTP, O,O-dimethylthiophosphate; DMP, O,O-dimethylphosphate.
It has been demonstrated that OPs can degrade in the environment to DAPs and that DAPs are present in raw food stuffs at molar levels that approach or exceed those of the parent OP (Slotkin et al., 2009; Sakellarides et al., 2003; Hong and Pehkonen, 1998; Lu et al., 2005; Zhang et al., 2008). It has also been demonstrated that the ethylated DAP hydrolysis products of chlorpyrifos are excreted in the urine of rats orally dosed with O,O-diethylphosphate, DEP, and O,O-diethylthiophosphate, DETP (Shafik et al., 1971; Timchalk et al., 2007; Busby-Hjerpe et al., 2010). Additionally, the afore-mentioned ethyl DAP toxicokinetic studies demonstrated that exposure to equal molar levels of ethylated DAPs and chlorpyrifos consistently resulted in higher urinary ethyl DAP excretion rates and cumulative urine levels in rats receiving oral doses of ethyl DAPs (Timchalk et al., 2007).
There were three objectives in the current study; (1) to assess the metabolic stability of methyl DAPs to rat and human hepatic microsomes, (2) to investigate the absorption, metabolism and elimination of methyl DAPs in a rat model and (3) determine methyl DAP pharmacokinetic parameters following oral and I.V. dosing. Integration of results from the current study with those for the ethyl DAPs will provide quantitative information necessary for interpretation of biomonitoring data that employ the DAPs as chemical markers of OP insecticide exposure.
Materials and methods
Chemicals
O,O-dimethylphosphoric acid sodium salt (DMP, ≥ 95%) and O,O-dimethylthiophosphoric acid sodium salt (DMTP, ≥ 96%) were purchased from AppliChem GmbH (Darmstradt, Germany) and O,O-dimethlyphosphoric acid was purchased from AccuStandard (New Haven, CT, USA). Tramadol-hydrochloride (T-HC) was obtained from Sigma-Aldrich Corp (St. Louis, MO, USA). HPLC-grade solvents were purchased from Fisher Scientific Co. (Fairlawn, NJ, USA). All other reagent chemicals were obtained from commercial sources and used without further purification.
Metabolic stability assays
The metabolic stability of DMP was determined using rat and pooled human hepatic microsomes (BD Biosciences, San Jose, CA, USA). Incubation mixtures were prepared starting with 2455 μL of phosphate buffer (50 mM, pH = 7.4) and adding sequentially 225 μL of microsomal protein (20 mg mL−1), 300 μL of NADPH (10 mM) and 20 μL of DMP (75 mM) to yield a solution with a final volume of 3 mL and DMP concentration of 0.5 mM. After a 0, 0.25, 0.5, 1, 2-h incubation at 37 °C with shaking, the reaction was terminated with an equal volume solution of ice-cold methanol with 0.5% (v/v) formic acid and DMTP (internal standard) at 0.5 mM. Proteins were precipitated by centrifugation at 12 000 g for 15 min. Supernatant was diluted 1:50 for a final dilution of 1:100 with methanol and the resulting solutions were stored at −20 °C until analysis. The experiment was carried out with four replicates. Positive control incubations were conducted with T-HC, a drug with a known metabolic profile, in the presence of all other reported incubation components (metabolism expected), while negative controls were conducted in the absence of NADPH (no metabolism expected). DMP and control incubations were carried out in tandem and underwent similar sample preparation.
Animal study
All procedures using animals were reviewed and approved by the Institutional Animal and Care Use Committee of Oregon State University. Male Sprague-Dawley rats with jugular catheters attached were purchased from Charles River Laboratories and Simonsen Laboratories and were acclimated to laboratory conditions for three to four days in metabolic cages designed to separate feces from urine. Rats were maintained on a 12-h light/dark cycle at the Oregon State University Laboratory Animal Resource Center. During acclimation rats were allowed free access to water and food (Teklad 8640). At the time of dosing, weight among rats ranged from 233 – 274 g.
Dose selection and preparation
Weight specific dosing solutions (20 mg kg−1) were prepared by dissolving DMP in an appropriate amount of normal saline (0.9 % NaCl) such that rats received 100 μL and 500 μL dosing volumes during I.V. and oral dose administration respectively. Control rats were administered normal saline vehicle at the same dosing volumes as DMP treated rats. The 20 mg kg−1 dose level was chosen to increase the ability to detect chemicals in rat blood and urine over the time-course of the study.
Pharmacokinetic study
Rats were denied access to food from 1-h prior to dosing to 2-h post-dosing. DMP was administered intravenously via jugular catheter to manually restrained rats or by gavage to rats lightly anesthetized with isoflurane in a controlled chamber. Blood samples (~100 μL) from I.V. and orally dosed animals were collected at 0, 5, 10, 20, 30, 40, 60, 75, 90, 120 min and 0, 20, 30, 40, 60, 90, 120, 150, 180, 240 min post-dosing respectively from jugular vein catheters. The blood volume removed was replaced with an equal volume of normal saline followed by cannula blocking solution. Time-course urine samples were collected at 0, 1.5, 4.5, 7.5, 14, 24, 36 and 48-h post dosing into 15 mL screw-top vials and excretion volumes were recorded. Average urine collection volumes for time points 1.5 – 4.5 h and 7.5 – 48 h were 1.1 ± 0.9 mL and 2.7 ± 2.0 mL respectively. Rats were humanely sacrificed via CO2 asphyxiation following the final urine time point collection. Plasma and urine samples were placed on ice during sample collection and subsequently stored at −20 °C until sample preparation.
