Abstract
Background
Currently used indicators of iron status have limitations. Hepcidin, a key regulator of iron metabolism, is reduced in iron deficiency. We sought to determine the properties of hepcidin as a diagnostic test of iron deficiency.
Design and Methods
Sera from female, non-anemic, whole blood donors were analyzed for hepcidin (enzyme-linked immunosorbent assay), ferritin, soluble transferrin receptor and C-reactive protein. Iron deficiency was defined as (i) serum ferritin less than 15 ng/mL or (ii) soluble transferrin receptor /log(ferritin) index greater than 3.2 if the C-reactive protein concentration was less than 10 mg/L, or greater than 2.2 if the C-reactive protein concentration was greater than 10 mg/L). Receiver operating characteristic curves were plotted to determine the overall utility and identify optimal cut-points of hepcidin as a test of iron deficiency.
Results
In 261 blood donors the prevalence of iron deficiency defined by ferritin concentration was 59/261 [22.6% (17.5, 27.7)], whereas defined by soluble transferrin receptor/log(ferritin) index it was 53/261 [20.4% (15.4, 25.2)]. The 95% reference range of hepcidin concentration in the iron-replete population was 8.2–199.7 ng/mL. The area under the receiver operating characteristic curve for hepcidin compared with ferritin concentration less than 15 ng/mL was 0.87 (0.82, 0.92), while that compared with the soluble transferrin receptor /log(ferritin) index was 0.89 (95% CI 0.84, 0.93). For a diagnosis of iron deficiency defined by the soluble transferrin receptor/log(ferritin) index, hepcidin less than 8 ng/mL had a sensitivity of 41.5% and a specificity of 97.6%, while hepcidin less than 18 ng/mL had a sensitivity of 79.2% and a specificity of 85.6%.
Conclusions
Serum hepcidin concentration may be a useful indicator of deficient iron stores. Further studies are required to evaluate the role of hepcidin in the diagnosis of iron deficiency in other groups of patients.
Keywords: serum hepcidin, iron deficiency, diagnostic test, blood donors, iron status, premenopausal females
Introduction
Iron deficiency remains a major public health problem, affecting over 1.5 billion people worldwide.1 Widely used tests of iron status include serum ferritin, soluble transferrin receptor (sTfR), and other indices such as transferrin and transferrin saturation. Each of these has limitations: for example, ferritin is an indicator of iron stores,2 but may be elevated in patients with coexistent inflammation, while sTfR reflects tissue iron deficiency, but is influenced by erythropoietic activity.
Hepcidin, a 25-amino acid peptide secreted by the liver, is a key regulator of iron metabolism which down-regulates duodenal iron absorption and macrophage iron release.3 Hepcidin levels are reduced in iron deficiency, and measurement of blood or urine hepcidin levels may enable evaluation of iron requirements and provide a powerful indicator of physiological iron deficiency.4 Mass-spectrometry5 and, more recently, immunoassays for quantitation of hepcidin in serum, plasma and urine have been developed.6,7 Many clinical applications for hepcidin measurement in patients have been proposed, including the diagnosis of anemia of chronic disease, anemia associated with chronic kidney disease and hemodialysis,8,9 genetic hemochromatosis,7 and iron deficiency.10 However, how hepcidin may add to the existing repertoire of iron indices is yet to be determined.11 In particular, the diagnostic properties of serum hepcidin concentration as an index of iron deficiency have not been well characterized in clinical populations, nor have studies included adequate samples of healthy and iron-deficient individuals to enable estimation of a reference range. The sensitivity and specificity of various cut-offs for serum hepcidin in diagnosing iron deficiency are yet to be determined.
Blood donors, especially premenopausal women, are at high risk of iron deficiency,12 and preventing and alleviating this problem is a major concern for blood services.13 Thus, donors are a good population in which to evaluate novel tests of iron deficiency. In a population of healthy female blood donors we evaluated the diagnostic properties of hepcidin concentration as a test of iron deficiency.
Design and Methods
Subjects
We recruited non-anemic (capillary hemoglobin >120 g/L, HemoCue, Angelholm, Sweden) females who were eligible for and underwent whole blood donation at two Australian Red Cross Blood Service donation centers; first time and returned donors were eligible for recruitment (the minimum routine inter-donation interval in Australia is 12 weeks).14 Donors who were taking iron supplementation, had undergone menopause, or had a personal or family history of genetic hemochromatosis were excluded. In order to minimize confounding effects due to potential diurnal variation in hepcidin,15 and to facilitate specimen processing during standard working hours, only donors presenting to collection centers during morning sessions were recruited.
