Abstract
Background
Cases of mantle cell lymphoma with indolent behavior have been reported, but are poorly identified by current clinical risk models. Early studies found peripheral blood involvement to be an adverse prognostic factor; however, cases of a seemingly indolent variant of mantle cell lymphoma, characterized by peripheral blood involvement and minimal nodal disease, have been incompletely described, particularly with regard to bone marrow findings. We report a series of leukemic phase mantle cell lymphomas with a non-progressive or slowly progressive course.
Design and Methods
Cases presenting with mantle cell lymphoma limited to the peripheral blood/bone marrow from 2000–2010 were identified. Diagnoses were established by morphology, flow cytometric analysis and requisite evidence of IGH-CCND1@ by fluorescence in-situ hybridization or t(11;14)(q13;q32) by cytogenetics. Patients with lymphadenopathy, splenomegaly and gastrointestinal symptomatology were excluded.
Results
Patients (n=8, median age 60.5 years) were asymptomatic with mild lymphocytosis (8.7×109/L; range, 4.5–14.2×109/L) and cytology typical of mantle cell lymphoma. Flow cytometric analysis showed that all expressed CD5, CD19, CD20, variable CD23, and a striking kappa immunoglobulin light chain restriction (7/8 cases). Bone marrow biopsy at diagnosis showed interstitial single or small lymphoid aggregates with similar patterns of CD20 and cyclin D1 immunostaining which were not readily discernable by hematoxylin and eosin stain. SOX11 was negative (4/5) or only weakly expressed (1/5). The median follow-up was 27 months (range, 5–109 months) and all patients, but one, are alive with no clinical evidence of disease. The prevalence of indolent mantle cell lymphoma presenting only with lymphocytosis, among all mantle cell lymphomas diagnosed during the same period, was 3%.
Conclusions
Leukemic mantle cell lymphoma limited to blood and bone marrow is an indolent variant characterized by mild-moderate lymphocytosis, interstitial low-level bone marrow involvement, simple karyotype, kappa light chain expression, cyclin D1 expression with lack of SOX11, and slow or absent clinical progression. Some cases may represent a mantle cell lymphoma counterpart to chronic lymphocytic leukemia – phenotype monoclonal B-cell lymphocytosis. Recognition of this variant could inform treatment decisions and possibly avoid unnecessary treatment.
Keywords: mantle cell lymphoma, indolent, monoclonal B-cell lymphocytosis, SOX11, leukemia
Introduction
Mantle cell lymphoma (MCL) accounts for approximately 6% of cases of non-Hodgkin’s lymphoma.1 It characteristically occurs in middle-aged to older adults with the median age at presentation being 60 years; there is a male predominance. Patients usually present with lymphadenopathy and advanced stage disease. Hepatosplenomegaly and involvement of other extranodal sites is common. MCL is generally considered to be an incurable disease, with the median survival of affected patients being 3–5 years.2 The hallmark genetic abnormality is the t(11;14)(q13;q32) leading to over-expression of cyclin D1. This leads to dysregulation of the cell cycle and, combined with chromosomal instability and activation of cell survival mechanisms, contributes to the pathogenesis of MCL.3
Recently, it has been recognized that the clinical behavior of MCL is heterogeneous, and that the disease course is particularly indolent in some patients. Case reports of patients with lymphadenopathy and minimal involvement by MCL document prolonged survival even without therapy.4,5 Indeed, selected asymptomatic patients may be managed expectantly, without risk of worsening their long-term prognosis.7 There is immunological and genomic evidence that two distinct subgroups of mantle cell leukemia exist with different clinical courses (aggressive versus indolent clinical behavior).8,9 Subsequent studies identified MCL patients with non-nodal disease and mutated IGHV genes who had a good prognosis.10 These patients often presented with blood involvement and varying lymphocyte count (ranging from normal to markedly elevated), variable CD5 expression, and splenomegaly. However, such cases remain incompletely characterized, especially with regard to bone marrow findings.
We report a retrospective analysis of patients with indolent MCL presenting with lymphocytosis but lacking lymphadenopathy, splenomegaly, or evidence of gastrointestinal or splenic involvement and, on the basis of our findings, discuss the concept of a MCL-type of monoclonal B-cell lymphocytosis.