Rat plasma and urine sample preparation
Time-course blood samples were dispensed into heparinized microcentrifuge tubes (50 units of heparin mL−1 blood) and underwent plasma separation via centrifugation at 10 000 g for 15 min. Plasma proteins were precipitated via a 1:5 dilution with methanol, vortexing for 10 s and centrifugation at 15 000 g for 15 min. Plasma samples were subsequently diluted with methanol for a final dilution of 1:100 – 1:1000 and spiked with DMTP as an internal standard.
Urine samples were vortexed for 10 s and centrifuged for 10 min at 10 000 g. One hundred μL of the supernatant was diluted 1:10 with methanol, vortexed for 10 s and centrifuged for 10 min at 10 000 g. The resulting supernatant was diluted to a final dilution of 1:100 – 1:1000 with methanol and spiked with DMTP as an internal standard. Prepared samples were stored at −20 °C until analysis.
Analytical instrumentation
All samples were analyzed using an Applied Biosystems/MDS Sciex 4000 Q-trap mass spectrometer (Applied Biosystems, Foster City, CA) equipped with a TurboV electrospray ionization source, Shimadzu Prominence HPLC solvent delivery system and a Shimadzu SIL-HTc autosampler (Columbia, MD, USA). Optimization of instrument sensitivity was achieved by performing infusion experiments with DMP, DMTP and T-HC and generating enhanced product ion mass spectrums in combination with Applied Biosystems Analyst 1.4.2 software (Foster City, CA, USA). The analytical methods for DMP, DMTP and T-HC and T-HC metabolites were adapted from Hernandez et al. and Wu et al. respectively (2004 (2002). Optimized instrument parameters are presented in Table 1. Samples were chromatographically resolved using a 150 × 3 mm Phenomenex Luna C18 column with a 4.6 μm internal diameter and 100 Å particle size (Torrence, CA, USA).
Table 1.
Mass spectrometry parameters used in the determination of DMP, DMTP, tramadol-HCl and O-desmethyl tramadol-HCl
| Compound | Precursor ion (m/z) | Product ion (m/z) | Declustering potential | Entrance potential | Collision energy | Collision cell exit potential |
|---|---|---|---|---|---|---|
| DMP | 125ac | 79 | −35 | −10 | −35 | −5 |
| 125ad | 63 | −35 | −10 | −35 | −5 | |
| DMTP | 141ac | 126 | −46 | −10 | −20 | −5 |
| 141ad | 95 | −46 | −10 | −36 | −5 | |
| 141ad | 79 | −46 | −10 | −20 | −5 | |
| Tramadol-HCl | 264bc | 58 | 46 | 10 | 27 | 9 |
| O-desmethyl tramadol-HCl | 250bc | 58 | 46 | 10 | 27 | 9 |
Mass spectrometer operated in negative mode; source temperature, 550 °C; ion spray voltage, −4200 V.
Mass spectrometer operated in positive mode; source temperature, 450 °C; ion spray voltage, 5500 V.
Quantification transition
Confirmation transition
LC-MS/MS analyses
Microsome incubation samples
DMP and DMTP were chromatographically resolved from microsome samples using gradient elution with aqueous (A) and methanol (B) solutions of formic acid (0.1%, v/v). The elution program was as follows: 0 – 6 min, 20 – 50% B; 6 – 7 min, 50 – 90% B; 7 – 10 min, 90–90% B; 10 – 10.1 min, 90 – 20% B; 10.1 – 15 min, 20 – 20% B at a flow rate of 300 μL min−1 and an injection volume of 1 μL. DMP standard curves were prepared in methanol and spiked with the DMTP internal standard similarly to samples.
T-HC and its major metabolites were chromatographically resolved using a 0 – 15 min, 10 – 90% B; 15 – 16 min, 90 – 10% B; 16 – 22 min, 10 – 10% B gradient elution program at a flow rate of 300 μL min−1 and an injection volume of 10 μL. Metabolism of T-HC was assessed in terms of % T-HC remaining and presence of T-HC’s major metabolite O-desmethyl tramadol-hydrochloride (transition followed m/z 250 → 58). The analysis of DMP, DMTP, T-HC and O-desmethyl tramadol-hydrochloride were performed with the instrument in MRM mode following the transitions presented in Table 1.
Rat plasma and urine samples
DMP and DMTP were chromatographically resolved from rat plasma and urine samples using the microsome HPLC gradient elution program modified to equilibrate from 10.1 to 18 min and with an injection volume ranging from 1–10 μL. For quantification, matrix matched standards were prepared from control plasma and blood samples. The instrument was run in MRM mode monitoring transitions described previously. The LC-MS/MS instrumental range for all DMP analyses was 0.001 – 1 μg mL−1 and yielded correlation coefficients greater than 0.999.
