Abstract
Acute periods of pulmonary exacerbation are the single most important cause of morbidity in cystic fibrosis patients, and may be associated with a loss of lung function. Intervening prior to the onset of a substantially increased inflammatory response may limit the associated damage to the airways. While a number of biomarker assays based on inflammatory markers have been developed, providing useful and important measures of disease during these periods, such factors are typically only elevated once the process of exacerbation has been initiated. Identifying biomarkers that can predict the onset of pulmonary exacerbation at an early stage would provide an opportunity to intervene before the establishment of a substantial immune response, with major implications for the advancement of cystic fibrosis care. The precise triggers of pulmonary exacerbation remain to be determined; however, the majority of models relate to the activity of microbes present in the patient's lower airways of cystic fibrosis. Advances in diagnostic microbiology now allow for the examination of these complex systems at a level likely to identify factors on which biomarker assays can be based. In this article, we discuss key considerations in the design and testing of assays that could predict pulmonary exacerbations.
Keywords: biomarkers, cystic fibrosis, exhaled breath, inflammation, molecular diagnostics, predictive biomarkers, proteomics, pulmonary exacerbation, quantitative PCR, sputum, trace metals
The need for biomarkers of pulmonary exacerbation in cystic fibrosis
Cystic fibrosis (CF) lung disease is characterized by a self-perpetuating cycle of airway obstruction, chronic bacterial infection and vigorous inflammation that results in bronchiectasis. Over 90% of people with CF die as a consequence of progressive lung damage resulting from bacterial infection [1,2]. CF patients typically have periods of clinical remission interspersed with acute episodes of increased respiratory symptoms, known as cystic fibrosis pulmonary exacerbation (CFPE). CFPEs are a major cause of morbidity for CF patients [3] and are often accompanied by a decline in lung function that may not be recovered following treatment [4,5]. CFPEs and associated lung damage have a major impact on quality of life [6–9], short-term mortality [10–13] and healthcare expenditure [10]. However, the lack of a specific disease marker or a unifying, commonly-accepted indicator to initiate treatment for a CFPE hampers the improvement of patient care. There is, therefore, substantial effort directed towards finding a marker to enable prompt diagnosis and treatment of CFPEs before irreversible lung damage occurs.
A key problem in diagnosing CFPE is the lack of a consensus definition of what it constitutes. Diagnosis typically revolves around a clinician's decision to treat a constellation of symptoms [14], including cough, sputum production, dyspnea, decreased energy level and appetite, weight loss and decreases in spirometric parameters [15]. There is a continuing debate as to which combination of these measures most accurately represents a significant clinical change necessitating intervention [16]. CF patients differ widely in the way in which they manifest their condition [17] and the large number of treatment options may have side effects that mask or mimic some clinical signs [18–20]. Increasingly, patients who appear or feel ‘not quite right’ are being treated with antibiotics to prevent the development of more worrying symptoms [16]. The treatment of patients with mild symptoms that do not yet meet typical CFPE definitions might, in some cases, reduce the incidence of CFPE over the long term [21,22]. However, the consequentially increased antibiotic use and the serious accompanying side effects of such an approach are a significant cause for concern.
Patient-reported symptoms might be an important complement to physician-assessed clinical signs in the diagnosis of CFPE, and newly developed diaries are currently being validated for this purpose [15,17,23,24]. However, patient-reported outcome scores are not without drawbacks. Subjective judgments about symptom severity differ significantly between patients [25], and could potentially be influenced by the desire to more firmly control therapy.
It would be ideal if biomarkers could be identified that had the capability of directly reflecting objective disease activity rather than relying entirely on clinical markers. Factors involved early in the CFPE initiation pathway could prove useful as predictive markers, allowing earlier intervention. The aim of early intervention would be to quickly reduce the development of a full inflammatory response and to shorten and reduce the severity of the CFPE, with the hope of preventing the development of irreversible lung damage. In this way, an effective and reproducible diagnostic marker for the early stages of a CFPE could offer the exciting possibility of disease-modifying therapy that could prevent permanent loss of lung function and the associated morbidity and mortality.
Bacterial infections in the CF airways are currently monitored through routine microbiology. While useful in the long-term surveillance of disease progression, these data are unlikely to provide sufficient detail to be predictive of CFPE onset. The application of molecular diagnostics to monitor biomarkers of bacterial infection continues to expand [26–28]. The increasing availability of commercial assays, in conjunction with a growing recognition of the utility of such molecular diagnostics in the characterization of clinical infections, means that routine surveillance in the management of CF respiratory disease using these methods may now be possible. However, which biomarkers are to be measured requires careful consideration.
In this article, we examine the potential of microbial factors to provide biomarkers for the early detection of CFPE onset, as well as the challenges that must be overcome for such molecular diagnostic surveillance to be implemented routinely.
Cystic fibrosis pulmonary exacerbation
The reasons for the occurrence of periods of CFPE are often not known, although a number of potential causes have been suggested (Box 1). These include the acquisition of new strains of bacterial species [29], the expansion of existing bacterial populations in the airways [29], blooms of planktonic bacterial cells released from biofilm populations [30], the expression of bacterial virulence factors [31], viral infections [32,33] and ambient air pollution (Box 1) [34]. Despite the lack of clarity regarding the triggers of CFPE, once initiated, the typical increase in severity of respiratory signs and symptoms is thought to reflect an upregulation of local inflammatory responses. As highlighted by a number of review articles, this inflammatory response is complex and multifactorial [17,23,24,35]. Broadly, CF airway disease is considered to be dominated by persistent neutrophilic infiltration, with elevated IL-8 and neutrophil elastase in airway secretions [36–41]. There is evidence that proinflammatory cytokines and other mediators are abnormally elevated in CF patients, relative to the burden of infection [42]; it has been suggested that dysregulation directly results from the underlying CF defect [41,43,44]. However, inflammation is increased by local airway epithelium–pathogen interactions and is elevated during CFPE [41].
Box 1. Hypothesized triggers of cystic fibrosis pulmonary exacerbation.
Bacterial
Environmental
Viral
Fungal
Allergic bronchopulmonary aspergillosis [127]
Colonization by Exophiala dermatitidis [131] or Candida albicans [132]
These triggers do not include structural changes in the airways, such as lobar/segmental collapse, or nonadherence to treatment for factors outlined.
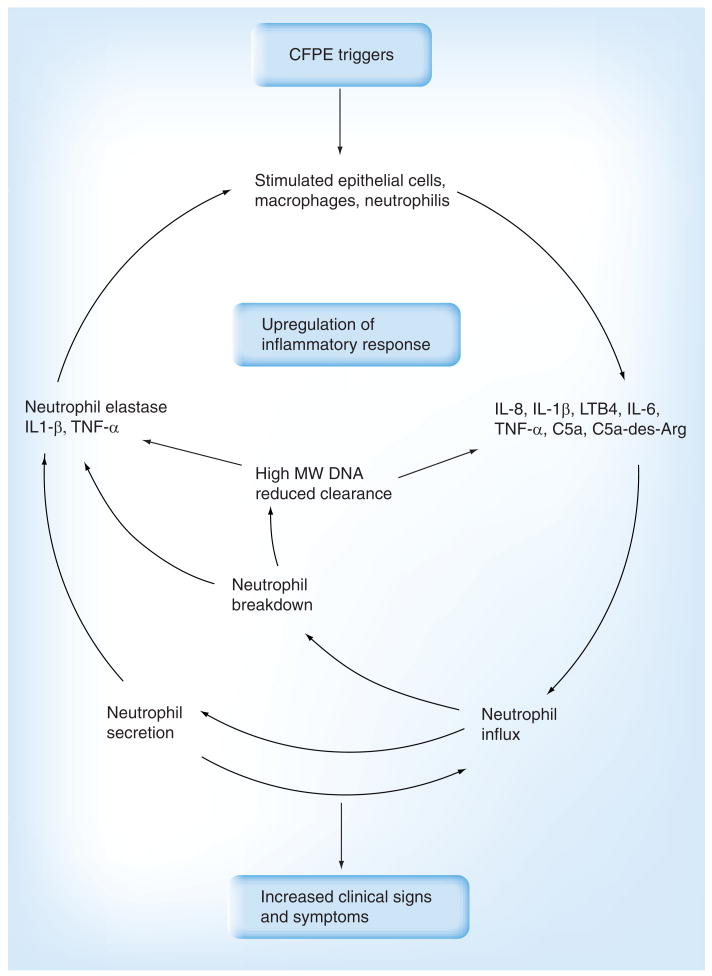
The elevated inflammatory response that characterizes CFPE forms a positive-feedback loop (Figure 1). Activated neutrophils release large amounts of elastase and other proteases that over-whelm the local host defenses [15]. Furthermore, as these neutrophils break down, they release large amounts of high-molecular-weight DNA that further increases the viscosity of the endobronchial secretions, which hinders mucociliary clearance [45]. In this way, a vicious cycle of chronic infection and inflammation occurs that encourages persistence of pathogens, promotes obstruction of the airway lumen and causes the destruction of airway wall architecture [15,46].
