Abstract
Umbilical cord blood transplantation has been increasingly used over the past years for both malignant and non-malignant hematologic and other diseases as an alternative to mismatched-related or matched-unrelated bone marrow or peripheral blood hematopoietic stem cell transplantation. A disadvantage of cord blood is its low cell content which limits cord blood transplantation to generally low weight recipients, such as children. Various alternatives have been used to overcome this limitation, including co-infusion of two partially HLA-matched cord blood units.
According to Eurocord Registry data, this strategy has been applied in approximately 993 adult patients with hematologic diseases since the first double umbilical cord blood transplantation in 1999. In fact, since 2005, the number of adult patients receiving double umbilical cord blood transplantation has surpassed the number of adults transplanted with single cord blood units. The engraftment rate is comparable for both single and double umbilical cord blood transplantation, although the latter is accompanied by a higher incidence of grade II acute graft-versus-host disease and lower leukemia relapse for patients in first complete remission. In the majority of patients undergoing double umbilical cord blood transplantation, transient chimerism, due to the presence of cells from both donor units early post transplant, is replaced by sustained dominance of one unit from which long-term hematopoiesis is derived. Although the biology and the factors that determine unit dominance have not been clarified, the implication of immune-mediated mechanisms has been reported.
Preliminary data have demonstrated the safety of double umbilical cord blood transplantation. Ongoing clinical trials and prolonged follow up of the patients will clarify the immunology and determine the efficacy of this approach. We present here a brief overview of the clinical experience on double umbilical cord blood transplantation and its underlying biology.
Keywords: double, umbilical, cord blood, transplant
Introduction
Umbilical cord blood transplantation (UCBT), as an alternative to mismatched-related or matched-unrelated bone marrow or peripheral blood stem cell transplantation, has been increasingly used over the past years for both malignant and non-malignant hematologic and other diseases. Since the first UCBT in 1988 for Fanconi’s anemia, more than 20,000 have been performed, mainly in children.1–4 The major factor which compromises the application of unrelated UCBT in adults is the relatively low number of progenitor cells present in umbilical cord blood and HLA disparity.3,5 Increasing cell dose has been reported to improve engraftment, especially in adults, and partially overcome the influence of HLA disparities (up to 2).1,2,3,6,7,8,9 On the other hand, HLA compatibility is crucial for the survival of patients undergoing UCBT for non-malignant diseases, but there is still controversy over HLA disparity being associated with a decrease in relapse in the case of malignancies due to the possible increased alloreactivity to both the host and tumor cells, which is known as graft-versus-leukemia (GvL) effect.6,9
To overcome the low cell content of single UCB units, various alternatives have been used. Multidonor UCBTs of up to 12 units have shown that crossed immunological reaction between the units does not occur.10–16 Equally encouraging were the results of a study involving co-infusion of a related umbilical cord blood graft and a haploidentical or third-party donor peripheral blood graft (dual transplant) in patients with high-risk hematologic malignancies.17,18 However, with this approach, the potential development of severe acute graft-versus-host disease (GvHD) that could be triggered by the haploidentical CD34+ cells is a major concern. Within the context of dual transplant, the co-infusion of unrelated umbilical cord blood with mobilized hematopoietic stem cells (HSCs), whether or not these are T-cell depleted, from third-party donors has been used in clinical practice.19 Compared to single UCBT, this approach is accompanied by shorter periods of neutropenia due to early engraftment of the mobilized hematopoietic stem cells, and therefore a lower incidence of graft-versus-host disease. Studies involving in vitro expansion of a fraction of the umbilical cord blood with cytokines and subsequent co-infusion of the expanded and non-expanded units reported engraftment, but there was no difference in median time to neutrophil recovery to that observed with non-expanded umbilical cord blood.20 The infusion of a CD34+ selected and ex vivo expanded unit via the Notch signaling pathway unit alongside an unmanipulated umbilical cord blood unit has also been reported with improved time to neutrophil recovery compared to the infusion of 2 unmanipulated units.21 The simultaneous transplantation of 2 partially HLA-matched umbilical cord blood units (double umbilical cord blood transplantation, dUCBT) has also been used to overcome cell dose limitations. We present here a brief literature overview of the clinical experience from dUCBT and its underlying biology.
