Abstract
Background
In the 2008 World Health Organization classification, small lymphocytic lymphoma is defined as a neoplasm with the tissue morphology and immunophenotype of chronic lymphocytic leukemia, but with absence of leukemia. Minimal criteria of tissue involvement to separate small lymphocytic lymphoma from monoclonal B-cell lymphocytosis have not been defined.
Design and Methods
We reviewed the clinicopathological features of 36 patients with extramedullary tissue biopsies containing chronic lymphocytic leukemia-type cells and less than 5×109/L peripheral blood monoclonal B cells. Pathological features (extent and patterns of involvement, architectural preservation, presence of proliferation centers) as well as cytogenetic and radiological findings were examined in relation to clinical outcome.
Results
The biopsies were performed to evaluate lymphadenopathy in 20 patients and for other reasons (most frequently staging of a non-hematologic neoplasm) in 16 patients. At latest follow-up (median 23 months), 21 untreated patients had no or stable lymphadenopathy, 3 had regressed lymphadenopathy, and 12 had developed progressive lymphadenopathy and/or received therapy for chronic lymphocytic leukemia/small lymphocytic lymphoma. Features associated with progression/treatment included lymph nodes 1.5 cm or greater on imaging studies (P=0.01) and presence of proliferation centers in the biopsied tissue (P=0.004). Neither the size nor extent of involvement of the excised lymph node correlated with progression/treatment.
Conclusions
Our findings suggest that biopsies containing chronic lymphocytic leukemia-type cells, but lacking proliferation centers and with non-enlarged or only slightly enlarged lymph nodes on imaging, represent a very indolent disease that may best be considered a tissue equivalent of monoclonal B-cell lymphocytosis rather than overt small lymphocytic lymphoma. We propose that such cases be designated as tissue involvement by chronic lymphocytic leukemia/small lymphocytic lymphoma-like cells of uncertain significance.
Keywords: chronic lymphocytic leukemia, small lymphocytic lymphoma, monoclonal B-cell lymphocytosis, MBL
Introduction
The 2008 World Health Organization (WHO) classification definition of chronic lymphocytic leukemia (CLL) requires 5×109/L or more peripheral blood (PB) monoclonal B cells (MBC) with a CLL phenotype.1 The 2008 International Workshop on Chronic Lymphocytic Leukemia (IWCLL) allows the diagnosis to be made also with lower MBC counts if the patient has cytopenias or symptoms attributable to the CLL.2 Patients who in the past would have been diagnosed with CLL but who no longer fulfill these new criteria are now classified as having monoclonal B-cell lymphocytosis (MBL) with a CLL phenotype (CLL-type MBL). CLL-type MBL has been identified in up to 12% of adults with normal blood counts using highly sensitive flow cytometry techniques.3–6 Patients in whom PB MBC are detected because of a medical work-up (so-called clinical MBL) tend to have higher MBC counts than those with MBL detected in population screening studies.7 The biological association between clinical MBL and CLL is suggested by the similar proportion of deletion 13q14 and trisomy 12 detected in clinical CLL-type MBL and in CLL and the finding that the vast majority of patients with CLL have B-cell clones detectable by flow cytometric or molecular genetic analyses up to 77 months before diagnosis.4–9 Compared to patients with CLL, patients with clinical MBL have superior treatment-free survival and longer lymphocyte doubling times.10–12 While blood counts remain stable over time in the vast majority of patients with clinical MBL, progression to CLL occurs in a small proportion of patients at a rate that has been estimated to be approximately 1–2% per year.4,5,13 The most important predictor of outcome in clinical MBL appears to be the B-cell count at diagnosis, with several studies demonstrating that the B-cell count predicts progression to CLL, treatment-free survival, and overall survival as a continuous variable.5,9–11 Currently, a PB MBC count of 5×109/L is the WHO criterion that differentiates MBL from CLL; however, there are differences in the literature regarding the optimal B-cell thresholds that best predict the risk of progression, treatment-free survival and overall survival, with these thresholds ranging from 1.2×109/L to 11 ×109/L.8,10–12
Although a distinction between clinical MBL and CLL has been defined in PB, criteria for the diagnosis based on involvement of bone marrow or extramedullary tissue have not been extensively investigated. The IWCLL report states that lymphocytes typically account for more than 30% of nucleated cells in the bone marrow aspirate in patients with CLL. However, bone marrow examination is not recommended at the time of the diagnosis of CLL, and the guidelines do not specify a level of bone marrow involvement that would discriminate between MBL and CLL.14 According to the 2008 WHO classification, the diagnosis of small lymphocytic lymphoma (SLL) is used for non-leukemic cases with the tissue morphology and immunophenotype of CLL. Whereas the IWCLL requires a PB MBC count of less than 5×109/L for a diagnosis of SLL, the WHO classification does not provide a specific PB MBC count definition of non-leukemic disease.1,2 The histological and immunophenotypic features of SLL are indistinguishable from those of lymph nodes involved by CLL, and in the WHO classification, CLL and SLL are, therefore, considered as a single disease entity (CLL/SLL).1 Neither the WHO nor IWCLL provides guidelines defining the minimal level of tissue involvement that would qualify for a diagnosis of SLL. While palpable lymphadenopathy (LAD) and/or splenomegaly are considered exclusion criteria for MBL,7 these criteria are not part of the WHO or IWCLL definitions of SLL and the diagnosis of SLL can, therefore, be rendered independent of the extent of nodal involvement or presence of LAD.
With the frequent use of flow cytometry and immunohistochemistry to evaluate lymph nodes, it is possible to identify subtle populations of CLL-type cells in tissues. While a PB MBC count threshold is being used to distinguish MBL from CLL, it is uncertain whether there should be a threshold of tissue involvement that would define SLL. Recently, in situ proliferations of follicular lymphoma cells and mantle cell lymphoma cells that are not considered equivalent to overt lymphoma and that have a relatively indolent behavior compared to their bona fide lymphomatous counterparts have been recognized;15–17 no analogous type of CLL/SLL with minimal tissue involvement is currently recognized. We studied the clinicopathological features of 36 extramedullary tissue biopsies containing MBC with a CLL phenotype, but from patients with a PB MBC count of less than 5×109/L, in order to determine whether a subset of these cases might represent a more indolent disorder than SLL (i.e. a tissue equivalent of MBL).