Design and Methods
Patients
The Institutional Review Boards of the Cleveland Clinic (Cleveland, OH, USA) and the Cross Cancer Institute (Edmonton, Alberta, Canada) granted approval for this study before its initiation. Cases of incidental leukemic MCL discovered at the Cleveland Clinic and Cross Cancer Institute between January 2000 and December 2010 were retrospectively identified by initially using a search for patients with CD5+ lymphoproliferative disorders characterized as MCL at presentation. The diagnoses were established by peripheral blood morphology review, flow cytometry, and cytogenetic and/or fluorescence in situ hybridization (FISH) analysis confirming a t(11;14)(q13;q32) or IGH@/CCND1. Patients with lymphadenopathy, gastrointestinal involvement or splenic involvement were excluded. Routine endoscopic staging examination was not performed on all eight patients, so we cannot exclude subclinical gastrointestinal involvement in all patients. In order to estimate the prevalence of pure leukemic and non-nodal indolent MCL at Cleveland Clinic, a companion search was performed to identify additional cases of low-stage disease that may have been initially excluded by specifically searching for non-nodal patients with t(11:14) lymphocytosis who may have additionally had gastrointestinal or splenic involvement (thus meeting criteria for non-nodal MCL as described by Orchard et al.).10 The results of this search are described in the body of the article. Bone marrow biopsies performed at the time of diagnosis were reviewed, if available. A chart review was performed to collect the patients’ clinical characteristics, follow-up, and laboratory values.
Immunohistochemistry
For selected cases, formalin-fixed, paraffin-embedded tissues from available diagnostic bone marrow trephine biopsies were characterized at the time of diagnosis by immunohistochemistry using the following antibodies: L26-CD20 (Dako, Carpinteria, CA, USA), 2GV6-CD3 (Ventana Medical Systems, Tucson, AZ, USA), 4C7-CD5 (Biogenex, San Ramon, CA, USA), and SP4-cyclin D1 (Thermo Scientific, Waltham, MA, USA). Stains were performed on an automated stainer using heat induced epitope retrieval (CC1 standard, Ventana Benchmark) and 3,3′-diaminobenzidine detection (DAB, Ventana). Double-labeling immunohistochemistry was performed using an antibody cocktail for L26-CD20 (1:100 dilution) and rabbit polyclonal-SOX11 (Sigma, St. Louis, MO, USA; Lot#B33103 1:50 dilution) using DAB detection for CD20 and fast red for detection of SOX11 (Ventana). SOX11 protein expression was identified as strong nuclear staining in the lymphocytes as originally described.9
Flow cytometry
Flow cytometric immunophenotyping was performed as part of the diagnostic work-up using standard methods on a FACSCalibur (before July 2007) or a FACSCanto instrument (after July 2007; BD Bioscience, San Jose, CA, USA) using antibodies to: (i) CD3, CD4, CD5, CD8, CD10, CD13, CD19, CD20, CD23, CD45, FMC7, CD79b, CD16/56 and kappa/lambda light chains (4-color panel) and (ii) CD2, CD3, CD4, CD5, CD7, CD8, CD10, CD13, CD16/56, CD19, CD20, CD23, CD45, CD79b, CD123, FMC7 and kappa/lambda light chains (6-color panel). CD38 was performed for three patients. All antibodies were directly conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll protein complex (PerCP), PerCP-cyanine-5.5 (PerCP-Cy5.5), PE-cyanine 7 (PE-Cy7), allophycocyanin (APC), or APC-Cy7 (all from BD Biosciences).
Karyotyping
Conventional chromosome analysis was performed using G-banded metaphases obtained from stimulated bone marrow cultures according to standard cytogenetic protocols in three bone marrow biopsies taken at diagnosis and on one biopsy performed several years after therapy had been initiated. The karyotypes are described according to the International System for Human Cytogenetic Nomenclature (ISCN). FISH analysis was performed on either cell suspensions from peripheral blood at the time of diagnosis or on paraffin-embedded tissue blocks using a dual color fusion FISH probe set for 11q13 (Vysis LSI CCND1) and 14q32 (Vysis LSI IGH) intended to detect the reciprocal translocation (Abbott, Abbott Park, IL, USA) using methods previously described.11 The cut-off for positive CCND1/IGH FISH was the presence of fusion signal in 10% of cells, based on laboratory established thresholds.