Double umbilical cord blood transplantation
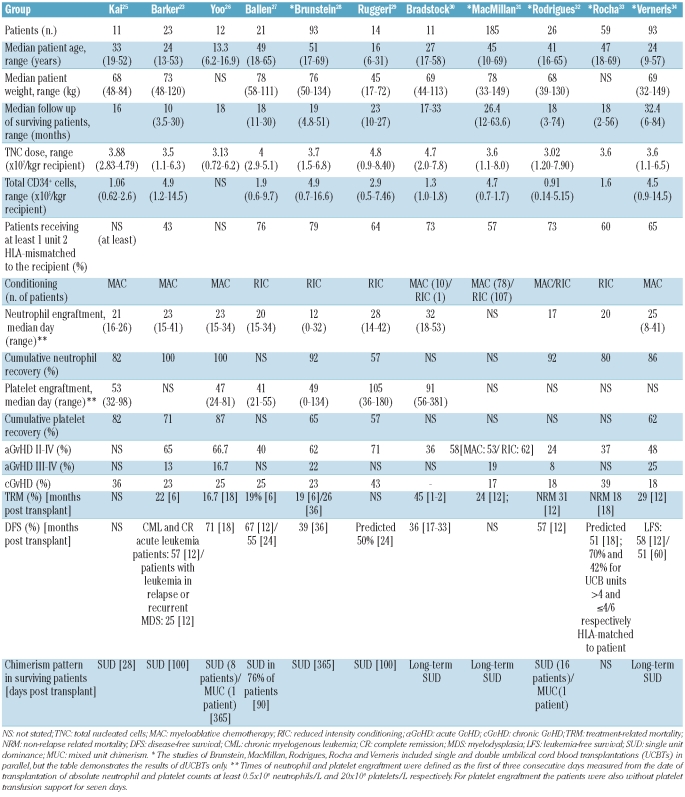
The first dUCBT was performed in Europe in 1999 on 2 adults with acute lymphoid and chronic myeologenous leukemia.22 Both patients had signs of donor engraftment but died three months post transplant; one from relapse and the other from hemorrhage (Eurocord, unpublished data, 2010).22 In 2001, the first case of dual donor chimerism was reported by Barker et al. following transplantation of 2 partially HLA-matched unrelated umbilical cord blood donors.13 In 2005, the same group published the safety and feasibility of dUCBT in 21 adults undergoing myeloablation for malignant diseases, and a few years earlier (2003) used dUCBT as a strategy for patients with no adequately sized units in a study regarding the outcome of UCBT with reduced intensity conditioning (RIC) regimens.23,24 The results were encouraging, as the percentage of engraftment was increased, whereas relapse and severe acute graft-versus-host disease were decreased. Since then, dUCBT has been performed mainly in patients with hematologic malignancies undergoing both non-myeloablative and myeloablative conditioning (Table 1). Amongst these, 84 dUCBTs were carried out on children (n=45) and adults (n=39) with non-malignant diseases, mainly with bone marrow failure syndromes.22 According to Eurocord reports, since 2005, the number of adult patients receiving dUCBT has surpassed the number of adults transplanted with single units.22
Table 1.
Double umbilical cord blood transplantation studies.
Conditioning regimens
Conditioning regimens of variable intensity have been used to reduce the complications associated with dUCBT and improve its outcome. In the context of myeloablation, high-dose total body irradiation (TBI) either alone or in combination with various chemotherapeutic agents (mainly cyclophosphamide and fludarabine) has been used.23,25,26,30,31,32,34 Reduced intensity conditioning regimens, along with immunosuppressants, have also been successfully used to minimize the undesirable toxic effects of myeloablation while producing sufficient immunosuppression to promote long-term engraftment.24,27–33 Majhail et al. recommended reduced intensity conditioning in patients over 55 years of age undergoing dUCBT.35 Such patients were previously excluded from transplant on the basis of age and lack of a suitable matched related or unrelated donor. Recently, Eurocord published a study involving single and double UCBT on 104 patients with lymphoid malignancies using both conditioning regimens and concluded that a better outcome was achieved in chemosensitive patients receiving low-dose total body irradiation and high cell dose.31,32
Graft characteristics
The current recommendations for UCBT are: i) cord blood units with no more than 2 HLA disparities and 3×107 or more nucleated cells/kg or 2x105 CD34+ or more cells/kg. For units that are HLA-matched (6/6 units) and mismatched (4-5/6) the minimum recommended cell dose is 3×107 or more and 4×107 or more nucleated cells/kg, respectively; ii) in cases in which the risk of rejection is higher, such as in non-malignant diseases, the cell dose should be higher than 3.5×107 or more nucleated cells/kg and HLA disparity between the units and the patient of not more than 1; iii) if no single unit meeting these requirements is available, it is recommended to use 2 units with a combined cell dose of 3×107 or more nucleated cells/kg and, if possible, no more than 2 HLA disparities between the units and the recipient or between the units.22,36,37,38
The role of HLA matching, and the interaction between cell dose and the extent of HLA disparity have not been fully clarified.9 Bearing in mind that HLA disparity is associated with survival and hematopoietic recovery in UCBT, higher cell dose can abrogate the effect of HLA mismatching, as long as there are no more than 2 HLA incompatibilities between the patient and each unit.9,33,39,40 There are few data on the effect of locus-specific HLA matching in UCBT beyond HLA-A, B and DR; usually allele-level typing for locus HLA-DRB1 and antigen-level typing for HLA-A and B.
In dUCBT, the units are administered intravenously either sequentially (within 30 min) or within 6 h apart, after confirming that the first unit has been successfully infused.41 Intrabonal administration has also been used and found to be well-tolerated, safe and comparable to the conventional intravenous administration of the 2 units.42,43
Chimerism
More than 85% of patients undergoing dUCBT demonstrate rapid skewing of donor engraftment and a single unit emerges as the ‘winner’ to sustain long-term hematopoiesis (i.e. at least 90% marrow reconstitution of recipient by donor).23–34,38,44 The time frame for determining dominance has not yet been clarified.45 Usually, by day 21 post transplant single unit dominance can be detected in over 80% of patients, although dominance as soon as 14 days post transplant has also been reported.38,46 There can also be mixed chimerism (i.e. presence of both donor units) at varying ratios, especially in patients undergoing reduced intensity conditioning regimens.47 Algorithms are available that can provide approximations of the chimerism pattern following dUCBT.48 Dominance reversion has rarely been reported, except for a 15-year old boy treated with dUCBT for acute lymphoblastic leukemia in whom long-term mixed donor chimerism and dominance reversion (on day 253 post transplant) were observed during follow up for more than 16 months (479 days post transplant).49 Furthermore, in a phase I dUCBT clinical trial involving patients with hematologic malignancies undergoing a reduced intensity conditioning regimen, a patient with 95% single donor chimerism on day 65 post transplant was reported to lose single unit dominance in favor of mixed donor chimerism over time.41 The patient died approximately 200 days post transplant from a lymphoproliferative disorder.
