Abstract
During 2009, a new strain of A/H1N1 influenza appeared and became pandemic. A prospective study was performed to collect data regarding risk factors and outcome of A/H1N1 in hematopoietic stem cell transplant recipients. Only verified pandemic A/H1N1 influenza strains were included: 286 patients were reported, 222 allogeneic and 64 autologous recipients. The median age was 38.3 years and the median time from transplant was 19.4 months. Oseltamivir was administered to 267 patients and 15 patients received zanamivir. One hundred and twenty-five patients (43.7%) were hospitalized. Ninety-three patients (32.5%) developed lower respiratory tract disease. In multivariate analysis, risk factors were age (OR 1.025; 1.01–1.04; P=0.002) and lymphopenia (OR 2.49; 1.33–4.67; P<0.001). Thirty-three patients (11.5%) required mechanical ventilation. Eighteen patients (6.3%) died from A/H1N1 infection or its complications. Neutropenia (P=0.03) and patient age (P=0.04) were significant risk factors for death. The 2009 A/H1N1 influenza pandemic caused severe complications in stem cell transplant recipients.
Keywords: H1N1, influenza, pandemic, HSCT
Introduction
A new influenza A/H1N1strain was recognized in early 2009 and developed into a pandemic with severe disease documented in risk groups.1–4 Hematopoietic stem cell transplant (HSCT) recipients were at greater risk for complications from seasonal influenza than the general population, especially before the introduction of neuraminidase inhibitors.5–7 Annual vaccination is, therefore, recommended for HSCT patients, their family, and the hospital staff caring for them.8 Neuraminidase inhibitors have become widely used for therapy, and higher doses and longer duration of antiviral treatment might be needed in transplant recipients.9
The WHO recently declared the end of the A/H1N1 pandemic. However, the pandemic influenza A/H1N1 virus is still circulating and is included in the 2010/2011 trivalent vaccine. Vaccine responses may be suboptimal after HSCT10–12 including the responses to the 2009 A/H1N1 influenza vaccine.13 In the summer of 2009, two separate surveys were designed to gather information on A/H1N1 infected HSCT patients to characterize the illness and determine risk factors for severe complications.
Design and Methods
Data was collected through two prospective surveys; one from the European Group for Blood and Marrow Transplantation (EBMT) and the other from the Infectious Complications Subcommittee of the Spanish Group of Haematopoietic Stem Cell Transplantation (GETH). All centers belonging to the two organizations were invited to take part. Data were collected from July 2009 through May 2010. While not identical, both surveys collected information through standardized case-collection forms regarding patients’ characteristics and their treatment, factors determining their immunosuppressed state, characteristics of the influenza infection, complications, vaccination status, antiviral therapy and outcome. All patients had given their consent to the data collection. Institutional review board approval was sought as required locally at participating centers. Only patients with molecular proof of pandemic influenza A/H1N1 were included. Oseltamivir resistance was assessed when feasible and as clinically indicated by nucleic acid sequencing of the A/H1N1 neuraminidase gene.
Lower respiratory tract (LRT) disease was defined as presence of pulmonary infiltrates and/or hypoxemia. Cause of death was assessed by the local investigator. Lymphopenia was defined as less than 0.3×109/L and neutropenia as less than 0.5×109/L. Data were collected in Stockholm for the EBMT and in Madrid for the GETH patients. All data were merged and analyzed in Stockholm. Proportions were compared using the χ2 test. Univariate analysis was used to identify factors that led to LRT disease, ventilator use or death. Multivariate analysis was performed with backwards stepwise logistic regression modeling using Statistica version 9.0.
Results and Discussion
Two hundred and eighty-six patients were included in the study; 222 had undergone allogeneic and 64 autologous HSCT. The median age was 38.3 years (range 3.3–72.3). Age distribution is shown in Table 1. Forty-seven patients had received bone marrow, 231 peripheral stem cells, and 8 cord-blood grafts. One hundred and thirty patients had HLA-identical sibling donors, 11 other family donors, and 81 unrelated donors.