Analytical methods
Venous blood samples were collected at the beginning of venesection. Separated serum was frozen to −70° Celsius for storage and transport, and later thawed and analyzed in a single batch. Serum was analyzed for ferritin (chemiluminescent microparticle immunoassay, Abbott Laboratories, Abbott Park, IL, USA), soluble transferrin receptor and C-reactive protein (both Roche Tinaquant Immunoturbidometric assays, Roche Diagnostics, Mannheim, Germany), all performed on the Abbott Architect ci8200 (Abbott Laboratories, Abbott Park, IL, USA).
Serum hepcidin was measured by a previously described competitive enzyme-linked immunoassay (C-ELISA) (Intrinsic Life Sciences, La Jolla, CA, USA). All participants’ samples were assayed in duplicate. Results from the C-ELISA were determined from standard curves developed from calibrators run simultaneously with study samples. This assay has been validated technically and physiologically.16–18
Statistical analysis
Assuming a 20% prevalence of iron deficiency in this population, a sample size of 250 would enable a calculation of specificity of at least 85% with a 95% confidence interval of ±5%, and a determination of sensitivity of at least 85% with a 95% confidence interval of ±10%.19 For iron indices with a logarithmic distribution, geometric means were calculated. Undetectably low hepcidin values (<5.4 ng/mL) were defined as ‘0’. Hepcidin was logarithmically transformed following addition of 1 (as logarithmic transformation of a ‘0’ value is not possible) to convert to a normal distribution. Mean values were calculated for iron indices by exponentiating mean values of logarithmically transformed variables (geometric means),20 with subtraction of 1 from the final value in the case of hepcidin. The association between hepcidin and standard iron indices was estimated using linear regression on transformed variables. Mean values of logarithmically transformed values were compared using the t-test.
Receiver operating characteristic (ROC) curves were calculated for hepcidin concentration as a test of iron deficiency as compared with surrogate gold standards: serum ferritin less than 15 ng/mL2 and sTfR/log(ferritin) ratio (sTfR-F index) greater than 3.2 if the CRP was less than 10 mg/L and greater than 2.2 if the CRP was greater than 10 mg/L).21 The sTfR-F ratio has been reported to have almost perfect utility for diagnosing iron deficiency when compared with bone marrow iron stores.22 The cut-offs of sTfR-F to define iron deficiency used in this study are based on a study utilizing this index to define iron deficiency, functional iron deficiency and anemia of chronic disease, with reticulocyte hemoglobin as the gold standard.23 The sensitivity and specificity of hepcidin as an indicator of iron deficiency were determined for each possible cut-off of hepcidin, and the area under the curve for ROC curves (AUCROC) was generated. The curves were inspected to identify suitable hepcidin cut-offs. The Youden index [(sensitivity/100+specificity/100)-1] was calculated for each value of hepcidin to assist selection of an optimal cut-off.24 The AUCROC for samples collected before and after the median time of collection was compared.25 Statistical significance was defined as P values less than 0.05.
Data were entered into database software (Microsoft Access, Microsoft Corporation, Redmond, WA, USA), and transferred to statistical software for analysis (STATA 11, Statacorp, College Station, TX, USA).
Ethics
Informed consent was obtained from all subjects. The study was approved by the Human Research Ethics Committee of the Blood Service. Iron-deficient subjects were provided with the results of their iron studies once results became available and referred to their family physician.
Results
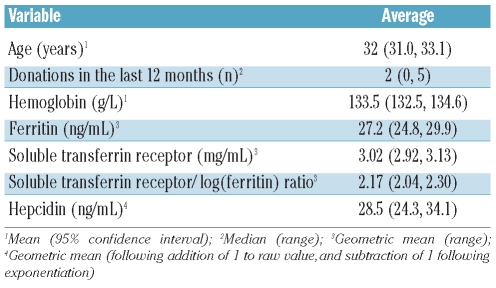
Two hundred and sixty-one donors were recruited between October 2009 and January 2010 (Figure 1). Age, hemoglobin and iron indices are summarized in Table 1. The prevalence of iron deficiency among donors was as follows: 59/261 [22.6% (95% CI 17.5, 27.7)] defined by ferritin concentration and 53/261 [20.4% (15.4, 25.2)] defined by the sTfR-F index. The mean CRP concentration was 1.23 mg/L; three donors had CRP levels greater than 10 mg/L, one of whom had a sTfR-F index greater than 2.2; 30 donors (11.5%) had CRP levels between 5 and 10 mg/L.
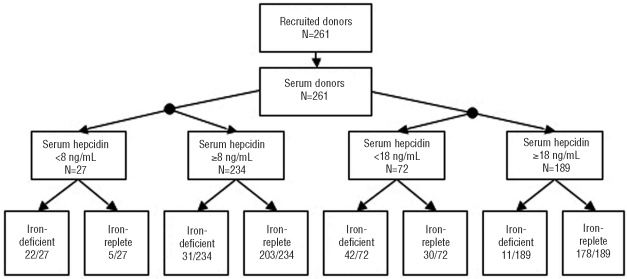
Figure 1.