The parameters that influence umbilical cord blood predominance in dUCBT have not been clarified. There is no correlation between dominance and the number of nucleated cells, CD34+, CD3+, degree of HLA/sex mismatch, high resolution HLA matching, ABO group, viability, order and route of infusion.38,44 Preliminary evidence, however, suggests that cord blood units with low CD34+ cell viability (<75%) have low probability of engraftment upon co-infusion with a unit of high CD34+ cell viability (>75%).50 Whether dominance is influenced by intrinsic features of the units, host-versus-graft and/or graft-versus-graft interactions must still be determined. It is possible that the non-engrafted unit might facilitate/activate the engraftment of the sustained unit. On the other hand, the use of 2 units might simply increase the chances of infusing a unit with engrafting potential.38 Predicting the ‘winning’ unit might, therefore, be impossible (e.g. “atmospheric noise” theory).51 Interestingly, patients with mixed chimerism following reduced intensity conditioning dUCBT tend to be more prone to chronic graft-versus-host disease at one year post transplantation, which suggests that the conditioning regimen might interfere with the chimerism pattern.27 This observation could also indicate graft-versus-graft interactions and is consistent with recent in vivo evidence demonstrating that naive CD8+ T cells in one unit expanded and differentiated into IFN-γ secreting effector T cells that specifically recognized the non-engrafting unit and mediated its rejection.46 These cells were only detected transiently in the peripheral blood of dUCBT recipients with single unit dominance and are, therefore, not likely to be the sole cause of rejection. Further evidence in favor of a T-cell mediated graft-versus-graft effect is provided by studies on both mice and patients. According to these, the infusion of 2 CD34+ units resulted in mixed chimerism, whereas the addition of the corresponding mononuclear cells or CD34− cells restored single unit dominance.52,53 Bearing in mind that the 2 units are mismatched at one or more HLA alleles, the alloreactive response could be specific for major or minor allogeneic determinants, such as minor H antigens that are shared between umbilical cord blood units. This hypothesis can explain the enhanced graft-versus-leukemia effect associated with dUCBT if the major or minor H antigens expressed on hematopoietic stem cells of the non-engrafting unit are common with those expressed on leukemic stem cells, although it is not clear whether HLA disparity also contributes. The identification of the antigens expressed on hematopoietic stem cells to which T cells of the dominant unit are responsive is ongoing. On the other hand, mixed chimerism can be explained on the basis of CD4+ T cells that develop in utero and promote tolerance to non-inherited maternal alloantigens that are by chance shared by the other umbilical cord blood unit.46,54 The contribution of other immune-related effector mechanisms, such as killer immunoglobulin-like receptor-ligand differences and NK-cell activation, are under study.55,56
Co-transplantation of mesenchymal stem cells (MSCs) from various sources (bone marrow, placenta) has been shown to marginally improve the engraftment of hematopoietic stem cells in clinical studies.31,57–59 In murine models, dUCBT combined with co-transplantation of placental or bone marrow mesenchymal stem cells resulted in improved engraftment and reduced dominance of a single unit in favor of mixed chimerism.57,61 Mesenchymal stem cells secrete a broad range of immune-related bioactive molecules, such as cytokines, growth factors and chemokines that have both autocrine and paracrine activities, such as turning off T-cell surveillance (i.e. trophic activity).60 Also, culture-expanded mesenchymal stem cells do not express MHC class II surface markers and co-stimulatory molecules and cannot, therefore, function as antigen-presenting cells; they are invisible to the host’s immune system.62–64 They could, therefore, interfere with immunosurveillance and modify graft-versus-graft interactions so that, although one donor unit still dominates, the degree of dominance is reduced.57,61
It has been reported that when the units are infused intravenously within 3.5–4.5 h apart, the predominant unit is usually the first to be infused.27,41 It has also been demonstrated that non-lineage hematopoietic stem cells can home to the endosteal region of the niche in under five hours.65 Even a short interval in infusion may, therefore, confer advantage to the unit infused first, making it more likely to fill the niche space, which is limited to maintain tight long-term regulation between proliferation and quiescence of the resident stem cells.27,41,66 The combination of intravenous and intrabone administration was not shown to confer selective advantage in predominance of engraftment.43
These studies could provide evidence in favor of both graft-versus-graft interactions between the 2 units. Gutman et al. observed a great variation in vitro regarding the proliferative potential of CD34+ cells from umbilical cord blood.46 It is, therefore, likely that intrinsic factors concerning the stem cells which are not yet fully understood, such as homing to the niche, as well as prior therapy, intensity of conditioning regime, trophic effects and host factors, are all likely to contribute to the pattern of chimerism in dUCBT.