Design and Methods
Case selection and clinical review
The study was approved by the institutional review boards of the University of Pittsburgh and Massachusetts General Hospital (MGH). The records of the pathology departments of the University of Pittsburgh Medical Center (2000–2009) and MGH (2004–2009) were searched for lymph node and other extramedullary tissue biopsy specimens on which a diagnosis of SLL had been made, or that contained MBC with a CLL immunophenotype, and for which the patients had a PB MBC count less than 5×109/L. Thirty-six extramedullary tissue biopsies (34 lymph nodes and 2 extranodal sites) were identified. All available clinical data were reviewed. When sufficient clinical data were available, patients were staged using the Rai and Binet staging systems and the International Prognostic Index (IPI).18–20 An additional cohort of ten consecutive patients from MGH diagnosed with clinical MBL in 2009 by PB flow cytometry and who underwent radiological staging studies was evaluated for comparison.
Morphological and immunophenotypic review
All routine hematoxylin and eosin (H&E)-stained sections, existing immunohistochemical stains, and flow cytometric immunophenotypic studies performed on the extramedullary tissue biopsies were reviewed. The following pathological features were examined in all lymph node specimens: lymph node size (greatest diameter), extent and patterns of lymph node involvement by cells with a CLL phenotype, preservation of normal lymph node architectural features (sinuses, mantles, and germinal centers), presence or absence of proliferation centers, number of germinal centers, the presence of any additional pathological processes, and percentage of CLL-type cells in the tissue as evaluated by flow cytometry. The aforementioned features were scored together by three observers (SEG, SHS, RPH) and separately by a fourth observer (NLH); any discrepancies were resolved by discussion and a consensus score determined for each parameter. All observers were blinded to the clinical outcome of the cases. In addition, one observer (NLH) blinded to clinical outcome and immunohistochemical/flow cytometry studies evaluated whether an abnormal infiltrate could be detected on the H&E-stained sections. The morphological and immunophenotypic features of concurrent and/or subsequent PB and bone marrow specimens were also reviewed when available.
Classical cytogenetic and fluorescence in situ hybridization studies
All classical cytogenetic studies and fluorescence in situ hybridization (FISH) studies performed for routine clinical purposes on PB, bone marrow, and/or extramedullary tissue biopsies were reviewed. Additional FISH studies were performed on formalin-fixed, paraffin-embedded 5 μm whole tissue sections from 13 extramedullary tissue biopsies without prior cytogenetic studies using the following probes: Vysis LSI p53/LSI ATM multi-color, LDI D13S319/CEP12 multicolor, LSI IGH dual color break apart, and LSI MYB (6q23) SpectrumAqua (Abbott Molecular, Des Plaines, IL, USA). The signal patterns from a minimum of 200 cells were scored. Cut-offs to determine positive samples were established for each probe based on individual laboratory experience with clinical specimens.
Follow-up
Progression was defined as new or increased LAD detected by radiological studies or physical examination after the time of diagnosis. Dates and types of treatments and clinical responses were recorded. Patients who progressed or were treated for CLL/SLL were compared to patients with at least 1 year of follow-up who showed no progression and were not treated. Overall and disease-specific survivals were determined for all patients.
Statistical analysis
Statistical analyses, including two-tailed t-tests, two-tailed Fisher’s exact tests, Mann-Whitney tests, and survival curves (log rank test) were calculated using the Prism software package, version 5 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Clinical features
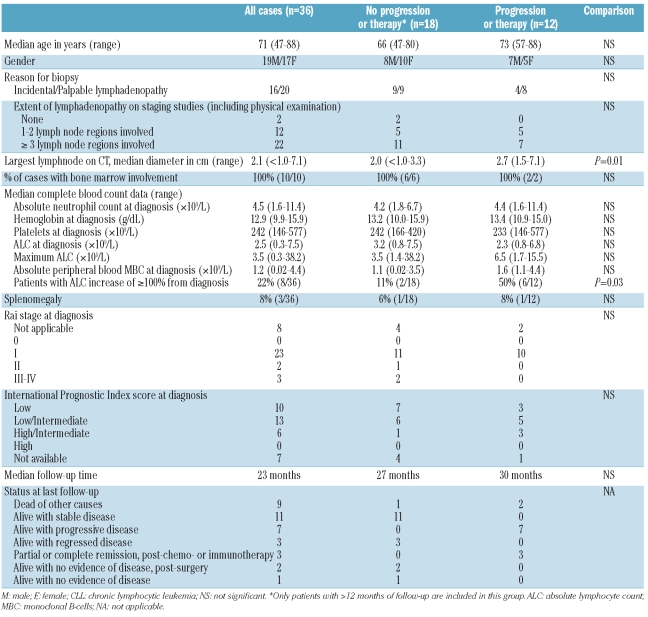
The 36 patients included 19 men and 17 women with a median age of 71 years (range, 47–88 years) (Table 1). In 16 patients, the tissue was biopsied for reasons other than to evaluate palpable LAD. These tissues included nine lymph nodes removed during staging for carcinoma or melanoma, four removed during surgery for benign diseases, and one removed when LAD was detected on radiological studies performed to evaluate another process, as well as two extranodal tissue biopsies (one nasal biopsy in a patient with nasal obstruction without a mass lesion and one breast biopsy performed to evaluate breast calcifications without a mass lesion). Palpable LAD was subsequently discovered on physical examination after the tissue diagnosis in eight of these 16 patients. Twenty patients underwent lymph node biopsies because of palpable LAD or splenomegaly. Palpable LAD was localized in 14 cases and more extensive in six cases. On radiological staging studies [chest and abdominal computed tomography (CT) scans], LAD (>1 cm in smallest dimension) was detected in 32/36 (90%) patients and involved three or more lymph node regions in 22/36 patients (61%). In comparison, only 2/10 (20%) patients in the cohort with clinical MBL diagnosed on PB flow cytometry had any LAD detected on chest and abdominal CT scans (P<0.0001 compared with the patients in the current study), and none of these MBL patients had LAD involving more than two lymph node regions (P<0.0001 compared with the patients in the current study). Combining the findings of physical examination and radiological studies, 34/36 patients (94%) had detectable LAD. Three patients had splenomegaly, while hepatomegaly was not identified in any patients.