Figure 1. Proposed path of cystic fibrosis pulmonary exacerbation onset.
Conventionally, CFPE is diagnosed on the basis of an increase in the severity of clinical signs and symptoms. Changes in these markers follow an upregulation of the local inflammatory response. While molecular diagnostic assays based on components of the inflammatory response offer an earlier and more specific indication of the onset of CFPE, the ability to monitor factors associated with triggers of CFPE could allow intervention prior to the infliction of airway damage.
CFPE: Cystic fibrosis pulmonary exacerbation; MW: Molecular weight.
CFPE biomarkers
Biomarkers enable objective measurement and evaluation of “normal biologic processes, pathogenic processes, and pharmacologic responses to a therapeutic intervention” [47]. They have been shown to be useful in the diagnosis and characterization of a wide range of clinical conditions, including cardiovascular, renal and metabolic diseases, as well as sepsis and cancer [48–50]. Biomarkers should reflect biological activity and correlate with established clinical outcome measures, such as how a patient feels, functions or survives [51–53]. Their potential use in CF is particularly attractive given the diverse manifestations of CF airway disease and the response to therapy among individual CF patients.
Key biomarker characteristics that must be considered when designing assays for CFPE generally have been set out by Mayer-Hamblett et al. [53]. However, some of these considerations are more relevant to the use of biomarkers as early indicators of CFPE onset. Biomarkers useful in this context must be considered on the basis of two key areas; the degree to which they reflect biologically informative processes predictive of CFPE onset, and their suitability for use as a routine diagnostic tool.
Reflection of biologically informative processes predictive of CFPE onset
Biomarkers must consistently show a change in level prior to the onset of CFPE when compared with levels during clinical stability, at least among a subset of patients or exacerbations. Determining such a change relies on defining the point at which a patient is experiencing CFPE. However, in the absence of a consensus definition for CFPE, biomarker levels can only be correlated with an increase in accepted clinical markers and symptom scores. Although measurements of sputum inflammatory biomarkers obtained from the same patient on different occasions have been shown to be reproducible (although not specifically as predictors of CFPE), they can vary considerably between patients [54,55]. It is therefore important that the baseline values of such clinical markers are recognized as specific to individual patients, and that the assay can cover the effective range of clinically relevant test results.
Useful biomarkers should have a turnover rate that allows them to reflect short-term changes within the airways, but be sufficiently stable for accurate and reproducible measurement. Biomarkers that are highly unstable may be present for a very short period of time and may therefore only be detectable through frequent sampling. Furthermore, such instability could lead to appreciable biomarker degradation during sample handling. By contrast, highly stable biomarkers may accumulate over time in the airways in such a way that they do not reflect short-term changes in disease or return to baseline levels following successful treatment.
Suitability for use as a routine diagnostic tool
It is essential that the biomarker being measured is easily available from samples that can be routinely collected from patients, such as blood, urine, exhaled breath (EB), saliva or sputum. Inflammation in the CF lung appears to be primarily driven by local stimuli, mediators and chemoattractants, and is not the local effect of a systemic inflammatory reaction [56]. As such, systemic indicators of inflammation are likely to have low sensitivity and show only modest increases during acute exacerbations. Blood-based biomarkers are therefore less appropriate for diagnosing the onset of CFPE. Sputum is readily obtainable in the vast majority of adult CF patients and has been shown to be a useful basis for characterizing CF airway infection [57–59]. However, it should be noted that owing to the uneven distribution of infection within the CF lower airways, performing frequent assays is necessary in order to minimize sampling bias [60,61]. EB and EB condensate (EBC) have been used as samples for measuring biomarkers in a range of respiratory conditions [62,63]. While some concerns have been raised about the sensitivity and reproducibility of these sample types [63], they are noninvasive and easily collectable. EB and EBC samples have been used for the measurement of a number of biomarkers in CF patients, including nitric oxide [64–66], condensate acidity, nitrate, nitrite, 8-isoprostane, hydrogen peroxide and IFN-γ [67].
Since analysis will not be performed immediately in most instances, biomarkers must be stable at room temperature or have the capacity to be stabilized with the addition of specific reagents or by freezing. This is particularly important in the case of highly unstable molecules, such as mRNA. By contrast, although data regarding protein markers are sparse, for the majority of such proteins the process of freezing of samples for batch processing does not appear to affect levels observed [68].
Assays must be sufficiently inexpensive, both to carry out and in terms of equipment, expertise or reagents, in order to allow repeated use in routine surveillance.
The process of identifying clinically informative biomarkers typically involves comparison with an existing measure that is considered to be the ‘gold standard’ (something that definitively identifies a condition) [27]. However, in the context of CFPE, no such gold standard exists. This situation greatly complicates the process of evaluating potential assays. Nevertheless, the central role of the local inflammatory response in CFPE has led to the investigation of a wide array of inflammatory mediators as biomarkers (as discussed by Sagel et al. in 2007 [54]). Particular attention has been given to IL-8 [24,38,53,69–75], neutrophil elastase [38,53,69–71,75,76] and myeloperoxidase [39,69,77–81] in sputum samples. In each study, biomarker levels were elevated during CFPE, then decreased following the initiation of antibiotic therapy. In addition to immunoenzymatic assays, techniques such as 18F-fluorodeoxyglucose PET/CT imaging allow the direct determination of the physical distribution of neutrophilic inflammation [82]. However, this method remains too expensive and technically complex for routine use at this time.
As before, these studies have primarily sought to identify biomarkers that can provide accurate and reliable measurement of the biologic activity surrounding CFPE and/or as a sensitive marker of inflammation, primarily for use in assessing treatment strategies or disease progression. Here, a wide range of host-derived biomarkers have the potential to be useful. An increase in levels of these factors could be detectable prior to an elevation of clinical signs and symptoms. However, this is likely only to occur once an upregulation in inflammatory response has been established. As such, while having the potential to be informative in the diagnosis of CFPE, disease progression and the evaluation of therapy, these inflammatory biomarkers will be limited in their predictive power, because the point at which inflammation is elevated may be too late to prevent irreversible lung damage. Instead, in order to indicate the likely onset of CFPE, biomarkers that are associated with triggers of inflammatory response upregulation must be identified. Consideration must therefore be given to where such novel predictive biomarkers might be found.
CFPE-predictive biomarkers
A wide range of potential triggers have been proposed for the increased neutrophilic influx that characterizes CFPE. Such a diversity of potential triggers may translate into a number of different types of CFPE, each with different characteristics. For example, it has been suggested that episodes of CFPE that result from bacterial and viral infection represent distinct phenomena [83]. As such, separate diagnostic strategies may be required.