Pharmacokinetic analysis
DMP pharmacokinetic parameters were estimated using a two-compartment model to fit the experimental results following oral and I.V. administration. The oral (Eq. 1) and I.V. (Eq. 2) models were defined by the following equations:
Oral administration:
| (Eq. 1) |
Intravenous administration:
| (Eq. 2) |
where α is the first-order distribution rate constant, β is the first-order elimination rate constant and ka is the absorption rate constant; A and B are the intercepts for the respective phases and C(t) represents the concentration of DMP at a given time t. Maximum plasma concentration (Cmax) and the time to reach Cmax (tmax) were determined from the plasma concentration-time curves. Optimized estimates for the model parameters were obtained using nonlinear regression analysis (WinNonlin® 5.0.1; Pharsight, Mountain View, CA, USA). Estimates were further used to calculate values for apparent volume of distribution (Vd) and the apparent half-life of each phase (t1/2 α, β). Additionally, the total amount of DMP excreted in urine when excretion was complete (DMPU∞) was estimated by Method IIA (Wagner and Ayres, 1977). Bioavailability (F) was determined as the quotient of total DMPU∞ after oral and I.V. DMP administration, appropriately correcting for dose, and assuming that clearance remained constant.
Results
Metabolic stability
Quality control incubations were carried out in tandem with DMP incubations. In positive controls, the % T-HC decreased 41 – 50% over the 2-h rat hepatic microsome incubation and the total peak area for T-HC decreased by ~ 35%, while it increased for its major reported O-desmethyl metabolite. Conversely, there was no statistically significant decrease in T-HC over the 2-h incubation and no metabolites detected in negative rat controls. The same procedure was carried out for pooled human hepatic microsomes. The percentage of T-HC remaining for positive controls after two hours of incubation was 86 – 90%, while its percentage of total peak area decreased by ~ 5% and its major metabolite increased ~ 5%. Similarly to the rat hepatic microsomes, there was no statistically significant decrease in T-HC over the 2-h incubation and no metabolites detected in negative human controls.
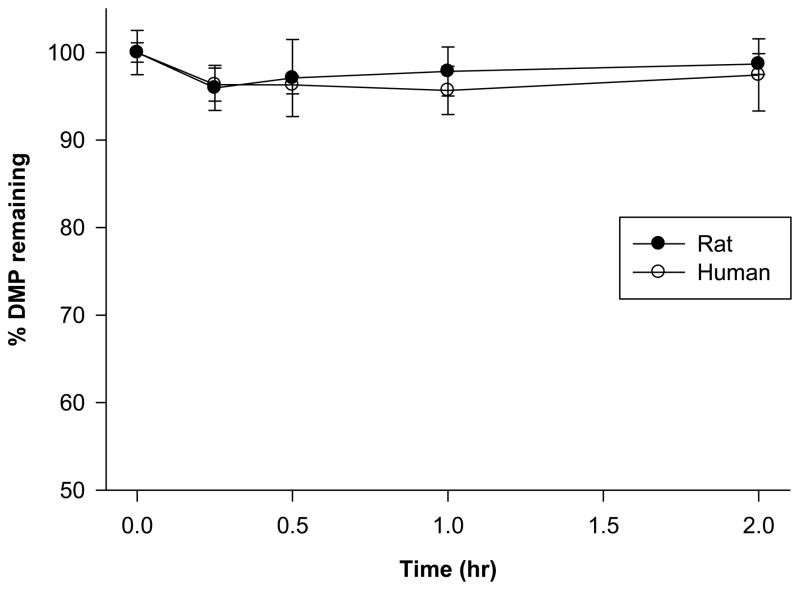
The percentage of DMP remaining in rat hepatic microsome incubation mixtures was followed for two hours and assessed by LC-MS/MS (Fig. 2). No statistically significant difference was detected between time points (p-value = 0.35 from a one-way ANOVA with 19 d.f.). Similarly, the percentage of DMP remaining in pooled human microsome incubations did not display a trend that suggested it was being metabolized (p-value = 0.14 from a one-way ANOVA with 19 d.f.). Additionally, there was no statistically significant difference detected between the mean percentage of DMP remaining in rat and human DMP microsome incubations (p-value = 0.15 from a two sample t-test with 38 d.f.).
Fig. 2.
Percentage of DMP remaining in male Sprague-Dawley and pooled human hepatic microsome incubations. Error bars represent S.D. of four replicates. Statistical significance for intraspecies and interspecies differences, *p < 0.05.
DMP pharmacokinetics
The concentrations of DMP in plasma following both oral and I.V. administration are shown in Table 2, while the time-course of DMP excreted in urine from both dosing groups are presented in Figures 3A and B. Pharmacokinetic models (two-compartment) were developed to describe the time-course of DMP in plasma and the best fitting model parameter values are listed in Table 3. The volume of distribution (Vd) suggested extensive distribution of DMP in the body (6970 ± 905 mL kg−1 for I.V. and 6973 ± 691 mL kg−1 for oral dose). Following oral administration, peak plasma DMP levels of 3.3 ± 0.27 mg mL−1 were reached within 0.5-h post dosing, and DMP was undetectable 2-h post dosing. The plasma time-course of DMP was bi-exponential with a rapid distribution phase (α) and a slower elimination phase (β). The half-lives for each phase were estimated to be 0.17 h (t 1/2, α) and 6.2 h (t 1/2, β), respectively. The absolute bioavailability of DMP was determined to be 107 ± 39%. In comparison to DMP, we analyzed the DEP and DETP plasma concentration time curve data from Timchalk’s et al. (2007) paper using our methods of WinNonlin® software to estimate the pharmacokinetic parameters of these metabolites (Table 3).
