Abstract
Background
The expression levels of LPL, ZAP70, TCL1A, CLLU1 and MCL1 have recently been proposed as prognostic factors in chronic lymphocytic leukemia. However, few studies have systematically compared these different RNA-based markers.
Design and Methods
Using real-time quantitative PCR, we measured the mRNA expression levels of these genes in unsorted samples from 252 newly diagnosed chronic lymphocytic leukemia patients and correlated our data with established prognostic markers (for example Binet stage, CD38, IGHV gene mutational status and genomic aberrations) and clinical outcome.
Results
High expression levels of all RNA-based markers, except MCL1, predicted shorter overall survival and time to treatment, with LPL being the most significant. In multivariate analysis including the RNA-based markers, LPL expression was the only independent prognostic marker for overall survival and time to treatment. When studying LPL expression and the established markers, LPL expression retained its independent prognostic strength for overall survival. All of the RNA-based markers, albeit with varying ability, added prognostic information to established markers, with LPL expression giving the most significant results. Notably, high LPL expression predicted a worse outcome in good-prognosis subgroups, such as patients with mutated IGHV genes, Binet stage A, CD38 negativity or favorable cytogenetics. In particular, the combination of LPL expression and CD38 could further stratify Binet stage A patients.
Conclusions
LPL expression is the strongest RNA-based prognostic marker in chronic lymphocytic leukemia that could potentially be applied to predict outcome in the clinical setting, particularly in the large group of patients with favorable prognosis.
Keywords: LPL, RNA-based markers, chronic lymphocytic leukemia, prognosis
Introduction
Chronic lymphocytic leukemia (CLL) accounts for roughly 30% of all leukemias in Western countries and is characterized by a heterogeneous clinical course.1,2 Recognizing this, the Rai and Binet staging systems were developed approximately three decades ago to stratify patients according to disease burden and the degree of cytopenia.3–5 However, these staging systems have a limited capacity to predict clinical outcome at an early stage of the disease. In the past decade, several biomarkers have been suggested as potential prognostic factors in CLL. These include the mutational status of the immunoglobulin heavy variable (IGHV) genes6,7 and certain recurrent genomic aberrations.8 Additionally, flow-cytometry analysis of CD38 and Zeta-chain-associated protein kinase 70 (ZAP70) are considered to be independent prognostic markers in CLL.9,10 Given the difficulties in standardization of flow cytometry methods for ZAP70 measurement, analysis of mRNA expression levels has been proposed as a promising alternative.11,12
In recent years, several additional markers with prognostic potential in CLL have emerged. Lipoprotein lipase (LPL), initially identified in gene expression profiling of CLL, is one of the most differentially expressed genes in IGHV mutated versus unmutated CLL, where it is significantly higher expressed in unmutated cases.13–15 In addition, T-cell leukemia/lymphoma 1 (TCL1A),16,17 CLL-upregulated gene-1 (CLLU1)18,19 and myeloid cell factor-1 (MCL1)20 have also been found to display higher expression in unmutated CLL patients. Moreover, high expression of these markers at the protein and/or mRNA transcription level has been associated with inferior treatment-free and overall survival (OS) in a number of independent CLL cohorts.19–26
The current study aimed to validate and further investigate the prognostic strength of LPL, ZAP70, TCL1A, CLLU1 and MCL1 mRNA expression in CLL prognosis, either as single markers or in combination with established markers. Herein, we measured the mRNA expression levels in non-purified tumor samples from 252 newly diagnosed CLL patients from a Scandinavian population-based cohort. In summary, we found LPL expression to be the strongest RNA-based prognostic marker in CLL. In addition, we noted that high LPL expression was associated with poor outcome in favorable prognosis subgroups, including patients with Binet stage A, mutated IGHV genes, CD38 negativity or favorable cytogenetics.
Design and Methods
Patients
Two hundred and fifty-two CLL patients were included from the Swedish cohort of the Scandinavian Lymphoma Etiology (SCALE) study, a population-based case-control study including patients aged 18–74 years.27 All cases were classified according to recently revised criteria and displayed the typical CLL immunophenotype (CD5+/CD19+/CD23+).28,29 The CLL samples, collected over the period from 1999 to 2002,27 were obtained from peripheral blood and contained 70% or more tumor cells. Median time for sample collection was three months from the date of diagnosis. The study included 160 men and 92 women with a median age at diagnosis of 64 years (quartile range 57–69 years). The median follow-up time for the cohort was 102 months (quartile range 77–113 months). Survival data was available for all patients, while treatment data was obtained in 223 cases (88%) of whom 114 were treated. Binet stage was available in 239 cases (95%): stage A n=188, stage B n=39, and stage C n=12. Informed consent was obtained according to the Helsinki declaration and the study was approved by the local Ethics Review Committees.