Table 1.
Clinical and laboratory features of tissue infiltrates of CLL/SLL cells.
At diagnosis, the patients had a median absolute lymphocyte count (ALC) of 2.5×109/L (range, 0.3–7.5×109/L). Flow cytometric immunophenotypic studies performed on PB specimens in 18 patients demonstrated CLL-type MBC in all patients, with a median absolute MBC count of 1.2×109/L (range, 0.02–4.4×109/L). All three cases with an ALC count greater than 5×109/L underwent PB flow cytometry and had absolute MBC counts of 3.0, 3.5, and 4.4×109/L, while all 18 cases in which flow cytometry was not performed had ALC of less than 5×109/L. Bone marrow involvement was identified in all ten patients who underwent a bone marrow biopsy. The extent of bone marrow involvement varied from less than 5% to over 50% (median, 30%) of the biopsy cellularity. Twenty-eight of 36 (78%) patients had palpable LAD or splenomegaly and would, therefore, be considered to have SLL by IWCLL criteria. Among the remaining eight (22%) patients, all four examined by PB flow cytometry would have been classified as having clinical MBL according to the IWCLL (because LAD or splenomegaly was absent or only detectable on imaging studies), while flow cytometry was not performed in the other four patients. Among the 28 patients with palpable LAD, 23 had Rai stage I disease, two had stage II, and three had stage III (although 5 patients were anemic with hemoglobin <11 g/dL, in 2 cases the anemia was attributable to causes other than CLL/SLL). Twenty-five patients were in Binet stage A and three in Binet stage B. Rai and Binet staging was not applicable in the eight patients lacking palpable LAD.
Gross and microscopic features of the extramedullary tissue biopsies
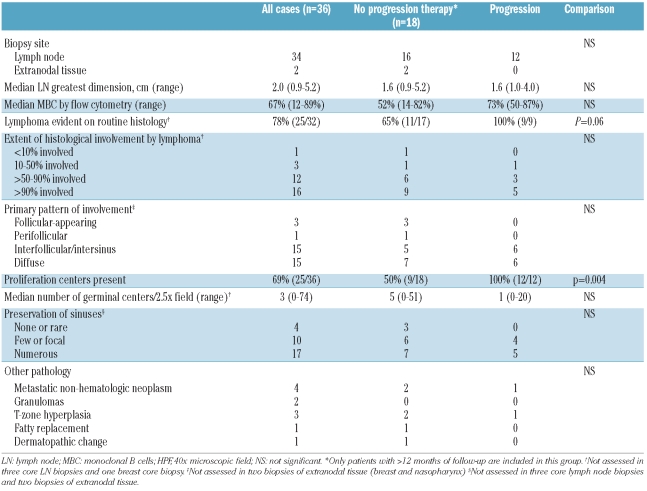
The specimens included 31 excisional lymph node biopsies, three lymph node core biopsies, one nasopharyngeal tissue biopsy, and one breast core biopsy (Table 2). Excisional lymph node biopsies performed in 31 patients had a median greatest dimension of 2.0 cm (range, 0.9–5.2 cm). In four cases, the lymph node appeared enlarged as a result of another pathology, including metastatic tumor, paracortical (T-zone) hyperplasia, granulomas, and/or fatty replacement. Lymphoma was evident on routine H&E-stained sections in 25/32 (78%) evaluable cases (excluding 3 core biopsies of lymph node and 1 core biopsy of breast, in which tissue architecture could not be reliably ascertained). These cases exhibited morphological features typical of CLL/SLL, manifesting as a diffuse infiltrate of small lymphoid cells with round nuclear contours and clumped chromatin (Online Supplementary Figure S1). In these 25 cases, as well as in another three cases after using immunohistochemistry to highlight CD20+/CD5+ cells, the neoplastic B cells were found to involve more than 50% of the lymph node area. Most cases had either a predominantly diffuse pattern, in which sinuses were obliterated, or an interfollicular and intersinus pattern, in which follicles and/or sinuses were preserved (Online Supplementary Figure S1). Three cases had a predominantly follicular pattern of growth, in which the neoplastic B cells infiltrated existing germinal centers, while in one case the neoplastic cells showed a predominantly perifollicular growth pattern (Figure 1). Seven cases with mainly diffuse or interfollicular/intersinus growth patterns had follicular (2 cases) or perifollicular (5 cases) patterns as prominent secondary growth patterns. Proliferation centers were identified in 25/36 (69%) cases; when present, the proliferation centers were usually scattered, but were perifollicular in distribution in the case with a predominantly perifollicular growth pattern. Interobserver reproducibility was high for assessing extent of nodal involvement (94% agreement) and presence or absence of proliferation centers (92% agreement), but was lower for assessing the primary pattern of nodal involvement (79% agreement).
Table 2.
Gross and microscopic features of tissue infiltrates of CLL/SLL cells.
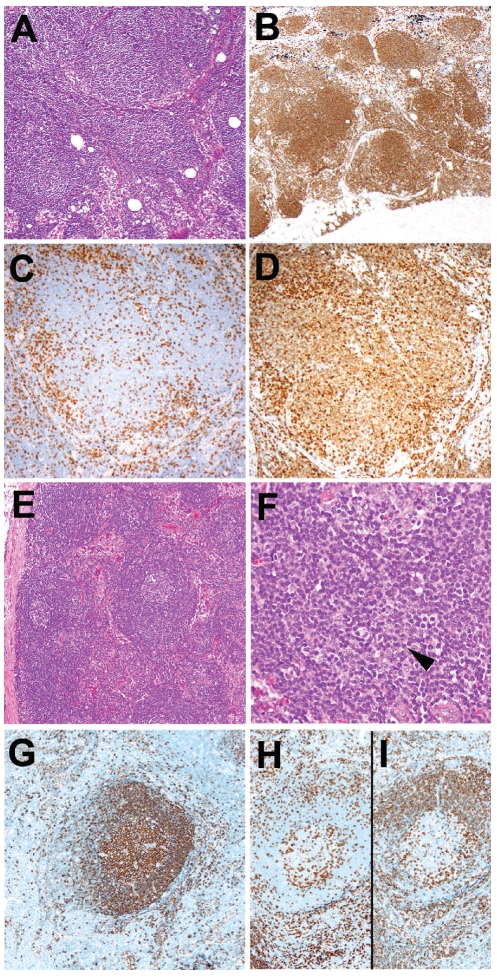
Figure 1.