Results
Clinical features
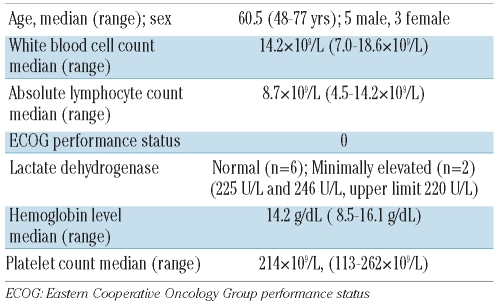
Six patients from Cleveland Clinic had isolated leukemic MCL (with or without bone marrow involvement) without overt tissue involvement (nodal, splenic, or gastrointestinal). Search results from Cross Cancer Institute yielded an additional two cases who met these criteria. Thus a total of eight patients (5 males, 3 females) with a median age of 60.5 years (range, 48–77 years) were discovered to have mild to moderate absolute lymphocytosis with features of mantle cell leukemia and formed the basis of this study. The white blood cell counts were normal to mildly elevated (median, 14.2×109/L; range, 7.0–18.6×109/L). The median lymphocyte count was 8.7×109/L (range, 4.5–14.2×109/L). All patients were asymptomatic at the time of presentation. Splenomegaly and lymphadenopathy were not present on computed tomography scans or detectable by palpation at the time of diagnosis in any of the patients. The ECOG performance status was 0 for all patients and the lactate dehydrogenase concentration was minimally elevated in two patients (225 U/L and 246 U/L; upper limit of normal = 220 U/L). Red cell indices and hemoglobin (Hb) were normal in all patients with the exception of one who had macrocytic anemia, attributable to alcohol abuse and poor nutrition (Hb median, 14.2 g/dL; range, 8.5–16.1 g/dL). Platelet counts were normal in seven of the eight cases at presentation (median, 214×109/L; range, 113–262×109/L). One patient was mildly thrombocytopenic. The basic clinical and laboratory features of the patients are shown in Table 1.
Table 1.
Clinical features of indolent mantle cell leukemia at presentation in patients (n=8) selected without nodal disease.
Pathological features
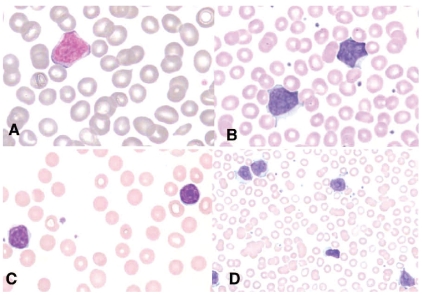
The morphology of circulating lymphoma cells was classified according to the French-American-British criteria.12 The peripheral blood smears demonstrated the presence of atypical small-to-medium-size lymphocytes with high nuclear:cytoplasmic ratios, slight nuclear clefts or irregularities and hyperchromatic condensed chromatin in the majority of cases (classic type). Two cases showed small-cell (chronic lymphocytic leukemia-like) morphology. One patient had prolymphocytic cells (Figure 1D).
Figure 1.
Spectrum of peripheral blood morphologies. (A-B) Classic type with high nuclear:cytoplasmic ratios, slight nuclear clefts or irregularities and hyperchromatic condensed chromatin. (C) Small cell or chronic lymphocytic leukemia-like morphology. (D) One case had prolymphocytic morphology. Wright’s stain, Olympus BX50F4, oil immersion, magnification x 1000 A-C, magnification x 500, D; colors corrected after acquisition with Adobe photoshop.
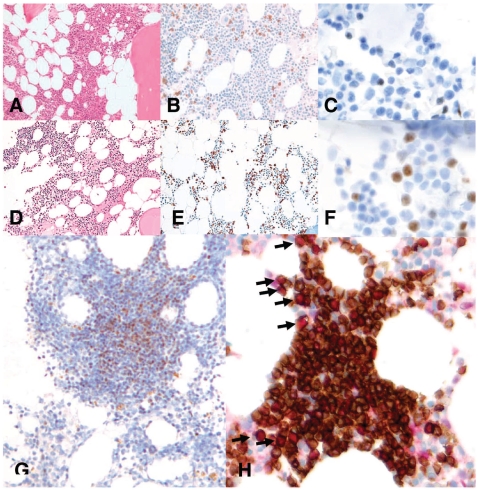
Bone marrow biopsy was performed at diagnosis in six of the eight cases. Routine hematoxylin and eosin-stained sections of the trephine biopsy showed normocellular bone marrow with trilineage hematopoiesis in all cases. Biopsies were also evaluated by immunohistochemistry for CD3, CD20, CD5, and cyclinD1. The five cases with no obvious infiltrates on hematoxylin and eosin-stained sections showed interstitial involvement by scattered lymphocytes, singly or in small clusters, with similar patterns of CD20 and cyclinD1 immunostaining, estimated at 1–5% of bone marrow cellularity (Figure 2). The pattern of involvement in the remaining case was small interstitial aggregates of CD20+/cyclinD1+ lymphocytes which constituted 10% or less of total cellularity (Table 2, Figure 2).
Figure 2.