Flow of donors and classification of disease (as defined by sTfR-F index) using two cut-offs for hepcidin. A total of 261 donors were recruited into the study. When a hepcidin cut-off of <8 ng/mL was selected, 10.3% were considered iron-deficient, of whom 81.5% were correctly classified; of the 89.7% considered iron replete, 86.8% were correctly classidied. When a hepcidin cut-off of <18 ng/mL was selected, 27.6% of this population were considered iron-deficient, of whom 58.3% were correctly classified; of the 72.4% not considered iron-deficient, 94.2% were correctly classified.
Table 1.
Summary of donors’ characteristics and iron status.
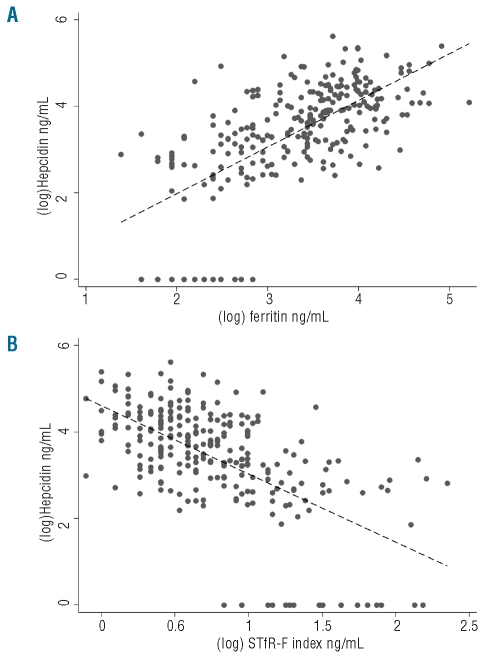
The geometric mean hepcidin concentration was 28.5 ng/mL. Twenty-one donors [8.0% (4.7, 11.4)] had undetectably low serum hepcidin (<5.4 ng/mL). The 95% reference range for the overall population was less than 5.4 ng/mL to 174.6 ng/mL. The 95% reference range for the population considered iron replete (non-deplete sTfR-F index) and with a CRP less than 10 mg/mL, was 8.2–199.7 ng/mL (n=206). The mean hepcidin concentration was lower in iron-deficient donors than in iron-replete ones, whether based on ferritin (7.3 ng/mL versus 44.4 ng/mL, P<0.001), sTfR (7.4 ng/mL versus 34.6 ng/mL) or sTfR-F index (6.8 ng/mL versus 42.9 ng/mL, P<0.001). By linear regression, log(hepcidin) was associated with log(ferritin) (coefficient +1.08, P<0.001); log(sTfR) (−2.02, P<−0.001) and log(sTfR-F index) (−1.58, P<0.001), as depicted in Figure 2. There was no association between log(hepcidin) and log(CRP) (P=0.835), although only three donors had elevated CRP.
Figure 2.
Associations between (log)hepcidin and (A) log(ferritin), and (B) log(sTfR-F index). By linear regression, log(hepcidin) was associated with log(ferritin) (coefficient +1.08, P<0.001) and log(sTfR-F index) (−1.58, P<0.001). Correlation: log(hepcidin) with log(ferritin) (coefficient 0.66, P<0.001) and log(hepcidin) with log(sTfR-F index) (−0.61, P<0.001).
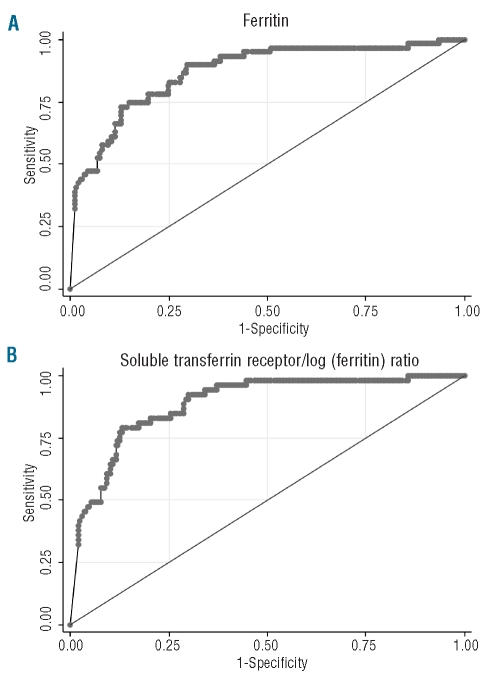
Figure 3 shows the ROC curves for hepcidin as a diagnostic test for iron deficiency as defined by the ferritin concentration and sTfR-F index. The AUCROC for hepcidin compared with sTfR-F index was 0.89 (95% CI 0.84, 0.93), and the AUCROC for hepcidin compared with ferritin less than 15 ng/mL was 0.87 (0.82, 0.92). In this dataset, the AUCROC of sTfR as a test of iron deficiency as defined by ferritin was 0.87 (0.82, 0.92). When the analysis was restricted to cases with CRP less than 5 mg/L, the AUCROC for hepcidin compared with ferritin less than 15 ng/mL was 0.86 (0.81, 0.92).