IGHV gene mutational analysis and cell surface CD38 expression
IGHV subgroup-specific polymerase chain reaction (PCR) and sequence analysis was performed on genomic DNA as previously described.30 Sequences were aligned using the IMGT/V-QUEST tool in the IMGT database.31,32 IGHV sequences with less than 98% identity to germline were classified as mutated, whereas cases with 98% or more identity were considered unmutated. Immunophenotyping for CD38-positivity was performed as previously described.33 A 7% cut off was applied to delineate CD38 positive from negative samples.
Analysis of recurrent genomic aberration
High-resolution genomic screening to detect del(17)(p13), +12, del(11)(q22), and del(13)(q14) was performed on 244 cases (97%) using Affymetrix 250K SNP-arrays from which data on known recurrent genomic aberrations were extracted, as described in a previous study.34
Expression analysis of RNA-based markers
RNA was isolated from peripheral blood mononuclear cells (PBMCs) using spin-column technology (Qiagen, Hilden, Germany). RNA integrity and quality was assessed using the 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). cDNA was synthesized from 400 ng total RNA using M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA, USA). The transcription levels of LPL, TCL1A, ZAP70, CLLU1 and MCL1 were quantified using real-time quantitative PCR (RQ-PCR) and gene specific Taqman MGB® probes (Applied Biosystems, Foster City, CA, USA) (Online Supplementary Table S1) and the data were normalized against internal beta-2-microglobulin (β2M) expression levels using the comparative Ct method. The reactions were run on a Stratagene Mx 3005 instrument (La Jolla, CA, USA).
Statistical analysis
Receiver operating characteristics (ROC) curve analysis was applied to calculate the expression cut-off value for each RNA-based marker. The cut-off value predicting survival as above or below cohort median with the highest sensitivity and specificity was used for further analysis. Shapiro-Wilk’s test was applied to assess normal distribution, the Mann-Whitney U test was used to compare data in subgroups while the χ2 test was