The predominant bone marrow pattern of involvement for indolent mantle cell leukemia patients. No infiltrates are readily discernable on hematoxylineosin stain (A, D) (Olympus BX50F4, magnification x 200, colors corrected after acquisition with Adobe photoshop), but immunostaining showed interstitial involvement by scattered single lymphocytes with a similar patterns of CD20 (B, E) and cyclinD1 (C, F) immunostaining, estimated at 1% of bone marrow cellularity (magnification x 200–1000, Discovery, Ventana Medical Systems). Patient n. 4 (G) had small interstitial lymphoid aggregates with numerous cyclinD1–positive lymphocytes (Olympus BX50F4, magnification x 200, Discovery, Ventana Medical Systems). Double immunolabeling for CD20/SOX11 (H) shows positive co-expression of SOX11 (red nuclear stain) at a low level in approximately 10% of B cells indicated by CD20+ brown cytoplasmic staining (Olympus BX50F4, magnification x 400).
Table 2.
Pathological features of indolent leukemic variant MCL: Peripheral blood and bone marrow.
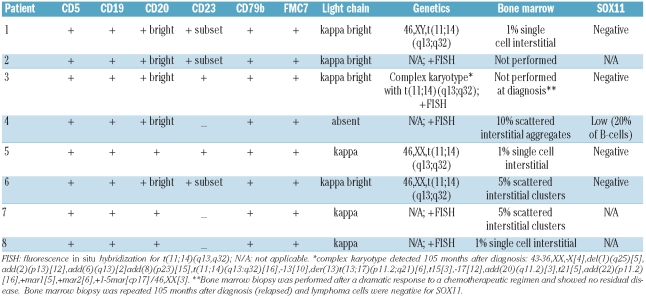
Because of recent data showing decreased SOX11 expression in indolent MCL,13 CD20 and SOX11 double immunolabeling was performed in five of the six cases with isolated peripheral blood or bone marrow involvement and showed that four cases lacked concomitant expression of SOX11. The one case which was positive showed nuclear reactivity in a minority (20%) of CD20+ B cells. For comparison, we investigated the expression of SOX11 by immunohistochemistry in a series of 37 conventional nodal MCL and found strong nuclear expression (in more than 50% of tumor cells) in 91% of cases. Thus, considering levels above 20% as positive, the expression of SOX11 is significantly lower in cases of indolent leukemic MCL than in typical nodal MCL (P<0.00001, Fisher’s exact test) (Table 2). All patients had evidence of IGH@-CCND1 translocation by FISH or conventional cytogenetics, or both. The three patients who underwent conventional karyotyping at diagnosis showed a simple karyotype with isolated t(11;14)(q13;q32). The cytogenetic analysis in patient n.3 was performed 8 years after diagnosis and the patient was found to have a complex karyotype in addition to t(11;14)(q13;q32) (Table 2).
Flow cytometry of the peripheral blood was conducted in all cases. The median absolute abnormal B-cell count at diagnosis was 6.351×109/L (range, 1.072–12.780×109/L). In all cases the B-lymphocytes expressed CD5, CD19, CD20 (bright), CD79b and FMC7. CD22 was expressed in all three patients in whom it was examined. CD38 was negative (<20%) in the three patients for whom data were available. CD23 expression was examined in all eight patients and showed at least a subset of positive cells (>20% of cells) in five cases (63%). Strikingly, seven of eight cases demonstrated kappa monotypic surface immunoglobulin light chain with the remaining case lacking detectable surface light chain expression (Table 2).
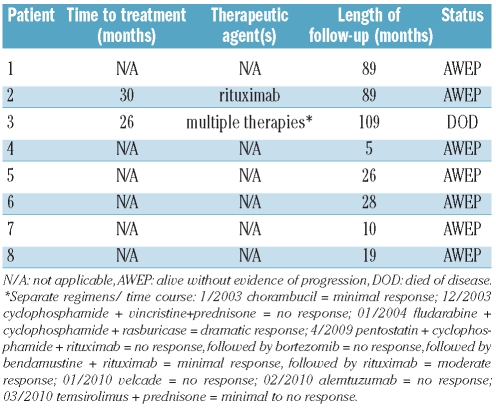
Clinical follow-up
Details of the patients’ clinical follow-up are shown in Table 3. The median follow-up for all patients was 27 months, while that of the seven survivors was 26 months (range, 5–89 months) (Table 3). All survivors are alive with no evidence of overt disease (i.e. currently lacking lymphadenopathy or splenomegaly) and with stable lymphocytosis. In our follow-up, we estimated lymphocyte doubling time as the number of months from the first discovered lymphocytosis to the first documented doubling of the absolute lymphocyte count. Five patients doubled their counts over a median of 18 months (range, 6–24 months). The remaining three patients have been followed for 5, 26 and 89 months without a doubling of their count.
