Abstract
Background
Although typically derived from the contour of arterial pressure waveform, augmentation index (AIx) may also be derived from the digital pulse volume waveform using finger plethysmography (peripheral arterial tonometry, PAT). Little is known regarding the physiologic correlates of AIx derived from PAT. In this study, we investigated the relation of PAT-AIx with measures of ventricular-vascular coupling.
Methods
Pulse volume waves were measured via PAT and used to derive AIx. Using 2-dimensional echocardiography, effective arterial elastance index (EaI) was estimated as end systolic pressure / stroke volume index. Left ventricular (LV) end-systolic elastance index (ELVI) was calculated as end systolic pressure / end systolic volume index. Ventricular-vascular coupling ratio was defined as EaI/ELVI.
Results
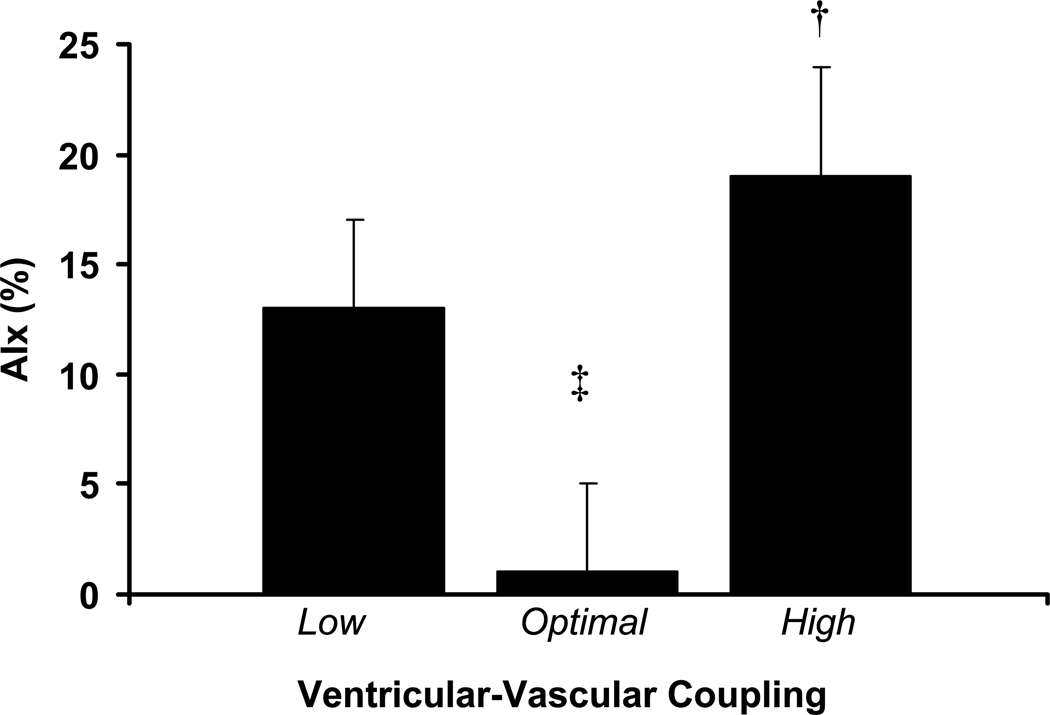
Given the bi-directional nature of ventricular-vascular uncoupling as measured by echocardiography, patients were separated into 3 groups: low EaI/ELVI (<0.6, n = 21), optimal EaI/ELVI (mean 0.6–1.2, n = 16) and high EaI/ELVI (>1.2, n = 10). Adjusting for potential confounders (age, mean arterial pressure, height, and heart rate) patients with optimal EaI/ELVI had lower AIx (1±4%, p<0.05) compared to those with low EaI/ELVI (13±4%) and high EaI/ELVI (19±5%).
Conclusions
Abnormal ventricular-vascular coupling, arising from either increased effective arterial elastance or increased ventricular elastance, is associated with increased AIx as measured by PAT. Additional research is needed to examine other vascular correlates of PAT-AIx.