Infections caused by common respiratory viruses are associated with disease progression in CF patients [33,83–86]. While such infections are symptomatically acute, there is evidence that respiratory viruses may persist for extended periods in the airways of patients with a range of chronic respiratory disease [87,88]. Therefore, it may be most appropriate to analyze their presence in respiratory secretions through the enumeration of viral particles, with data compared against a threshold level associated with respiratory symptoms, rather than a simple presence/absence assay. Increasingly sophisticated molecular diagnostic strategies are being developed for such analysis [89–92], making the application of these assays a realistic proposition in the surveillance of CF patients.
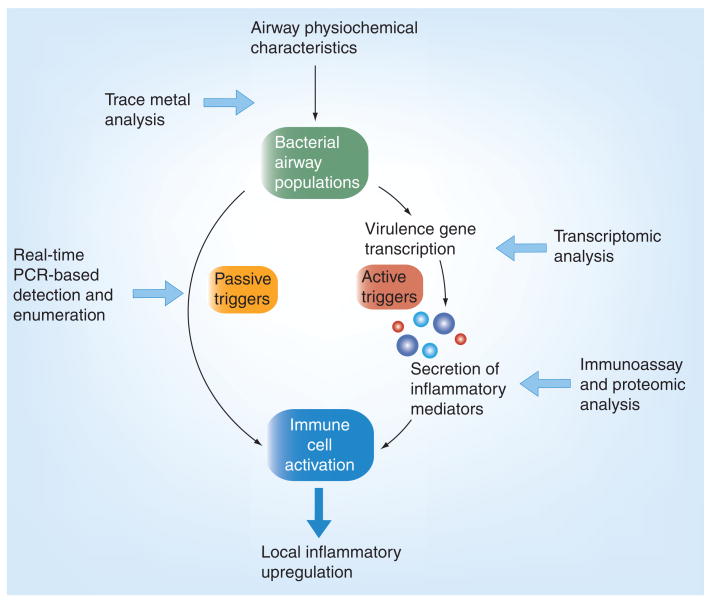
Bacterial infections of the CF airways are typically chronic. The interactions between these bacteria and the host, which may give rise to episodes of CFPE, therefore, are again unlikely to be characterizable by presence/absence assays, and would require more detailed analysis. For example, characterization of factors such as relative species abundance and gene-expression profiles may be necessary [93,94]. However, in each instance, the response of the host to changes in airway bacterial populations (and the resulting upregulation of the inflammatory response among leukocytes and/or epithelial cells) should be the focus of analysis (attempts to identify predictive biomarkers form part of the wider effort to determine the nature of the relationship between bacteria and their products in the CF lower airways, and the occurrence of CFPE). From a bacterial perspective, potential trigger mechanisms may occur through different routes. Such mechanisms could include the activation of neutrophils and epithelial cells when detecting the presence of bacterial cells in the airways. Other mechanisms may involve the upregulation of the immune response through the secretion of specific compounds by bacteria in the airways. In the case of the former inflammatory response triggers, assays must focus on the detection and enumeration of bacterial cells. For the other triggers, assays must be directed towards the measurement of levels of either secreted compounds in the airway or the transcription of the genes that encode them by bacterial cells (Figure 2).
Figure 2. Passive and active potential microbial triggers for cystic fibrosis pulmonary exacerbation, with suggested analytical points (white arrows) for onset prediction.
Traditional, culture-based diagnostic microbiology is of limited use in reporting subtle changes in airway bacterial populations because of the presence of slow-growing bacterial variants [95,96] and potentially viable but nonculturable bacterial cells [97–100]. However, the application of culture-independent quantitative PCR (Q-PCR) potentially offers a more accurate means by which changes in bacterial numbers prior to the onset of CFPE can be characterized [101], regardless of whether species are refractory to in vitro culture [102]. Q-PCR technology is well validated and used widely in the characterization of bacteria in clinical samples [103]. However, where clearance of material from the site of infection is poor, as in the lower CF airways [104,105], the presence of nonviable cells and extra cellular DNA can have a substantial impact on the accuracy of quantification by molecular methods. In order to address this problem, new molecular protocols have been developed that exclude from analysis any DNA not derived from viable bacterial cells [106]. These protocols, based on the use of propidium monoazide photo-crosslinking chemistry to block extracellular DNA or DNA from nonviable cells from serving as a template for PCR reactions, have been adapted to the CF respiratory context [107]. Furthermore, they have been shown to be capable of identifying short-term changes in bacterial levels in sputum not detected by standard Q-PCR techniques, such as a reduction in bacterial density in CF respiratory secretions following the intravenous administration of certain antibiotics [108]. Such approaches are rapid, relatively inexpensive and can be performed using automated systems. While requiring further validation, such pretreatment of samples could provide a basis for the routine surveillance of either total bacterial density in airway secretions, or the densities of specific species or strains of particular interest using multiplex PCR reactions [109].
It may be that it is the expression of particular traits by bacteria, rather than simply their presence in the airways, that is key in triggering the onset of CFPE. In such circumstances, early indications of CFPE onset might be derived from the determination of levels of virulence behavior. Specific immunoenzymatic assays (ELISA) have previously been used to directly determine levels of bacterially secreted compounds, such as Pseudomonas aeruginosa elastase, exotoxin A and alkaline protease, in CF airway secretions [31]. While not yet available as commercial kits, such assays may provide a rapid and relatively inexpensive routine assay of bacterial virulence biomarkers. Real-time PCR assays could be used to determine the transcription rates of virulence genes [110,111]. However, owing to the instability of mRNA, such assays would be dependent on sample handling and, as such, would be inappropriate for routine surveillance.
In some cases, changes in the behavior of bacteria can be driven by alterations in the physiochemical characteristics of the airways. Trace metals required for bacterial growth may be particularly important in determining the way in which bacteria grow. For example, lactoferrin, an iron-chelating protein abundant in human external secretions, blocks the formation of biofilms by P. aeruginosa. Biofilms are a bacterial growth phenotype implicated in CF respiratory pathogenesis [112,113]. Scavenging of trace metals by secreted molecules such as lactoferrin may represent an important host antimicrobial strategy [72]. Direct spectrometric measurement of copper, zinc and iron has revealed elevated levels in CF sputum compared with controls; furthermore, zinc levels in sputum decrease following treatment of CFPE and derangements of this defense may impact lung disease in CF [72]. While requiring significant technological investment, elemental level analyses have the advantage of not being affected by the activity of proteases [41,114]. However, further investigation will be required in order to assess the clinical value of these measurements.
A number of secreted peptides that confer antimicrobial effects through the chelation of trace metals may provide the basis for a more practical biomarker assay than trace-metal measurement. For example, the neutrophil-secreted zinc chelator, calprotectin, is present in high levels in CF airway secretions, and correlates with disease markers, including neutrophil count [77,115,116]. The assays required to quantify calprotectin are comparatively simple. If this biomarker is predictive of CFPE, the relative ease of quantification would provide a range of practical benefits.
Expert commentary
As yet, there has been little investigation into whether inflammatory or microbial biomarkers can provide an early indication of the likely onset of CFPE. This is partly owing to the fact that many patients can remain stable for months or even years without experiencing a CFPE episode. Substantial efforts are required to assemble the comprehensive data sets needed to study and validate candidate markers by taking multiple airway secretion samples during periods of pulmonary stability leading up to CFPE.
Several assays have now been validated analyzing potentially informative inflammatory biomarkers in the assessment of CF sputum. The development of microbial biomarkers is far less advanced. The utility of both types of measurement in predicting CFPE when applied to longitudinally collected sample sets must now be investigated.
Owing to a variation in the way in which CFPEs manifest themselves between patients, and even within the same patient [70], a panel of biomarkers may be more predictive than a single measure. The use of composite biomarker panels has been shown to provide improved diagnostic power in other contexts, including in the prediction of cardiovascular disease risk [117] in relation to myocardial infarction and death [118]. Furthermore, since the mechanisms by which CFPE occurs are not understood, it is potentially problematic to select biomarkers on the basis of proposed mechanistic models. A composite CF lung biomarker panel may therefore be more appropriate for CFPE prediction than any individual biomarker [119].