Table 2.
Concentration of DMP in plasma following single I.V. and oral administration of 20 mg kg−1 dose to male Sprague-Dawley rats (μg mL−1)
| Time post-dosing (h) | Plasma concentration (μg mL−1)
|
|
|---|---|---|
| Intravenous | Oral | |
| 0.08 | 138 ± 99.6 | - |
| 0.17 | 93.7 ± 57.7 | ND |
| 0.33 | 36.1 ± 21.9 | 2.05 ± 0.65 |
| 0.5 | 18.9 ± 11.8 | 1.54 ± 0.63 |
| 0.67 | 4.71 ± 3.85 | 0.86 ± 0.55 |
| 1 | 4.05 ± 1.88 | 1.25 ± 0.66 |
| 1.25 | 1.41 ± 1.15 | - |
| 1.5 | 1.14 ± 0.93 | ND |
| 2 | 0.59 ± 0.53 | 0.73 ± 0.63 |
| 4 | ND | ND |
| 8 | ND | - |
ND, non-detected; (−), no sample
Data represents mean ± S.E. for n = 3–5.
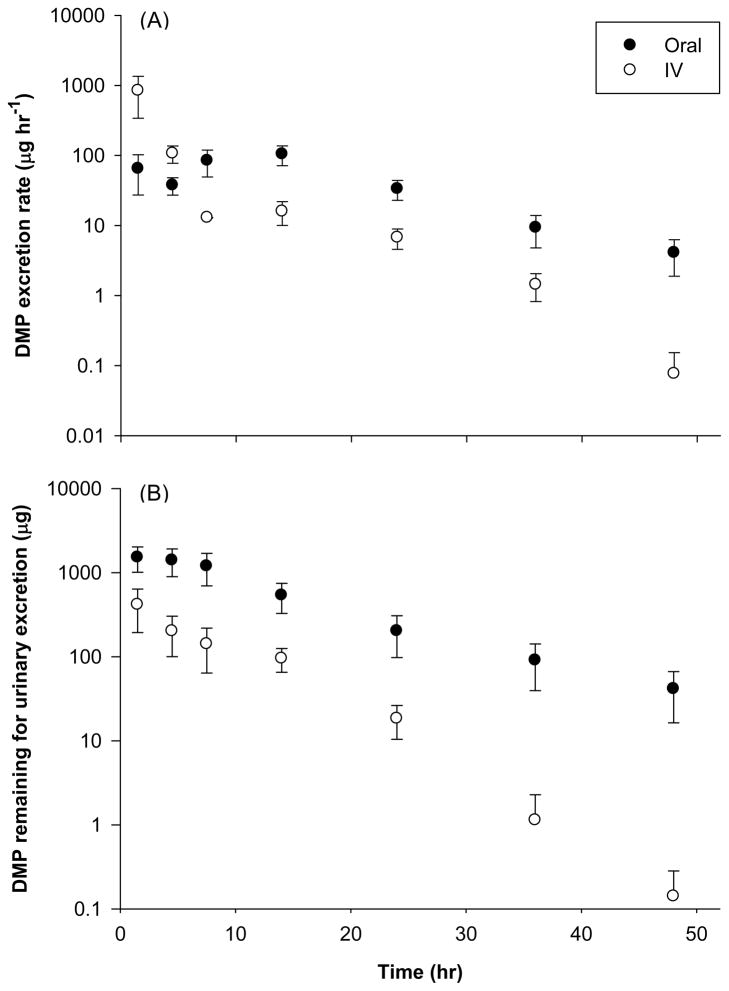
Fig. 3.
Time-course of DMP excretion rate (A) and amount of DMP remaining to be excreted in urine (B) following oral and IV administration of 20 mg kg−1 dose to male Sprague-Dawley rats. Data represents mean ± S.E. of 3 – 4 rats.
Table 3.
Pharmacokinetic parameters of DMP following a single I.V. and oral dose of 20 mg kg−1 to male Sprague-Dawley rats in comparison with results from Timchalk et al. (2007) DEP (dose = 21.6 mg kg−1) and DETP (dose = 23.8 mg kg−1) data obtained from male Sprague-Dawley rats.
| Parameters | Units | Intravenous | DMP (Oral) | DEP (Oral)† | DETP (Oral)† |
|---|---|---|---|---|---|
| α (Distribution) | h−1 | 4.5 ± 0.79 | 4.6 ± 0.79 | 3.5 | 0.17 |
| t 1/2, α | h | 0.17 ± 0.03 | 0.17 ± 0.03 | 0.20 | 4.2 |
| β (Elimination) | h−1 | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.013 | 0.12 |
| t 1/2, β | h | 6.2 ± 0.62 | 6.2 ± 0.62 | 52 | 5.8 |
| ka (Absorption) | h−1 | NA | 0.26 ± 0.08 | 1.3 | 4.2 |
| t 1/2, a | h | NA | 3.6 ± 1.0 | 0.51 | 0.16 |
| Vd/F | mL kg−1 | 6970 ± 905 | 6973 ± 691 | 29 680 | 10 086 |
| Cmax | μg mL−1 | NA | 3.3 ± 0.27 | 0.31 | 2.0 |
| tmax | h | NA | 0.39 ± 0.06 | 1.0 | 0.80 |
NA, not applicable.
