This animal study demonstrates that single-port robotic surgery using the VeSPA platform can allow the performance of technically challenging procedures within acceptable operative times and without complications or insertion of additional trocars.
Keywords: Single-port surgery, Robotic single-port
Abstract
Background and Objectives:
The purpose of this study was to evaluate the feasibility and validity of a dedicated da Vinci single-port platform in the porcine model in the performance of gynecologic surgery.
Methods:
This pilot study was conducted in 4 female pigs. All pigs had a general anesthetic and were placed in the supine and flank position. A 2-cm umbilical incision was made, through which a robotic single-port device was placed and pneumoperitoneum obtained. A data set was collected for each procedure and included port placement time, docking time, operative time, blood loss, and complications. Operative times were compared between cases and procedures by use of the Student t test.
Results:
A total of 28 surgical procedures (8 oophorectomies, 4 hysterectomies, 8 pelvic lymph node dissections, 4 aorto-caval nodal dissections, 2 bladder repairs, 1 uterine horn anastomosis, and 1 radical cystectomy) were performed. There was no statistically significant difference in operating times for symmetrical procedures among animals (P=0.3215).
Conclusions:
This animal study demonstrates that single-port robotic surgery using a dedicated single-site platform allows performing technically challenging procedures within acceptable operative times and without complications or insertion of additional trocars.
INTRODUCTION
Innovations in minimally invasive surgical technology, such as multi-channel ports, articulating instruments, and flexible high-definition endoscopes, have allowed laparoscopic surgeons to perform increasingly complex surgeries through smaller incisions. An emerging area in minimally invasive surgery is single-port laparoscopy, or laparoendoscopic single-site surgery (LESS). Single-port laparoscopy entails performing laparoscopic surgery utilizing a multi-channel port system, typically placed through a single umbilical skin incision. Preliminary advances in LESS, as applied to gynecologic surgery, demonstrate that the techniques are feasible provided that both laparoscopic surgical expertise and optimal instrumentation are available.1,2 While this approach is innovative and perhaps the newest frontier in laparoscopic surgery, it presents some unique challenges, such as instrument crowding, loss of depth of perception, and need for significant laparoscopic skills. Robotic surgery has greatly improved surgeon dexterity, surgical precision, visualization, ergonomics, and has allowed procedures that were performed by laparotomy to be performed by laparoscopy. However, robotic surgery has substantially increased the number and size of ports required compared with conventional laparoscopy. Fusion of both concepts of single-site surgery and robotic technology is the next step in the evolution of minimally invasive surgery. The purpose of this study was to evaluate the feasibility and validity of a dedicated single-port robotic platform in the porcine model in the performance of gynecologic surgery.
METHODS
This pilot training study was performed at the Cleveland Clinic, Atrial Fibrillation Innovation Center, Cleveland, Ohio, USA. All procedures performed in this training protocol have been approved by the Institutional Animal Care and Use Committee (IACUC) at the Cleveland Clinic, Protocol #(2009-0086). A data set was collected for each procedure and included port placement time, docking time, operative time, blood loss, and complications. Operative times were compared between cases and procedures by use of the Student t test, Fisher's exact test, or the Wilcoxon Rank-Sum Test (utilized when variables were not normally distributed). A Pearson product-moment correlation coefficient was computed to assess the relationship between docking time and number of animals/pigs.
Single-Port Robotic Platform
The Intuitive Surgical single-site Instruments and Accessories are intended to be used with the IS3000 da Vinci Si Surgical System to perform single incision laparoscopic surgery. Since the single-site Instruments and Accessories are used with the IS3000 da Vinci Si Surgical System, they maintain the same core clinical capabilities as the currently marketed and cleared IS3000 da Vinci Si Surgical System with EndoWrist Instruments and Accessories. Unlike the current EndoWrist Instruments and Accessories that require up to 4 separate incisions, the single-site instruments and accessories can be delivered through a single incision.