Five-year view
Informing treatment strategies
The identification of biomarkers that provide an early indication of CFPE would represent a major advancement in the management of CF airway infections. However, the impact that such biomarkers could have is dependent on their ability to inform clinical decision-making, and thus improve patient outcome. Current treatment strategies center around long-term maintenance therapy to retard disease progression and short-term therapy during periods of CFPE to reduce symptoms. However, little consideration has been given to what the most appropriate treatment would be if an impending pulmonary exacerbation was detected prior to the upregulation of the local immune response. As efforts to measure biomarkers in CF airway disease increase, such considerations will be necessary.
Microbial community
In addition to recognized CF pathogens, the CF lower airways often contain a complex polymicrobial community [92–94,120–121]. It is therefore important to consider if and how this wider community, consisting of bacteria, fungi, and eukaryotic viruses and phage species, contributes to triggering CFPE, and, as such, presents potential targets for diagnostic biomarker design. Microbial species may act as triggers, either through direct interaction with the host, or through interactions with recognized CF pathogens such as P. aeruginosa [92,122,123]. The potential complexity of these multispecies interactions represents a significant challenge to predictive biomarker design and further elucidation is required in order for them to inform this process.
Biomarker assay roll out
The value of performing certain mainstays of routine sputum diagnostics, such as the determination of the antibiotic susceptibility of clinical isolates, are being called into question [201]. The process of re-evaluating the components of CF sputum analysis provides an opportunity to apply resources to emerging assays that may be clinically informative. Furthermore, the expanding role being played by molecular diagnostics within healthcare laboratories provides a skills base and infrastructure for the performance of increasingly complex routine biomarker assays. Where assays can take advantage of sample types already provided on a regular basis (e.g., for routine diagnostic microbiology), surveillance through novel strategies might be possible without substantial disruption of existing patient routines. However, while an exciting prospect, the identification of appropriate predictive CFPE biomarkers requires further investigation.
Key issues.
A biomarker that objectively reflects disease activity, rather than relying on clinical markers, would be a major advantage in the management of cystic fibrosis pulmonary exacerbations.
The identification of biomarkers that are predictive of pulmonary exacerbation might allow early intervention prior to the establishment of an elevated inflammatory response.
The suspected infective nature of these events suggests that microbe-derived biomarkers may be most useful.
Potential assays can be based on the detection and enumeration of microbes, or on measures of expression of clinically significant microbial traits.
Both the accurate enumeration of key bacterial species and their gene-expression markers are now possible through the application of molecular genetic assays.
While still in its infancy, this work has the potential to be useful in the management of cystic fibrosis respiratory disease.
Footnotes
Financial & competing interests disclosure: The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Ramsey BW. Management of pulmonary disease in patients with cystic fibrosis. N Engl J Med. 1996;335(Suppl. 3):179–188. doi: 10.1056/NEJM199607183350307. [DOI] [PubMed] [Google Scholar]
- 2.Konstan MW, Berger M. Current understanding of the inflammatory process in cystic fibrosis: onset and etiology. Pediatr Pulmonol. 1997;24(Suppl. 2):137–142. doi: 10.1002/(sici)1099-0496(199708)24:2<137::aid-ppul13>3.0.co;2-3. discussion 159–161. [DOI] [PubMed] [Google Scholar]
- 3.Balfour-Lynn IA, Elborn JS. Respiratory disease: infection. In: Hodson M, Geddes D, Bush A, editors. Cystic Fibrosis. 3rd. Hodder Arnold; London, UK: 2007. pp. 137–158. [Google Scholar]
- 4.Sanders DB, Bittner RCL, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med. 2010;182:627–632. doi: 10.1164/rccm.200909-1421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanders DB, Bittner RCL, Rosenfeld M, Redding GJ, Goss CH. Pulmonary exacerbations are associated with subsequent FEV1 decline in both adults and children with cystic fibrosis. Ped Pulmonol. 2010 doi: 10.1002/ppul.21374. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Bradley J, McAlister O, Elborn S. Pulmonary function, inflammation, exercise capacity and quality of life in cystic fibrosis. Eur Respir J. 2001;17(Suppl. 4):712–715. doi: 10.1183/09031936.01.17407120. [DOI] [PubMed] [Google Scholar]
- 7.Orenstein DM, Pattishall EN, Nixon PA, et al. Quality of well-being before and after antibiotic treatment of pulmonary exacerbation in patients with cystic fibrosis. Chest. 1990;98(Suppl. 5):1081–1084. doi: 10.1378/chest.98.5.1081. [DOI] [PubMed] [Google Scholar]
- 8.Britto MT, Kotagal UR, Hornung RW, et al. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest. 2002;121(Suppl. 1):64–72. doi: 10.1378/chest.121.1.64. [DOI] [PubMed] [Google Scholar]
- 9.Yi MS, Tsevat J, Wilmott RW, et al. The impact of treatment of pulmonary exacerbations on the health-related quality of life of patients with cystic fibrosis: does hospitalization make a difference? J Pediatr. 2004;144(Suppl. 6):711–718. doi: 10.1016/j.jpeds.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 10.Liou TG, Adler FR, Fitzsimmons SC, et al. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153(Suppl. 4):345–352. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emerson J, Rosenfeld M, McNamara S, et al. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34(Suppl. 2):91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 12.Ellaffi M, Vinsonneau C, Coste J, et al. One-year outcome after severe pulmonary exacerbation in adults with cystic fibrosis. Am J Respir Crit Care Med. 2005;171(Suppl. 2):158–164. doi: 10.1164/rccm.200405-667OC. [DOI] [PubMed] [Google Scholar]
- 13.Mayer-Hamblett N, Rosenfeld M, Emerson J, et al. Developing cystic fibrosis lung transplant referral criteria using predictors of 2-year mortality. Am J Respir Crit Care Med. 2002;166(Suppl. 12, Pt 1):1550–1555. doi: 10.1164/rccm.200202-087OC. [DOI] [PubMed] [Google Scholar]
- 14.Ramsey BW, Boat TF. Outcome measures for clinical trials in cystic fibrosis Summary of a Cystic Fibrosis Foundation consensus conference. J Pediatr. 1994;124(Suppl. 2):177–192. doi: 10.1016/s0022-3476(94)70301-9. [DOI] [PubMed] [Google Scholar]
- 15.Goss CH, Burns JL. Exacerbations in cystic fibrosis 1: epidemiology and pathogenesis. Thorax. 2007;62(Suppl. 4):360–367. doi: 10.1136/thx.2006.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bush A. Treatment of cystic fibrosis: time for a new paradigm? Chest. 2009;136(Suppl. 5):1197–1199. doi: 10.1378/chest.09-1523. [DOI] [PubMed] [Google Scholar]