Examples of lymph nodes with subtle follicular and perifollicular involvement by CLL/SLL cells in patients with <5x109/L peripheral blood monoclonal B-cells. (A) Enlarged lymph node taken at the time of lung cancer staging, with preserved architecture and numerous follicular structures resembling primary follicles (follicular pattern)(H&E, original magnification x100), highlighted by an immunohistochemical stain for CD20 (B), original magnification x40). CD3 (C) and CD5 (D) reveal that the CD20+ CLL/SLL cells in the follicles weakly express CD5 (original magnification x 200). (E) This non-enlarged inguinal lymph node shows preserved architecture (H&E, original magnification x40). (F) Focal perifollicular proliferation centers are noted (arrowhead; H&E, original magnification x400). Immunohistochemical stains for CD20 (G), CD3 (H), and CD5 (I) highlight the subtle perifollicular distribution of neoplastic CD20+, CD5+ CLL/SLL cells, surrounding the non-neoplastic CD20+, CD5− germinal center (original magnification x100).
Many normal architectural features were often present in the lymph node biopsies. Residual follicles with germinal centers were identified in 23/32 (72%) evaluable lymph node biopsies, and in 20 cases at least three residual germinal centers were noted. Mantle zones were thinned (<3 cells wide) in five cases and absent in 11 of the 20 cases with sufficient germinal centers for evaluation; the remaining 4 cases had unremarkable-appearing mantle zones. In the 11 cases with absent mantle zones, the neoplastic B cells surrounded the normal residual germinal centers. Lymph node sinuses were intact in 16/30 (53%) evaluable cases, while sinuses were entirely absent or rare in only 4/30 cases (13%).
Immunophenotypic and cytogenetic features
All 36 cases showed expression of CD20 (which was dim to intermediate in intensity in the 15 cases studied with flow cytometry) and were CD5-positive. Thirty of 32 cases (94%) were positive for CD23, while all evaluated cases were negative for FMC7 (0/10 cases) and cyclin D1 (0/33 cases). There was dim surface immunoglobulin (sIg) light chain expression in all 15 cases evaluated by flow cytometry. CD38 was expressed in 10/22 cases (45%), and ZAP-70 was positive in 4/15 cases (27%). Cytogenetic abnormalities were assessed by conventional cytogenetics and/or FISH in 27 cases. The most common abnormality identified was deletion 13q14.3 (9/26 cases, isolated in 8 cases and together with trisomy 12 in 1 case), followed by trisomy 12 (5/24 cases), deletion 11q22.3 (2/26 cases), and deletion 17p13.1 (2/27 cases). No cases with deletion 6q23 were identified (0/27 cases).
Follow-up
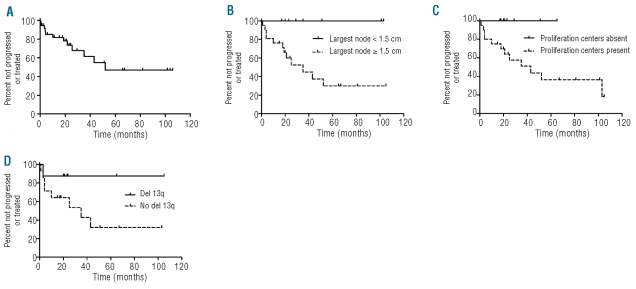
Five patients were treated for CLL/SLL: two were treated within 1 month of diagnosis due to symptomatic disease (progressive myopathy attributed to CLL cell infiltration in 1 patient and bulky LAD in 1 patient) and three were treated for disease progression at 5, 19, and 19 months following diagnosis. Treatment included rituximab monotherapy, rituximab with bendamustine, cyclophosphamide or fludarabine, or a combination of cyclophosphamide and prednisone. Two patients achieved a complete response, one a partial response, one had persistently progressive LAD, and one died of other causes within 1 month of therapy. After a median follow-up of 26 months, another seven patients developed progressive disease (new or increasing LAD on physical examination and/or radiological studies) but were not treated during the follow-up period. The median time to lymph node progression in these seven patients and the three patients treated for progression was 22 months (range, 4–53 months) (Figure 2A). One untreated patient with progression died of other causes 13 months after diagnosis. At last follow-up, 24 patients had no evidence of progressive LAD and remained untreated. These included 17 patients who were alive [median follow-up 27 months, including 3 patients without any detectable LAD (2 with post-surgical excision of a single enlarged lymph node), 3 patients with regression of LAD without therapy, and 11 patients with stable LAD] and seven patients who died of other causes 0.5–21 months after diagnosis. No patients died due to CLL/SLL. Among the 24 patients with no progression or treatment, 18 had a follow-up of at least 1 year and constituted the no progression/treatment group used for comparison with the group of patients who progressed or required therapy.
Figure 2.
Outcome of patients with CLL/SLL tissue infiltrates and <5x109/L peripheral blood monoclonal B cells. (A) The median follow-up for all 36 patients was 23 months (range, 0.5–106 months) and median time to progression of lymphadenopathy or treatment was 52 months. (B) Patients with at least one lymph node ≥1.5 cm in diameter identified on CT staging studies were more likely to progress or be treated (median time 35 months) than patients lacking lymphadenopathy or with smaller lymph nodes on staging studies (median time not reached, P=0.02). (C) Patients with proliferation centers in the biopsied involved tissue were more likely to progress or be treated (median time 43 months) than patients lacking identifiable proliferation centers (median time not reached, P=0.03). (D) Patients lacking a del(13q) cytogenetic abnormality had a median time to progression or treatment of 35 months as compared to patients with a del(13q) cytogenetic abnormality (median not reached), but this was not reach statistically significant (P=0.13) (Figures 2B-D exclude patients with <1 year of progression/therapy-free follow-up).