utilized to investigate the association between the RNA-based markers and other prognostic markers. Kaplan-Meier analysis was performed to construct survival curves. OS was measured from the date of diagnosis to either the last follow-up date (defined as censored) or death (all deaths included), whereas time to first treatment (TTT) was defined as date of diagnosis until the starting date of initial treatment or last follow up. A multivariate log rank test was used to assess differences. Cox’s proportional hazards model was applied to evaluate independent associations between single risk factors and OS as well as TTT. All statistical analyses were carried out using Statistica version 9.1 (Stat Soft, Tulsa, OK, USA).
Results
Prognostic markers and clinical outcome
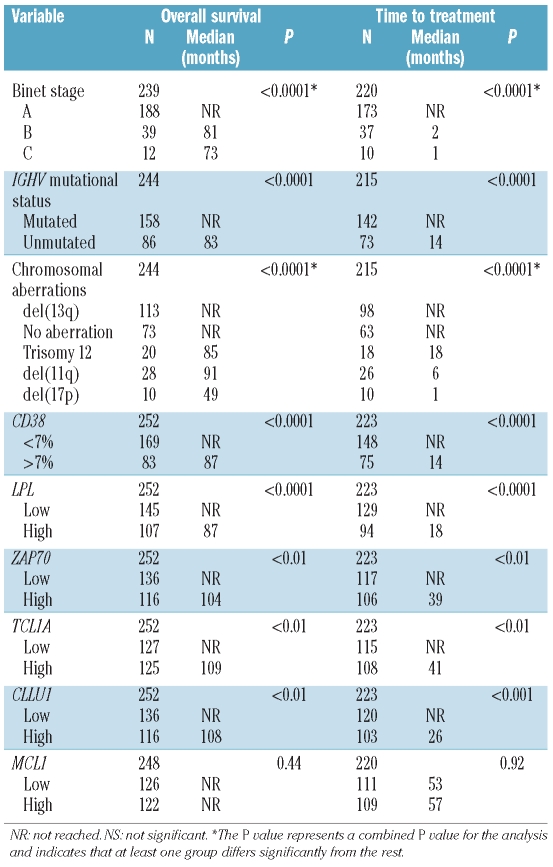
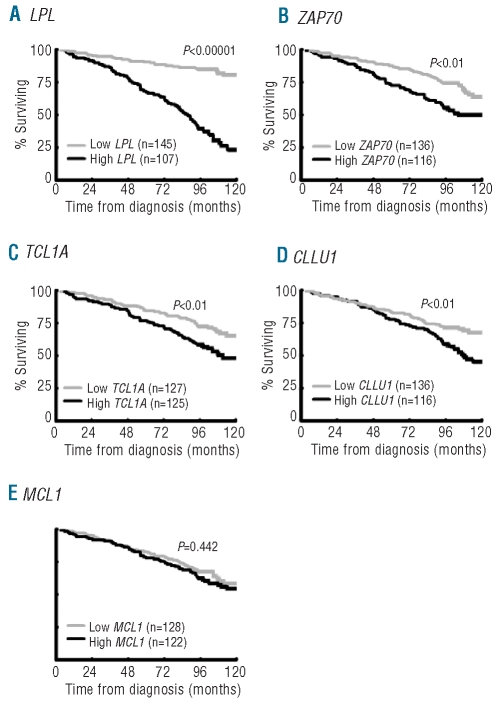
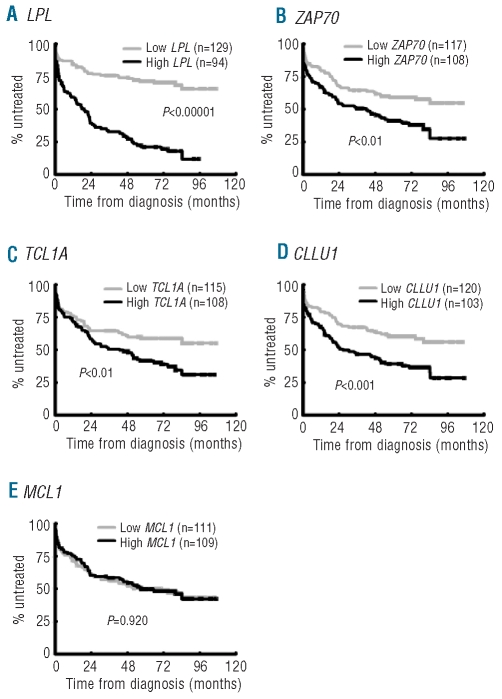
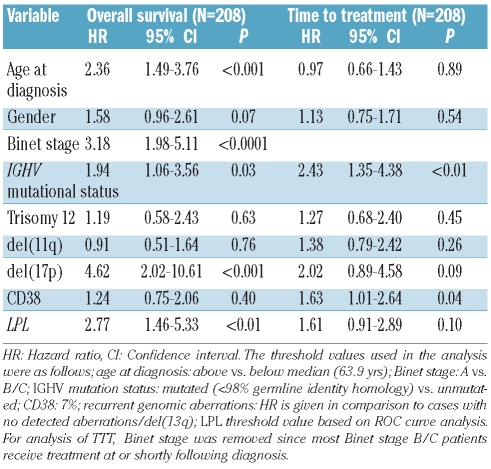
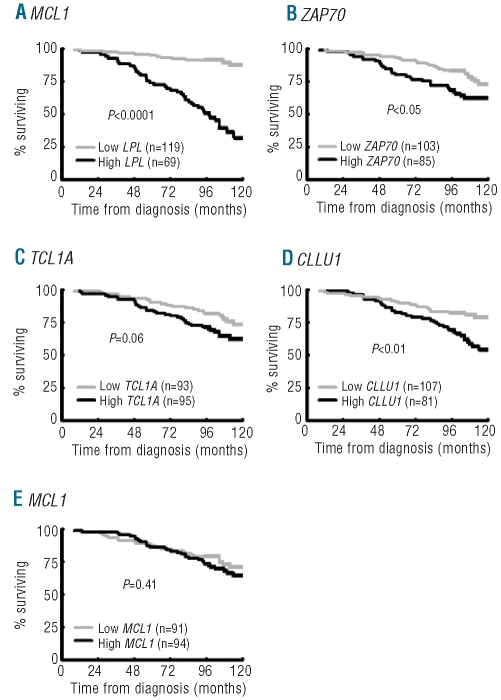
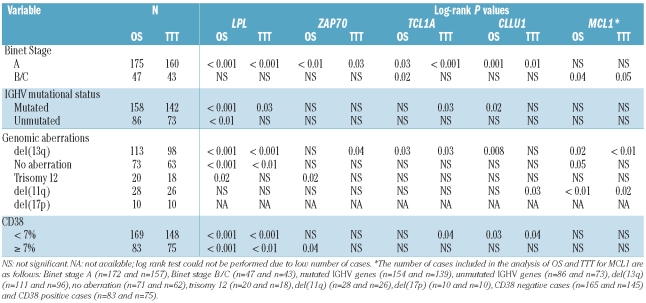
As expected, Binet staging, IGHV gene mutational status, recurrent genomic aberrations and CD38 expression were all significant prognostic factors of clinical outcome (Table 1).4–8 For the RNA-based markers, mRNA expression analysis of LPL, ZAP70, CLLU1 and TCL1A was performed in all 252 cases, while MCL1 expression was measured in 248 of the cases included in this study. The expression cut off for each RNA-based marker was defined using ROC curve analysis as follows: 6.90×10−5 for LPL, 1.25×10−2 for ZAP70, 6.97×10−3 for TCL1A, 1.75×10−3 for CLLU1 and 3.02×10−1 for MCL1. High expression of LPL, ZAP70, TCL1A and CLLU1 was associated with shorter OS and TTT, while no significant difference in outcome was observed for MCL1 expression (Table 1, Figures 1 and 2). LPL was most significant in delineating OS and TTT between low and high expressing groups (P<0.00001). In sub-analyses excluding patients treated within six months of diagnosis to distinguish between progressive disease and advanced disease at diagnosis, LPL, ZAP70, CLLU1 and TCL1A all remained prognostic of time to first treatment (Online Supplementary Figure S1).