Keywords: augmentation index, effective arterial elastance, Left ventricular end-systolic elastance
As a measure of late systolic load and systemic vascular function (Borlaug, et al. 2007) the augmentation index (AIx) may be a useful therapeutic target and secondary endpoint (Hashimoto, et al. 2007). AIx is associated with numerous cardiovascular morbidities (Nurnberger, et al. 2002), coronary artery disease (Hayashi, et al. 2002; Weber, et al. 2004), albuminuria (Tsioufis, et al. 2003), and predicts future cardiovascular events (Weber, et al. 2005) and mortality (London, et al. 2001). Moreover, the augmentation of systolic blood pressure owing to wave reflections (i.e. increased late systolic load) reduces left ventricular (LV) systolic and diastolic function (Borlaug, Melenovsky, Redfield, Kessler, Chang, Abraham & Kass 2007), increases LV pressure effort, increases myocardial oxygen demand and reduces coronary perfusion (Hashimoto, Imai & O'Rourke 2007; Hashimoto, et al. 2007; Hashimoto, et al. 2008). This resultant ventricular-vascular mismatch impairs cardiac metabolic efficiency and causes LV hypertrophy (Hashimoto, Nichols, O'Rourke & Imai 2008). Although typically derived from the contour of central and peripheral arterial pressure waveforms (Chen, et al. 1997), AIx can also be derived from digital pulse volume waveforms using finger plethysmography (peripheral arterial tonometry, PAT). Little is known regarding the systemic vascular correlates of AIx derived from PAT. The digital pulse volume waveform is influenced by wave reflections (Chowienczyk, et al. 1999) and correlates with pressure waveforms measured at the finger, radial artery and carotid artery, and changes in the digital volume pulse with perturbation have been shown to be very similar to changes in the pressure waveforms (Millasseau, et al. 2000; Millasseau, et al. 2003). Thus, it is reasonable to speculate that digital AIx derived from PAT may also serve as a proxy of ventricular-vascular load but this has yet to be empirically demonstrated.
Ventricular-vascular coupling can be quantified via examination of the ratio of effective arterial elastance and LV end-systolic elastance derived either invasively from catheterization-derived pressure-volume loops or non-invasively from conventional blood pressure measurement and echocardiography (Chen, et al. 2001). Ventricular-vascular uncoupling is associated with low exercise capacity (Chen, et al. 1999; Wong, et al.), has been implicated in the etiology of numerous pathologies (Kass 2005) and has also recently been shown to be associated with B-type natriuretic peptide (BNP) in patients after myocardial infarction and predicts long-term CV mortality in this setting (Antonini-Canterin, et al. 2009). The purpose of this study was to examine the association between PAT-AIx and ventricular-vascular coupling. We hypothesized that elevated PAT-AIx would reflect abnormal ventricular-vascular coupling.
Methods
Forty-seven individuals, recruited from the Tufts Medical Center preventive cardiology clinic, participated in this study. Exclusion criteria included ongoing myocardial ischemia, severe valvular disease, peripheral arterial disease (ankle-brachial index < 0.9), LDL-cholesterol > 100 mg/dl, unstable cardiac symptoms, renal insufficiency (serum creatinine > 2 mg/dL), active liver disease, chronic obstructive pulmonary disease, bronchial asthma, uncontrolled hypertension defined as BP > 190/100 mmHg, Raynaud’s disease and/or finger deformities. The presence or absence of the following cardiovascular risk factors was assessed in each subject: gender, hypertension (SBP > 140mm Hg or DBP > 90mm Hg or being on an antihypertensive medication), hyperlipidemia (total serum cholesterol > 240mg/dl or taking lipid lowering medication), diabetes mellitus (fasting blood glucose level>140mg/dl or on oral hypoglycemics or insulin), coronary artery disease (defined as the presence of ischemia or infarction on single-photon emission computed tomographic nuclear myocardial perfusion imaging or > 50% stenosis of an epicardial coronary artery by angiography), family history of CAD (having first or second degree relatives with CAD), and current smoking status (having smoked at least five times per day within the last month). This study was approved by the Institutional Review Board at Tufts Medical Center. Written informed consent was obtained from all the study participants prior to obtaining vascular measures.