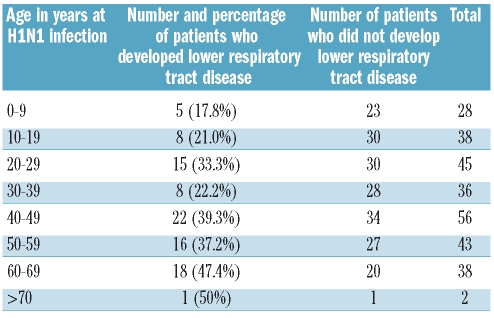
Table 1.
Distribution of the frequency of lower respiratory tract disease by patient age group.
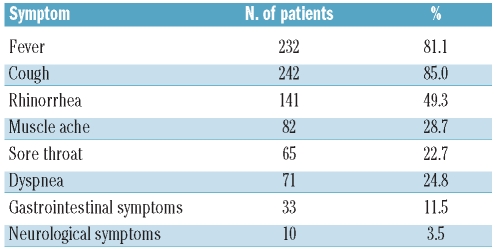
The median time from HSCT was 19.4 months (0–204.9). Symptoms of the infection are shown in Table 2. One hundred and twenty-five patients (43.7%) were or became hospitalized due to the A/H1N1 infection. Thirty-three patients were reported as having developed the A/H1N1 infection while in hospital (nosocomial infection).
Table 2.
Symptoms of H1N1 infection.
One hundred and five patients (36.7%) had received influenza vaccine: 94 patients (32.9%) had been vaccinated against seasonal influenza. Thirty-three patients (11.5%) had received at least one dose of a 2009 A/H1N1 vaccine; 6 had received 2 doses. Twenty-two patients (7.0%) had received both seasonal and A/H1N1 vaccines. Two hundred and sixty-seven patients (93.3%) received oseltamivir therapy. Fifteen patients were given zanamivir (5.2%).
Lower respiratory tract disease occurred in 93 patients (32.5%); 68 of 222 allogeneic and 25 of 64 autologous patients. The frequency in children under the age of ten years was 17.8%. The risk thereafter increased with age so that LRT developed in 19 of 40 (47.5%) patients over the age of 60 years (Table 1). In univariate analysis, age, lymphopenia, neutropenia, nosocomial acquisition, and ongoing immunosuppressive therapy were significant factors for LRT disease while type of transplant (allogeneic vs. autologous), donor type, and time between HSCT and A/H1N1 infection analyzed as a continuous variable had no significant impact. However, patients infected with H1N1 less than 12 months after HSCT had a higher risk for LRT (49%) compared to those who developed H1N1 later (23%). When analyzed together in univariate analysis, vaccination against A/H1N1 and/or seasonal influenza lowered the risk for LRT (P=0.045) but when exposure to either A/H1N1 or seasonal influenza vaccine was analyzed separately there was no impact.
In multivariate analysis, patient age (OR 1.025; 1.01–1.04; P=0.002) and lymphopenia (OR 2.49; 1.33–4.67; P<0.001) were significant risk factors for LRT disease. Vaccination had no protective effect in the multivariate model (OR 0.98 (CI 0.52; 1.83); P=NS). Splitting the cohorts into allogeneic and autologous HSCT recipients, significant risk factors in the allogeneic group were age (OR 1.02; 1.01–1.04; P=0.006), lymphopenia (OR 2.8; 1.4–3.9; P<0.001), and having an unrelated or mis-matched family donor (OR 1.8; 1.1–3.0; P=0.02), No significant risk factor was found among autologous HSCT recipients.
Thirty-three patients required mechanical ventilation (11.5%): 25 of the 222 allogeneic transplant patients and 8 of the 64 autologous transplant patients. The patients requiring mechanical ventilation were significantly older than those who did not (median age 53.5 vs. 35.0 years; P=0.007). In the total cohort, age, lymphopenia, nosocomial acquisition, and neutropenia were risk factors for mechanical ventilation in univariate analysis. Age (OR 1.02; 1.00–1.04; P=0.03), and nosocomial acquisition (OR 1.7; 1.03–2.9; P=0.04) remained independent risk factors in the multivariate analysis while lymphopenia were of borderline significance (1.7; 1.0–2.8; P=0.050). In the allogeneic HSCT recipient cohort, age (P=0.039) and lymphopenia (P=0.002) were significant risk factors in the multivariate analysis.