Table 1.
Overall survival and time to treatment in a Swedish cohort of CLL patients according to established and RNA-based prognostic markers.
Figure 1.
Expression status of RNA-based markers and overall survival. Kaplan-Meier analysis of overall survival of CLL cases according to the expression status of (A) LPL, (B) ZAP70, (C) TCL1A, (D) CLLU1 and (E) MCL1.
Figure 2.
Expression status of the RNA-based markers and time to treatment. Kaplan-Meier analysis of time to treatment of CLL cases according to the expression status of (A) LPL, (B) ZAP70, (C) TCL1A, (D) CLLU1 and (E) MCL1.
Multivariate analysis of prognostic markers
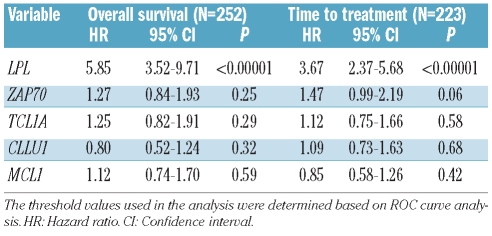
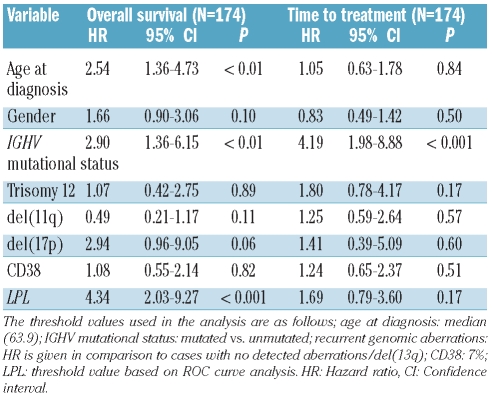
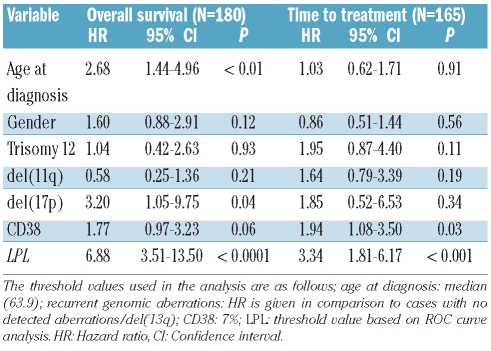
In multivariate analysis including only the RNA-based markers, LPL expression was the only significant independent prognostic factor for OS as well as TTT (Table 2). This finding also held true when studying Binet stage A patients only (Online Supplementary Table S2). In a multivariate model including LPL expression and the established markers, LPL expression remained a strong marker for OS, but was not formally significantly associated with TTT (Table 3A). LPL expression is strongly associated with IGHV mutational status (Figure 3), and if IGHV mutational status was excluded from the analysis, LPL was highly predictive also for TTT (Online Supplementary Table S3). Similar associations were observed when only Binet stage A patients were considered (Tables 3B and 3C).
Table 2.
Multivariate Cox regression analysis of RNA-based markers.
Table 3A.
Multivariate Cox regression analysis of LPL and established markers.
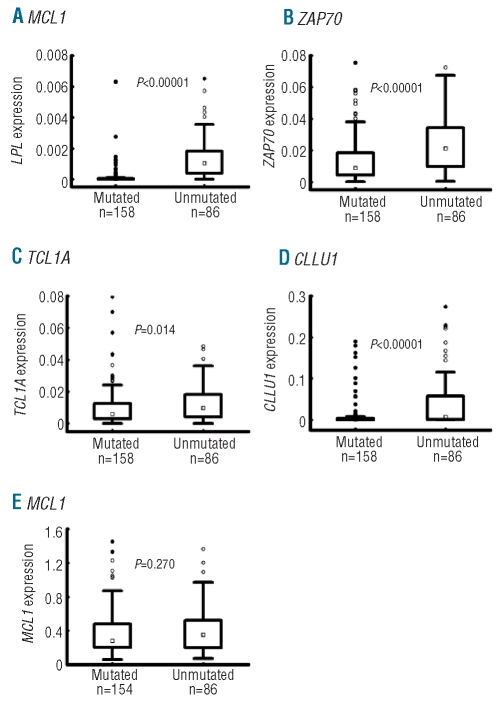
Figure 3.
The expression levels of RNA-based markers in relation to IGHV mutation status. Box plots depict the expression levels of (A) LPL, (B) ZAP70, (C) TCL1A, (D) CLLU1 and (E) MCL1 within CLL cases carrying mutated and unmutated IGHV genes. The box plots show median, 25 and 75 percentile values, non-outlier ranges, outliers and extremes. P values are derived from Student’s t-test.