Figure 3.
ROC curves for hepcidin as diagnostic tests of (A) ferritin, (B) sTfR-F index. The AUCROC for hepcidin compared with ferritin <15ng/mL was 0.87. The AUCROC for hepcidin compared with sTfR-F index >3.2 was 0.89.
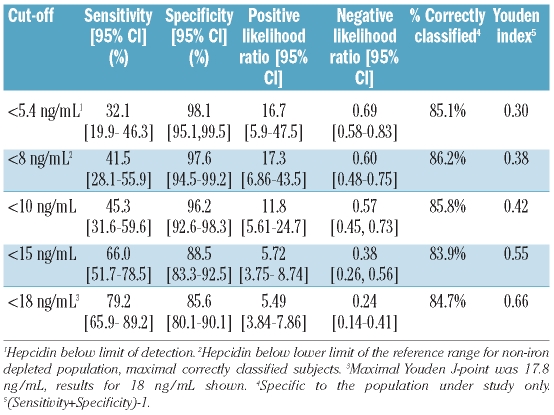
The diagnostic properties (sensitivity, specificity and positive and negative likelihood ratios) of several potential cut-offs (below the level of detection, the lower limit of the 95% range, and significant points on the ROC curve) of hepcidin for identifying iron deficiency (as defined by sTfR-F index) are shown in Table 2. A hepcidin level below the cut-off of 8 ng/mL achieved maximal correct classification (86.2%) of donors; each of the other cut-offs presented correctly classified at least 80% of subjects. A hepcidin level below the cut-off of 17.8 ng/mL achieved the maximal Youden index (0.66) with a sensitivity of 79.3% and a specificity of 86.5%.
Table 2.
Properties of different cut-offs of hepcidin as a diagnostic test of iron deficiency (defined by sTfR-F index).
The performance of hepcidin to determine different cutoffs of ferritin, with and without a very strict definition of inflammation (CRP<5 mg/L), was then evaluated. When a ferritin concentration less than 10 ng/mL was adopted as the gold standard, the AUCROC for hepcidin was 0.86 (0.80, 0.93), the hepcidin cut-off with the optimal Youden index (0.68) was 17.9 ng/mL and, at this cut-off, the sensitivity and specificity of hepcidin concentration for diagnosing a ferritin level of less than 10 ng/mL were 86.7% and 80.5%, respectively; using a hepcidin cut off of 8 ng/mL, the sensitivity and specificity were 46.7% and 94.4%, respectively. When only donors with a CRP level less than 5 mg/L were considered, the AUCROC for hepcidin was 0.87 (0.80, 0.94). If a ferritin concentration less than 30 ng/mL was adopted as the gold standard, the AUCROC for hepcidin was 0.82 (0.77, 0.87), the cut-off with the optimal Youden index (0.53) was 28.8 ng/mL and at this cut-off, the sensitivity and specificity of hepcidin concentration for diagnosing a ferritin level less than 30 ng/mL were 72.1% and 78.4%, respectively; using a hepcidin cut-off of 8 ng/mL, the sensitivity and specificity were 22.1% and 100.0%, respectively. When the analysis was restricted to donors with a CRP level less than 5 mg/L, the AUCROC for hepcidin was 0.81 (0.75, 0.87). Among subjects with a ferritin concentration in the indeterminate range (15–30 ng/mL) 14.1% had a hepcidin level below 8 ng/mL and 39.1% had a hepcidin level less than 18 ng/mL.
The median time of sample collection was 10:56 a.m. (range, 07:33–13:49). Dividing the samples according to whether they were collected before or after the median time of collection, those collected before the median time had a significantly lower ferritin concentration (24.4 ng/mL versus 29.9 ng/mL, P<0.05), higher sTfR (2.93 mg/mL versus 3.14 mg/mL, P<0.05) and higher sTfR-F index (2.33 versus 2.03, P<0.02). Hepcidin levels were lower among samples collected before the median time (21.3 ng/mL) than among samples collected after this time (37.2 ng/mL) (P<0.05). The AUCROC for hepcidin compared with sTfR-F index was 0.85 (0.78–0.93) for samples collected before the median time of collection, and 0.92 (0.87–0.97) for samples collected after the median time (P=0.157 for difference in AUCROC).