Overall, 26 patients died during follow up. Eighteen (6.3%) died from A/H1N1 infection or its complications: 11 of the 222 allogeneic transplant patients and 7 of the 64 autologous transplant patients (P=NS). Sixteen patients died from respiratory failure while 2 patients died due to neurological disease. The median time from HSCT in the cases of fatality was 13 months (0–184 months). Univariate risk factors for death were age, neutropenia, nosocomial acquisition, and lymphopenia. In the multivariate analysis, neutropenia (P<0.01) and patient age (P=0.04) were significant risk factors for death from A/H1N1 influenza.
Seven strains of 75 tested (9.3% of tested strains; 2.4% of the entire population) were shown to be oseltamivir-resistant. Six of these 7 patients had LRT disease (P=0.005), 5 required mechanical ventilation (P<0.001), and 5 patients died, 3 of them from A/H1N1 infection or its complications (P<0.01).
Kumar et al. has reported data on 237 solid organ transplant (SOT) recipients with pandemic A/H1N1 infection.3 Their results showed a high risk (31.7%) of pneumonia with 15.6% of recipients requiring admission to intensive care units. Our results in HSCT recipients were similar with 32.5% of patients developing pneumonia and 11.5% needing mechanical ventilation. The risk of death from A/H1N1 infection or its complications was, however, somewhat higher in our study than that reported by Kumar et al. (6.3% vs. 4.2%). A small study from Australia showed a high mortality rate (21.9%) in hospitalized allogeneic HSCT patients and patients with malignancy.14 Our corresponding proportion was 14.5%. Before the availability of neuraminidase inhibitors, mortality from influenza A in HSCT recipients was reported to be up to 15%.5,7 In more recent studies, both morbidity and mortality rates have fallen, suggesting a positive effect of neuraminidase-inhibitor therapy.6,15 The rapid increase in the rate of oseltamivir resistance in non-pandemic H1N1 has, however, become a major concern.16
The design for both the EBMT and the GETH studies probably identified the more severe cases, since patients with milder infections presumably did not seek medical attention as often as those with more significant symptoms. The denominator of A/H1N1-infected transplant recipients is, therefore, difficult to determine. It is, however, likely that the allogeneic HSCT recipients were more likely to be diagnosed and reported than those who had undergone autologous HSCT: we received five times as many reports for the former, despite the fact that similar numbers of allogeneic and autologous transplants are performed annually.17 It is likely that the fact that we found no difference in outcome between the two cohorts represents a reporting bias. Many patients were diagnosed quite late after HSCT. This could be due to several reasons, including the general advice given to these patients to contact their physicians with the onset of significant infections and the fact that these patients are prone to more severe infections. In fact, the longest time from transplant to fatal A/H1N1 infection was 184 months after HSCT.
We found age and lymphopenia to be risk factors for pneumonia, mechanical ventilation and death. Studies show that the likelihood of severe complications from the pandemic increased with age, presumably representing the presence of concomitant diseases. Similar results were found in the Kumar study.3 Two small studies of children with malignancies infected with the A/H1N1 pandemic strain also suggest relatively mild disease.18,19 Lymphopenia has been identified as a risk factor for LRT disease, both for influenza and for infections with other respiratory viruses.5 Although we found that neutropenia was a significant risk factor for death, this was not the case for pneumonia or for mechanical ventilation. This could be due to an increased risk of secondary bacterial infections. Among allogeneic patients, a transplant from an unrelated or mismatched family donor was a risk factor for LRT disease. This is recognized in other viral infections due to delayed immune reconstitution as compared with patients receiving HLA-matched sibling donor grafts. Interestingly, having an unrelated donor in allogeneic HSCT and lower lymphocyte counts were recently found to be associated with poor antibody response to 2009 A/H1N1 vaccination.13 In our study, it was not possible to analyze in sufficient detail the impact of acute or chronic graft-versus-host disease which may, of course, influence the likelihood of lymphopenia. The same probably applies to ongoing immunosuppressive therapy, which was a risk factor in our univariate but not in our multivariate analysis.
Oseltamivir therapy initiated within 48 h of symptom onset was associated with reduced risk both for pneumonia3,4 and for the need for intensive care.3 While most patients received antiviral therapy, we have no information about time to initiation. It is logical, however, that rapid antiviral treatment is at least as important after HSCT as after organ transplantation, despite the absence of formal, prospective, controlled studies. Although the rates of oseltamivir resistance have until now been quite low for the pandemic A/H1N1 strain,20 it is worthy of note that we found a relatively high incidence and this also seemed to affect the severity of the disease in these patients. However, it is likely that severe disease prompted testing for resistance. Thus, our finding does not necessarily imply that oseltamivir resistant pandemic A/H1N1 strains are more pathogenic. Another report shows a high incidence of oseltamivir resistance in hematology/oncology patients21 suggesting that immunosuppressed patients are more at risk of having resistant strains.