Table 3B.
Multivariate Cox’s regression analysis of LPL and established markers within Binet A subgroup.
Table 3C.
Multivariate Cox regression analysis of LPL and established markers excluding IGHV mutational status within Binet A subgroup.
RNA-based markers in relation to other prognostic markers
We investigated the expression distribution of the RNA-based markers in relation to IGHV gene mutational status, genomic aberrations, Binet stage and other prognostic markers (Online Supplementary Table S4). Briefly, all markers except MCL1 were differentially expressed when comparing IGHV mutated and unmutated cases (Figure 3), whereas high expression of all markers except TCL1A was associated with high CD38 expression. Furthermore, LPL and CLLU1 displayed differential expression with regard to Binet stage and recurrent genomic aberrations, while no significant differences in expression were found for any marker when studying patient gender and age at diagnosis.
When studying Binet stage A patients, high expression of LPL, ZAP70 and CLLU1 was associated with shorter OS, while high expression of LPL, ZAP70, CLLU1 and TCL1A was found to add prognostic information in the analysis of TTT (Figure 4, Online Supplementary Figure S2). The additive prognostic information of the different RNA-based markers in subgroups of the established markers is summarized in Table 4. Notably, high LPL expression also added significant prognostic information to favorable-prognostic subgroups such as patients with mutated IGHV genes, CD38 negativity or favorable recurrent genomic aberrations.
Figure 4.
Overall survival of patients within Binet A subgroup. Kaplan-Meier analysis of overall survival of CLL cases according to the expression status of (A) LPL, (B) ZAP70, (C) TCL1A, (D) CLLU1 and (E) MCL1.
Table 4.
The prognostic information of RNA-based markers in subgroups of established markers
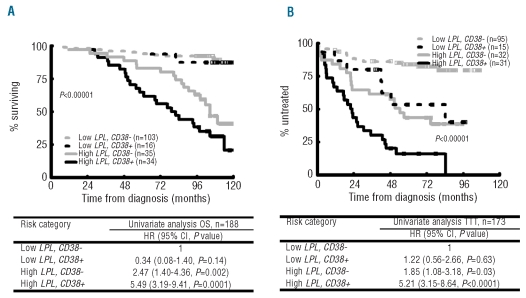
Further analysis of Binet stage A patients showed that LPL expression stratified CD38+ cases in terms of OS as well as TTT (Figure 5). Within the CD38– group of patients, LPL expression was again found to further subdivide clinical outcome (Figure 5). Cox’s regression analysis revealed the highest risk of death and highest risk of treatment initiation for the group with high LPL expression and CD38 positivity (Figure 5).
Figure 5.
LPL and CD38 expression status subdivides overall survival and time to treatment in the Binet A subgroup. Kaplan-Meier curves analysis of overall survival (A) and time to treatment (B) within the Binet A subgroup of patients according to LPL and CD38 expression status. Cox’s univariate analysis indicates hazard ratios (HR) for different marker combinations for overall survival and time to treatment analysis. The threshold values used in the analysis are as follows; LPL threshold value based on ROC curve analysis; CD38: 7%.
Discussion
This study set out to identify the most reliable prognostic factor among recently proposed RNA-based markers. In addition, we aimed to investigate the suitability of RNA-based markers in the clinical setting. Since sorting of tumor cells may not be feasible in routine diagnostic laboratories, we chose to analyze unsorted samples. There are several lines of evidence that provide further support for our approach. For example, LPL is not expressed in normal B cells and found in very low levels in CD19 negative cells in CLL patients.15,22 Moreover, differences found in TCL1A expression were negligible when comparing sorted versus unsorted CLL samples from the same patients.17 Finally, CLLU1 expression has previously been reported to be restricted to CLL tumor cells.18
In this study, the expression levels of LPL, ZAP70, TCL1A and CLLU1 predicted OS and time to first treatment in the current cohort of CLL, where high expression of these markers was significantly associated with unfavorable outcome. Particularly, LPL expression was found to be the most significant marker for analysis of OS and time to first treatment. In contrast to earlier reports on smaller cohorts,24,25 the prognostic potential of MCL1 expression could not be confirmed in this study. In addition, we analyzed TTT excluding patients treated within six months of diagnosis to evaluate disease progression rather than advanced disease at diagnosis. Using this approach, LPL, ZAP70, TCL1A and CLLU1 all remained significant for the analysis of TTT, where LPL appeared to be most informative. Our findings are in contrast to a recent study in which ZAP70 expression in sorted tumor cells was shown to be a powerful prognostic factor.12 Since we have performed our analysis on unsorted CLL samples, the expression of ZAP70 by other immune cells, including T cells, NK cells and normal B cells, may interfere with ZAP70 quantification in the tumor population,35 thus ZAP70 provides less information than LPL in non-purified cells.36
Among the RNA-based markers, LPL expression was the only significant independent prognostic factor for OS and TTT in multivariate analysis. In subsequent analysis, when including LPL and the established markers, LPL remained an independent prognostic factor for overall survival, also among Binet stage A patients only. When studying TTT, LPL lost its significance as an independent factor, probably due to its close relation to the IGHV mutational status,12,36 but regained its significance when the mutational status was excluded. Accordingly, a 73-fold higher median LPL expression was found in unmutated versus mutated cases (Figure 3), making a clear distinction between the two groups of patients. Therefore, LPL expression could be a useful surrogate marker for IGHV gene mutational status.