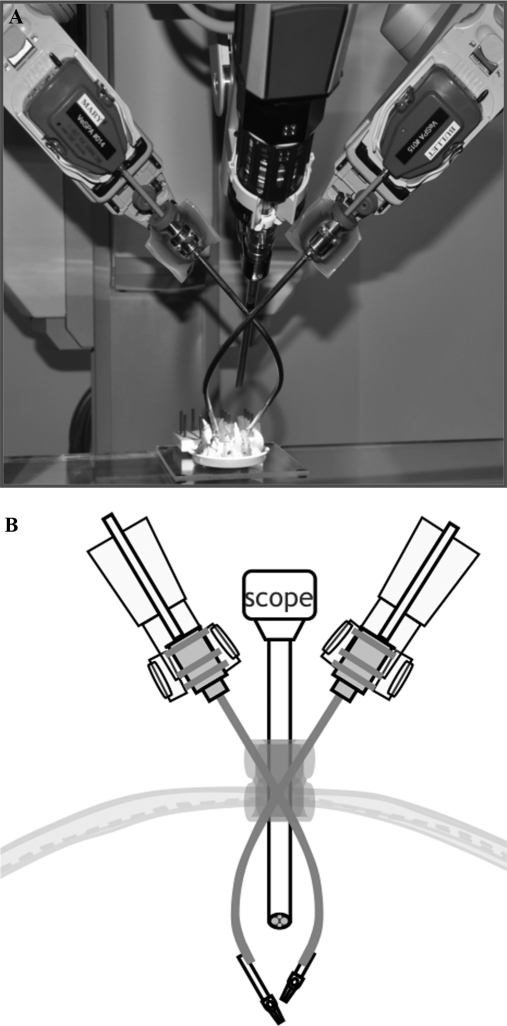
The robotic single-site instruments and accessories are of similar construction to existing IS3000 da Vinci Si EndoWrist Instruments except they do not have a wrist at the distal end of the instrument, and the shaft of the instrument is semi-rigid allowing them to be inserted through the curved cannulae. The single-site instruments and accessories include a needle driver, cadiere grasper, right-angle Maryland retractor, curved scissors, hook, clip applier, and suction irrigator for laparoscopic manipulation of tissue, including grasping, cutting, blunt and sharp dissection, approximation, ligation, electrocautery, and suturing (Figure 1).
Figure 1.
Single-Site Platform.
To enable robotic single incision surgery, the curved cannulae are placed in the swine umbilicus (or other suitable location), with the curves of the cannulae crossing over each other at the distal end (Figure 1). This allows alignment of the remote center and effectively re-creates triangulation of the instruments. When the single-site instruments are docked into the IS3000 da Vinci Si System, they are automatically reassigned so the right hand of the Surgeon's Control will control the left instrument and vice versa. From the Surgeon's Console, the surgeon controls the movement and position of each individual instrument's arm as necessary to perform the surgical procedure just as with the existing EndoWrist Instruments.
Surgical Procedures
All participants underwent orientation and approval by IACUC as well as 2-dry laboratory sessions with the da Vinci Si System single-site instrumentation. The study was performed on 4 healthy female pigs. Normal health status was determined preoperatively by physical examination, blood chemistry, and quarantine (according to the IACUC). Food was withheld from the pigs for 24 hours before surgery. All animals were provided water ad libitum. Preoperative care, anesthesia, and euthanasia were overseen by a staff veterinarian.
After induction, all pigs were positioned in a dorsal lithotomy for the surgical procedures. At the conclusion of the laboratory session, the pigs remained under general anesthesia and were humanely euthanized. A 2.5-cm to 2.8-cm stab incision was made at the umbilicus. An open laparoscopy technique (Hasson) was thus used to create an incision that allowed insertion of a single-port robotic trocar system 2.5-mm to 2.8-mm trocar-cannula system (Intuitive Surgical System, USA). The abdominal cavity was insufflated directly through the port system with CO2 to an intraabdominal pressure of 15mm Hg. The robotic camera used for all procedures was an 8.5-mm high-definition camera. Then the surgical table was tilted to a 35° Trendelenburg position to displace the abdominal viscera cranially. The urinary bladder was then drained to ease the visualization of the reproductive system and pelvis.