Survival (GvHD, GvL, infectious complications)
dUCBT is accompanied by increased acute graft-versus-host disease which varies according to the conditioning regimen and immunoprophylaxis, while the incidence of chronic graft-versus-host disease is roughly similar in all studies (Table 1). There is also a trend for reduced intensity conditioning dUCBT patients with mixed chimerism to be more prone to chronic graft-versus-host disease at one year post transplantation.32 Furthermore, there is increasing evidence to suggest improved leukemia-free survival for leukemia patients undergoing dUCBT in remission at the time of transplant.28,32,33,34,38,67
HLA matching is important for engraftment and to minimize risk of relapse, especially for leukemia patients.9,34 However, high resolution HLA matching does not affect either overall survival nor disease-free survival, nor can it predict dominance in dUCBT.40 The effect of HLA disparities, especially of the engrafting unit to the recipient with respect to acute graft-versus-host disease onset has not been fully clarified. Following dUCBT under reduced intensity conditioning, Delaney et al. reported that close HLA-DR matching was associated with a trend for lower risk of incidence of acute graft-versus-host disease, and HLA-B matching was associated with faster neutrophil and platelet engraftment.40
Reporting on infectious complications following dUCBT is limited, due to small cohort size and availability of only short-term patient follow-up data. In general, the prolonged neutropenia and monocytopenia that accompany UCBT increase susceptibility to bacterial, fungal and opportunistic viral infections. Approximately 30% of the deaths following dUCBT can be attributed to infections, amongst which a relatively higher incidence of BK-virus, Epstein Barr virus, adenovirus and HHV6 have been reported.68 In fact, HHV6 virus was detected almost systematically in blood samples from dUCBT patients, with no clinical manifestations.68 Prophylactic citomegalovirus treatment in dUCBT patients is generally not considered necessary.69 It has been suggested that as long as a sufficient dose of total nucleated cells is infused and sustained neutrophil recovery is established, infection-related mortality is not excessively high.68 Other complications are rarely reported.70 The main cause of death is the progression and relapse of the original disease. Myelogenous malignancies of donor origin have also been reported to occur, suggesting that careful donor selection is necessary for all patients with suspected relapse.71
Clinical experience with dUCBT
From 1999 to March 2010, 1,152 dUCBTs, combined with conditioning of various intensities, have been performed in patients with hematologic malignancies who could not find suitable unrelated donors.22 The combination of reduced intensity conditioning and dUCBT in particular have extended the availability of transplantation to older or heavily treated patients who have an increased risk of treatment-related mortality.24,27–29,31–33,35 More than 300 patients, both children and adults who had no alternative treatment option for leukemia, have also received dUCBT at the University of Minnesota since 2001.27
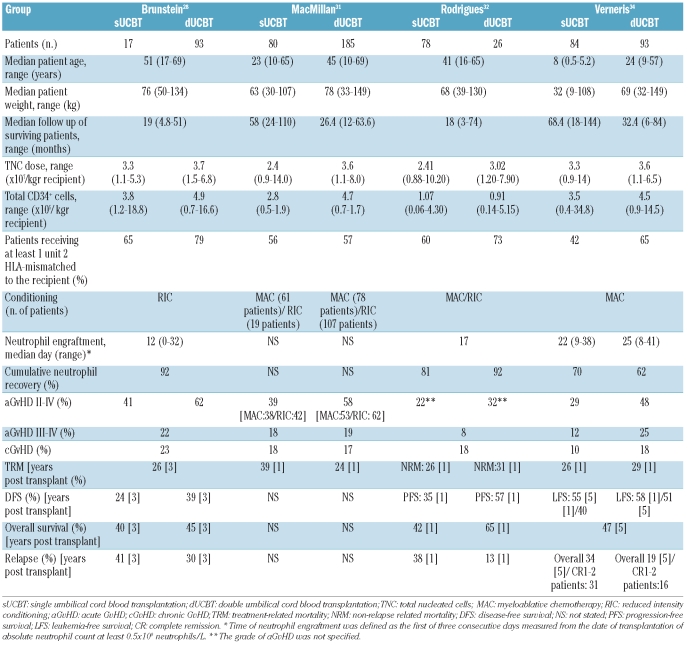
The preliminary encouraging data on the safety and feasibility of dUCBT have resulted in the launch of randomized clinical trials, investigating either the outcome of dUCBT or comparing single versus double UCBT in children and adults with hematologic malignancies. However, little published evidence is currently available on the long-term immune reconstitution and clinical benefit of dUCBT. Although similar neutrophil engraftment kinetics have been observed in a small study involving adults undergoing dUCBT and children undergoing single UCBT, an overview of studies on relatively large cohorts of patients comparing the outcome of single versus double UCBT in parallel suggests that dUCBT is accompanied by: i) a higher incidence of acute graft-versus-host disease grade II, though not higher treatment-related mortality or chronic graft-versus-host disease; ii) lower leukemia relapse for patients with good disease status (complete remission 1–2), indicating a potentially higher graft-versus-leukemia effect (Table 2).28,31,32,34,72,73 MacMillan et al. also reported that acute graft-versus-host disease occurred sooner in dUCBT recipients (median day 28 vs. 36).31 In the same study, treatment-related mortality after the onset of acute graft-versus-host disease was significantly lower in dUCBT patients (20% vs. 39% one year post transplant).31
Table 2.