Table 3.
Follow-up data.
Two patients required treatment. Treatment was initiated for patient #3 at 26 months because of rising peripheral lymphocyte counts. Multiple chemotherapeutic regimens, including rituximab, were used over the course of approximately 6 years with the longest observed remission of 5 years. However, the patient ultimately died of disease after 109 months. Single agent rituximab (8 cycles) was initiated for patient n. 2 at 30 months because of rising lymphocyte counts as well as fatigue and night sweats. This patient responded to therapy, was alive and well at 89 months, with minimal absolute lymphocytosis, no symptoms and no adenopathy or splenomegaly. Of note, this patient was characterized as having prolymphocyte-like MCL morphology.
Estimated incidence of indolent leukemic mantle cell lymphoma and comparison with other non-nodal mantle cell lymphomas
During the period in which the six cases of indolent leukemic MCL were identified at Cleveland Clinic, a total of 207 new cases of MCL were retrospectively identified (allowing an estimation of the prevalence of indolent, isolated leukemic MCL of 3%). To understand the distribution of indolent MCL disease at Cleveland Clinic, we found only two patients with non-nodal MCL (as defined by Orchard et al.)10 in this group of 207 cases. Neither patient had lymphocytosis at the time of presentation. One patient presented with leukopenia and splenomegaly and underwent elective splenectomy approximately 18 months after diagnosis. The spleen showed involvement by MCL, which was also present in low levels in the peripheral blood and in small non-paratrabecular aggregates in the bone marrow. This female patient delayed chemotherapy for 24 months but then went on to develop a rapidly progressive, therapy-resistant disease requiring hematopoietic progenitor cell transplantation and ultimately died due to disease at 101 months. SOX11 expression, determined by immunohistochemistry, was strongly positive in the splenectomy specimen. The second patient had gastrointestinal involvement, discovered incidentally by routine colonoscopy with polypectomy. He had low-level bone marrow involvement but lacked peripheral blood, lymph node and/or splenic involvement and has been followed for 46 months without disease progression and on no therapy. This patient showed strong SOX11 expression, by immunohistochemistry, in the polypectomy specimens.
Discussion
MCL is considered a lymphoma with a moderately aggressive behavior and affected individuals have a median survival of only 3–5 years.2 Peripheral blood involvement in MCL is variable but common, depends on initial staging techniques applied, and is reported to occur in 13–92% of patients with newly diagnosed nodal disease.15–18 Early studies found that leukemic involvement was associated with aggressive disease and a poor prognosis;13–19 however, a growing body of research has identified patients with an indolent form of MCL with relatively long survival even without therapy.4–5,7,9–10 Features that may identify these patients include non-nodal disease and a lack of clinical symptoms.6,10 Detailed pathological features and bone marrow findings in these indolent MCL patients are lacking. We report our experience with eight patients with isolated lymphocytosis due to MCL cells and describe their immunophenotype and pattern of bone marrow involvement.
The median age in our series and slight male predominance of these patients with isolated lymphocytosis are similar to those seen in MCL in general.15 All patients except one were alive without clinical evidence of disease progression at 26 months (median follow-up of surviving patients). All examined patients had subtle, low-level bone marrow involvement. Thus, these patients are a cohort with indolent MCL presenting with incidentally discovered systemic low tumor burden disease. In recent years, it has been recognized that despite the generally observed poor prognosis of MCL, a subset of patients may have indolent disease and a long survival. The features of our cohort resemble those of a prior series of cases of non-nodal MCL.10 In that study, the male:female ratio was 2.0 and the mean age of the patients was 65 years. However, in contrast to our patients, the majority of those patients (75%) had splenomegaly and/or gastrointestinal involvement at presentation. Such patients were excluded from our series. A feature noted by the investigators was somatic hypermutation of the IGVH genes, reported in chronic lymphocytic leukemia to portend a favorable prognosis.21 We did not have sufficient samples to perform this analysis.
Another series of indolent non-nodal, leukemic MCL, characterized by hypermutated IGVH genes and a non-complex karyotype, has also been reported.9 Again, splenomegaly or gastrointestinal involvement was common.
In an effort to characterize this specific and unique subset of leukemic MCL, we searched for additional cases at Cleveland Clinic which might have been excluded due to splenomegaly or gastrointestinal involvement. Additional cases were identified by searching for all non-nodal cases as a surrogate for indolent disease as done by Orchard et al.10 Two additional cases (comparison group) were identified using this search parameter. Thus, it appears the predominant “indolent” MCL patient population seen at our center consists of patients with a mild leukemic presentation lacking splenomegaly and gastrointestinal involvement, contrasting with the previously described patients with indolent MCL.9 This may reflect the consolidated nature and integrated (bone marrow, blood and tissue) use of flow cytometry within the hematopathology service at our institutions.