All RNA-based markers, albeit to varying degrees, were found to add prognostic information to the established markers. Here, LPL expression out-performed the other markers being able to significantly stratify patients with mutated IGHV genes, Binet stage A, favorable recurrent genomic aberrations and CD38 expression for both OS and TTT. Furthermore, CLLU1 expression successfully stratified patients with Binet stage A while MCL1 expression was the only significant marker for the analysis of OS and TTT in Binet stage B/C patients (Table 4). This latter finding, however, needs to be confirmed in a larger cohort of patients.
Although several reports have investigated individual RNA-based markers in CLL prognosis, few studies have systematically compared the relative prognostic strength between multiple RNA-based markers. van’t Veer et al. demonstrated that LPL expression was the best predictive marker for survival among ten different RNA-based markers in unsorted CLL samples, equaling IGHV gene mutational status in strength and out-performing ZAP70 expression.36 Furthermore, in two recent studies, LPL expression in CD19 sorted CLL samples was again shown to be a strong predictor of clinical outcome in different panels of RNA-based markers.37,38 Interestingly, our results agree with these findings, even though there is little overlap in genes analyzed between the studies, and further support the prognostic strength of LPL expression in CLL.
The vast majority of CLL patients have indolent disease at diagnosis but may still experience progression. Thus, prognostic markers in Binet stage A patients are particularly useful. Analysis of the IGHV gene mutational status is quite laborious and our identification of LPL expression as a surrogate marker has a potential clinical role. We also found that LPL expression in combination with CD38 expression could further subdivide CLL patients. Binet A patients with high expression of both LPL and CD38 showed the worst clinical outcome, while those with low expression of both markers appeared to have the most favorable clinical outcome; patients with mixed pattern showed an intermediate outcome for time to first treatment (Figure 5). Similarly, ZAP70 and CD38+ Binet stage A patients recently showed the highest risk for needing treatment.39 These data provide prognostic stratification for good-prognosis patients which might be useful in a clinical setting.
In summary, our current study verifies the prognostic value of the transcriptional status of LPL, ZAP70, TCL1A and CLLU1 with respect to OS and TTT. Furthermore, we report that the LPL expression status is the strongest RNA-based marker for clinical outcome using unsorted CLL cells. We also demonstrate that LPL expression in combination with CD38 expression can further subdivide Binet stage A patient outcome. Our data thus imply that RQ-PCR analysis of LPL could be used as an important prognostic marker in clinical routine practice, especially for indolent cases of CLL. The fact that no cell sorting appears necessary and the distinct differences in expression between poor versus good prognosis patients are additional advantages which may facilitate the analysis in diagnostic laboratories. RQ-PCR is routinely being applied for diagnostic and prognostic purposes in leukemia, e.g. in chronic myelogeneous leukemia.40 In spite of this, further efforts are needed to standardize the LPL measurement techniques. In addition, LPL expression during the course of disease still needs to be investigated. Large collaborative efforts to address these issues are, therefore, required.