- 17•.Dakin C, Henry RL, Field P, et al. Defining an exacerbation of pulmonary disease in cystic fibrosis. Pediatr Pulmonol. 2001;31(Suppl. 6):436–442. doi: 10.1002/ppul.1072. Examination of the basis of cystic fibrosis pulmonary exacerbation (CFPE) diagnosis. [DOI] [PubMed] [Google Scholar]
- 18.Amsden GW. Anti-inflammatory effects of macrolides – an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J Antimicrob Chemother. 2005;55(Suppl. 1):10–21. doi: 10.1093/jac/dkh519. [DOI] [PubMed] [Google Scholar]
- 19.Mallon P, Murphy P, Elborn S. Fever associated with intravenous antibiotics in adults with cystic fibrosis. Lancet. 1997;350(9092):1676–1677. doi: 10.1016/S0140-6736(05)64274-2. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Boado YS, Rubin BK. Macrolides as immunomodulatory medications for the therapy of chronic lung diseases. Curr Opin Pharmacol. 2008;8(Suppl. 3):286–291. doi: 10.1016/j.coph.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Kabra SK, Pawaiya R, Lodha R, et al. Long-term daily high and low doses of azithromycin in children with cystic fibrosis: a randomized controlled trial. J Cyst Fibros. 2010;9(Suppl. 1):17–23. doi: 10.1016/j.jcf.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Florescu DF, Murphy PJ, Kalil AC. Effects of prolonged use of azithromycin in patients with cystic fibrosis: a metaanalysis. Pulm Pharmacol Ther. 2009;22(Suppl. 6):467–472. doi: 10.1016/j.pupt.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Rosenfeld M, Emerson J, Williams-Warren J, et al. Defining a pulmonary exacerbation in cystic fibrosis. J Pediatr. 2001;139(Suppl. 3):359–365. doi: 10.1067/mpd.2001.117288. [DOI] [PubMed] [Google Scholar]
- 24.Rabin HR, Butler SM, Wohl ME, et al. Pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol. 2004;37(Suppl. 5):400–406. doi: 10.1002/ppul.20023. [DOI] [PubMed] [Google Scholar]
- 25.Meyer KB, Clayton KA. Measurement and analysis of patient-reported outcomes. Methods Mol Biol. 2009;473:155–169. doi: 10.1007/978-1-59745-385-1_9. [DOI] [PubMed] [Google Scholar]
- 26.Christ-Crain M, Muller B. Biomarkers in respiratory tract infections: diagnostic guides to antibiotic prescription, prognostic markers and mediators. Eur Respir J. 2007;30(Suppl. 3):556–573. doi: 10.1183/09031936.00166106. [DOI] [PubMed] [Google Scholar]
- 27.Marshall JC, Reinhart K International Sepsis Forum. Biomarkers of sepsis. Crit Care Med. 2009;37(Suppl. 7):2290–2298. doi: 10.1097/CCM.0b013e3181a02afc. [DOI] [PubMed] [Google Scholar]
- 28.Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010;14(Suppl. 1):R15. doi: 10.1186/cc8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aaron SD, Ramotar K, Ferris W, et al. Adult cystic fibrosis exacerbations and new strains of Pseudomonas aeruginosa. Am J Respir Crit Care Med. 2004;169(Suppl. 7):811–815. doi: 10.1164/rccm.200309-1306OC. [DOI] [PubMed] [Google Scholar]
- 30.VanDevanter DR, Van Dalfsen JM. How much do Pseudomonas biofilms contribute to symptoms of pulmonary exacerbation in cystic fibrosis? Pediatr Pulmonol. 2005;39(Suppl. 6):504–506. doi: 10.1002/ppul.20220. [DOI] [PubMed] [Google Scholar]
- 31••.Jaffar-Bandjee MC, Lazdunski A, Bally M, et al. Production of elastase, exotoxin A, and alkaline protease in sputa during pulmonary exacerbation of cystic fibrosis in patients chronically infected by Pseudomonas aeruginosa. J Clin Microbiol. 1995;33(Suppl. 4):924–929. doi: 10.1128/jcm.33.4.924-929.1995. Analysis of Pseudomonas aeruginosa exoprotein concentrations in cystic fibrosis (CF) sputum over the course of treatment for CFPE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiatt PW, Grace SC, Kozinetz CA, et al. Effects of viral lower respiratory tract infection on lung function in infants with cystic fibrosis. Pediatrics. 1999;103(Suppl. 3):619–626. doi: 10.1542/peds.103.3.619. [DOI] [PubMed] [Google Scholar]
- 33.de Almeida MB, Zerbinati RM, Tateno AF, et al. Rhinovirus C and respiratory exacerbations in children with cystic fibrosis. Emerg Infect Dis. 2010;16(Suppl. 6):996–999. doi: 10.3201/eid1606.100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goss CH, Newsom SA, Schildcrout JS, et al. Effect of ambient air pollution on pulmonary exacerbations and lung function in cystic fibrosis. Am J Respir Crit Care Med. 2004;169(Suppl. 7):816–821. doi: 10.1164/rccm.200306-779OC. [DOI] [PubMed] [Google Scholar]
- 35.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168(Suppl. 8):918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 36.Konstan MW, Hilliard KA, Norvell TM, et al. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am J Respir Crit Care Med. 1994;150(Suppl. 2):448–454. doi: 10.1164/ajrccm.150.2.8049828. [DOI] [PubMed] [Google Scholar]
- 37.Bonfield TL, Panuska JR, Konstan MW, et al. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med. 1995;152(Suppl. 6 Pt 1):2111–2118. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- 38.Sagel SD, Kapsner R, Osberg I, et al. Airway inflammation in children with cystic fibrosis and healthy children assessed by sputum induction. Am J Respir Crit Care Med. 2001;164(Suppl. 8 Pt 1):1425–1431. doi: 10.1164/ajrccm.164.8.2104075. [DOI] [PubMed] [Google Scholar]
- 39•.Ordonez CL, Henig NR, Mayer-Hamblett N, et al. Inflammatory and microbiologic markers in induced sputum after intravenous antibiotics in cystic fibrosis. Am J Respir Crit Care Med. 2003;168(Suppl. 12):1471–1475. doi: 10.1164/rccm.200306-731OC. Assessment of the usefulness of microbiologic and inflammatory markers in CF-induced sputum as outcome measures. [DOI] [PubMed] [Google Scholar]
- 40•.Colombo C, Costantini D, Rocchi A, et al. Cytokine levels in sputum of cystic fibrosis patients before and after antibiotic therapy. Pediatr Pulmonol. 2005;40(Suppl. 1):15–21. doi: 10.1002/ppul.20237. Assessment of changes in cytokine levels in CF sputum in response to antibiotic therapy. [DOI] [PubMed] [Google Scholar]
- 41.Chmiel JF, Berger M, Konstan MW. The role of inflammation in the pathophysiology of CF lung disease. Clin Rev Allergy Immunol. 2002;23(Suppl. 1):5–27. doi: 10.1385/CRIAI:23:1:005. [DOI] [PubMed] [Google Scholar]
- 42.Pfeffer KD, Huecksteadt TP, Hoidal JR. Expression and regulation of tumor necrosis factor in macrophages from cystic fibrosis patients. Am J Respir Cell Mol Biol. 1993;9(Suppl. 5):511–519. doi: 10.1165/ajrcmb/9.5.511. [DOI] [PubMed] [Google Scholar]
- 43•.Muhlebach MS, Stewart PW, Leigh MW, et al. Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. Am J Respir Crit Care Med. 1999;160(Suppl. 1):186–191. doi: 10.1164/ajrccm.160.1.9808096. Evidence of an increased inflammatory response in CF patients. [DOI] [PubMed] [Google Scholar]
- 44.Noah TL, Black HR, Cheng PW, et al. Nasal and bronchoalveolar lavage fluid cytokines in early cystic fibrosis. J Infect Dis. 1997;175(Suppl. 3):638–647. doi: 10.1093/infdis/175.3.638. [DOI] [PubMed] [Google Scholar]
- 45.Shak S, Capon DJ, Hellmiss R, et al. Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proc Natl Acad Sci USA. 1990;87(Suppl. 23):9188–9192. doi: 10.1073/pnas.87.23.9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berger M. Inflammation in the lung in cystic fibrosis. A vicious cycle that does more harm than good? Clin Rev Allergy. 1991;9(Suppl. 1–2):119–142. doi: 10.1007/978-1-4612-0475-6_8. [DOI] [PubMed] [Google Scholar]