Finger pulse wave amplitude
Beat-by-beat pulse wave amplitude was captured in all patients using finger arterial tonometry (EndoPAT, Itamar Medical Ltd., Israel) as previously described in detail (Kuvin, et al. 2003). With patients in the supine position, plethysmographic finger cuffs were placed on the index fingers of both hands. A computerized algorithm automatically identified peak volume and inflection points using a 4th order derivative as previously described by Kelly et al. (Kelly, et al. 1989) and Takazawa et al. (Takazawa, et al. 1995). Augmentation index was calculated from PWA waveforms as the ratio of the difference between the early and late systolic peaks of the waveform relative to the early peak expressed as a percentage (P2 – P1/P1 * 100). This method has been shown to correlate well with other methods of AIx derivation (Haller, et al. 2007).
Doppler Echocardiography
Cardiac dimensions were assessed using standard 2-dimensional echocardiographic techniques (Simpson’s method) in 47 separate patients. End systolic pressure (Esp) was estimated as 0.9 * systolic blood pressure (Kelly, et al. 1992). Effective arterial elastance index (EaI) was estimated as end systolic pressure / stroke volume (Kelly, Ting, Yang, Liu, Maughan, Chang & Kass 1992). This was indexed to body surface area. LV systolic elastance index (ELVI) was calculated as end systolic pressure / end systolic volume (Najjar, et al. 2004). This too was indexed to body surface area. Vascular-ventricular coupling index was defined as EaI/ ELVI (Najjar, Schulman, Gerstenblith, Fleg, Kass, O'Connor, Becker & Lakatta 2004). Stroke work index was calculated as mean arterial pressure * SV/EDV and taken as a load-independent index of cardiac contractility (Borlaug, Melenovsky, Redfield, Kessler, Chang, Abraham & Kass 2007). Fractional shortening (FS, %) was calculated as 100 × (LVID – LVIDs)/LVID. Ejection fraction (EF, %) was calculated as 100 × (LV diastolic volume - LV systolic volume)/LV diastolic volume.
Statistical analysis
It has been suggested that “optimal” ventricular-vascular coupling in the resting state is in the range of 0.6–1.2 as this confers the near-optimal balance between mechanical efficacy and energetic efficiency and this range is conserved across species (Chantler, et al. 2008). Given the bi-directional nature of ventricular-vascular uncoupling as measured by echocardiography, patients were separated into 3 groups: low EaI/ELVI (mean 0.38±0.03, n = 21), optimal EaI/ELVI (mean 0.90±0.05, n = 16) and high EaI/ELVI (2.1±0.2, n = 10). Normality of distribution was assessed using Kolmogorov-Smirnof and Shapiro-Wilk tests. Group comparisons were made using analysis of variance with Tukey post hoc testing where appropriate. Chi-square tests were used to compare categorical variables. If significant group differences in potential confounders existed, analysis of covariance was used to statistically remove the influence of these parameters of outcome variables of interest. Pearson’s correlation coefficients were used to assess relationships between variables of interest. All data are reported as means ± SEM. A priori significance was set at p < 0.05.