The planned single-port robotic curriculum included pig positioning, single-port placement, and docking single-site in different positions (supine, flank). Surgical procedures included oophorectomy (left, right), hysterectomy, cystotomy repair in 2-layers, uterine horn anastomosis, ureteral dissection, and radical cystectomy, as well as pelvic (left, right) and paraaortic lymphadenectomy. Single-site cadiere graspers, Maryland bipolar graspers (30 watts coagulation), curved scissors, and electrocautery (monopolar energy, 30 watts coagulation/30 watts cut) were used for lymph node dissections and hysterectomies/oophorectomies. Iliac artery and vein were first identified, retroperitoneum entered and developed in a caudal fashion, then lymphatic tissue was removed and skeletonized using the cadiere graspers and monopolar energy.
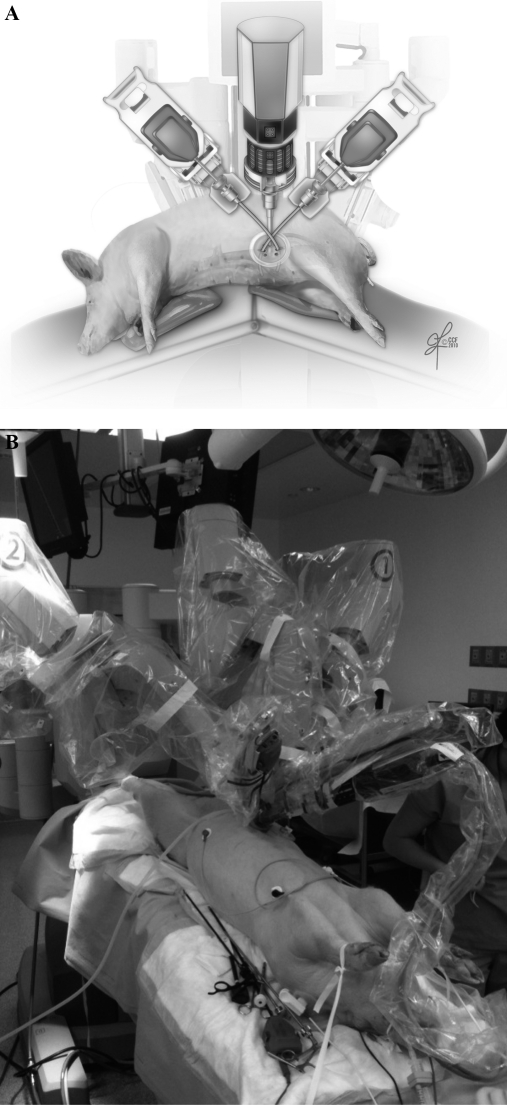
Repair of the bladder and uterine horn anastomosis were performed using single-site needle drivers and 4-0 Vicryl suture (SH needle) in a 2-layer fashion. Needles were placed and removed from the peritoneum through the assistant channel within the robotic-single port device using a laparoscopic 5-mm Maryland dissector. No accessory trocar that was not part of the single-site device was inserted. For aortic nodal dissections, the pigs were placed in a flank position; the da Vinci was then docked in from the backside of the pig (Figure 2). A cadiere grasper, curved scissors, and monopolar energy were used for grasping and removing nodal tissue from the bifurcation of iliacs (artery and vein) all the way up the left renal vein and posterior vena cava. The assistant surgeon helped retract bowel by using a standard 5-mm bowel grasper through the assistant channel within the robotic single-port device.
Figure 2.
System docking.