Studies of SOX11 in MCL have suggested a complex role for this transcription factor. SOX11 is aberrantly expressed in several hematologic malignancies including MCL, but the exact molecular mechanism responsible for its up-regulation and/or pathogenic function is unclear. Functional data have identified a subset of SOX11-responsive genes in MCL cell lines22 and have demonstrated a possible tumor suppressor role for SOX11 in MCL.23 Wang and colleagues demonstrated shorter survival in 5/53 patients with SOX11 cytoplasmic protein expression compared to 48/53 with SOX11 nuclear protein expression.24 Consistent with other reports that stated that 78–93% of cases are positive, we found that the great majority (91%) of nodal MCL cases express SOX11; however, in our cohort of indolent leukemic MCL cases only one of five showed low-level positivity.9,11,21,25 This was statistically different from typical nodal MCL. Interestingly, gene expression analysis identified a set of genes that may be useful in identifying indolent MCL cases. In particular, SOX11 may be an important biomarker, as SOX11-negative tumors tended to be non-nodal with better survival.9 Our findings are more in keeping with those of the study by Fernandez et al., who showed a favorable outcome in SOX11-negative cases.9 These data further support SOX11 as a potential prognostic biomarker in MCL assessable by immunohistochemistry. While we cannot fully reconcile our data with a putative tumor suppressor role for SOX11, one may speculate that in experimental systems and cell lines non-physiological high levels of SOX11 expression might act in an anti-proliferative fashion. However, at more physiological levels in mature cells, SOX11 is not a primary driver of a malignant phenotype and a low level or lack of expression as a favorable marker might be an epiphenomenon, albeit a useful one for recognizing the favorable phenotype.
Patterns of bone marrow involvement in stage IV conventional MCL are quite variable but are usually apparent on routine histopathology with hematoxylin and eosin staining. Non-paratrabecular aggregates are seen more commonly than diffuse or paratrabecular infiltrates.15,17,26 Bone marrow involvement in cases categorized as indolent-behavior MCL is reportedly common (76–92%.)7,9 The pattern has not been well characterized but appears to have no prognostic relevance.24 Bone marrow findings have not been described in the type of patients with leukemic, non-nodal disease who are presented here. We show that there is uniformly low tumor burden in the marrow with sparsely distributed interstitial single cyclin D1+ lymphocytes or, uncommonly, subtle interstitial aggregates, representing less than 5% of cellularity. This low tumor burden is in keeping with the generally indolent nature of the disease course.
Lack of CD5 expression has been reported in a small subset of patients with indolent disease, all of whom had associated tissue (nodal) involvement.4,5,10 We found CD5 expression in all cases, accompanied by CD19, CD20 (bright), FMC7 and CD79b. CD38 expression has been shown to predict lymph node involvement in MCL in that it is less often positive in patients who lack lymphadenopathy,10 and was negative in all of our patients with available data (n=3). While flow cytometry data for a prior series have been reported,10 immunoglobulin light chain usage has not been described. As opposed to the typical lambda predominance seen in MCL, we found a striking kappa light chain predominance in our series (7 of 8, with the eighth case being negative for surface immunoglobulin). While this appears to be a near uniform feature in these leukemic, non-nodal, clinically indolent cases, we are uncertain as to the underlying biology that would select for kappa over lambda light chains. Whether this reflects an antigen-driven preference is also uncertain. Further analysis of the IGH@ V gene usage may shed light on this.
From a practical standpoint, clinical prognostic systems such as the International Prognostic Index have repeatedly shown limitations when applied to MCL. For this reason, the Mantle Cell International Prognostic Index (MIPI)27 was developed. Among our cohort, five of the eight patients would be categorized as intermediate- to high-risk, with an expected median survival of 29 to 51 months. However, application of the MIPI to this subtype of disease is probably not appropriate though our follow-up time is relatively short. Whether the lymphocyte doubling time is useful for monitoring patients is also not known. Interestingly, doubling of the lymphocyte count has not occurred in three of eight patients, in the absence of treatment, and this may be a relevant prognostic marker.