Results
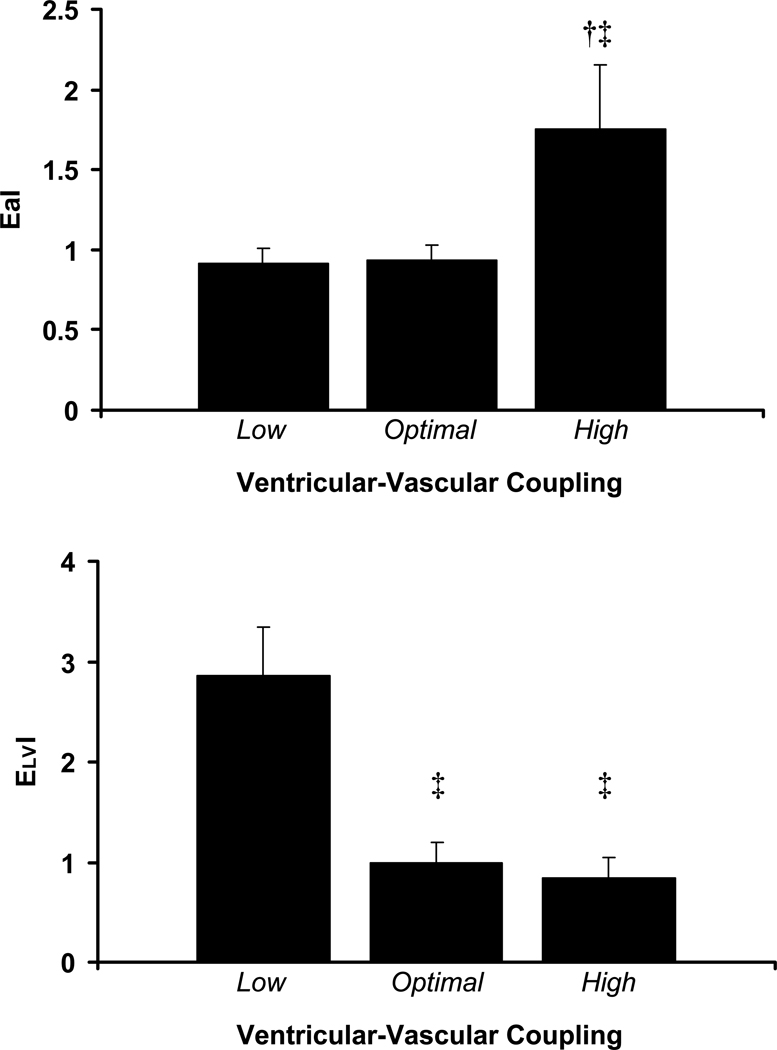
Patient descriptive characteristics are presented in Table 1. Groups were separated according to echocardiographically-derived ventricular-vascular coupling as aforementioned. There were no differences in age, systolic blood pressure, diastolic blood pressure, mean arterial pressure, pulse pressure, height, heart rate, lipids, medication history, or gender across groups. There were significant group differences in ejection fraction, fractional shortening and stroke work index (Table 1, p<0.05). As seen in figure 1, adjusting for potential confounders (age, gender, mean arterial pressure, height, and heart rate) patients with optimal EaI/ELVI had lower PAT-AIx (1±4%, p<0.05) compared to those with low EaI/ELVI (13±4%) and high EaI/ELVI (19±5%). Adjusting for ejection fraction in addition to aforementioned co-variates did not affect group differences in PAT-AIx (p<0.05) as patients with optimal EaI/ELVI still had lower PAT-AIx (0±%, p<0.05) compared to those with low EaI/ELVI (15±6%) and high EaI/ELVI (16±10%). PAT-AIx did not differ between those with low EaI/ELVI versus high EaI/ELVI. As seen in figure 2a, those with high coupling had significantly higher EaI than those with optimal coupling and low coupling (p<0.05). EaI was similar between those with optimal coupling and those with low coupling (p>0.05). As seen in figure 2b, those with low coupling had significantly higher ELVI than those with optimal coupling and high coupling (p<0.05). ELVI was similar between those with optimal coupling and those with high coupling (p>0.05).
Table 1.
Patient descriptive characteristics.