RESULTS
The mean weight of the 4 female pigs was 46.8kg (range, 38.6 to 65). The 4 pigs had a total of 28 surgical procedures (8 oophorectomies, 4 hysterectomies, 8 pelvic lymph node dissections, 4 aorto-caval nodal dissections, 2 bladder repairs, 1 uterine horn anastomosis, and 1 radical cystectomy) with no intraoperative complications, conversion to laparotomy or laparoscopy, or placement of additional ports that were not part of the umbilical device. The mean time of incision (Hasson approach) into the peritoneal cavity and insertion of the single-port device into the pigs was 8 minute (range, 5 to 11).
The mean operative times for the respective procedures are summarized in Table 1. The mean (range) operative duration for a 2-layer cystotomy repair and uterine-horn anastomosis was 19 minutes (range, 18 to 20) and 30 minutes, respectively. This included swapping robotic instrumentation (ie, needle drivers, and others) and introducing the suture by the assistant surgeon as well as removal of needles. The mean procedure time for aortic lymph node dissection was 39.3 minutes (range, 30 to 47). This operative time did not include changing the pig into a flank position, repositioning the robotic cart or redocking.
Table 1.
Surgical Procedures and Operating Times
| Surgical Procedure | N | Operating Time Mean Min (range) | P |
|---|---|---|---|
| Oophorectomy | |||
| Left | 4 | 14 (12–15) | 0.3215 |
| Right | 4 | 15 (14–17) | |
| Pelvic lymphadenectomy | |||
| Left | 4 | 22.4 (18–25) | 0.2747 |
| Right | 4 | 19.5 (15–24) | |
| Aortic lymphadenectomy | 4 | 39.3 (30–47) | — |
| Cystotomy repair (2 layers) | 2 | 19 (18–20) | — |
| Radical cystectomy | 1 | 35 | — |
| Uterine-horn anastomosis | 1 | 30 | — |
| Hysterectomy | 4 | 23.4 (18–27) | — |
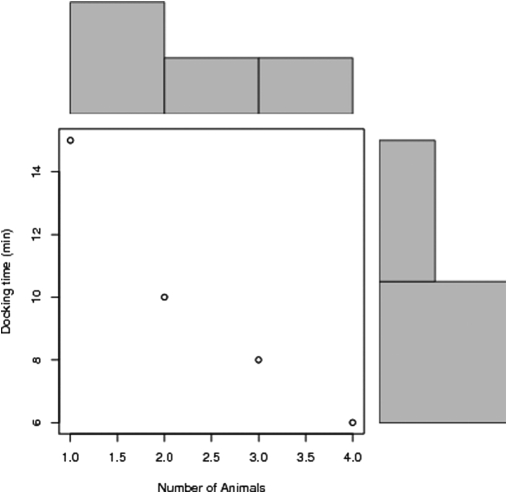
There was no statistically significant difference in operating times for symmetrical procedures (ie, oophorectomy, pelvic lymphadenectomy) among animals (P=0.3215). A Pearson product-moment correlation coefficient was computed to assess the relationship between docking time and number of animals. There was a strong correlation between the 2 variables [r=−0.82, P=0.034, (Figure 3)].
Figure 3.
Relationship between docking time and number of animals.
DISCUSSION
We present the first gynecologic study using a dedicated robotic single-site platform. Our laboratory animal data indicate that single-port robotic surgery using the novel single-site platform is feasible and safe for a variety of gynecologic procedures. No difference in mean operating times was noted between the sides of the procedure (ie, right oophorectomy vs. left oophorectomy). Complex procedures such as lymph node dissections and anastomosis were feasible without the addition of extra trocars, or marionette techniques, or both of these together. Finally, our data demonstrated that after proper training of surgeons (didactics and dry laboratory), robotic single-port insertion and docking times in the animal model decreases with the number of procedures performed.
In the last decade, numerous studies have demonstrated that laparoscopic approaches to various gynecologic conditions including oncology is feasible and results in shorter hospital stays, improved quality of life and comparable surgical outcomes to those of laparotomy.3–6 Single-port laparoscopic surgery has been introduced as a further development of laparoscopy. The concept of multiple instruments and optics operating through a single incision give rise to specific challenges and unique ergonomic problems not previously encountered with conventional laparoscopy.