Over the follow-up period, the PB ALC remained less than 5.0×109/L in 25/36 patients (69%), all of whom had complete blood count results available at the latest follow-up. In eight of the remaining 11 patients, the ALC at least doubled at a median of 27 months after diagnosis (range, 3–104 months). Although flow cytometric immunophenotypic studies were not performed to determine whether the absolute MBL count qualified for a diagnosis of CLL, the ALC in these eight patients ranged from 8.6–38.2×109/L and represented 228–1091% of the original ALC at diagnosis. These eight patients comprised two who did not develop progressive LAD and six who had been treated and/or developed progressive LAD.
Correlation of pathological and clinical features
The following parameters showed no association with progression/treatment: age; gender; Rai or Binet stage; number of involved lymph node sites; palpable LAD on physical examination; splenomegaly; or IPI score. The three patients whose LAD regressed spontaneously all had more than one site of LAD at diagnosis. There was no association between any hematologic parameter, including ALC and absolute PB MBC count at diagnosis (Table 1), and progression/treatment. In 4/12 patients (33%) who progressed or required therapy, the CLL infiltrate was discovered incidentally in a biopsy performed for reasons other than LAD, and palpable LAD as a reason for the biopsy was not associated with progression/treatment. There were no statistically significant differences in the size of the biopsied lymph node between patients with and without progression/treatment, nor were maximal lymph node diameters of greater than 1 cm, 1.5 cm, or 3 cm associated with progression or treatment. However, detection of any lymph node of 1.5 cm or more in diameter on radiological staging studies was associated with progression/treatment (P=0.02, log rank test for time to progression/treatment) and the largest lymph node size measured on CT at diagnosis was greater in patients who subsequently experienced progression or were treated (P=0.01, Mann-Whitney test) (Figure 2B). Of note, the size of the largest lymph node detected by CT on staging studies was not correlated with the diameter of the biopsied lymph node.
The degree of histological or flow cytometric involvement of the biopsied tissue by CLL/SLL was not associated with progression/treatment. An abnormal infiltrate characteristic of CLL/SLL was identified on routine histology in all nine patients with evaluable biopsy tissue who progressed or required therapy, versus in 11/17 (65%) patients with evaluable biopsy material and a follow-up of at least 12 months who did not progress or require therapy (P=0.06, Fisher’s exact test). The only histological parameter that was associated with progression/treatment was the presence of proliferation centers, which were found in all 12 patients who progressed or required therapy versus in 9/18 (50%) of patients who did not progress (P=0.004, Fisher’s exact test and P=0.03, log rank test for time to progression/therapy) (Figure 2C). There was no significant difference in CD38 or ZAP-70 expression between patients who did or did not progress or require therapy. Deletion 13q14.3 was identified in only 1/8 (13%) patients who progressed or required therapy versus in 7/13 (54%) of patients with adequate follow-up who did not progress (P=0.08, Fisher’s exact test and P=0.13 log rank test for time to progression/treatment) (Figure 2D). No other cytogenetic/FISH abnormalities correlated with progression/therapy.
Discussion
The concept of MBL is relatively new, having evolved from the recognition that small MBC populations with a CLL-type phenotype can be identified in healthy adults with normal PB counts.3–6 Although a recent study suggested that CLL is almost always preceded by a period of MBL, it has been estimated that the rate of progression of clinical CLL-type MBL to CLL is only 1–2% per year.4,5,8,13 Several studies have demonstrated that the B-cell count predicts progression to CLL/SLL, treatment-free survival, and overall survival as a continuous variable.5,9–11 While the likelihood of treatment in clinical MBL is lower than in Rai stage 0 CLL, it remains uncertain whether there is a clinically significant difference in survival between patients with clinical MBL and those with Rai stage 0 CLL with a relatively low MBC count.9
Criteria for the diagnosis of MBL are based solely on investigation of MBC in PB specimens, with few existing studies of bone marrow involvement10,21 and no studies to date on the appearance of extramedullary tissues. Although the IWCLL report states that lymphoid cells usually account for over 30% of the nucleated cells in the bone marrow aspirate in patients with CLL, this cut-off has not been validated in subsequent studies.2 Indeed, one recent study found a subset of patients with clinical MBL who had more extensive bone marrow involvement in spite of clinically stable disease, thus implying that, unlike the PB MBC count, the degree of bone marrow involvement may not affect the prognosis of clinical MBL.21 According to the 2008 WHO criteria, any extramedullary tissue involvement by CLL-type MBC not fulfilling the PB criteria for CLL is defined as SLL.1 However, the IWCLL definition of SLL requires palpable LAD and/or splenomegaly.2 The current recommendations are that patients diagnosed with MBL and lacking clinically evident LAD do not require radiological staging, implying that non-palpable LAD would not exclude a diagnosis of MBL, or that it would only rarely be found.7 In this study, we investigated the clinicopathological features of 36 extramedullary tissue biopsies fulfilling the current WHO criteria for SLL, but with less than 5×109/L PB MBC, to better characterize SLL in the MBL era and to determine whether or not there may be a tissue equivalent of MBL that should not be diagnosed as overt SLL. Although 16 of our cases were found incidentally, the clinicopathological features and outcome of these cases did not differ significantly from those of the cases biopsied for LAD. In a small cohort of ten patients with clinical MBL who underwent CT staging at diagnosis, we found that two had radiologically detectable LAD. The influence of radiologically detectable LAD on progression of patients with clinical MBL is unknown; however, one recent study found that CT-detected abdominal LAD in patients with Rai Stage 0 CLL predicted progression and earlier treatment requirement.22 Even though up to 15% of patients with clinical MBL are reported to develop LAD or eventually progress to SLL, the proportion of patients who develop LAD with stable PB MBC counts is unknown.5,9 A subset of the patients in the study by Rawstron et al. had LAD during follow-up, but this feature was reported only in patients with progressive lymphocytosis.5 In contrast, Rossi et al. identified 19 patients (15%) with clinical MBL that progressed to SLL after a median of 42.7 months.10 Although the outcome of this group of patients was not specified, the authors did speculate that some of the cases could have been SLL with very low disease burden at diagnosis.10
The disease burden in MBL is presumably reflected by the PB MBC count, which has been shown to correlate directly with progression to CLL/SLL and/or requiring therapy in multiple studies.5,9–11 Disease burden is more difficult to assess in lymph node-based disease. In our series of patients with tissue-based disease, the ALC was not associated with progressive LAD or treatment. However, the presence of any enlarged lymph node at least 1.5 cm in diameter on CT staging at diagnosis correlated with progression/treatment. In contrast, the size of the biopsied lymph node correlated with either progression/treatment or the size of the largest lymph node detected on radiological staging, suggesting that a single lymph node sampled for biopsy may not be representative of overall lymph node enlargement. There was also no correlation between palpable LAD and progression/treatment, a finding that challenges the notion that SLL should be defined by palpable LAD and suggests that CT staging of patients with CLL/SLL cells identified in tissue biopsies may help in identifying patients more likely to progress.