- 47.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(Suppl. 3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 48.Moriates C, Maisel A. The utility of biomarkers in sorting out the complex patient. Am J Med. 2010;123(Suppl. 5):393–399. doi: 10.1016/j.amjmed.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 49.Anderson R, Schmidt R. Front Biosci. Elite. Vol. 2. 2010. Clinical biomarkers in sepsis; pp. 504–520. [DOI] [PubMed] [Google Scholar]
- 50.Duffy MJ, Crown J. A personalized approach to cancer treatment: how biomarkers can help. Clin Chem. 2008;54(Suppl. 11):1770–1779. doi: 10.1373/clinchem.2008.110056. [DOI] [PubMed] [Google Scholar]
- 51.Bucher H, Guyatt G, Cook D, et al. Surrogate outcomes. In: Guyatt G, Rennie D, editors. The Users' Guide to the Medical Literature: A Manual for Evidence-based Clinical Practice. AMA Publications; IL, USA: 2002. [Google Scholar]
- 52.Wittes J, Lakatos E, Probstfield J. Surrogate endpoints in clinical trials: cardiovascular diseases. Stat Med. 1989;8(Suppl. 4):415–425. doi: 10.1002/sim.4780080405. [DOI] [PubMed] [Google Scholar]
- 53•.Mayer-Hamblett N, Aitken ML, Accurso FJ, et al. Association between pulmonary function and sputum biomarkers in cystic fibrosis. Am J Respir Crit Care Med. 2007;175(Suppl. 8):822–828. doi: 10.1164/rccm.200609-1354OC. Correlation between expectorate sputum biomarkers and lung function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54••.Sagel SD, Chmiel JF, Konstan MW. Sputum biomarkers of inflammation in cystic fibrosis lung disease. Proc Am Thorac Soc. 2007;4(Suppl. 4):406–417. doi: 10.1513/pats.200703-044BR. Review of biomarkers of inflammation in CF lung disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laguna TA, Wagner BD, Luckey HK, et al. Sputum desmosine during hospital admission for pulmonary exacerbation in cystic fibrosis. Chest. 2009;136:1561–1568. doi: 10.1378/chest.09-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferkol T, Rosenfeld M, Milla CE. Cystic fibrosis pulmonary exacerbations. J Pediatr. 2006;148(Suppl. 2):259–264. doi: 10.1016/j.jpeds.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 57.Thomassen MJ, Klinger JD, Badger SJ, et al. Cultures of thoracotomy specimens confirm usefulness of sputum cultures in cystic fibrosis. J Pediatr. 1984;104(Suppl. 3):352–356. doi: 10.1016/s0022-3476(84)81094-x. [DOI] [PubMed] [Google Scholar]
- 58.Aaron SD, Kottachchi D, Ferris WJ, et al. Sputum versus bronchoscopy for diagnosis of Pseudomonas aeruginosa biofilms in cystic fibrosis. Eur Respir J. 2004;24(Suppl. 4):631–637. doi: 10.1183/09031936.04.00049104. [DOI] [PubMed] [Google Scholar]
- 59.Gilljam H, Malmborg AS, Strandvik B. Conformity of bacterial growth in sputum and contamination free endobronchial samples in patients with cystic fibrosis. Thorax. 1986;41(Suppl. 8):641–646. doi: 10.1136/thx.41.8.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rubin BK. Mucus structure and properties in cystic fibrosis. Paediatr Respir Rev. 2007;8(Suppl. 1):4–7. doi: 10.1016/j.prrv.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 61.Rogers GB, Skelton S, Serisier DJ, et al. Determining CF lung microbiology: a comparison of spontaneous and serially induced sputum samples using T-RFLP profiling. J Clin Microbiol. 2009;48(1):78–86. doi: 10.1128/JCM.01324-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kharitonov SA, Barnes PJ. Exhaled biomarkers. Chest. 2006;130:1541–1546. doi: 10.1378/chest.130.5.1541. [DOI] [PubMed] [Google Scholar]
- 63.Barnes PJ, Chowdhury B, Kharitonov SA, et al. Pulmonary biomarkers in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174:6–14. doi: 10.1164/rccm.200510-1659PP. [DOI] [PubMed] [Google Scholar]
- 64.Keen C, Gustafsson P, Lindblad A, Wennergren G, Olin AC. Low levels of exhaled nitric oxide are associated with impaired lung function in cystic fibrosis. Ped Pulmonol. 2010;45:241–248. doi: 10.1002/ppul.21137. [DOI] [PubMed] [Google Scholar]
- 65.Barnes PJ, Dweik RA, Gelb AF, et al. Exhaled nitric oxide in pulmonary diseases: a comprehensive review. Chest. 2010;138:682–692. doi: 10.1378/chest.09-2090. [DOI] [PubMed] [Google Scholar]
- 66.Hofer M, Mueller L, Rechsteiner T, Benden C, Boehler A. Extended nitric oxide measurements in exhaled air of cystic fibrosis and healthy adults. Lung. 2009;187:307–313. doi: 10.1007/s00408-009-9160-8. [DOI] [PubMed] [Google Scholar]
- 67.Robroeks CMHHT, Roozeboom MH, de Jong PA, et al. Structural lung changes, lung function, and non-invasive inflammatory markers in cystic fibrosis. Pediatr Allergy Immunol. 2010;21:493–500. doi: 10.1111/j.1399-3038.2009.00872.x. [DOI] [PubMed] [Google Scholar]
- 68.Tirelli AS, Colombo C, Torresani E, Cariani L, Arnaboldi E, Conese M. Validation of an automated immunoassay for quantification of cytokines in the sputum of cystic fibrosis patients. Clin Chem Lab Med. 2007;45:108–111. doi: 10.1515/CCLM.2007.019. [DOI] [PubMed] [Google Scholar]
- 69.Saiman L, Marshall BC, Mayer-Hamblett N, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290(Suppl. 13):1749–1756. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 70•.Ordonez CL, Kartashov AI, Wohl ME. Variability of markers of inflammation and infection in induced sputum in children with cystic fibrosis. J Pediatr. 2004;145(Suppl. 5):689–692. doi: 10.1016/j.jpeds.2004.06.054. Analysis of the reproducibility of inflammatory marker concentrations in induced sputum. [DOI] [PubMed] [Google Scholar]
- 71.Sagel SD, Sontag MK, Wagener JS, et al. Induced sputum inflammatory measures correlate with lung function in children with cystic fibrosis. J Pediatr. 2002;141(Suppl. 6):811–817. doi: 10.1067/mpd.2002.129847. [DOI] [PubMed] [Google Scholar]
- 72.Gray RD, Imrie M, Boyd AC, et al. Sputum and serum calprotectin are useful biomarkers during CF exacerbation. J Cyst Fibros. 2010;9(3):193–198. doi: 10.1016/j.jcf.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 73.Salva PS, Doyle NA, Graham L, et al. TNF-α, IL-8, soluble ICAM-1, and neutrophils in sputum of cystic fibrosis patients. Pediatr Pulmonol. 1996;21(Suppl. 1):11–19. doi: 10.1002/(SICI)1099-0496(199601)21:1<11::AID-PPUL2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 74.Henig NR, Tonelli MR, Pier MV, et al. Sputum induction as a research tool for sampling the airways of subjects with cystic fibrosis. Thorax. 2001;56(Suppl 4):306–311. doi: 10.1136/thorax.56.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McGarvey LP, Dunbar K, Martin SL, et al. Cytokine concentrations and neutrophil elastase activity in bronchoalveolar lavage and induced sputum from patients with cystic fibrosis, mild asthma and healthy volunteers. J Cyst Fibros. 2002;1(Suppl. 4):269–275. doi: 10.1016/s1569-1993(02)00098-x. [DOI] [PubMed] [Google Scholar]
- 76.Downey DG, Martin SL, Dempster M, et al. The relationship of clinical and inflammatory markers to outcome in stable patients with cystic fibrosis. Pediatr Pulmonol. 2007;42(Suppl. 3):216–220. doi: 10.1002/ppul.20553. [DOI] [PubMed] [Google Scholar]