For instance, triangulation is needed for proper dissection while providing effective traction and countertraction during surgery; a task that is easily achievable with conventional laparoscopy is by far more difficult with single-port laparoscopy. External and internal conflicts also represent an important challenge. Instrument crowding is perhaps the most frustrating aspect of this new modality. This has improved somewhat with the development of streamlined profile flexible camera systems and by using instruments of different lengths and articulation.
Robotics may overcome some of the technical limitations of single-port laparoscopy. The da Vinci robotic surgical system (Intuitive Surgical, Inc.) has gained tremendous popularity among gynecologists as an adjunctive tool for minimally invasive gynecologic surgery. Robotic-assisted surgery offers advantages, such as 3-dimensional visualization, scaling of movement, and range of motion superior to that with conventional laparoscopy.
Initial experience with robotic LESS was reported by Haber et al.7 Subsequently, Kaouk et al8 reported the first robotic single-port transumbilical surgery in urology by performing a successful radical prostatectomy and nephrectomy. Our group reported the first experience with robotic-assisted single-port surgery utilizing the da Vinci-S platform.9,10 Advantages like 3-dimensional visualization of the operative field, decreasing tension tremor of the surgeon, and added wrist motion for improved dexterity and greater surgical precision allowed the completion of multiple gynecologic procedures including hysterectomy, oophorectomy, and lymph node dissections. However, we believe there are several factors and limitations intrinsic to using the da Vinci-S or Si platform for single-port surgery that merit further discussion.
First, although the capabilities of the da Vinci S and Si platforms to dock at different positions and target different organs is well established, it follows the aforementioned classical surgical concepts (ie, triangulation of target anatomy by placement of multiple robotic trocars at different angles, etc). Second, hardware (robotic trocars, cannulas, instrumentation, optics) and software were not designed for single-incision surgery. Inverting controls of the robotic systems (right to left and vice versa) may allow the surgeon to operate without crossing the hands at the console; however, the robotic arms are crossed internally. This internal crossing presents a limitation of movement during pelvic surgery especially when working laterally (ie, side wall) or with large uteri. Finally, commercially available port systems were not designed for single-port robotics. Docking and advancing cross-rigid robotic instruments is inherently dangerous and may cause disruption of the port system.
Clearly, several objections can be raised to the arguments we have presented. Surgeons proficient in single-port operative laparoscopy performed all of the cases. However, as with most technology, a learning curve is required to become proficient. There are no published data for single-port operative laparoscopy, but it is expected that robotics will enable more surgeons to adopt this approach to surgery. This article demonstrates the potential for this new technology. Although the robotic single-site platform clearly offers advantages over conventional single-port laparoscopy in terms of 3-dimensional visualization of the operative field, and decreasing tension tremor of the surgeon, it lacks wrist motion for improved dexterity and surgical precision. This major limitation is akin to conventional laparoscopy or single-port surgery. Whether this platform would be superior in terms of performance and surgical outcomes to conventional single-port laparoscopy remains to be seen in clinical trials.
Benefits beyond cosmesis of single-port laparoscopy over conventional laparoscopy are beginning to emerge. Recent data demonstrated improved blood loss, hospital stay, and pain scores in women who underwent single-port hysterectomy.11,12 Nevertheless, initial learning curve studies suggest that the learning curve for complex single-port procedures is similar to that of conventional laparoscopic cases.13 Further prospective studies will be required to confirm these results.
CONCLUSION
This animal study demonstrates that single-port robotic surgery using this novel platform allows performing technically challenging procedures such as lymph node dissection and uterine horn anastomosis within acceptable operative times and without complications or insertion of additional trocars. This new fusion technology of single-port laparoscopy and robotics may be the next step in the evolution of minimally invasive gynecologic surgery.
Contributor Information
Pedro F. Escobar, Women's Institute, Cleveland Clinic, Cleveland, Ohio, USA..