Comparison of selected parallel single versus double umbilical cord blood transplantation studies.
Future
Umbilical cord blood is being increasingly used as a source of hematopoietic stem cells in allogeneic transplantations for patients who do not have an available HLA-matched donor. To overcome the low cell number, the co-transplantation of 2 umbilical cord blood units (dUCBT) is a promising strategy with comparable engraftment rate and potentially higher graft-versus-leukemia effect compared to single UCBT.
The biology underlying dUCBT and the factors that determine unit dominance are not yet fully understood. The implication of immune-mediated mechanisms raises the question that stem cells HLA-matched to the recipient unit, even of low cell dose, could be combined with a higher cell dose unit to improve the outcome in dUCBT. This could also be of significance in the context of leukemia; if the dominant unit can be predicted prior to transplantation, a non-engrafting unit sharing host antigens not present on the engrafting unit can be selected to promote the graft-versus-leukemia effect.
A reasonable limitation of dUCBT is the cost of 2 umbilical cord blood units, especially from unrelated donors, and the costs of hospitalization due to the low engraftment rate.22,74 Various approaches to improve engraftment and enhance immune reconstitution after dUCBT are being evaluated, such as infusions of cells (NK cells and cytotoxic T lymphocytes) with antiviral and antileukemic specificities, co-culture or co-transplantation with mesenchymal stem cells, as well as ex vivo expansion with cytokines and/or homing factors.75 The ongoing clinical trials will clarify the therapeutic benefit of dUCBT in a variety of malignant and non-malignant diseases in children and adults without an HLA-matched sibling donor.
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Gluckman E, Rocha V. Cord blood transplantation: state of the art. Haematologica. 2009;94(4):451–4. doi: 10.3324/haematol.2009.005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornetta K, Laughlin M, Carter S, Wall D, Weinthal J, Delaney C, et al. Umbilical cord blood transplantation in adults: results of the prospective Cord Blood Transplantation (COBLT) Biol Blood Marrow Transplant. 2005;11(2):149–60. doi: 10.1016/j.bbmt.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 3.Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100(5):1611–8. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 4.Michel G, Rocha V, Chevret S, Arcese W, Chan KW, Filipovich A, et al. Unrelated cord blood transplantation for childhood acute myeloid leukemia: a Eurocord Group analysis. Blood. 2003;102(13):4290–7. doi: 10.1182/blood-2003-04-1288. [DOI] [PubMed] [Google Scholar]
- 5.Gluckman E, Rocha V, Arcese W, Michel G, Sanz G, Chan KW, et al. Eurocord Group. Factors associated with outcomes of unrelated cord blood transplant: guidelines for donor choice. Exp Hematol. 2004;32(4):397–407. doi: 10.1016/j.exphem.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Delaney M, Ballen KK. The role of HLA in umbilical cord blood transplantation. Best Pract Res Clin Haematol. 2010;23(2):179–87. doi: 10.1016/j.beha.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio AR, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339(22):1565–77. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 8.Laughlin MJ, Barker J, Bambach B, Koc ON, Rizzieri DA, Wagner JE, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med. 2001;344(24):1815–22. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 9.Rocha V, Gluckman E Eurocord-Netcord registry and European Blood and Marrow Transplant group. Improving outcomes of cord blood transplantation: HLA matching, cell dose and other graft- and transplantation-related factors. Br J Haematol. 2009;147(2):262–74. doi: 10.1111/j.1365-2141.2009.07883.x. [DOI] [PubMed] [Google Scholar]
- 10.Shen BJ, Hou HS, Zhang HQ, Sui XW. Unrelated, HLA-mismatched multiple human umbilical cord blood transfusion in four cases with advanced solid tumors: initial studies. Blood Cells. 1994;20(2–3):285–92. [PubMed] [Google Scholar]
- 11.Weinreb S, Delgado JC, Clavijo OP, Yunis EJ, Bayer-Zwirello L, Polansky L, et al. Transplantation of unrelated cord blood cells. Bone Marrow Transplant. 1998;22(2):193–6. doi: 10.1038/sj.bmt.1701309. [DOI] [PubMed] [Google Scholar]
- 12.Wiktor-Jedrzejczak W, Rokicka M, Urbanowska E, Torosjan T, Gronkowska A, Graczyk-Pol E, et al. Simultaneous Transplantation of Three Cord Blood Units in Adults with High Risk Acute Leukemia. 2005;106:Abstract 5457. [Google Scholar]
- 13.Barker JN, Weisdorf DJ, Wagner JE. Creation of a double chimera after the transplantation of umbilical-cord blood from two partially matched unrelated donors. N Engl J Med. 2001;344(24):1870–1. doi: 10.1056/NEJM200106143442417. [DOI] [PubMed] [Google Scholar]
- 14.De Lima M, St John LS, Wieder ED, Lee MS, McMannis J, Karandish S, et al. Double-chimerism after transplantation of two human leucocyte antigen mismatched, unrelated cord blood units. Br J Haematol. 2002;119(3):773–6. doi: 10.1046/j.1365-2141.2002.03893.x. [DOI] [PubMed] [Google Scholar]