Discussion
In this study of healthy, female, non-anemic whole blood donors, we evaluated the performance of serum hepcidin concentration as a diagnostic test of iron deficiency, and proposed a clinical reference range and cut-offs for one assay.
In this study, hepcidin concentration performed well as a diagnostic test of iron deficiency.26 In our study population, the AUCROC for hepcidin was identical to that of sTfR if iron deficiency was defined as a ferritin concentration less than 15 ng/mL. Other studies in women found an AUCROC of 0.83 for sTfR versus ferritin.27 A study of reticulocyte hemoglobin in women found an AUCROC of 0.89 for identifying non-anemic iron deficiency defined by ferritin concentration,28 and in infants, an AUCROC of 0.85 when iron deficiency was defined by transferrin saturation.29 We found that serum hepcidin, when compared with the sTfR-F index gold standard, had an AUCROC of 0.89, and when compared with ferritin, the AUCROC was 0.87. Hepcidin is, therefore, at least as promising as other recently introduced tests of iron deficiency.
As with all tests that yield values on a continuous scale but aim to provide a binary disease classification, cut-offs must balance sensitivity and specificity.30 The determination of optimal cut-offs is based on the intended clinical use of the test and the costs associated with false positive (inadequate specificity) and false negative (inadequate sensitivity) results.31 In non-anemic patients, costs of a false positive diagnosis of iron deficiency might include unnecessary iron supplementation and even endoscopic investigation; and for blood services, deferral from donation with potential impacts on blood supply and donor retention. Costs of a false negative classification might include subsequent development of anemia in untreated patients and failure to detect underlying nutritional disorders or bleeding (including malignancy).32 In this population, a hepcidin concentration less than 8 ng/mL appears to classify the largest proportion of subjects into their true status, and has satisfactory sensitivity with excellent specificity, while a hepcidin concentration of less than 18 ng/mL achieves the highest combined sensitivity and specificity.
The specificity of hepcidin concentration as an index of iron status reflects its physiology. Iron deficiency and erythropoiesis are each associated with suppressed hepatic hepcidin release,6,33 facilitating increased intestinal iron absorption17 and release from macrophage stores3 through intact membrane ferroportin on the basolateral aspect of enterocytes34,35 and macrophages.35 Thus, reduced hepcidin is an essential part of the physiological response to an iron deficit. Since ferritin (an index of iron stores), sTfR (an index of erythropoietic marrow iron depletion) and hepcidin (a signal that increased iron is needed) each reflect different aspects of iron metabolism, combined evaluation of these indices may provide complementary clinical information. A low hepcidin concentration may predict efficient intestinal absorption of supplemental iron,17 potentially identifying patients who would benefit most from oral iron therapy.
Iron deficiency is an important risk in blood donation. Addressing donor iron deficiency is an important part of the Australian Red Cross Blood Service’s strategy to optimize donors’ health, and integral to maintaining the national blood supply.36 In Australia, currently, only hemoglobin concentration is routinely measured during pre-donation assessment for whole blood collection. A point-of-care (POC) instrument to detect iron deficiency in non-anemic donors could contribute greatly to optimizing donors’ health, by preventing venesection of donors who have depleted iron stores but have not yet developed anemia. Hepcidin is secreted in urine, where it can be detected by immunological and mass spectrometric techniques.4 This raises the possibility of development of a POC device for non-invasive diagnosis of iron deficiency, which could be very useful in the blood donation setting. A POC test could also be of value in developing countries where high burdens of anemia are attributable to iron deficiency and infectious diseases such as malaria,37 and where targeted therapy (iron supplementation or antimicrobials) for the anemia is advisable.38 A potential limitation of this approach is the higher pre-analytical variability associated with urine (as compared with serum) hepcidin measurements.15 Further studies are required to evaluate hepcidin in other donor populations (for example, male and post-menopausal female whole blood donors, and apheresis donors).
A potential limitation of hepcidin concentration as a diagnostic test is its apparent diurnal variation.10,15 However, we did not identify a significant difference in the hepcidin AUCROC of donors tested earlier or later in the morning. The lower hepcidin concentration in donors attending earlier in the morning may reflect lower iron stores, as suggested by the lower ferritin and higher sTfR-F index in this group.