Vaccination is the key preventive measure against influenza and is recommended both by health authorities and by scientific organizations.8,22 Experience from the A/H1N1 pandemic, however, shows the difficulty of rapidly producing and delivering a pandemic strain vaccine. An important issue is, therefore, whether the seasonal vaccine had any effect. Unfortunately, our multivariate analysis did not show any protective effect from either the seasonal or the A/H1N1 vaccines against the more severe complications. The possibility of a protective effect of the seasonal vaccine has been suggested by an early study from Mexico23 and findings of cross-reacting antibodies in some but not all studies.24 It should be noted that most patients receiving A/H1N1 vaccine only received a single vaccination and the interval between vaccination and infection was short (data not shown). No conclusions regarding efficacy can, therefore, be drawn.
Our study shows that new influenza A strains can cause significant morbidity and even mortality in HSCT recipients, and its impact would have been devastating had the strain been more pathogenic. Strategies must, therefore, be in place as soon as possible during the early phase of influenza pandemics and large outbreaks of seasonal influenza for management of high-risk individuals.
Acknowledgments
We are grateful to the investigators and institutions contributing data to the study (see Online Appendix) and to Carmen Ruiz de Elvira and Virginie Chesnel for providing data management support from the EBMT registry.
Appendix
Participating investigators and institutions
M Abecasis, Inst. Portugues Oncologia, Lisbon, Portugal; A Ahmed, King Hussein Cancer Centre, Amman, Jordan; M Batle Massana, Hospital Universitari Germans Trias i Pujol, Barcelona, Spain; T Carrascosa Vallejo, Hospital Galdakao, Vizcaya, Spain; L Castagna, Institut Paoli Calmettes, Marseille, France; C Cordonnier, Henri Mondor Teaching Hospital, Créteil, France; L Cudillo, Policlinico Universitario Tor Vergata, Rome, Italy; E Dammann, Hannover Medical School, Hannover, Germany; R de la Camara, J Cannata-Ortiz, A García Noblejas, Hospital “La Princesa, Madrid, Spain; N de las Heras, Hospital de León, León, Spain; J de la Rubia, University Hospital “La Fe”, Valencia, Spain; W Dengel, Universitätsklinikum, Ulm, Germany; A Devos, Antwerp University Hospital, Antwerp, Belgium; H Einsele, Julius Maximilian University, Würzburg, Germany; D Engelhard, R Or, Hadassah University Hospital, Jerusalem, Israel; I Espigado, F de la Cruz-Vicente, on behalf of the Andalucia SCT Commission, Hospital Universitario Virgen del Rocío, Spain; J Finke, University Hospital, Freiburg, Germany; C Girmenia, Univ. “La Sapienza”, Rome, Italy; J Gaertner, Klinikum Nürnberg, Nürnberg, Germany; M Gonzalez-Vicent, Á Lassaletta, University Hospital “Niño Jesús”, Madrid, Spain; A Grassi, Ospedali Riuniti, Bergamo, Italy; B Patriño Guiterrez, Hospital Duran i Reynals, Barcelona, Spain; J Halter, H Hirsch, Kantonspital, Basel, Switzerland; M Helweg, University Hospital Schleswig-Holstein Kiel Campus, Kiel, Germany; E Holler, University Hospital, Regensburg, Germany; M Itälä-Remes, University Hospital, Tampere, Finland; S Leveille, Hôpital Robert Debre, Paris, France; P Ljungman, L Perez-Bercoff, Karolinska University Hospital, Stockholm, Sweden; J López, University Hospital “Ramón y Cajal”, Madrid, Spain; J Maertens, University Hospital Gasthuisberg, Leuven, Belgium; J Martínez, University Hospital “Doce de Octubre”, Madrid, Spain; R Martino, Hospital Santa Creu i Sant Pau, Barcelona, Spain; B Mohty, University Hospital, Geneva, Switzerland; J Monserrat, University Hospital “Virgen de la Arrixaca”, Murcia, Spain; JB Nieto Campuzano, I Heras, University