The extent of lymph node involvement by CLL/SLL cells did not correlate with progression/treatment and nearly half of the cases in this series demonstrated a predominant interfollicular/intersinus growth pattern. An interfollicular growth pattern, with preservation of reactive follicles and open sinuses, has been described in 8–17% of CLL/SLL involving lymph nodes.23,24 Similar to the cases in our study, the majority of interfollicular CLL/SLL cases have been reported to have low PB ALC at diagnosis.25–27 However, this interfollicular/intersinus pattern is likely not helpful in predicting clinical behavior, as it has been described in both leukemic (CLL) and non-leukemic (SLL) cases and in cases with both focal and extensive LAD25–28 and was not correlated with progression in our series. In contrast, although perifollicular proliferation centers can occur in interfollicular CLL/SLL,25 perifollicular and follicular growth patterns are unusual in CLL/SLL and are more often seen in marginal zone and mantle cell lymphomas. These latter possibilities were excluded in our cases by the characteristic CLL phenotype (CD20dim, sIgdim, CD5+, CD23+) and lack of cyclin D1 expression. Perifollicular and follicular growth patterns were relatively common in this series, manifesting as the predominant or secondary infiltration patterns in 33% of assessable cases. These findings suggest that perifollicular and follicular infiltration patterns may identify a unique subset of CLL/SLL cases that often lack a significant leukemic involvement (<5×109/L circulating MBC). The only pathological feature that correlated with progression or treatment in this series was the presence of proliferation centers. The clinical significance of this latter finding is uncertain, as the majority of previous studies did not show a correlation between the extent of proliferation centers and clinical behavior in CLL/SLL.23,29–31 However, one recent study did suggest that expanded and confluent proliferation centers predict a worse outcome in patients with CLL/SLL.32
While the proportion of cases with deletion 13q14.3 was relatively lower in our study than in other reports on CLL/SLL and clinical MBL, the proportions of trisomy 12 and deletions of 11q22.3, 17p13.1 and 6q23 were similar.5,11,33–34 Cytogenetic abnormalities are important predictors of disease progression and survival in CLL and have been shown to predict treatment-free survival in clinical MBL.11,34 There was a trend to more frequent identification of deletion 13q14.3 in patients who did not progress or require therapy in our series, but 1/8 patients with deletion 13q14.3 did progress and this finding did not reach statistical significance. Among the four cases with the poor prognostic markers deletion 17p13.1 or deletion 11q22.3, only two developed progressive LAD or required therapy. The clinical variability of these high-risk cytogenetic abnormalities was also recently suggested in a study of 99 CLL patients with deletion 17p13.1, in which approximately half of the patients had relatively stable disease for up to 70 months of follow-up.35
An unusual finding in our study was the observation that LAD regressed in three patients without treatment. Spontaneous clinical regression of CLL has been previously described in the literature, although a recent study suggested that a residual MBC population remains detectable by flow cytometry or molecular analysis in such patients.36,37 While the three patients in our study with regression had no evidence of an absolute lymphocytosis, LAD, or splenomegaly at last follow-up, PB specimens were not available to evaluate for the presence of a persistent clonal B-cell population by flow cytometry. Continued follow-up is necessary to determine whether these patients will remain in clinical remission.
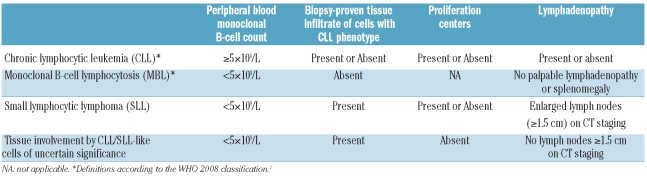
In summary, we identified 36 extramedullary tissue biopsies fulfilling the WHO criteria for SLL, nearly half of which were diagnosed in lymph nodes removed for incidental reasons other than evaluation of unexplained palpable LAD. Most patients had disseminated disease and low-level PB involvement was present in all patients tested. The majority of cases, including those diagnosed incidentally, were associated with variably enlarged lymph nodes that were usually extensively involved by lymphoma, resembling overt SLL. In a significant subset of cases, however, the abnormal lymphoid population was more subtle and/or focal, and could not be recognized without the use of immunohistochemistry and/or flow cytometry. Most patients had stable LAD and did not develop lymphocytosis over the follow-up period and a minority of patients either never manifested LAD or showed spontaneous regression of lymph nodes that were enlarged at initial diagnosis. Although the incidence of progression or treatment appears to be greater in the cases reported here than in many cases of clinical MBL as currently defined, only five of the 36 patients in our series required treatment. Our data suggest that some CLL/SLL-like tissue infiltrates may represent an indolent or even potentially regressing disease and may be better considered as a tissue equivalent to clinical MBL rather than full-fledged lymphoma (SLL). An absence of lymph nodes of at least 1.5 cm on radiological staging studies and an absence of proliferation centers were correlated with freedom from progression or treatment in our series. Cases with these features may be more appropriately diagnosed as “involvement by CLL/SLL-like cells of uncertain significance” rather than SLL, analogous to terminology proposed for the “in situ” versions of follicular lymphoma and mantle cell lymphoma and as suggested at the recent European Association of Hematopathologists/Society for Hematopathology 2010 Workshop (Table 3).38 A 13q14.3 deletion was more common in patients who did not progress, but this parameter did not attain statistical significance. Achieving an appropriate definition for “involvement by CLL/SLL-like cells of uncertain significance” will require larger validation studies with longer follow-up. The results of our study suggest that cases lacking recognizable proliferation centers in patients who do not have LAD of at least 1.5 cm on CT scans are the most likely candidates for such a diagnosis.