Hepcidin has been explored as an indicator of iron status in more complex clinical scenarios. The hepcidin immunoassay utilized in this study has been shown to detect inflammation, iron deficiency and hereditary hemochromatosis.6 Hepcidin has been proposed as a potential marker of iron bioavailability for erythropoiesis in chronic kidney disease8 and also for identifying coexisting iron deficiency in patients with concomitant anemia of chronic disease,39–41 and has been proposed as an alternative to the sTfR-F index for discriminating between iron deficiency and anemia of chronic disease.42 A comparative study of different assays for hepcidin analysis found that although the absolute value for results at each laboratory differed significantly, results for samples correlated well and analytical variance was generally low.5 Development of reference preparations of hepcidin would enable inter-laboratory comparison of assays and standardization of units and reference ranges, facilitating clinical use of this index.
Our study should be interpreted in the context of its strengths and limitations. We recruited a large sample of female, non-anemic, blood donors; thus, our findings may be generalized to this population and other healthy women of reproductive age. However, further work is required to evaluate the performance of this test among other risk groups, particularly young children, adolescents, pregnant women and the elderly. We excluded anemic donors for two reasons. Firstly, we wanted to evaluate the relationship between hepcidin and iron status without confounding from the altered erythropoietic state due to anemia which may have independently influenced hepcidin levels.43 Secondly, we wanted to evaluate, in the blood donation setting, the performance of hepcidin concentration as a test of iron deficiency among donors who, having passed their initial hemoglobin screen, would be accepted for venesection and thus be at risk of exacerbating iron deficiency, if present. Inclusion of participants with more severe iron deficiency and anemia, and who presumably would have low serum hepcidin concentration, might have increased the AUCROC, but further studies are required to evaluate this. Another potential limitation is the selection of non-invasive, biochemical indices as gold standards (the sTfR-F index and ferritin), however, it would not have been feasible to evaluate bone marrow iron stores in volunteer blood donors as part of this study. The sTfR-F index and ferritin are well-established indices of iron status, and both correlate closely with bone marrow iron stores,22,44 although cut-offs to define iron deficiency are debated. Finally, while we cannot be certain that no subjects with hereditary hemochromatosis or thalassemia trait were included in this study, certainty regarding exclusion of donors with iron-loading conditions would have likely resulted in an improved AUCROC of hepcidin. However, our results reflect the utility of hepcidin in a ‘real life’ setting. In clinical practice, management algorithms should ensure that subjects with low hepcidin who are found to have elevated ferritin receive further investigation for iron-loading conditions.
The discovery of hepcidin, the master regulator of iron metabolism, has led to an exciting decade of advances in the understanding of iron disorders. We have evaluated hepcidin concentration as a test of iron deficiency in a large sample of blood donors at high risk for this condition, and found that it shows considerable promise as a diagnostic test of iron deficiency and appears to perform at least as well as recent additions to the repertoire of available iron indices such as sTfR and reticulocyte hemoglobin. Urine hepcidin measurements may offer a unique opportunity for non-invasive screening for iron status, for example in blood donors and for patients in developing countries in which the population burden of iron deficiency is immense.11 We have defined clinically useful reference ranges and potential cut-offs of hepcidin as an indicator of iron deficiency, for this immunoassay. These results may be beneficial for the interpretation of clinical samples, and for guidance in defining appropriate reference ranges with inter-laboratory collaboration and standardization. In view of our findings, further studies in additional groups of patients and using other assays are now warranted to develop the diagnostic value of hepcidin in the assessment of iron deficiency.
Acknowledgments
The authors thank the Australian Red Cross Blood Service staff at the Southbank, Clarke Street and Bourke Street centers for recruiting subjects, collecting specimens and processing study samples. We are grateful to Associate Professor Damien Jolley, Senior Biostatistician, Monash Institute of Health Services Research, for assistance with the statistical analysis.