- 15.Lister J, Gryn JF, McQueen KL, Harris DT, Rossetti JM, Shadduck RK. Multiple unit HLA-unmatched sex-mismatched umbilical cord blood transplantation for advanced hematological malignancy. Stem Cells Dev. 2007;16(1):177–86. doi: 10.1089/scd.2006.06500-HB. [DOI] [PubMed] [Google Scholar]
- 16.Schöttker B, Feuchtinger T, Schumm M, Klinker E, Handgretinger R, Einsele H, et al. Five donors-one recipient: modeling a mosaic of granulocytes, natural killer and T cells from cord-blood and third-party donors. Nat Clin Pract Oncol. 2008;5(5):291–5. doi: 10.1038/ncponc1105. [DOI] [PubMed] [Google Scholar]
- 17.Fernández MN, Regidor C, Cabrera R, García-Marco JA, Forés R, Sanjuán I, et al. Unrelated umbilical cord blood transplants in adults: Early recovery of neutrophils by supportive co-transplantation of a low number of highly purified peripheral blood CD34+ cells from an HLA-haploidentical donor. Exp Hematol. 2003;31(6):535–44. doi: 10.1016/s0301-472x(03)00067-5. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalo-Daganzo R, Regidor C, Martín-Donaire T, Rico MA, Bautista G, Krsnik I, et al. Results of a pilot study on the use of third-party donor mesenchymal stromal cells in cord blood transplantation in adults. Cytotherapy. 2009;11(3):278–88. doi: 10.1080/14653240902807018. [DOI] [PubMed] [Google Scholar]
- 19.Fernández MN. Improving the outcome of cord blood transplantation: use of mobilized HSC and other cells from third party donors. Br J Haematol. 2009;147(2):161–76. doi: 10.1111/j.1365-2141.2009.07766.x. [DOI] [PubMed] [Google Scholar]
- 20.Shpall EJ, Quinones R, Giller R, Zeng C, Baron AE, Jones RB, et al. Transplantation of ex vivo expanded cord blood. Biol Blood Marrow Transplant. 2002;8(7):368–76. doi: 10.1053/bbmt.2002.v8.pm12171483. [DOI] [PubMed] [Google Scholar]
- 21.Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16(2):232–6. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rocha V, Crotta A, Ruggeri A, Purtill D, Boudjedir K, Herr AL, et al. Double cord blood transplantation: extending the use of unrelated umbilical cord blood cells for patients with hematological diseases. Best Pract Res Clin Haematol. 2010;23(2):223–9. doi: 10.1016/j.beha.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105(3):1343–7. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 24.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS, Wagner JE. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003;102(5):1915–9. doi: 10.1182/blood-2002-11-3337. [DOI] [PubMed] [Google Scholar]
- 25.Kai S, Misawa M, Iseki T, Takahashi S, Kishi K, Hiraoka A, et al. Double-unit cord blood transplantation in Japan. Blood. 2004;104:a5166. [Google Scholar]
- 26.Yoo KH, Kang HJ, Lee SH, Jung HL, Sung KW, Koo HH, et al. Double unit cord blood transplantation in children with acute leukemia. Blood. 2005;106:a2043. [Google Scholar]
- 27.Ballen KK, Spitzer TR, Yeap BY, McAfee S, Dey BR, Attar E, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant. 2007;13(1):82–9. doi: 10.1016/j.bbmt.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110(8):3064–70. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruggeri A, de Latour RP, Rocha V, Larghero J, Robin M, Rodrigues CA, et al. Double cord blood transplantation in patients with high risk bone marrow failure syndromes. Br J Haematol. 2008;143(3):404–8. doi: 10.1111/j.1365-2141.2008.07364.x. [DOI] [PubMed] [Google Scholar]
- 30.Bradstock K, Hertzberg M, Kerridge I, Svennilson J, George B, McGurgan M, et al. Single versus double unrelated umbilical cord blood units for allogeneic transplantation in adults with advanced haematological malignancies: a retrospective comparison of outcomes. Internal Medicine Journal. 2009;39:744–51. doi: 10.1111/j.1445-5994.2008.01825.x. [DOI] [PubMed] [Google Scholar]
- 31.MacMillan ML, Weisdorf DJ, Brunstein CG, Cao Q, DeFor TE, Verneris MR, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2009;113(11):2410–5. doi: 10.1182/blood-2008-07-163238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodrigues CA, Sanz G, Brunstein CG, Sanz J, Wagner JE, Renaud M, et al. Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: a study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2009;27(2):256–63. doi: 10.1200/JCO.2007.15.8865. [DOI] [PubMed] [Google Scholar]
- 33.Rocha V, Mohty M, Gluckman E, Rio B. Reduced intensity conditioning regimens before unrelated cord blood transplantation in adults with acute leukemia and other hematological malignancies. Curr Opin Oncol. 2009;21(Suppl 1):S31–S34. doi: 10.1097/01.cco.0000357473.58411.1b. [DOI] [PubMed] [Google Scholar]
- 34.Verneris MR, Brunstein CG, Barker J, MacMillan ML, DeFor T, McKenna DH, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood. 2009;114(19):4293–9. doi: 10.1182/blood-2009-05-220525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Majhail NS, Brunstein CG, Tomblyn M, Thomas AJ, Miller JS, Arora M, et al. Reduced-intensity allogeneic transplant in patients older than 55 years: unrelated umbilical cord blood is safe and effective for patients without a matched related donor. Biol Blood Marrow Transplant. 2008;14(3):282–9. doi: 10.1016/j.bbmt.2007.12.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gluckman E. Ten years of cord blood transplantation: from bench to bedside. Br J Haematol. 2009;147(2):192–9. doi: 10.1111/j.1365-2141.2009.07780.x. [DOI] [PubMed] [Google Scholar]