Table 3.
Proposed classification scheme for CLL/SLL cells in blood and tissues.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Muller-Hermelink HK, Montserrat E, Catovsky D, Campo E, Harris NL, Stein H. Chronic lymphocytic leukaemia/small lymphocytic lymphoma. In: Swerdlow SH, Campo E, Harris NL, Jaffe E, Pileri S, Stein H, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: World Health Organization Classification of Tumours; 2008. pp. 180–2. [Google Scholar]
- 2.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–56. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghia P, Prato G, Scielzo C, Stella S, Geuna M, Guida G, et al. Monoclonal CD5+ and CD5-B-lymphocyte expansions are frequent in the peripheral blood of the elderly. Blood. 2004;103(6):2337–42. doi: 10.1182/blood-2003-09-3277. [DOI] [PubMed] [Google Scholar]
- 4.Rawstron AC, Bennett F, Hillmen P. The biological and clinical relationship between CD5+23+ monoclonal B-cell lymphocytosis and chronic lymphocytic leukaemia. Br J Haematol. 2007;139(5):724–9. doi: 10.1111/j.1365-2141.2007.06863.x. [DOI] [PubMed] [Google Scholar]
- 5.Rawstron AC, Bennett FL, O’Connor SJ, Kwok M, Fenton JA, Plummer M, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med. 2008;359(6):575–83. doi: 10.1056/NEJMoa075290. [DOI] [PubMed] [Google Scholar]
- 6.Nieto WG, Almeida J, Romero A, Teodosio C, Lopez A, Henriques AF, et al. Increased frequency (12%) of circulating chronic lymphocytic leukemia-like B-cell clones in healthy subjects using a highly sensitive multicolor flow cytometry approach. Blood. 2009;114(1):33–7. doi: 10.1182/blood-2009-01-197368. [DOI] [PubMed] [Google Scholar]
- 7.Shanafelt TD, Ghia P, Lanasa MC, Landgren O, Rawstron AC. Monoclonal B-cell lymphocytosis (MBL): biology, natural history and clinical management. Leukemia. 2010;24(3):512–20. doi: 10.1038/leu.2009.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landgren O, Albitar M, Ma W, Abbasi F, Hayes RB, Ghia P, et al. B-cell clones as early markers for chronic lymphocytic leukemia. N Engl J Med. 2009;360(7):659–67. doi: 10.1056/NEJMoa0806122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shanafelt TD, Kay NE, Rabe KG, Call TG, Zent CS, Maddocks K, et al. Brief report: natural history of individuals with clinically recognized monoclonal B-cell lymphocytosis compared with patients with Rai 0 chronic lymphocytic leukemia. J Clin Oncol. 2009;27(24):3959–63. doi: 10.1200/JCO.2008.21.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossi D, Sozzi E, Puma A, De Paoli L, Rasi S, Spina V, et al. The prognosis of clinical monoclonal B cell lymphocytosis differs from prognosis of Rai 0 chronic lymphocytic leukaemia and is recapitulated by biological risk factors. Br J Haematol. 2009;146(1):64–75. doi: 10.1111/j.1365-2141.2009.07711.x. [DOI] [PubMed] [Google Scholar]
- 11.Shanafelt TD, Kay NE, Jenkins G, Call TG, Zent CS, Jelinek DF, et al. B-cell count and survival: differentiating chronic lymphocytic leukemia from monoclonal B-cell lymphocytosis based on clinical outcome. Blood. 2009;113(18):4188–96. doi: 10.1182/blood-2008-09-176149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shanafelt T, Hanson CA. Monoclonal B-cell lymphocytosis: definitions and natural history. Leuk Lymphoma. 2009;50(3):493–7. doi: 10.1080/10428190902763483. [DOI] [PubMed] [Google Scholar]
- 13.Fung SS, Hillier KL, Leger CS, Sandhu I, Vickars LM, Galbraith PF, et al. Clinical progression and outcome of patients with monoclonal B-cell lymphocytosis. Leuk Lymphoma. 2007;48(6):1087–91. doi: 10.1080/10428190701321277. [DOI] [PubMed] [Google Scholar]
- 14.Molica S, Mauro F, Giannarelli D, Lauria F, Cortelezzi A, Brugiatelli M, et al. Differentiating chronic lymphocytic leukemia from monoclonal B-lymphocytosis according to the clinical outcome: on behalf of the GIMEMA Chronic Lymphoproliferative Diseases Working Group. Haematologica. 2010;96(2):277–83. doi: 10.3324/haematol.2010.030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montes-Moreno S, Castro Y, Rodriguez-Pinilla SM, Garcia JF, Mollejo M, Castillo ME, et al. Intrafollicular neoplasia/in situ follicular lymphoma: review of a series of 13 cases. Histopathology. 2010;56(5):658–62. doi: 10.1111/j.1365-2559.2010.03529.x. [DOI] [PubMed] [Google Scholar]
- 16.Cong P, Raffeld M, Teruya-Feldstein J, Sorbara L, Pittaluga S, Jaffe ES. In situ localization of follicular lymphoma: description and analysis by laser capture microdissection. Blood. 2002;99(9):3376–82. doi: 10.1182/blood.v99.9.3376. [DOI] [PubMed] [Google Scholar]
- 17.Pileri SA, Falini B. Mantle cell lymphoma. Haematologica. 2009;94(11):1488–92. doi: 10.3324/haematol.2009.013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Binet JL, Auquier A, Dighiero G, Chastang C, Piguet H, Goasguen J, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48(1):198–206. doi: 10.1002/1097-0142(19810701)48:1<198::aid-cncr2820480131>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 19.Rai KR, Han T. Prognostic factors and clinical staging in chronic lymphocytic leukemia. Hematol Oncol Clin North Am. 1990;4(2):447–56. [PubMed] [Google Scholar]