- 77.Gray RD, MacGregor G, Noble D, et al. Sputum proteomics in inflammatory and suppurative respiratory diseases. Am J Respir Crit Care Med. 2008;178(Suppl. 5):444–452. doi: 10.1164/rccm.200703-409OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78••.Sloane AJ, Lindner RA, Prasad SS, et al. Proteomic analysis of sputum from adults and children with cystic fibrosis and from control subjects. Am J Respir Crit Care Med. 2005;172(Suppl. 11):1416–1426. doi: 10.1164/rccm.200409-1215OC. Proteomic analysis of biomarkers of inflammation relating to CFPE. [DOI] [PubMed] [Google Scholar]
- 79.Regelmann WE, Siefferman CM, Herron JM, et al. Sputum peroxidase activity correlates with the severity of lung disease in cystic fibrosis. Pediatr Pulmonol. 1995;19(Suppl. 1):1–9. doi: 10.1002/ppul.1950190102. [DOI] [PubMed] [Google Scholar]
- 80.Meyer KC. Neutrophils, myeloperoxidase, and bronchiectasis in cystic fibrosis: green is not good. J Lab Clin Med. 2004;144(Suppl. 3):124–126. doi: 10.1016/j.lab.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 81.Sagel SD, Kapsner RK, Osberg I. Induced sputum matrix metalloproteinase-9 correlates with lung function and airway inflammation in children with cystic fibrosis. Pediatr Pulmonol. 2005;39(Suppl. 3):224–232. doi: 10.1002/ppul.20165. [DOI] [PubMed] [Google Scholar]
- 82.Klein M, Cohen-Cymberknoh M, Armoni S, et al. 18F-fluorodeoxyglucose-PET/CT imaging of lungs in patients with cystic fibrosis. Chest. 2009;136(Suppl. 5):1220–1228. doi: 10.1378/chest.09-0610. [DOI] [PubMed] [Google Scholar]
- 83.Wat D, Gelder C, Hibbitts S, et al. The role of respiratory viruses in cystic fibrosis. J Cyst Fibros. 2008;7(Suppl. 4):320–328. doi: 10.1016/j.jcf.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Ewijk BE, van der Zalm MM, Wolfs TF, et al. Viral respiratory infections in cystic fibrosis. J Cyst Fibros. 2005;4(Suppl. 2):31–36. doi: 10.1016/j.jcf.2005.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang EE, Prober CG, Manson B, et al. Association of respiratory viral infections with pulmonary deterioration in patients with cystic fibrosis. N Engl J Med. 1984;311(Suppl. 26):1653–1658. doi: 10.1056/NEJM198412273112602. [DOI] [PubMed] [Google Scholar]
- 86.Collinson J, Nicholson KG, Cancio E, et al. Effects of upper respiratory tract infections in patients with cystic fibrosis. Thorax. 1996;51(Suppl. 11):1115–1122. doi: 10.1136/thx.51.11.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kalu SU, Loeffelholz M, Beck E, et al. Persistence of adenovirus nucleic acids in nasopharyngeal secretions: a diagnostic conundrum. Pediatr Infect Dis J. 2010;29:746–750. doi: 10.1097/INF.0b013e3181d743c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sikkel MB, Quint JK, Mallia P, et al. Respiratory syncytial virus persistence in chronic obstructive pulmonary disease. Pediatr Infect Dis J. 2008;27:S63–S70. doi: 10.1097/INF.0b013e3181684d67. [DOI] [PubMed] [Google Scholar]
- 89.Arens MQ, Buller RS, Rankin A, et al. Comparison of the eragen multi-code respiratory virus panel with conventional viral testing and real-time multiplex pcr assays for respiratory viruses. J Clin Microbiol. 2010;48(7):2387–2395. doi: 10.1128/JCM.00220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gadsby NJ, Hardie A, Claas EC, et al. Comparison of the Luminex RVP Fast assay with in-house real-time PCR for respiratory viral diagnosis. J Clin Microbiol. 2010;48(6):2213–2216. doi: 10.1128/JCM.02446-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lassauniere R, Kresfelder T, Venter M. A novel multiplex real-time RT-PCR assay with FRET hybridization probes for the detection and quantitation of 13 respiratory viruses. J Virol Methods. 2010;165(Suppl. 2):254–260. doi: 10.1016/j.jviromet.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Willner D, Furlan M, Haynes M, et al. Metagenomic analysis of respiratory tract DNA viral communities in cystic fibrosis and non-cystic fibrosis individuals. PLoS One. 2009;4(Suppl. 10):e7370. doi: 10.1371/journal.pone.0007370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rogers GB, Stressmann FA, Walker AW, et al. Lung infections in cystic fibrosis: deriving clinical insight from microbial complexity. Expert Rev Mol Diagn. 2010;10(2):187–196. doi: 10.1586/erm.09.81. [DOI] [PubMed] [Google Scholar]
- 94.Rogers GB, Carroll MP, Bruce KD. Studying bacterial infections through culture-independent approaches. J Med Microbiol. 2009;58(Suppl. Pt 11):1401–1418. doi: 10.1099/jmm.0.013334-0. [DOI] [PubMed] [Google Scholar]
- 95.Proctor RA, von Eiff C, Kahl BC, et al. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol. 2006;4(Suppl. 4):295–305. doi: 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- 96.Schneider M, Muhlemann K, Droz S, et al. Clinical characteristics associated with isolation of small-colony variants of Staphylococcus aureus and Pseudomonas aeruginosa from respiratory secretions of patients with cystic fibrosis. J Clin Microbiol. 2008;46(Suppl. 5):1832–1834. doi: 10.1128/JCM.00361-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Colwell RR. Viable but nonculturable bacteria: a survival strategy. J Infect Chemother. 2000;6(Suppl. 2):121–125. doi: 10.1007/pl00012151. [DOI] [PubMed] [Google Scholar]
- 98.Anderson M, Bollinger D, Hagler A, et al. Viable but nonculturable bacteria are present in mouse and human urine specimens. J Clin Microbiol. 2004;42(Suppl. 2):753–758. doi: 10.1128/JCM.42.2.753-758.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rogers GB, Daniels TT, Tuck A, et al. Studying bacteria in respiratory specimens by using conventional and molecular microbiological approaches. BMC Pulm Med. 2009;9(Suppl. 1):14. doi: 10.1186/1471-2466-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bittar F, Richet H, Dubus JC, et al. Molecular detection of multiple emerging pathogens in sputa from cystic fibrosis patients. PLoS One. 2008;3(Suppl. 8):e2908. doi: 10.1371/journal.pone.0002908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fenollar F, Raoult D. Molecular diagnosis of bloodstream infections caused by non-cultivable bacteria. Int J Antimicrob Agents. 2007;30(Suppl. 1):S7–S15. doi: 10.1016/j.ijantimicag.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 102.Nadkarni MA, Martin FE, Jacques NA, et al. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology. 2002;148(Suppl. Pt 1):257–266. doi: 10.1099/00221287-148-1-257. [DOI] [PubMed] [Google Scholar]
- 103.Espy MJ, Uhl JR, Sloan LM, et al. Real-time PCR in clinical microbiology: applications for routine laboratory testing. Clin Microbiol Rev. 2006;19(Suppl. 1):165–256. doi: 10.1128/CMR.19.1.165-256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Robinson M, Bye PT. Mucociliary clearance in cystic fibrosis. Pediatr Pulmonol. 2002;33(Suppl. 4):293–306. doi: 10.1002/ppul.10079. [DOI] [PubMed] [Google Scholar]
- 105.Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Intern Med. 2007;261(Suppl. 1):5–16. doi: 10.1111/j.1365-2796.2006.01744.x. [DOI] [PubMed] [Google Scholar]