Acknowledgments
This study was supported by grants from the Nordic Cancer Union, Swedish Cancer Society, the Swedish Society for Medical Research and Lion's Cancer Research Foundation, Uppsala and grant 1 R03 CA 101496-01 from the National Institutes of Health (SCALE study). We thank the Swedish CLL group for their support during the collection of clinical data.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352(8):804–15. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56(2):106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 3.Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46(2):219–34. doi: 10.1182/blood-2016-08-737650. [DOI] [PubMed] [Google Scholar]
- 4.Binet JL, Lepoprier M, Dighiero G, Charron D, D'Athis P, Vaugier G, et al. A clinical staging system for chronic lymphocytic leukemia: prognostic significance. Cancer. 1977;40(2):855–64. doi: 10.1002/1097-0142(197708)40:2<855::aid-cncr2820400239>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Binet JL, Auquier A, Dighiero G, Chastang C, Piguet H, Goasguen J, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48(1):198–206. doi: 10.1002/1097-0142(19810701)48:1<198::aid-cncr2820480131>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94(6):1848–54. [PubMed] [Google Scholar]
- 7.Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94(6):1840–7. [PubMed] [Google Scholar]
- 8.Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, Bullinger L, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26):1910–6. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 9.Rassenti LZ, Huynh L, Toy TL, Chen L, Keating MJ, Gribben JG, et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med. 2004;351(9):893–901. doi: 10.1056/NEJMoa040857. [DOI] [PubMed] [Google Scholar]
- 10.Rassenti LZ, Jain S, Keating MJ, Wierda WG, Grever MR, Byrd JC, et al. Relative value of ZAP-70, CD38, and immunoglobulin mutation status in predicting aggressive disease in chronic lymphocytic leukemia. Blood. 2008;112(5):1923–30. doi: 10.1182/blood-2007-05-092882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catherwood MA, Matthews C, Niblock R, Dobbin E, Morris TC, Alexander HD. ZAP-70 mRNA quantification in B-cell chronic lymphocytic leukaemia. Eur J Haematol. 2006;76(4):294–8. doi: 10.1111/j.1600-0609.2005.00619.x. [DOI] [PubMed] [Google Scholar]
- 12.Stamatopoulos B, Meuleman N, Haibe-Kains B, Duvillier H, Massy M, Martiat P, et al. Quantification of ZAP70 mRNA in B cells by real-time PCR is a powerful prognostic factor in chronic lymphocytic leukemia. Clin Chem. 2007;53(10):1757–66. doi: 10.1373/clinchem.2007.089326. [DOI] [PubMed] [Google Scholar]
- 13.Rosenwald A, Alizadeh AA, Widhopf G, Simon R, Davis RE, Yu X, et al. Relation of gene expression phenotype to immunoglobulin mutation genotype in B cell chronic lymphocytic leukemia. J Exp Med. 2001;194(11):1639–47. doi: 10.1084/jem.194.11.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Bockstaele F, Pede V, Janssens A, Callewaert F, Offner F, Verhasselt B, et al. Lipoprotein lipase mRNA expression in whole blood is a prognostic marker in B cell chronic lymphocytic leukemia. Clin Chem. 2007;53(2):204–12. doi: 10.1373/clinchem.2006.076331. [DOI] [PubMed] [Google Scholar]
- 15.Mansouri M, Sevov M, Fahlgren E, Tobin G, Jondal M, Osorio L, et al. Lipoprotein lipase is differentially expressed in prognostic subsets of chronic lymphocytic leukemia but displays invariably low catalytical activity. Leuk Res. 2010;34(3):301–6. doi: 10.1016/j.leukres.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 16.Herling M, Patel KA, Khalili J, Schlette E, Kobayashi R, Medeiros LJ, et al. TCL1 shows a regulated expression pattern in chronic lymphocytic leukemia that correlates with molecular subtypes and proliferative state. Leukemia. 2006;20(2):280–5. doi: 10.1038/sj.leu.2404017. [DOI] [PubMed] [Google Scholar]
- 17.Mansouri MR, Sevov M, Aleskog A, Jondal M, Merup M, Sundstrom C, et al. IGHV3-21 gene usage is associated with high TCL1 expression in chronic lymphocytic leukemia. Eur J Haematol. 2010;84(2):109–16. doi: 10.1111/j.1600-0609.2009.01369.x. [DOI] [PubMed] [Google Scholar]
- 18.Buhl AM, Jurlander J, Jorgensen FS, Ottesen AM, Cowland JB, Gjerdrum LM, et al. Identification of a gene on chromosome 12q22 uniquely overexpressed in chronic lymphocytic leukemia. Blood. 2006;107(7):2904–11. doi: 10.1182/blood-2005-07-2615. [DOI] [PubMed] [Google Scholar]
- 19.Josefsson P, Geisler CH, Leffers H, Petersen JH, Andersen MK, Jurlander J, et al. CLLU1 expression analysis adds prognostic information to risk prediction in chronic lymphocytic leukemia. Blood. 2007;109(11):4973–9. doi: 10.1182/blood-2006-11-054916. [DOI] [PubMed] [Google Scholar]
- 20.Pepper C, Lin TT, Pratt G, Hewamana S, Brennan P, Hiller L, et al. Mcl-1 expression has in vitro and in vivo significance in chronic lymphocytic leukemia and is associated with other poor prognostic markers. Blood. 2008;112(9):3807–17. doi: 10.1182/blood-2008-05-157131. [DOI] [PubMed] [Google Scholar]