- 20.A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329(14):987–94. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 21.Herrick JL, Shanafelt TD, Kay N. Monoclonal B-cell lymphocytosis (MBL): a bone marrow study of an indolent form of chronic lymphocytic leukemia. Mod Pathol. 2008;21(suppl 1):256A. [Google Scholar]
- 22.Muntanola A, Bosch F, Arguis P, Arellano-Rodrigo E, Ayuso C, Gine E, et al. Abdominal computed tomography predicts progression in patients with Rai stage 0 chronic lymphocytic leukemia. J Clin Oncol. 2007;25(12):1576–80. doi: 10.1200/JCO.2006.08.4194. [DOI] [PubMed] [Google Scholar]
- 23.Ben-Ezra J, Burke JS, Swartz WG, Brownell MD, Brynes RK, Hill LR, et al. Small lymphocytic lymphoma: a clinicopathologic analysis of 268 cases. Blood. 1989;73(2):579–87. [PubMed] [Google Scholar]
- 24.Evans HL, Butler JJ, Youness EL. Malignant lymphoma, small lymphocytic type: a clinicopathologic study of 84 cases with suggested criteria for intermediate lymphocytic lymphoma. Cancer. 1978;41(4):1440–55. doi: 10.1002/1097-0142(197804)41:4<1440::aid-cncr2820410432>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 25.Bahler DW, Aguilera NS, Chen CC, Abbondanzo SL, Swerdlow SH. Histological and immunoglobulin VH gene analysis of interfollicular small lymphocytic lymphoma provides evidence for two types. Am J Pathol. 2000;157(4):1063–70. doi: 10.1016/S0002-9440(10)64620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellison DJ, Nathwani BN, Cho SY, Martin SE. Interfollicular small lymphocytic lymphoma: the diagnostic significance of pseudofollicles. Hum Pathol. 1989;20(11):1108–18. doi: 10.1016/0046-8177(89)90231-1. [DOI] [PubMed] [Google Scholar]
- 27.Gupta D, Lim MS, Medeiros LJ, Elenitoba-Johnson KS. Small lymphocytic lymphoma with perifollicular, marginal zone, or interfollicular distribution. Mod Pathol. 2000;13(11):1161–6. doi: 10.1038/modpathol.3880214. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen DT, Diamond LW, Schwonzen M, Bohlen H, Diehl V. Chronic lymphocytic leukemia with an interfollicular architecture: avoiding diagnostic confusion with monocytoid B-cell lymphoma. Leuk Lymphoma. 1995;18(1–2):179–84. doi: 10.3109/10428199509064940. [DOI] [PubMed] [Google Scholar]
- 29.Asplund SL, McKenna RW, Howard MS, Kroft SH. Immunophenotype does not correlate with lymph node histology in chronic lymphocytic leukemia/small lymphocytic lymphoma. Am J Surg Pathol. 2002;26(5):624–9. doi: 10.1097/00000478-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Garcia CF, Hunt KE, Kang H, Babb A, Gale JM, Vasef MA, et al. Most morphologic features in chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) do not reliably predict underlying FISH genetics or immunoglobulin heavy chain variable region somatic mutational status. Appl Immunohistochem Mol Morphol. 2010;18(2):119–27. doi: 10.1097/PAI.0b013e3181bbd5d5. [DOI] [PubMed] [Google Scholar]
- 31.Bonato M, Pittaluga S, Tierens A, Criel A, Verhoef G, Wlodarska I, et al. Lymph node histology in typical and atypical chronic lymphocytic leukemia. Am J Surg Pathol. 1998;22(1):49–56. doi: 10.1097/00000478-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Gine E, Martinez A, Villamor N, Lopez-Guillermo A, Camos M, Martinez D, et al. Expanded and highly active proliferation centers identify a histological subtype of chronic lymphocytic leukemia (“accelerated” chronic lymphocytic leukemia) with aggressive clinical behavior. Haematologica. 2010;95(9):1526–33. doi: 10.3324/haematol.2010.022277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flanagan MB, Sathanoori M, Surti U, Soma L, Swerdlow SH. Cytogenetic abnormalities detected by fluorescence in situ hybridization on paraffin-embedded chronic lymphocytic leukemia/small lymphocytic lymphoma lymphoid tissue biopsy specimens. Am J Clin Pathol. 2008;130(4):620–7. doi: 10.1309/H9AREV6E2JTMEC6J. [DOI] [PubMed] [Google Scholar]
- 34.Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, Bullinger L, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26):1910–6. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 35.Tam CS, Shanafelt TD, Wierda WG, Abruzzo LV, Van Dyke DL, O’Brien S, et al. De novo deletion 17p13.1 chronic lymphocytic leukemia shows significant clinical heterogeneity: the M. D. Anderson and Mayo Clinic experience. Blood. 2009;114(5):957–64. doi: 10.1182/blood-2009-03-210591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Del Giudice I, Chiaretti S, Tavolaro S, De Propris MS, Maggio R, Mancini F, et al. Spontaneous regression of chronic lymphocytic leukemia: clinical and biologic features of 9 cases. Blood. 2009;114(3):638–46. doi: 10.1182/blood-2008-12-196568. [DOI] [PubMed] [Google Scholar]
- 37.Han T, Ozer H, Gavigan M, Gajera R, Minowada J, Bloom ML, et al. Benign monoclonal B cell lymphocytosis--a benign variant of CLL: clinical, immunologic, phenotypic, and cytogenetic studies in 20 patients. Blood. 1984;64(1):244–52. [PubMed] [Google Scholar]
- 38.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011 Feb 7; doi: 10.1182/blood-2011-01-293050. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]