Data represents mean ± S.E. for n = 3 – 5.
The parameters presented were determined using the plasma concentration time curve data in Timchalk et al. (2007) paper and WinNonlin® software.
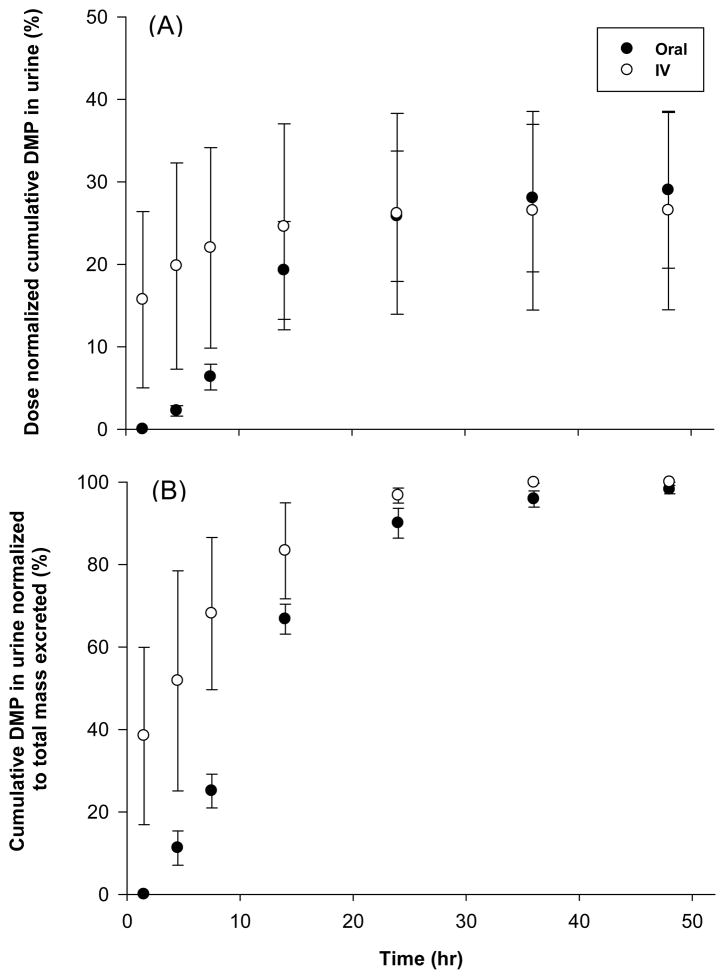
The interval urinary excretion rates (dDMP/dt), amount of DMP remaining to be excreted in urine (DMPU∞ - DMPU), dose normalized cumulative amount of DMP (DMPU/dose) and cumulative DMP in urine normalized to total mass excreted (DMPU/DMPU∞) for both administration routes are presented in Figures 3A, 3B, 4A and 4B respectively. In accordance with observed plasma pharmacokinetics, the urinary elimination of DMP following I.V. dosing also appears to be biphasic (Fig. 3A). Peak urinary DMP excretion rates (104 ± 32 μg h−1) were attained between 10 – 20 h post oral dosing (Fig. 3A) and excretion is almost complete after 40–50 h for both dosing groups (Fig. 4A and B). However, the estimated total recovery of DMP (DMPU∞/Dose) in the urine was relatively low for both oral (30 ± 9.9 %) and I.V. dosing (36 ± 15 %) as compared to the calculated bioavailability. This may indicate that urine is not the only elimination pathway for DMP.
Fig. 4.
Time-course of dose normalized cumulative amount of DMP in urine (A) and cumulative DMP in urine normalized to total mass excreted (B) following oral and IV administration of 20 mg kg−1 dose to male Sprague-Dawley rats. Data represents mean ± S.E. of 3 – 4 rats.
Discussion
General population level exposure to OP insecticides occurs mostly through the diet where the majority of chemical metabolism is facilitated by first pass hepatic metabolism. It is known that hepatic CYP450s play an integral role in phase I metabolism of environmental chemicals and drugs (Ackley et al., 2004; Wu and McKown, 2004; Yan et al., 2002). While it has been shown that cytochrome P450s, CYPs, participate in the detoxification of OPs via the hydrolysis of the OP phospho ester bond to yield DAPs, it has also been demonstrated that CYP-mediated bioactivation of OPs to oxons is a major contributor to overall OP toxicity (Poet et al., 2003; Buratti et al., 2003; Buratti et al., 2005; Busby-Hjerpe et al., 2010; Bharate et al., 2010; Casida and Quistad, 2004). In light of the importance of CYP-mediated bioactivation to the toxicity of OPs, it was hypothesized that they may play an equally important role in DAP metabolism. However, our results suggest that neither rat nor human hepatic microsomes are major contributors to the metabolism of DMP under CYP-mediated conditions. Additionally, our results provide an initial platform for comparing rat and human DAP metabolism.