Footnotes
Funding: this study was supported by research funding from the Australian Red Cross Blood Service.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Benoist B, McLean E, Egli I, Cogswell M. Worldwide prevalence of anaemia 1993–2005. Geneva: World Health Organization; 2008. [Google Scholar]
- 2.WHO/UNICEF/UNU. Iron Deficiency Anaemia: Assessment, Prevention, and Control A guide for programme managers. Geneva: World Health Organization; 2001. [Google Scholar]
- 3.Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102(3):783–8. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- 4.Kemna EH, Tjalsma H, Willems HL, Swinkels DW. Hepcidin: from discovery to differential diagnosis. Haematologica. 2008;93(1):90–7. doi: 10.3324/haematol.11705. [DOI] [PubMed] [Google Scholar]
- 5.Kroot JJ, Kemna EH, Bansal SS, Busbridge M, Campostrini N, Girelli D, et al. Results of the first international round robin for the quantification of urinary and plasma hepcidin assays: need for standardization. Haematologica. 2009;94(12):1748–52. doi: 10.3324/haematol.2009.010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112(10):4292–7. doi: 10.1182/blood-2008-02-139915. [DOI] [PubMed] [Google Scholar]
- 7.Koliaraki V, Marinou M, Vassilakopoulos TP, Vavourakis E, Tsochatzis E, Pangalis GA, et al. A novel immunological assay for hepcidin quantification in human serum. PLoS One. 2009;4(2):e4581. doi: 10.1371/journal.pone.0004581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swinkels DW, Wetzels JF. Hepcidin: a new tool in the management of anaemia in patients with chronic kidney disease? Nephrol Dial Transplant. 2008;23(8):2450–3. doi: 10.1093/ndt/gfn267. [DOI] [PubMed] [Google Scholar]
- 9.Malyszko J, Malyszko JS, Mysliwiec M. Serum prohepcidin and hepcidin in hemodialyzed patients undergoing iron therapy. Kidney Blood Press Res. 2009;32(4):235–8. doi: 10.1159/000235747. [DOI] [PubMed] [Google Scholar]
- 10.Nemeth E. Targeting the hepcidin-ferro-portin axis in the diagnosis and treatment of anemias. Adv Hematol. 2010;2010:750643. doi: 10.1155/2010/750643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brugnara C. An immunoassay for human serum hepcidin at last: Ganz klar? Blood. 2008;112(10):3922–3. doi: 10.1182/blood-2008-09-176701. [DOI] [PubMed] [Google Scholar]
- 12.Badami KG, Taylor K. Iron status and risk-profiling for deficiency in New Zealand blood donors. NZ Med J. 2008;121(1274):50–60. [PubMed] [Google Scholar]
- 13.Boulton F. Evidence-based criteria for the care and selection of blood donors, with some comments on the relationship to blood supply, and emphasis on the management of donation-induced iron depletion. Transfus Med. 2008;18(1):13–27. doi: 10.1111/j.1365-3148.2007.00818.x. [DOI] [PubMed] [Google Scholar]
- 14.Guidelines for the selection of blood donors. Australian Red Cross Blood Service; 2010. [Google Scholar]
- 15.Kroot JJ, Hendriks JC, Laarakkers CM, Klaver SM, Kemna EH, Tjalsma H, et al. (Pre)analytical imprecision, between-subject variability, and daily variations in serum and urine hepcidin: implications for clinical studies. Anal Biochem. 2009;389(2):124–9. doi: 10.1016/j.ab.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 16.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112(10):4292–7. doi: 10.1182/blood-2008-02-139915. [DOI] [PubMed] [Google Scholar]
- 17.Young MF, Glahn RP, Ariza-Nieto M, Inglis J, Olbina G, Westerman M, et al. Serum hepcidin is significantly associated with iron absorption from food and supplemental sources in healthy young women. Am J Clin Nutr. 2009;89(2):533–8. doi: 10.3945/ajcn.2008.26589. [DOI] [PubMed] [Google Scholar]
- 18.Girelli D, Trombini P, Busti F, Campostrini N, Sandri M, Pelucchi S, et al. A time course of hepcidin response to iron challenge in HFE and TfR2 Haemochromatosis patients. Haematologica. 2010 Dec 22; doi: 10.3324/haematol.2010.033449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irwig L, Glasziou PP, Berry G, Chock C, Mock P, Simpson JM. Efficient study designs to assess the accuracy of screening tests. Am J Epidemiol. 1994;140(8):759–69. doi: 10.1093/oxfordjournals.aje.a117323. [DOI] [PubMed] [Google Scholar]
- 20.Kirkwood BR, Sterne JA. Essential Medical Statistics. 2nd ed. Oxford: Blackwell Science; 2003. [Google Scholar]
- 21.Thomas L, Thomas C, Lehmann P, Roeddiger R, Brugnara C. Iron Deficiency, Erythropoietic Status in Anemia, rHuEPO Therapy: New Diagnostic Approaches –The “Thomas-Plot”. Mannheim: Roche Diagnostics GmbH; 2005. [Google Scholar]