Georges-Pascal Haber, Glickman Urologic Institute, Cleveland Clinic, Cleveland, Ohio, USA..
Jihad Kaouk, Glickman Urologic Institute, Cleveland Clinic, Cleveland, Ohio, USA..
Matthew Kroh, Surgical Institute, Cleveland Clinic, Cleveland, Ohio, USA..
Sricharan Chalikonda, Surgical Institute, Cleveland Clinic, Cleveland, Ohio, USA..
Tommaso Falcone, Women's Institute, Cleveland Clinic, Cleveland, Ohio, USA..
References:
- 1. Kim TJ, Lee YY, Kim MJ, et al. Single port access laparoscopic adnexal surgery. J Minim Invasive Gynecol. 2009;16:612–615 [DOI] [PubMed] [Google Scholar]
- 2. Escobar PF, Bedaiwy MA, Fader AN, Falcone T. Laparoendoscopic single-site (LESS) surgery in patients with benign adnexal disease. Fertil Steril. 2010. April;93(6):2074.e7–10 Epub 2010 Jan 25 [DOI] [PubMed] [Google Scholar]
- 3. Eltabbakh GH, Shamonki MI, Moody JM, Garafano LL. Hysterectomy for obese women with endometrial cancer: laparoscopy or laparotomy? Gynecol Oncol. 2000;78:329–335 [DOI] [PubMed] [Google Scholar]
- 4. Cho YH, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Laparoscopic management of early uterine cancer: 10-year experience in Asan Medical Center. Gynecol Oncol. 2007;106:585–590 Epub 2007 Jun 20 [DOI] [PubMed] [Google Scholar]
- 5. Johnson N, Barlow D, Lethaby A, Tavender E, Curr E, Garry R. Surgical approach to hysterectomy for benign gynecological disease. Cochrane Database Syst Rev. 2006, Issue 2. Art. No: CD003677 [DOI] [PubMed] [Google Scholar]
- 6. Sculpher M, Manca A, Abbott J, Fountain J, Mason S, Garry R. Cost effectiveness of laparoscopic hysterectomy compared with standard hysterectomy: results from a randomized trial. BMJ. 2004;328:134–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haber GP, Crouzet S, Kamoi K, et al. Robotic NOTES (natural orifice translumenal endoscopic surgery) in reconstructive urology: initial laboratory experience. Urology. 2008;71:996–1000 [DOI] [PubMed] [Google Scholar]
- 8. Kaouk JH, Goel RK, Haber GP, Crouzet S, Stein RJ. Robotic single-port transumbilical surgery in humans: initial report. BJU Int. 2009;103:366–369 [DOI] [PubMed] [Google Scholar]
- 9. Escobar PF, Fader AN, Paraiso MF, Kaouk JH, Falcone T. Robotic-assisted laparoendoscopic single-site surgery in gynecology: initial report and technique. J Minim Invasive Gynecol. 2009. Sep-Oct;16(5):589–591 Epub 2009 Jul 8 [DOI] [PubMed] [Google Scholar]
- 10. Fader AN, Escobar PF. Laparoendoscopic single-site surgery (LESS) in gynecologic oncology: technique and initial report. Gynecol Oncol. 2009. August;114(2):157–61 Epub 2009 May 28 [DOI] [PubMed] [Google Scholar]
- 11. Yim GW, Jung YW, Paek J, et al. Transumbilical single-port access versus conventional total laparoscopic hysterectomy: surgical outcomes. Am J Obstet Gynecol. 2010;203:26.e1–6 Epub 2010 Apr 24 [DOI] [PubMed] [Google Scholar]
- 12. Escobar PF, et al. Single-Port, Notes. Fertil Steril. 2010;94(7):2497–2502 [DOI] [PubMed] [Google Scholar]
- 13. Fader AN, Rojas L, Ibeanu O, Grumbine F, Escobar PF. Multi-institutional evaluation of LESS in gynecology. Am J Obstet Gynecol. 2010;203(5):501.e1–6 [DOI] [PubMed] [Google Scholar]