| Variable | All | Low VVC | Optimal VVC | High VVC |
|---|---|---|---|---|
| n = 47 | n = 21 | n = 16 | n = 10 | |
| Age, yrs | 58 ± 2 | 59 ± 3 | 57 ± 3 | 58 ± 5 |
| Male, % | 72 | 57 | 88 | 80 |
| BMI, kg/m2 | 31 ± 1 | 31 ± 1 | 33 ± 1 | 28 ± 2 |
| Hypertension, % | 81 | 90 | 81 | 60 |
| SBP, mmHg | 122 ± 2 | 125 ± 3 | 121 ± 5 | 118 ± 6 |
| DBP, mmHg | 66 ± 2 | 65 ± 3 | 70 ± 3 | 60 ± 4 |
| MAP, mmHg | 85 ± 2 | 85 ± 3 | 86 ± 3 | 79 ± 4 |
| PP, mmHg | 56 ± 2 | 59 ± 3 | 51 ± 4 | 57 ± 5 |
| HR, bpm | 68 ± 2 | 65 ± 3 | 70 ± 3 | 71 ± 5 |
| Diabetes mellitus, % | 34 | 29 | 44 | 30 |
| Hyperlipidemia, % | 83 | 81 | 94 | 70 |
| Total cholesterol, mg/dl | 167 ± 7 | 165 ± 12 | 179 ± 11 | 154 ± 15 |
| LDL cholesterol, mg/dl | 95 ± 6 | 92 ± 8 | 96 ± 12 | 97 ± 15 |
| HDL cholesterol, mg/dl | 35 ± 1 | 35 ± 3 | 35 ± 3 | 36 ± 2 |
| Triglycerides, mg/dl | 174 ± 24 | 188 ± 42 | 208 ± 45 | 102 ± 13 |
| CAD, % | 79 | 86 | 75 | 70 |
| Current smoker, % | 23 | 29 | 12 | 30 |
| Family history CVD, % | 47 | 57 | 38 | 40 |
| Medications, % | ||||
| Aspirin | 23 | 29 | 6 | 40 |
| Statin | 25 | 33 | 6 | 40 |
| Anti-hypertensive agent | 52 | 57 | 44 | 50 |
| Ejection Fraction, % | 58 ± 2 | 73 ± 2 | 53 ± 2‡ | 34 ± 2‡ † |
| Fractional Shortening, % | 32 ± 2 | 43 ± 2 | 28 ± 1‡ | 16 ± 1‡ † |
| Stroke Work Index | 49 ± 3 | 62 ± 3 | 46 ± 3‡ | 26 ± 2‡ † |
Significantly different from optimal (p<0.05).
Significantly different from low (p<0.05).
Figure 1.
PAT-AIx in patients with low, optimal and high ventricular-vascular coupling. † Significantly different from optimal (p<0.05). ‡ Significantly different from low (p<0.05).
Figure 2.
a) Effective arterial elastance and b) levt ventricular end-systolic elastance in patients with low, optimal and high ventricular-vascular coupling. † Significantly different from optimal (p<0.05). ‡ Significantly different from low (p<0.05).
According to univariate correlations, AIx was associated with age (r = 0.23, p<0.05), MAP (r = 0.20, p<0.05), end diastolic volume (r = 0.25, p<0.05), stroke volume (r = 0.28, p<0.05), and inversely associated with heart rate (r = −0.21, p<0.05).
Discussion
The novel finding of the present investigation was that patients with ventricular-vascular uncoupling arising from either increased effective arterial elastance or increased ventricular elastance had significantly higher PAT-AIx compared to patients with optimal ventricular-vascular coupling. Thus PAT-AIx may offer clinical insight into ventricular-vascular uncoupling and cardiovascular risk. This supports previous findings suggesting that AIx is not simply a measure of arterial stiffness or wave reflection per se but may also reflect properties of cardiac performance and overall ventricular-vascular coupling (Sharman, et al. 2009).
Patients with elevated EaI had elevated PAT-AIx and this may be due to a combination of factors. EaI is a measure of the net load imposed on the LV due to systemic functional properties of the vascular tree (Kelly, Ting, Yang, Liu, Maughan, Chang & Kass 1992). Unlike other measures of LV afterload which only account for steady-state pressure-flow relationships, Ea takes into account the pulsatile component of blood pressure and flow due to vascular stiffness (Chantler, Lakatta & Najjar 2008). Ea increases with advancing age and has been shown to be elevated in numerous disease states (Chen, et al. 1998). Elevated Ea has also been implicated in blunted exercise capacity, LV hypertrophy, and the pathogenesis of heart failure and hypertension (Chantler, Lakatta & Najjar 2008; Najjar, Schulman, Gerstenblith, Fleg, Kass, O'Connor, Becker & Lakatta 2004). Integrating such measures as vascular resistance, compliance, characteristic impedance and systolic/diastolic time intervals, Ea correlates well with other measures of arterial load derived invasively from vascular input impedance (Kelly, Ting, Yang, Liu, Maughan, Chang & Kass 1992). Therefore, the elevated PAT-AIx in patients with elevated EaI may be due to increased arterial stiffness reducing travel time of forward and reflected pressure waves resulting in reflected pressure waves arriving early during systole, increasing augmented pressure and overall pulse pressure. Increased vascular resistance in this setting may also influence AIx via modulating peripheral reflection sites, increasing the magnitude of reflected pressure waves.