- 22.Suominen P, Punnonen K, Rajamaki A, Irjala K. Serum transferrin receptor and transferrin receptor-ferritin index identify healthy subjects with subclinical iron deficits. Blood. 1998;92(8):2934–9. [PubMed] [Google Scholar]
- 23.Thomas C, Thomas L. Biochemical markers and hematologic indices in the diagnosis of functional iron deficiency. Clin Chem. 2002;48(7):1066–76. [PubMed] [Google Scholar]
- 24.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47(4):458–72. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 25.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45. [PubMed] [Google Scholar]
- 26.Fan J, Upadhye S, Worster A. Understanding receiver operating characteristic (ROC) curves. CJEM. 2006;8(1):19–20. doi: 10.1017/s1481803500013336. [DOI] [PubMed] [Google Scholar]
- 27.Lin XM, Zhang J, Zou ZY, Long Z, Tian W. Evaluation of serum transferrin receptor for iron deficiency in women of child-bearing age. Br J Nutr. 2008;100(5):1104–8. doi: 10.1017/S0007114508966101. [DOI] [PubMed] [Google Scholar]
- 28.Luo D, Chen Y, Wu W, Zhang F, Xu J, Cui W, et al. Reticulocyte hemoglobin content in the diagnosis of iron deficiency in Chinese pre-menopausal women. Chin Med J (Engl) 2007;120(11):1010–2. [PubMed] [Google Scholar]
- 29.Ullrich C, Wu A, Armsby C, Rieber S, Wingerter S, Brugnara C, et al. Screening healthy infants for iron deficiency using reticulocyte hemoglobin content. JAMA. 2005;294(8):924–30. doi: 10.1001/jama.294.8.924. [DOI] [PubMed] [Google Scholar]
- 30.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39(4):561–77. [PubMed] [Google Scholar]
- 31.Remaley AT, Sampson ML, DeLeo JM, Remaley NA, Farsi BD, Zweig MH. Prevalence-value-accuracy plots: a new method for comparing diagnostic tests based on misclassification costs. Clin Chem. 1999;45(7):934–41. [PubMed] [Google Scholar]
- 32.Rockey DC, Cello JP. Evaluation of the gastrointestinal tract in patients with iron-deficiency anemia. N Engl J Med. 1993;329(23):1691–5. doi: 10.1056/NEJM199312023292303. [DOI] [PubMed] [Google Scholar]
- 33.Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108(12):3730–5. doi: 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–3. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 35.Delaby C, Pilard N, Goncalves AS, Beaumont C, Canonne-Hergaux F. Presence of the iron exporter ferroportin at the plasma membrane of macrophages is enhanced by iron loading and down-regulated by hepcidin. Blood. 2005;106(12):3979–84. doi: 10.1182/blood-2005-06-2398. [DOI] [PubMed] [Google Scholar]
- 36.Brittenham GM, Klein HG, Kushner JP, Ajioka RS. Preserving the national blood supply. Hematology Am Soc Hematol Educ Program. 2001:422–32. doi: 10.1182/asheducation-2001.1.422. [DOI] [PubMed] [Google Scholar]
- 37.Calis JC, Phiri KS, Faragher EB, Brabin BJ, Bates I, Cuevas LE, et al. Severe anemia in Malawian children. New Engl J Med. 2008;358(9):888–99. doi: 10.1056/NEJMoa072727. [DOI] [PubMed] [Google Scholar]
- 38.Tielsch JM, Khatry SK, Stoltzfus RJ, Katz J, LeClerq SC, Adhikari R, et al. Effect of routine prophylactic supplementation with iron and folic acid on preschool child mortality in southern Nepal: community-based, cluster-randomised, placebo-controlled trial. Lancet. 2006;367(9505):144–52. doi: 10.1016/S0140-6736(06)67963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malyszko J, Mysliwiec M. Hepcidin in anemia and inflammation in chronic kidney disease. Kidney Blood Press Res. 2007;30(1):15–30. doi: 10.1159/000098522. [DOI] [PubMed] [Google Scholar]
- 40.Cheng PP, Jiao XY, Wang XH, Lin JH, Cai YM. Hepcidin expression in anemia of chronic disease and concomitant iron-deficiency anemia. Clin Exp Med. 2001;11(1):33–42. doi: 10.1007/s10238-010-0102-9. [DOI] [PubMed] [Google Scholar]
- 41.Lasocki S, Baron G, Driss F, Westerman M, Puy H, Boutron I, et al. Diagnostic accuracy of serum hepcidin for iron deficiency in critically ill patients with anemia. Intensive Care Med. 2010;36(6):1044–8. doi: 10.1007/s00134-010-1794-8. [DOI] [PubMed] [Google Scholar]
- 42.Thomas C, Kobold U, Balan S, Roeddiger R, Thomas L. Serum hepcidin-25 may replace the ferritin index in the Thomas plot in assessing iron status in anemic patients. Int J Lab Hematol. 2011;32(2):187–93. doi: 10.1111/j.1751-553X.2010.01265.x. [DOI] [PubMed] [Google Scholar]
- 43.Wrighting DM, Andrews NC. Iron homeostasis and erythropoiesis. Curr Top Dev Biol. 2008;82:141–67. doi: 10.1016/S0070-2153(07)00006-3. [DOI] [PubMed] [Google Scholar]
- 44.Punnonen K, Irjala K, Rajamaki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89(3):1052–7. [PubMed] [Google Scholar]