Recently, it was demonstrated that some healthy individuals (up to 7%) harbor very low (10−7) levels of monoclonal B cells with an IGH@-CCND1 fusion.28 These cells may remain stable or increase over time but do not progress to overt MCL, despite the presence of genetic features such as IGHV gene usage and fusion breakpoints that are similar or identical to those in MCL. Thus, one might also consider applying the concept of screening and clinical MCL-type monoclonal B-cell lymphocytosis (MBL) to at least some of our cases, as has been proposed for chronic lymphocytic leukemia-type MBL. In fact, patient n. 1 might be considered as having an MCL-type MBL since this patient had a relatively low monoclonal B-cell count (<5.0×109/L) persistent for over 7 years without evidence of progression. Of course current definitions would preclude these cases from being considered as MBL since an IGH@-CCND1 translocation was present. However, it is interesting to consider that, since analysis for IGH@-CCND1 is not routine in the normal course of work-up for MBL (mainly for technical reasons of low level involvement), some cases of previously described MBL might indeed be cases of indolent leukemic MCL.
In summary, we report an indolent leukemic variant of MCL that can be recognized based on the constellation of incidentally discovered lymphocytosis with MCL peripheral blood morphology, lack of lymphadenopathy and hepatosplenomegaly, low level (usually <5%) interstitial bone marrow involvement, kappa light chain restriction, lack of SOX11 expression, and a simple karyotype. The outcome appears quite favorable within the limits of our current follow-up, and awareness of this variant will allow future characterization of such cases and avoid over-treatment of such patients.
Acknowledgments
The authors wish to acknowledge Neeraj Kumar for technical assistance
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89(11):3909–18. [PubMed] [Google Scholar]
- 2.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of the Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008. pp. 229–32. [Google Scholar]
- 3.Jares P, Colomer D, Campo E. Genetic and molecular pathogenesis of mantle cell lymphoma: perspectives for new targeted therapeutics. Nat Rev Cancer. 2007;7(10):750–62. doi: 10.1038/nrc2230. [DOI] [PubMed] [Google Scholar]
- 4.Espinet B, Sole F, Pedro C, Garcia M, Bellosillo B, Salido M, et al. Clonal proliferation of cyclin D1-positive mantle lymphocytes in an asymptomatic patient: an early-stage event in the development or an indolent form of a mantle cell lymphoma? Hum Pathol. 2005;36(11):1232–7. doi: 10.1016/j.humpath.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Nodit L, Bahler DW, Jacobs SA, Locker J, Swerdlow SH. Indolent mantle cell lymphoma with nodal involvement and mutated immunoglobulin heavy chain genes. Hum Pathol. 2003;34(10):1030–4. doi: 10.1053/s0046-8177(03)00410-6. [DOI] [PubMed] [Google Scholar]
- 6.A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329(14):987–94. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 7.Martin P, Chadburn A, Christos P, Weil K, Furman RR, Ruan J, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol. 2009;27(8):1209–13. doi: 10.1200/JCO.2008.19.6121. [DOI] [PubMed] [Google Scholar]
- 8.Vizcarra E, Martínez-Climent JA, Benet I, Marugan I, Terol MJ, Prosper F, et al. Identification of two subgroups of mantle cell leukemia with distinct clinical and biological features. Hematol J. 2001;2(4):234–41. doi: 10.1038/sj.thj.6200111. [DOI] [PubMed] [Google Scholar]
- 9.Fernàndez V, Salamero O, Espinet B, Solé F, Royo C, Navarro A, et al. Genomic and gene expression profiling defines indolent forms of mantle cell lymphoma. Cancer Res. 2010;70(4):1408–18. doi: 10.1158/0008-5472.CAN-09-3419. [DOI] [PubMed] [Google Scholar]
- 10.Orchard J, Garand R, Davis Z, Babbage G, Sahota S, Matutes E, et al. A subset of t(11;14) lymphoma with mantle cell features displays mutated IgVH genes and includes patients with good prognosis, nonnodal disease. Blood. 2003;101(12):4975–81. doi: 10.1182/blood-2002-06-1864. [DOI] [PubMed] [Google Scholar]
- 11.Ek S, Dictor M, Jerkeman M, Jirstom K, Borrebaeck CA. Nuclear expresison of the non-B cell lineage SOX11 transcription factor identifies mantle cell lymphoma. Blood. 2008;111(2):800–5. doi: 10.1182/blood-2007-06-093401. [DOI] [PubMed] [Google Scholar]