- 37.Gluckman E. History of cord blood transplantation. Bone Marrow Transplant. 2009;44(10):621–6. doi: 10.1038/bmt.2009.280. [DOI] [PubMed] [Google Scholar]
- 38.Delaney C, Gutman JA, Appelbaum FR. Cord blood transplantation for haematological malignancies: conditioning regimens, double cord transplant and infectious complications. Br J Haematol. 2009;147(2):207–16. doi: 10.1111/j.1365-2141.2009.07782.x. [DOI] [PubMed] [Google Scholar]
- 39.Kamani N, Spellman S, Hurley CK, Barker JN, Smith FO, Oudshoorn M, et al. State of the art review: HLA matching and outcome of unrelated donor umbilical cord blood transplants. Biol Blood Marrow Transplant. 2008;14(1):1–6. doi: 10.1016/j.bbmt.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Delaney M, Cutler CS, Haspel RL, Yeap BY, McAfee SL, Dey BR, et al. High-resolution HLA matching in double-umbilical-cord-blood reduced-intensity transplantation in adults. Transfusion. 2009;49(5):995–1002. doi: 10.1111/j.1537-2995.2008.02077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haspel RL, Kao G, Yeap BY, Cutler C, Soiffer RJ, Alyea EP, et al. Preinfusion variables predict the predominant unit in the setting of reduced-intensity double cord blood transplantation. Bone Marrow Transplant. 2008;41(6):523–9. doi: 10.1038/sj.bmt.1705933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frassoni F, Gualandi F, Podestà M, Raiola AM, Ibatici A, Piaggio G, et al. Direct intrabone transplant of unrelated cord-blood cells in acute leukaemia: a phase I/II study. Lancet Oncol. 2008;9(9):831–9. doi: 10.1016/S1470-2045(08)70180-3. [DOI] [PubMed] [Google Scholar]
- 43.Brunstein CG, Barker JN, Weisdorf DJ, Defor TE, McKenna D, Chong SY, et al. Intra-BM injection to enhance engraftment after myeloablative umbilical cord blood transplantation with two partially HLA-matched units. Bone Marrow Transplant. 2009;43(12):935–40. doi: 10.1038/bmt.2008.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Majhail NS, Brunstein CG, Wagner JE. Double umbilical cord blood transplantation. Curr Opin Immunol. 2006;18(5):571–5. doi: 10.1016/j.coi.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 45.Kang HJ, Kho SH, Jang MK, Lee SH, Shin HY, Ahn HS. Early engraftment kinetics of two units cord blood transplantation. Bone Marrow Transplant. 2006;38(3):197–201. doi: 10.1038/sj.bmt.1705423. [DOI] [PubMed] [Google Scholar]
- 46.Gutman JA, Turtle CJ, Manley TJ, Heimfeld S, Bernstein ID, Riddell SR, et al. Single unit dominance following double unit umbilical cord blood transplantation coincides with a specific CD8+ T cell response against the non-engrafted unit. Blood. 2010;115(4):757–67. doi: 10.1182/blood-2009-07-228999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berglund S, Okas M, Gertow J, Uhlin M, Mattsson J. Stable mixed donor-donor chimerism after double cord blood transplantation. Int J Hematol. 2009;90(4):526–31. doi: 10.1007/s12185-009-0398-y. [DOI] [PubMed] [Google Scholar]
- 48.Kristt D, Gesundheit B, Stein J, Shapira MY, Or R, Amar A, et al. Quantitative monitoring of multi-donor chimerism: a systematic, validated framework for routine analysis. Bone Marrow Transplant. 2010;45(1):137–47. doi: 10.1038/bmt.2009.120. [DOI] [PubMed] [Google Scholar]
- 49.Yen HJ, Chiou TJ, Hung GY, Chang CY, Hsieh MY, Tzeng CH, et al. Long-term mixed full-donor chimerism with dominance reversion after a double-unit cord blood transplant. Eur J Haematol. 2008;80(4):366–7. doi: 10.1111/j.1600-0609.2008.01033.x. [DOI] [PubMed] [Google Scholar]
- 50.Scaradavou A, Smith KM, Hawke R, Schaible A, Abboud M, Kernan NA, et al. Cord blood units with low CD34+ cell viability have a low probability of engraftment after double unit transplantation. Biol Blood Marrow Transplant. 2010;16(4):500–8. doi: 10.1016/j.bbmt.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verneris MR, Wagner JE. UCB and atmospheric noise. Blood. 2010;115(4):754–5. doi: 10.1182/blood-2009-11-251819. [DOI] [PubMed] [Google Scholar]
- 52.Yahata T, Ando K, Miyatake H, Uno T, Sato T, Ito M, et al. Competitive repopulation assay of two gene-marked cord blood units in NOD/SCID/gammac(null) mice. Mol Ther. 2004;10(5):882–91. doi: 10.1016/j.ymthe.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 53.Eldjerou LK, Chaudhury S, Baisre-de Leon A, He M, Arcila ME, Heller G, et al. An in vivo model of double-unit cord blood transplantation that correlates with clinical engraftment. Blood. 2010;116(19):3999–4006. doi: 10.1182/blood-2010-03-276212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mold JE, Michaëlsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322(5907):1562–5. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willemze R, Rodrigues CA, Labopin M, Sanz G, Michel G, Socié G, et al. Eurocord-Netcord and Acute Leukaemia Working Party of the EBMT. KIR-ligand incompatibility in the graft-versus-host direction improves outcomes after umbilical cord blood transplantation for acute leukemia. Leukemia. 2009;23(3):492–500. doi: 10.1038/leu.2008.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brunstein CG, Wagner JE, Weisdorf DJ, Cooley S, Noreen H, Barker JN, et al. Negative effect of KIR alloreactivity in recipients of umbilical cord blood transplant depends on transplantation conditioning intensity. Blood. 2009;113(22):5628–34. doi: 10.1182/blood-2008-12-197467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim DW, Chung YJ, Kim TG, Kim YL, Oh IH. Cotransplantation of third-party mesenchymal stromal cells can alleviate single-donor predominance and increase engraftment from double cord transplantation. Blood. 2004;103(5):1941–8. doi: 10.1182/blood-2003-05-1601. [DOI] [PubMed] [Google Scholar]
- 58.Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM, et al. Co-Transplantation of HLA-Haploidentical, Bone Marrow Derived Mesenchymal Stem Cells Prevents Graft Failure and Improves Hematological Recovery in T-Cell Depleted Haploidentical Stem Cell Transplantation. Blood (ASH Annual Meeting Abstracts) 2007;110:3073. [Google Scholar]
- 59.Wang CY, Tan H, Huang ZQ, Li HM, Liu D, Zheng RH. Study on Effect Hemopoiesis Reconstituted of Auto-Mesenchymal Stromal Cells or Allo-Mesenchymal Stromal Cells Transplantion. Blood (ASH Annual Meeting Abstracts) 2007;110:5056. [Google Scholar]
- 60.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–84. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 61.Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217(2):318–24. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 63.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 64.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 65.Nilsson SK, Johnston HM, Coverdale JA. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97(8):2293–9. doi: 10.1182/blood.v97.8.2293. [DOI] [PubMed] [Google Scholar]
- 66.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–6. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 67.Verneris MV, Brunstein C, DeFor TE, Barker J, Weisdorf DJ, Blazar BR, et al. Risk of relapse after umbilical cord blood transplantation (UCBT) in patients with acute leukemia: marked reduction in recipients of two units. Blood. 2005;106:305. [Google Scholar]
- 68.Cahu X, Rialland F, Touzeau C, Chevallier P, Guillaume T, Delaunay J, et al. Infectious complications after unrelated umbilical cord blood transplantation in adult patients with hematologic malignancies. Biol Blood Marrow Transplant. 2009;15(12):1531–7. doi: 10.1016/j.bbmt.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 69.Montesinos P, Sanz J, Cantero S, Lorenzo I, Martín G, Saavedra S, et al. Incidence, risk factors, and outcome of cytomegalovirus infection and disease in patients receiving prophylaxis with oral valganciclovir or intravenous ganciclovir after umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2009;15(6):730–40. doi: 10.1016/j.bbmt.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 70.Patel KJ, Rice RD, Hawke R, Abboud M, Heller G, Scaradavou A, et al. Pre-Engraftment Syndrome after Double Unit Cord Blood Transplantation: A Distinct Syndrome not Associated with Acute Graft-Versus-Host Disease. Biol Blood Marrow Transplant. 2010;16(3):435–40. doi: 10.1016/j.bbmt.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ballen KK, Cutler C, Yeap BY, McAfee SL, Dey BR, Attar EC, et al. Donor-derived second hematologic malignancies after cord blood transplantation. Biol Blood Marrow Transplant. 2010;16(7):1025–31. doi: 10.1016/j.bbmt.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goldstein G, Elhasid R, Bielorai B, et al. Similar engraftment kinetics of cord blood transplantations of double units in adults, versus single unit in children. Bone Marrow Transplant. 2009;43:S109–S110. [Google Scholar]
- 73.Brunstein CG, Gutman JA, DeFor TE. Reduced relapse and similar progression-free survival after Double Umbilical Cord Blood Transplantation (DUCBT): Comparison of outcomes between sibling, unrelated adult and unrelated DUCB hematopoietic stem cell (HSC) donors. Blood (ASH Annual Meeting Abstracts) 2009;114(22):622. [Google Scholar]
- 74.Majhail NS, Mothukuri JM, Macmillan ML, Verneris MR, Orchard PJ, Wagner JE, et al. Costs of hematopoietic cell transplantation: comparison of umbilical cord blood and matched related donor transplantation and the impact of posttransplant complications. Biol Blood Marrow Transplant. 2009;15(5):564–73. doi: 10.1016/j.bbmt.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 75.Micklethwaite KP, Savoldo B, Hanley PJ, Leen AM, Demmler-Harrison GJ, Cooper LJ, et al. Derivation of human T lymphocytes from cord blood and peripheral blood with antiviral and antileukemic specificity from a single culture as protection against infection and relapse after stem cell transplantation. Blood. 2010;115(13):2695–703. doi: 10.1182/blood-2009-09-242263. [DOI] [PMC free article] [PubMed] [Google Scholar]