All animals orally treated with DMP excreted DMP in their urine. As expected, no DMP was detected in any of the control animal blood or urine samples. These results, in conjunction with data showing extensive and rapid urinary excretion of DEP and DETP within 72 hours following DEP and DETP dosing, suggest that oral exposure to DAPs leads to significant urinary DAP levels (Timchalk et al., 2007). Evaluation of the time-course cumulative DMP in urine plot (Fig. 4A) suggests that approximately 30% of the oral dose was excreted in the urine within 48 hours, which could signify that DMP has additional elimination pathways. Observing that urine samples collected after 48 hours had levels of DMP below detection suggested that comparison of the % cumulative DMP in urine normalized to total amount of DMP collected over 48 hours (Fig. 4B) would be appropriate. Assessment of urinary DMP levels under these assumptions demonstrates that the majority of DMP excretion occurs within 24 hours and is terminal by 48 hours.
The distribution phase half-life for DMP (0.17 h) was comparable to the 0.2-h estimate previously reported for O,O-diethylphosphate (DEP), while the reported elimination half-life of DEP was greater (6 h versus 52 h), Table 3. The discrepancy in DMP and DEP elimination half-lives may be related to the ethoxy groups of DEP interacting to a greater degree with biomolecules than the methyl groups of DMP. However, this explanation fails to account for differences between DMP, DEP and DETP, where DETP has been reported to display single compartment pharmacokinetics and an elimination half-life that falls intermediate to DMP and DEP (Timchalk et al., 2007). Observing that DMP, DEP and DETP are partially excreted in the urine, it is reasonable to assume that differences in DAP pharmacokinetics may also be attributed to structure specific incorporation of the DAPs into in vivo systems (Timchalk et al., 2007).
The large volume of distribution, rapid distribution and slow elimination of DMP following oral dosing demonstrate that DMP is efficiently removed from plasma, distributed throughout the body and slowly and partially eliminated in the urine. Though pharmacokinetic results demonstrate that DAPs can remain in the body from hours to days, few studies have been published investigating the toxicity of DAPs. It has been shown that DAPs are cytotoxic and immunotoxic to human peripheral blood mononuclear cells and that DETP and DEDTP induce genotoxic effects in human embryonic hepatic and hepatocarcinoma cells under metabolic conditions when assessed by single cell gel electrophoresis (Lima and Vega, 2005; Vega et al., 2009). Considering the volume of OPs applied each year in the U.S. and that OPs degrade to DAPs in produce, a better understanding of human DAP pharmacokinetics and toxicodynamics is warranted.
Conclusion
The goal of the current work was to investigate the metabolic stability of a model DAP and characterize its pharmacokinetics in an animal model. Toward this end, the extent of DMP metabolism in rats and humans was investigated using hepatic microsomes. Results suggested that DMP was not metabolized over a 2-h incubation by rats or humans. Similarities in rat and human DMP metabolic stability profiles also suggest that rats and humans may display similar trends with regards to DMP pharmacokinetics. Additionally, it was demonstrated that DMP has high oral bioavailability and is excreted in the urine of rats following oral environmental exposure. Assuming that humans display similar pharmacokinetics, the measurement of DMP as a biomarker of OP insecticide exposure may lead to overestimation of human exposure.
Acknowledgments
The authors thank Mr. Glenn Wilson for analytical assistance, Mr. Nick Johnston for assistance with the in vitro assays and Mr. Steven O’Connell, Mr. Lane Tidwell and Mrs. Kristen Pierre for sample preparation assistance. We acknowledge the use of the OSU/EHSC mass spectrometry facility supported by the Oregon State University Environmental Health Science Center (NIH grant P30 ES000210).