PAT-AIx may also be influenced by factors related to LV contractility. Patients with increased ELVI also had elevated PAT-AIx, concomitant with low EaI. LV elastance reflects a combination structural (i.e. LV stiffness, hypertrophy, fibrosis) and functional (i.e. LV contractility/relaxation) properties of the LV (Kass 2005). High ventricular elastance may augment systolic pressure sensitivity to cardiac loading, increase cardiac energy cost and myocardial oxygen consumption to deliver SV, and further impair diastolic function (Chen, Nakayama, Nevo, Fetics, Maughan & Kass 1998; Lam, et al. 2007). In the present study, it is likely that increased ELVI was a manifestation of increased LV contractility as patients with increased ELVI also had higher ejection fraction, fractional shortening and stroke work index compared to the other groups. The ventricular-vascular coupling index is inversely related to ejection fraction according to the formula EaI/ELVI = (1/EF)-1 (Chantler, Lakatta & Najjar 2008; Cohen-Solal, et al. 1996). Several studies note an association between LV systolic/diastolic function and AIx (Borlaug, Melenovsky, Redfield, Kessler, Chang, Abraham & Kass 2007; Ikonomidis, et al. 2008; Weber, et al. 2007; Weber, et al. 2006; Weber, et al. 2008) although this has recently been disputed (Sharman, Davies, Jenkins & Marwick 2009). Whether the high AIx in the high VVC group was a cause or a consequence of low EF cannot be ascertained from this study design. Patients with heart failure and low EF may have elevated AIx. It has been posited that increased pressure from wave reflections serves to offset low forward wave pressure genesis in the failing heart, ultimately maintaining systemic perfusion pressure (Curtis, et al. 2007). In the present study, PAT-AIx was also associated with end diastolic volume, a reflection of preload. To our knowledge, this is the fist study to note an association with AIx and echo-derived preload. End diastolic volume accounted for 6% of the variance in PAT-AIx. Although this is a small percentage, PAT-AIx may still have marginal preload dependence and additional research is needed to examine the influence of preload on AIx. Overall these findings would suggest that PAT-AIx is sensitive to LV contractile state.
In conclusion, elevated PAT-AIx is novel reflection of ventricular-vascular uncoupling stemming from either altered ventricular function or altered vascular function. Future research is needed to examine other vascular correlates of PAT-AIx as well as the efficacy of PAT-AIx as a surrogate of ventricular-vascular coupling in the clinical and ambulatory settings.
REFERENCES
- Antonini-Canterin F, Enache R, Popescu BA, Popescu AC, Ginghina C, Leiballi E, Piazza R, Pavan D, Rubin D, Cappelletti P, Nicolosi GL. Prognostic value of ventricular-arterial coupling and B-type natriuretic peptide in patients after myocardial infarction: a five-year follow-up study. J Am Soc Echocardiogr. 2009;22:1239–1245. doi: 10.1016/j.echo.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Borlaug BA, Melenovsky V, Redfield MM, Kessler K, Chang HJ, Abraham TP, Kass DA. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll Cardiol. 2007;50:1570–1577. doi: 10.1016/j.jacc.2007.07.032. [DOI] [PubMed] [Google Scholar]
- Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol. 2008;105:1342–1351. doi: 10.1152/japplphysiol.90600.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–2034. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- Chen CH, Nakayama M, Nevo E, Fetics BJ, Maughan WL, Kass DA. Coupled systolic-ventricular and vascular stiffening with age: implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol. 1998;32:1221–1227. doi: 10.1016/s0735-1097(98)00374-x. [DOI] [PubMed] [Google Scholar]