- 12.Frater JL, Tsiftsakis EK, Hsi ED, Pettay J, Tubbs RR. Use of novel t(11;14) and t(14;18) dual-fusion fluorescence in situ hybridization probes in the differential diagnosis of lymphomas of small lymphocytes. Diagn Mol Pathol. 2001;10:214–22. doi: 10.1097/00019606-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, Sultan C. Proposals for the classification of chronic (mature) B and T lymphoid leukaemias: French-American-British (FAB) Cooperative Group. J Clin Pathol. 1989;42(6):567–84. doi: 10.1136/jcp.42.6.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Argatoff LH, Connors JM, Klasa RJ, Horsman DE, Gascoyne RD. Mantle cell lymphoma: a clinicopathologic study of 80 cases. Blood. 1997;89(6):2067–78. [PubMed] [Google Scholar]
- 15.Bosch F, Lopez-Guillermo A, Campo E, Ribera JM, Conde E, Piris MA, et al. Mantle cell lymphoma: presenting features, response to therapy, and prognostic factors. Cancer. 1998;82(3):567–75. doi: 10.1002/(sici)1097-0142(19980201)82:3<567::aid-cncr20>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 16.Cohan PL, Kurtin PJ, Donovan KA, Hanson CA. Bone marrow and peripheral blood involvement in mantle cell lymphoma. Br J Haematol. 1998;101(2):302–10. doi: 10.1046/j.1365-2141.1998.00684.x. [DOI] [PubMed] [Google Scholar]
- 17.Ferrer A, Salaverria I, Bosch F, Villamor N, Rozman M, Beà S, et al. Leukemic involvement is a common feature in mantle cell lymphoma. Cancer. 2007;109(12):2473–80. doi: 10.1002/cncr.22715. [DOI] [PubMed] [Google Scholar]
- 18.Oinonen R, Franssila K, Teerenhovi L, Lappalainen K, Elonen E. Mantle cell lymphoma: clinical features, treatment and prognosis of 94 patients. Eur J Cancer. 1998;34(3):329–36. doi: 10.1016/s0959-8049(97)10056-9. [DOI] [PubMed] [Google Scholar]
- 19.Samaha H, Dumontet C, Ketterer N, Moullet I, Thieblemont C, Bouafia F, et al. Mantle cell lymphoma: a retrospective study of 121 cases. Leukemia. 1998;12(8):1281–7. doi: 10.1038/sj.leu.2401121. [DOI] [PubMed] [Google Scholar]
- 20.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94(6):1848–54. [PubMed] [Google Scholar]
- 21.Dictor M, Ek S, Sundberg M, Warenholt J, György C, Sernbo S, et al. Strong lymphoid nuclear expression of SOX11 transcription factor defines lymphoblastic neoplasms, mantle cell lymphoma and Burkitt’s lymphoma. Haematologica. 2009;94(11):1563–8. doi: 10.3324/haematol.2009.008474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Björklund S, Wasik AM, Grandien A, Andersson P, Kimby E, et al. Gene expression profiling and chromoprecipitation identify DBN1, SETMAR, and HIG2 as direct targets of SOX11 in mantle cell lymphoma. PLoS One. 2010;5(11):e14085. doi: 10.1371/journal.pone.0014085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gustavsson E, Sernbo S, Andersson E, Brennan DJ, Dictor M, Jerkeman M, et al. SOX11 expression correlates to promoter methylation and regulates tumor growth in hematopoietic malignancies. Mol Cancer. 2010;9:187. doi: 10.1186/1476-4598-9-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Asplund AC, Porwit A, Flygare J, Smith CI, Christensson B, et al. The subcellular SOX11 distribution pattern identifies subsets of mantle cell lymphoma: correlation to overall survival. Br J Haematol. 2008;143(2):248–52. doi: 10.1111/j.1365-2141.2008.07329.x. [DOI] [PubMed] [Google Scholar]
- 25.Mozos A, Royo C, Hartmann E, De Jong D, Baró C, Valera A, et al. SOX11 expression is highly specific for mantle cell lymphoma and identifies the cyclin D1 negative subtype. Haematologica. 2009;94(11):1555–62. doi: 10.3324/haematol.2009.010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wasman J, Rosenthal NS, Farhi DC. Mantle cell lymphoma: morphologic findings in bone marrow involvement. Am J Clin Pathol. 1996;106(2):196–200. doi: 10.1093/ajcp/106.2.196. [DOI] [PubMed] [Google Scholar]
- 27.Hoster E, Dreyling M, Klapper W, Gisselbrecht C, van Hoof A, Kluin-Nelemans HC, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111(2):558–65. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- 28.Lecluse Y, Lebailly P, Roulland S, Gac A-C, Nadel B, Gauduchon P. t(11;14)-positive clones can persist over a long period of time in the peripheral blood of healthy individuals. Leukemia. 2009;23(6):1190–3. doi: 10.1038/leu.2009.31. [DOI] [PubMed] [Google Scholar]