References
- Ackley DC, Rockich KT, Baker TR. Metabolic stability assessed by liver microsomes and hepatocytes. In: Yan Z, Caldwell GW, editors. Methods in Pharmacology and Toxicology Optimization in Drug Discovery: In Vitro Methods. Totowa, NJ: Humana Press Inc; 2004. [Google Scholar]
- Aprea C, Sciarra G, Orsi D, Boccalon P, Sartorelli P, Sartorelli E. Urinary excretion of alkylphosphates in the general population (Italy) Sci Total Environ. 1996;177:37–41. doi: 10.1016/0048-9697(95)04857-x. [DOI] [PubMed] [Google Scholar]
- Aprea C, Strambi M, Novelli MT, Lunghini L, Bozzi N. Biologic monitoring of exposure to organophosphorus pesticides in 195 Italian children. Environ Health Perspect. 2000;116:521–25. doi: 10.1289/ehp.00108521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Bravo R, Weerasekera G, Caltabiano LM, Whitehead RD, Jr, Olsson AO, Caudill SP, Schober SE, Pirkle JL, Sampson EJ. Concentrations of dialkyl phosphate metabolites of organophosphorus pesticides in the US population. Environ Health Perspect. 2004;112:186–200. doi: 10.1289/ehp.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharate SB, Prins JM, George KM, Thompson CM. Thionate versus oxon: comparison of stability, uptake, and cell toxicity of (14CH3O)2-labeled methyl parathion and methyl paraoxon with SH-SY5Y cells. J Agric Food Chem. 2010;58:8460–566. doi: 10.1021/jf100976v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicker W, Lämmerhofer M, Genser D, Kiss H, Lindner W. A case study of acute human chlorpyrifos poisoning: Novel aspects on metabolism and toxicokinetics derived from liquid chromatography-tandem mass spectrometry analysis of urine samples. Toxicol Lett. 2005;159:235–51. doi: 10.1016/j.toxlet.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Bradman A, Eskenazi B, Barr DB, Bravo R, Castorina R, Chevrier J, Kogut K, Harnly ME, McKone TE. Organophosphate urinary metabolite levels during pregnancy and after delivery in women living in an agricultural community. Environ Health Perspect. 2005;113:1802–807. doi: 10.1289/ehp.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradway DE, Shafik TM. Malathion exposure studies. Determination of mono- and dicarboxylic acids and alkyl phosphates in urine. J Agric Food Chem. 1977;25:1342–344. doi: 10.1021/jf60214a002. [DOI] [PubMed] [Google Scholar]
- Buratti FM, D’Aniello A, Volpe MT, Meneguz A, Testai E. Malathion bioactivation in the human liver: the contribution of different cytochrome P450 isoforms. Drug Metab Disp. 2005;33:295–302. doi: 10.1124/dmd.104.001693. [DOI] [PubMed] [Google Scholar]
- Buratti FM, Volpe MT, Meneguz A, Vittozzi L, Testai E. CYP-specific bioactivation of four organophosphorothioate pesticides by human liver microsomes. Toxicol Appl Pharm. 2003;186:143–54. doi: 10.1016/s0041-008x(02)00027-3. [DOI] [PubMed] [Google Scholar]
- Busby-Hjerpe AL, Campbell JA, Smith JN, Lee S, Poet TS, Barr DB, Timchalk C. Comparative pharmacokinetics of chlorpyrifos versus its major metabolites following oral administration in the rat. Toxicology. 2010;268:55–63. doi: 10.1016/j.tox.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol. 2004;17:983–98. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- Castorina R, Bradman A, McKone TE, Barr DB, Harnly ME, Eskenazi B. Cumulative organophosphate pesticide exposure and risk assessment among pregnant women living in an agricultural community: a case study from the CHAMACOS cohort. Environ Health Perspect. 2003;111:1640–648. doi: 10.1289/ehp.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda SM, Pope CN. Neurochemical and neurobehavioral effects of repeated gestational exposure to chlorpyrifos in maternal and developing rats. Pharmacol Biochem Be. 1996;53:771–76. doi: 10.1016/0091-3057(95)02105-1. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Harley K, Bradman A, Weltzien E, Jewell NP, Barr DB, Furlong CE, Holland NT. Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ Health Perspect. 2004;112:1116–123. doi: 10.1289/ehp.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Bart DB, Johnson C, Morga N, Jewell NP. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115:792–98. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenske RA, Kissel JC, Lu C, Kalman DA, Simcox NJ, Allen EH, Keifer MC. Biologically based pesticide dose estimates for children in an agricultural community. Environ Health Perspect. 2000;108:515–20. doi: 10.1289/ehp.00108515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández F, Sancho JV, Pozo OJ. An estimation of the exposure to organophosphorus pesticides through the simultaneous determination of their main metabolites in urine by liquid chromatography–tandem mass spectrometry. J Chromatog B. 2004;808:229–39. doi: 10.1016/j.jchromb.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Hong F, Pehkonen S. Hydrolysis of phorate using simulated environmental conditions: rates, mechanisms, and product analysis. J Agric Food Chem. 1998;46:1192–199. doi: 10.1021/jf010339k. [DOI] [PubMed] [Google Scholar]