- Chen CH, Nakayama M, Talbot M, Nevo E, Fetics B, Gerstenblith G, Becker LC, Kass DA. Verapamil acutely reduces ventricular-vascular stiffening and improves aerobic exercise performance in elderly individuals. J Am Coll Cardiol. 1999;33:1602–1609. doi: 10.1016/s0735-1097(99)00052-2. [DOI] [PubMed] [Google Scholar]
- Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, Kass DA. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997;95:1827–1836. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- Chowienczyk PJ, Kelly RP, MacCallum H, Millasseau SC, Andersson TL, Gosling RG, Ritter JM, Anggard EE. Photoplethysmographic assessment of pulse wave reflection: blunted response to endothelium-dependent beta2-adrenergic vasodilation in type II diabetes mellitus. J Am Coll Cardiol. 1999;34:2007–2014. doi: 10.1016/s0735-1097(99)00441-6. [DOI] [PubMed] [Google Scholar]
- Cohen-Solal A, Caviezel B, Laperche T, Gourgon R. Effects of aging on left ventricular-arterial coupling in man: assessment by means of arterial effective and left ventricular elastances. J Hum Hypertens. 1996;10:111–116. [PubMed] [Google Scholar]
- Curtis SL, Zambanini A, Mayet J, Mc GTSA, Foale R, Parker KH, Hughes AD. Reduced systolic wave generation and increased peripheral wave reflection in chronic heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H557–H562. doi: 10.1152/ajpheart.01095.2006. [DOI] [PubMed] [Google Scholar]
- Haller MJ, Silverstein JH, Shuster JJ. Correlation between radial artery tonometry- and fingertip tonometry-derived augmentation index in children with type 1 diabetes. Diab Vasc Dis Res. 2007;4:66. doi: 10.3132/dvdr.2007.011. [DOI] [PubMed] [Google Scholar]
- Hashimoto J, Imai Y, O'Rourke MF. Indices of pulse wave analysis are better predictors of left ventricular mass reduction than cuff pressure. Am J Hypertens. 2007;20:378–384. doi: 10.1016/j.amjhyper.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Hashimoto J, Imai Y, O'Rourke MF. Monitoring of antihypertensive therapy for reduction in left ventricular mass. Am J Hypertens. 2007;20:1229–1233. doi: 10.1016/j.amjhyper.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Hashimoto J, Nichols WW, O'Rourke MF, Imai Y. Association Between Wasted Pressure Effort and Left Ventricular Hypertrophy in Hypertension: Influence of Arterial Wave Reflection. Am J Hypertens. 2008 doi: 10.1038/ajh.2007.49. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Nakayama Y, Tsumura K, Yoshimaru K, Ueda H. Reflection in the arterial system and the risk of coronary heart disease. Am J Hypertens. 2002;15:405–409. doi: 10.1016/s0895-7061(02)02260-4. [DOI] [PubMed] [Google Scholar]
- Ikonomidis I, Tzortzis S, Papaioannou T, Protogerou A, Stamatelopoulos K, Papamichael C, Zakopoulos N, Lekakis J. Incremental value of arterial wave reflections in the determination of left ventricular diastolic dysfunction in untreated patients with essential hypertension. J Hum Hypertens. 2008;22:687–698. doi: 10.1038/jhh.2008.39. [DOI] [PubMed] [Google Scholar]
- Kass DA. Ventricular arterial stiffening: integrating the pathophysiology. Hypertension. 2005;46:185–193. doi: 10.1161/01.HYP.0000168053.34306.d4. [DOI] [PubMed] [Google Scholar]
- Kelly R, Hayward C, Avolio A, O'Rourke M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation. 1989;80:1652–1659. doi: 10.1161/01.cir.80.6.1652. [DOI] [PubMed] [Google Scholar]
- Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–521. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, Karas RH, Udelson JE. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–174. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115:1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London GM, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38:434–438. doi: 10.1161/01.hyp.38.3.434. [DOI] [PubMed] [Google Scholar]