- 106.Nocker A, Sossa KE, Camper AK. Molecular monitoring of disinfection efficacy using propidium monoazide in combination with quantitative PCR. J Microbiol Methods. 2007;70(Suppl. 2):252–260. doi: 10.1016/j.mimet.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 107.Rogers GB, Stressmann FA, Koller G, et al. Assessing the diagnostic importance of nonviable bacterial cells in respiratory infections. Diagn Microbiol Infect Dis. 2008;62(Suppl. 2):133–141. doi: 10.1016/j.diagmicrobio.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 108.Rogers GB, Marsh P, Stressmann AF, et al. The exclusion of dead bacterial cells is essential for accurate molecular analysis of clinical samples. Clin Microbiol Infect. 2010;16(11):1656–1658. doi: 10.1111/j.1469-0691.2010.03189.x. [DOI] [PubMed] [Google Scholar]
- 109.da Silva Filho LV, Tateno AF, Velloso Lde F, et al. Identification of Pseudomonas aeruginosa, Burkholderia cepacia complex, and Stenotrophomonas maltophilia in respiratory samples from cystic fibrosis patients using multiplex PCR. Pediatr Pulmonol. 2004;37(Suppl. 6):537–547. doi: 10.1002/ppul.20016. [DOI] [PubMed] [Google Scholar]
- 110.da Silva Filho LV, Tateno AF, Martins KM, et al. The combination of PCR and serology increases the diagnosis of Pseudomonas aeruginosa colonization/infection in cystic fibrosis. Pediatr Pulmonol. 2007;42(Suppl. 10):938–944. doi: 10.1002/ppul.20686. [DOI] [PubMed] [Google Scholar]
- 111.Qin X, Emerson J, Stapp J, et al. Use of real-time PCR with multiple targets to identify Pseudomonas aeruginosa and other nonfermenting gram-negative bacilli from patients with cystic fibrosis. J Clin Microbiol. 2003;41(Suppl. 9):4312–4317. doi: 10.1128/JCM.41.9.4312-4317.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Singh PK, Parsek MR, Greenberg EP, et al. A component of innate immunity prevents bacterial biofilm development. Nature. 2002;417(6888):552–555. doi: 10.1038/417552a. [DOI] [PubMed] [Google Scholar]
- 113.Bjarnsholt T, Jensen PO, Fiandaca MJ, et al. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr Pulmonol. 2009;44(Suppl. 6):547–558. doi: 10.1002/ppul.21011. [DOI] [PubMed] [Google Scholar]
- 114.Pizzichini E, Pizzichini MM, Efthimiadis A, et al. Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med. 1996;154(Suppl. 2 Pt 1):308–317. doi: 10.1164/ajrccm.154.2.8756799. [DOI] [PubMed] [Google Scholar]
- 115.MacGregor G, Gray RD, Hilliard TN, et al. Biomarkers for cystic fibrosis lung disease: application of SELDI-TOF mass spectrometry to BAL fluid. J Cyst Fibros. 2008;7(Suppl. 5):352–358. doi: 10.1016/j.jcf.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 116•.Sagel SD, Sontag MK, Accurso FJ. Relationship between antimicrobial proteins and airway inflammation and infection in cystic fibrosis. Pediatr Pulmonol. 2009;44(Suppl. 4):402–409. doi: 10.1002/ppul.21028. Analysis of antimicrobial proteins in CF airway secretions, with correlation to airway inflammation and infection. [DOI] [PubMed] [Google Scholar]
- 117.Cummings DM, King DE, Mainous AG, et al. Combining serum biomarkers: the association of C-reactive protein, insulin sensitivity, and homocysteine with cardiovascular disease history in the general US population. Eur J Cardiovasc Prev Rehabil. 2006;13(Suppl. 2):180–185. doi: 10.1097/01.hjr.0000185973.59512.d3. [DOI] [PubMed] [Google Scholar]
- 118.Sabatine MS, Morrow DA, de Lemos JA, et al. Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation. 2002;105(Suppl. 15):1760–1763. doi: 10.1161/01.cir.0000015464.18023.0a. [DOI] [PubMed] [Google Scholar]
- 119.Robin X, Turck N, Hainard A, et al. Bioinformatics for protein biomarker panel classification: what is needed to bring biomarker panels into in vitro diagnostics? Expert Rev Proteomics. 2009;6(Suppl. 6):675–689. doi: 10.1586/epr.09.83. [DOI] [PubMed] [Google Scholar]
- 120.Sibley CD, Parkins MD, Rabin HR, et al. A polymicrobial perspective of pulmonary infections exposes an enigmatic pathogen in cystic fibrosis patients. Proc Natl Acad Sci USA. 2008;105(Suppl. 39):15070–15075. doi: 10.1073/pnas.0804326105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bouchara JP, Hsieh HY, Croquefer S, et al. Development of an oligonucleotide array for direct detection of fungi in sputum samples from patients with cystic fibrosis. J Clin Microbiol. 2009;47(Suppl. 1):142–152. doi: 10.1128/JCM.01668-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ramsey MM, Whiteley M. Polymicrobial interactions stimulate resistance to host innate immunity through metabolite perception. Proc Natl Acad Sci USA. 2009;106(Suppl. 5):1578–1583. doi: 10.1073/pnas.0809533106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sibley CD, Duan K, Fischer C, et al. Discerning the complexity of community interactions using a Drosophila model of polymicrobial infections. PLoS Pathog. 2008;4(10):e1000184. doi: 10.1371/journal.ppat.1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Aaron SD, Ramotar K, Ferris W, et al. Adult cystic fibrosis exacerbations and new strains of Pseudomonas aeruginosa. Am J Respir Crit Care Med. 2004;169(Suppl. 7):811–815. doi: 10.1164/rccm.200309-1306OC. [DOI] [PubMed] [Google Scholar]
- 125.Regelmann WE, Elliott GR, Warwick WJ, et al. Reduction of sputum Pseudomonas aeruginosa density by antibiotics improves lung function in cystic fibrosis more than do bronchodilators and chest physiotherapy alone. Am Rev Respir Dis. 1990;141(Suppl. 4 Pt 1):914–921. doi: 10.1164/ajrccm/141.4_Pt_1.914. [DOI] [PubMed] [Google Scholar]
- 126.Rogers GB, Hoffman LR, Whiteley M, et al. Revealing the dynamics of polymicrobial infections: implications for antibiotic therapy. Trends Microbiol. 2010;18:357–364. doi: 10.1016/j.tim.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Smyth A, Elborn JS. Exacerbations in cystic fibrosis: 3–management. Thorax. 2008;63(Suppl. 2):180–184. doi: 10.1136/thx.2006.060905. [DOI] [PubMed] [Google Scholar]
- 128.Hiatt PW, Grace SC, Kozinetz CA, et al. Effects of viral lower respiratory tract infection on lung function in infants with cystic fibrosis. Pediatrics. 1999;103(Suppl. 3):619–626. doi: 10.1542/peds.103.3.619. [DOI] [PubMed] [Google Scholar]
- 129.Wat D, Doull I. Respiratory virus infections in cystic fibrosis. Paediatr Respir Rev. 2003;4(Suppl. 3):172–177. doi: 10.1016/s1526-0542(03)00059-9. [DOI] [PubMed] [Google Scholar]
- 130.Scheithauer S, Haase G, Hausler M, et al. Association between respiratory and herpes viruses on pulmonary exacerbations in cystic fibrosis patients. J Cyst Fibros. 2010;9(3):234–236. doi: 10.1016/j.jcf.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Griffard EA, Guajardo JR, Cooperstock MS, et al. Isolation of Exophiala dermatitidis from pigmented sputum in a cystic fibrosis patient. Pediatr Pulmonol. 2010;45(Suppl. 5):508–510. doi: 10.1002/ppul.21187. [DOI] [PubMed] [Google Scholar]
- 132.Chotirmall SH, O'Donoghue E, Bennett K, et al. Sputum Candida albicans presages FEV decline and hospital-treated exacerbations in cystic fibrosis. Chest. 2010;138:1186–1195. doi: 10.1378/chest.09-2996. [DOI] [PubMed] [Google Scholar]
Website
- 201.Report of the UK Cystic Fibrosis Trust Microbiology Laboratory Standards Working Group. Cystic Fibrosis Trust; 2010. Laboratory standards for processing microbiological samples from people with cystic fibrosis. www.cftrust.org.uk/aboutcf/publications/consensusdoc/CD_Laboratory_Standards_%28for_web%29_4_Oct_2010.pdf. [Google Scholar]