- 21.Oppezzo P, Vasconcelos Y, Settegrana C, Jeannel D, Vuillier F, Legarff-Tavernier M, et al. The LPL/ADAM29 expression ratio is a novel prognosis indicator in chronic lymphocytic leukemia. Blood. 2005;106(2):650–7. doi: 10.1182/blood-2004-08-3344. [DOI] [PubMed] [Google Scholar]
- 22.Heintel D, Kienle D, Shehata M, Krober A, Kroemer E, Schwarzinger I, et al. High expression of lipoprotein lipase in poor risk B-cell chronic lymphocytic leukemia. Leukemia. 2005;19(7):1216–23. doi: 10.1038/sj.leu.2403748. [DOI] [PubMed] [Google Scholar]
- 23.Browning RL, Geyer SM, Johnson AJ, Jelinek DF, Tschumper RC, Call TG, et al. Expression of TCL-1 as a potential prognostic factor for treatment outcome in B-cell chronic lymphocytic leukemia. Leuk Res. 2007;31(12):1737–40. doi: 10.1016/j.leukres.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veronese L, Tournilhac O, Verrelle P, Davi F, Dighiero G, Chautard E, et al. Low MCL-1 mRNA expression correlates with prolonged survival in B-cell chronic lymphocytic leukemia. Leukemia. 2008;22(6):1291–3. doi: 10.1038/sj.leu.2405052. [DOI] [PubMed] [Google Scholar]
- 25.Veronese L, Tournilhac O, Verrelle P, Davi F, Dighiero G, Chautard E, et al. Strong correlation between VEGF and MCL-1 mRNA expression levels in B-cell chronic lymphocytic leukemia. Leuk Res. 2009;33(12):1623–6. doi: 10.1016/j.leukres.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Herling M, Patel KA, Weit N, Lilienthal N, Hallek M, Keating MJ, et al. High TCL1 levels are a marker of B-cell receptor pathway responsiveness and adverse outcome in chronic lymphocytic leukemia. Blood. 2009;114(21):4675–86. doi: 10.1182/blood-2009-03-208256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smedby KE, Hjalgrim H, Melbye M, Torrang A, Rostgaard K, Munksgaard L, et al. Ultraviolet radiation exposure and risk of malignant lymphomas. J Natl Cancer Inst. 2005;97(3):199–209. doi: 10.1093/jnci/dji022. [DOI] [PubMed] [Google Scholar]
- 28.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. IARC; Lyon: 2008. [Google Scholar]
- 29.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–56. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li AH, Rosenquist R, Forestier E, Holmberg D, Lindh J, Lofvenberg E, et al. Clonal rearrangements in childhood and adult precursor B acute lymphoblastic leukemia: a comparative polymerase chain reaction study using multiple sets of primers. Eur J Haematol. 1999;63(4):211–8. doi: 10.1111/j.1600-0609.1999.tb01880.x. [DOI] [PubMed] [Google Scholar]
- 31.Giudicelli V, Chaume D, Lefranc MP. IMGT/V-QUEST, an integrated software program for immunoglobulin and T cell receptor V-J and V-D-J rearrangement analysis. Nucleic Acids Res. 2004;32(Web Server issue):W435–40. doi: 10.1093/nar/gkh412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lefranc MP, Giudicelli V, Kaas Q, Duprat E, Jabado-Michaloud J, Scaviner D, et al. IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res. 2005;33(Database issue):D593–7. doi: 10.1093/nar/gki065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thunberg U, Johnson A, Roos G, Thorn I, Tobin G, Sallstrom J, et al. CD38 expression is a poor predictor for VH gene mutational status and prognosis in chronic lymphocytic leukemia. Blood. 2001;97(6):1892–4. doi: 10.1182/blood.v97.6.1892. [DOI] [PubMed] [Google Scholar]
- 34.Gunnarsson R, Staaf J, Jansson M, Ottesen AM, Goransson H, Liljedahl U, et al. Screening for copy-number alterations and loss of heterozygosity in chronic lymphocytic leukemia--a comparative study of four differently designed, high resolution micro-array platforms. Genes Chromosomes Cancer. 2008;47(8):697–711. doi: 10.1002/gcc.20575. [DOI] [PubMed] [Google Scholar]
- 35.Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992;71(4):649–62. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 36.van't Veer MB, Brooijmans AM, Langerak AW, Verhaaf B, Goudswaard CS, Graveland WJ, et al. The predictive value of lipoprotein lipase for survival in chronic lymphocytic leukemia. Haematologica. 2006;91(1):56–63. [PubMed] [Google Scholar]
- 37.Kienle D, Benner A, Laufle C, Winkler D, Schneider C, Buhler A, et al. Gene expression factors as predictors of genetic risk and survival in chronic lymphocytic leukemia. Haematologica. 2010;95(1):102–9. doi: 10.3324/haematol.2009.010298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stamatopoulos B, Meuleman N, De Bruyn C, Pieters K, Anthoine G, Mineur P, et al. A molecular score by quantitative PCR as a new prognostic tool at diagnosis for chronic lymphocytic leukemia patients. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morabito F, Cutrona G, Gentile M, Matis S, Todoerti K, Colombo M, et al. Definition of progression risk based on combinations of cellular and molecular markers in patients with Binet stage A chronic lymphocytic leukaemia. Br J Haematol. 2009;146(1):44–53. doi: 10.1111/j.1365-2141.2009.07703.x. [DOI] [PubMed] [Google Scholar]
- 40.Muller MC, Cross NC, Erben P, Schenk T, Hanfstein B, Ernst T, et al. Harmonization of molecular monitoring of CML therapy in Europe. Leukemia. 2009;23(11):1957–63. doi: 10.1038/leu.2009.168. [DOI] [PubMed] [Google Scholar]