- Millasseau SC, Guigui FG, Kelly RP, Prasad K, Cockcroft JR, Ritter JM, Chowienczyk PJ. Noninvasive assessment of the digital volume pulse. Comparison with the peripheral pressure pulse. Hypertension. 2000;36:952–956. doi: 10.1161/01.hyp.36.6.952. [DOI] [PubMed] [Google Scholar]
- Millasseau SC, Kelly RP, Ritter JM, Chowienczyk PJ. The vascular impact of aging and vasoactive drugs: comparison of two digital volume pulse measurements. Am J Hypertens. 2003;16:467–472. doi: 10.1016/s0895-7061(03)00569-7. [DOI] [PubMed] [Google Scholar]
- Najjar SS, Schulman SP, Gerstenblith G, Fleg JL, Kass DA, O'Connor F, Becker LC, Lakatta EG. Age and gender affect ventricular-vascular coupling during aerobic exercise. J Am Coll Cardiol. 2004;44:611–617. doi: 10.1016/j.jacc.2004.04.041. [DOI] [PubMed] [Google Scholar]
- Nurnberger J, Keflioglu-Scheiber A, Opazo Saez AM, Wenzel RR, Philipp T, Schafers RF. Augmentation index is associated with cardiovascular risk. J Hypertens. 2002;20:2407–2414. doi: 10.1097/00004872-200212000-00020. [DOI] [PubMed] [Google Scholar]
- Sharman JE, Davies JE, Jenkins C, Marwick TH. Augmentation index, left ventricular contractility, and wave reflection. Hypertension. 2009;54:1099–1105. doi: 10.1161/HYPERTENSIONAHA.109.133066. [DOI] [PubMed] [Google Scholar]
- Takazawa K, Tanaka N, Takeda K, Kurosu F, Ibukiyama C. Underestimation of vasodilator effects of nitroglycerin by upper limb blood pressure. Hypertension. 1995;26:520–523. doi: 10.1161/01.hyp.26.3.520. [DOI] [PubMed] [Google Scholar]
- Tsioufis C, Tzioumis C, Marinakis N, Toutouzas K, Tousoulis D, Kallikazaros I, Stefanadis C, Toutouzas P. Microalbuminuria is closely related to impaired arterial elasticity in untreated patients with essential hypertension. Nephron Clin Pract. 2003;93:c106–c111. doi: 10.1159/000069546. [DOI] [PubMed] [Google Scholar]
- Weber T, Auer J, Lamm G, O'Rourke MF, Eber B. Arterial stiffness, central blood pressures, and wave reflections in cardiomyopathy-implications for risk stratification. J Card Fail. 2007;13:353–359. doi: 10.1016/j.cardfail.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Weber T, Auer J, O'Rourke MF, Kvas E, Lassnig E, Lamm G, Stark N, Rammer M, Eber B. Increased arterial wave reflections predict severe cardiovascular events in patients undergoing percutaneous coronary interventions. Eur Heart J. 2005;26:2657–2663. doi: 10.1093/eurheartj/ehi504. [DOI] [PubMed] [Google Scholar]
- Weber T, Auer J, O'Rourke MF, Kvas E, Lassnig E, Berent R, Eber B. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109:184–189. doi: 10.1161/01.CIR.0000105767.94169.E3. [DOI] [PubMed] [Google Scholar]
- Weber T, Auer J, O'Rourke MF, Punzengruber C, Kvas E, Eber B. Prolonged mechanical systole and increased arterial wave reflections in diastolic dysfunction. Heart. 2006;92:1616–1622. doi: 10.1136/hrt.2005.084145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T, O'Rourke MF, Ammer M, Kvas E, Punzengruber C, Eber B. Arterial stiffness and arterial wave reflections are associated with systolic and diastolic function in patients with normal ejection fraction. Am J Hypertens. 2008;21:1194–1202. doi: 10.1038/ajh.2008.277. [DOI] [PubMed] [Google Scholar]
- Wong RC, Dumont CA, Austin BA, Kwon DH, Flamm SD, Thomas JD, Starling RC, Desai MY. Relation of ventricular-vascular coupling to exercise capacity in ischemic cardiomyopathy: a cardiac multi-modality imaging study. Int J Cardiovasc Imaging. 26:151–159. doi: 10.1007/s10554-009-9516-4. [DOI] [PubMed] [Google Scholar]