- Lima A, Vega L. Methyl-parathion and organophosphorous pesticide metabolites modify the activation status and interleukin-2 secretion of human peripheral blood mononuclear cells. Toxicol Lett. 2005;158:30–38. doi: 10.1016/j.toxlet.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Loewenherz C, Fenske RA, Simcox NJ, Bellamy G, Kalman D. Biological monitoring of organophosphorus pesticide exposure among children of agricultural workers in central Washington state. Environ Health Perspect. 1997;105:1344–353. doi: 10.1289/ehp.971051344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Bravo R, Caltabiano LM, Irish RM, Weerasekera G, Barr DB. The presence of dialkylphosphates in fresh fruit juices: implication for organophosphorus pesticide exposure and risk assessments. J Toxicol Env Heal A. 2005;68:209–27. doi: 10.1080/15287390590890554. [DOI] [PubMed] [Google Scholar]
- Lu C, Knutson DE, Fisker-Andersen J, Fenske RA. Biological monitoring survey of organophosphorus pesticide exposure among preschool children in the Seattle metropolitan area. Environ Health Perspect. 2001;109:299–303. doi: 10.1289/ehp.01109299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileson BE, Chambers JE, Chen WL, Dettbarn W, Ehrich M, Eldefrawi AT, Gaylor DW, Hamernik K, Hodgson E, Karczmar AG, Padilla S, Pope CN, Richardson RJ, Saunders DR, Sheets LP, Sultatos LG, Wallace KB. Common mechanism of toxicity: a case study of organophosphorus pesticides. Toxicol Sci. 1998;41:8–20. doi: 10.1006/toxs.1997.2431. [DOI] [PubMed] [Google Scholar]
- Poet TS, Wu H, Kousba AA, Timchalk C. In vitro rat hepatic and intestinal metabolism of the organophosphate pesticides chlorpyrifos and diazinon. Toxicol Sci. 2003;72:193–200. doi: 10.1093/toxsci/kfg035. [DOI] [PubMed] [Google Scholar]
- Sakellarides TM, Siskos MG, Albanis TA. Photodegradation of selected organophosphorus insecticides under sunlight in different natural waters and soils. Int J Environ An Ch. 2003;83:33–50. [Google Scholar]
- Shafik MT, Bradway D, Enos HF. Cleanup procedure for the determination of low levels of alkyl phosphates, thiophosphates, and dithiophosphates in rat and human urine. J Agric Food Chem. 1971;19:885–89. doi: 10.1021/jf60177a045. [DOI] [PubMed] [Google Scholar]
- Shafik T, Bradway DE, Enos HF, Yobs AR. Human exposure to organophosphorus pesticides. Modified procedure for the gas-liquid chromatographic analysis of alkyl phosphate metabolites in urine. J Agric Food Chem. 1973;21:625–29. doi: 10.1021/jf60188a036. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Wu C, Mackillop EA, Linden KG. Ultraviolet photolysis of chlorpyrifos: developmental neurotoxicity modeled in PC12 cells. Environ Health Perspect. 2009;117:338–43. doi: 10.1289/ehp.11592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timchalk C, Busby A, Campbell JA, Needham LL, Barr DB. Comparative pharmacokinetics of the organophosphorus insecticide chlorpyrifos and its major metabolites diethylphosphate, diethylthiophosphate and 3,5,6-trichloro-2-pyridinol in the rat. Toxicology. 2007;237:145–57. doi: 10.1016/j.tox.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Vega L, Valverde M, Elizondo G, Leyva JF, Rojas E. Diethylthiophosphate and diethyldithiophosphate induce genotoxicity in hepatic cell lines when activated by further biotransformation via cytochrome P450. Mutat Res. 2009;679:39–43. doi: 10.1016/j.mrgentox.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Wagner JG, Ayres JW. Bioavailability assessment: Methods to estimate total area (AUC0-∞) and total amount excreted and importance of blood and urine sampling scheme with application to digoxin. J Pharmacokinet Phar. 1977;5:533–57. doi: 10.1007/BF01061733. [DOI] [PubMed] [Google Scholar]
- Wessels D, Barr DB, Mendola P. Use of biomarkers to indicate exposure of children to organophosphate pesticides: implications for a longitudinal study of children’s environmental health. Environ Health Perspect. 2003;111:1939–946. doi: 10.1289/ehp.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney KD, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: cellular mechanisms. Toxicol Appl Pharm. 1995;134:53–62. doi: 10.1006/taap.1995.1168. [DOI] [PubMed] [Google Scholar]
- Wu WN, McKown LA. In vitro drug metabolite profiling using hepatic S9 and human liver microsomes. In: Yan Z, Caldwell GW, editors. Optimization in Drug Discovery: In Vitro Methods. Totowa, NJ: Humana Press Inc; 2004. [Google Scholar]
- Wu WN, McKown LA, Liao S. Metabolism of the analgesic drug ULTRAM (tramadol hydrochloride) in humans: API-MS and MS/MS characterization of metabolites. Xenobiotica. 2002;32:411–25. doi: 10.1080/00498250110113230. [DOI] [PubMed] [Google Scholar]
- Yan Z, Caldwell GW, Wu WN, McKown LA, Rafferty B, Jones W, Masucci JA. In vitro identification of metabolic pathways and cytochrome P450 enzymes involved in the metabolism of etoperidone. Xenobiotica. 2002;32:949–962. doi: 10.1080/00498250210163298. [DOI] [PubMed] [Google Scholar]
- Young JG, Eskenazi B, Gladstone EA, Bradman A, Pedersen L, Johnson C, Barr DB, Furlong CE, Holland NT. Association between in utero organophosphate pesticide exposure and abnormal reflexes in neonates. Neurotoxicology. 2005;26:199–209. doi: 10.1016/j.neuro.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Zhang X, Driver JH, Li Y, Ross JH, Krieger RI. Dialkylphosphates (DAPs) in fruits and vegetables may confound biomonitoring in organophosphorus insecticide exposure and risk assessment. J Agric Food Chem. 2008;56:10638–645. doi: 10.1021/jf8018084. [DOI] [PubMed] [Google Scholar]