Hospital “Morales Meseguer”, Murcia, Spain; M Paulussen University Childrens Hospital, Basel, Switzerland; H Petersen, Rigshospitalet, Copenhagen, Denmark; M Rovira, University Hospital “Clinica”, Barcelona, Spain; I Ruiz, T Olivé, University Hospital “Valle de Hebrón”, Barcelona, Spain; P Sedlacek, University Hospital Motol, Prague, Czech Republic; D Serrano, University Hospital “Gregorio Marañón”, Madrid, Spain; A Severino, Ospedale S. Camillo, Rome, Italy; S Sica, Universita Cattolica S. Cuore, Rome, Italy; A Sonet, UCL Mont-Godinne, Yvoir, Belgium; A Spyridonidis, University Medical School, Patras, Greece; J Styczynski, University Hospital, Bydgoszcz, Poland; G Sucak, Gazi University Hospital, Ankara, Turkey; K Takács, St. László Hospital, Budapest, Hungary; C Theuser, University Hospital, Dresden, Germany; P Topoglu, Ankara University School of Medicine, Ankara, Turkey; A Ullmann, Johannes-Gutenberg-University, Mainz, Germany; C Vallejo, University Hospital “Central de Asturias”, Oviedo, Spain; L Vázquez, University Hospital “Clínico”, Salamanca, Spain; A Wahlin, University Hospital, Umeå, Sweden; T van der Wal, University Medical Center, Groningen, The Netherlands; KN Ward, University College London, Windeyer Institute of Medical Sciences, London, UK; T Zuckerman, Rambam Medical Center, Haifa, Israel;
Footnotes
Funding: the study was funded by the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation (EBMT) and the Infectious Complications Subcommittee of the GETH (Spanish Group of Hematopoietic Stem-cell Transplantation). KNW acknowledges funding from University College London/University College London Hospitals Comprehensive Biomedical Research Centre. The study was presented at the 50th ICAAC, Boston, MA, USA, Sept 12-15, 2010.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Bautista E, Chotpitayasunondh T, Gao Z, Harper SA, Shaw M, Uyeki TM, et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. The New Engl J Med. 2010;362(18):1708–19. doi: 10.1056/NEJMra1000449. [DOI] [PubMed] [Google Scholar]
- 2.Hajjar LA, Mauad T, Galas FR, Kumar A, da Silva LF, Dolhnikoff M, et al. Severe novel influenza A (H1N1) infection in cancer patients. Ann Oncol. 2010;21(12):2333–41. doi: 10.1093/annonc/mdq254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar D, Michaels MG, Morris MI, Green M, Avery RK, Liu C, et al. Outcomes from pandemic influenza A H1N1 infection in recipients of solid-organ transplants: a multicentre cohort study. Lancet Infect Dis. 2010;10(8):521–6. doi: 10.1016/S1473-3099(10)70133-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi SM, Boudreault AA, Xie H, Englund JA, Corey L, Boeckh M. Differences in clinical outcomes following 2009 influenza A/H1N1 and seasonal influenza among hematopoietic cell transplant recipients. Blood. 2011;117(19):5050–6. doi: 10.1182/blood-2010-11-319186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ljungman P, Ward KN, Crooks BN, Parker A, Martino R, Shaw PJ, et al. Respiratory virus infections after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2001;28(5):479–84. doi: 10.1038/sj.bmt.1703139. [DOI] [PubMed] [Google Scholar]
- 6.Nichols WG, Guthrie KA, Corey L, Boeckh M. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis. 2004;39(9):1300–6. doi: 10.1086/425004. [DOI] [PubMed] [Google Scholar]
- 7.Whimbey E, Elting LS, Couch RB, Lo W, Williams L, Champlin RE, et al. Influenza A virus infections among hospitalized adult bone marrow transplant recipients. Bone Marrow Transplant. 1994;13(4):437–40. [PubMed] [Google Scholar]
- 8.Ljungman P, Cordonnier C, Einsele H, Englund J, Machado CM, Storek J, et al. Vaccination of hematopoietic cell transplant recipients. Bone Marrow Transplant. 2009;44(8):521–6. doi: 10.1038/bmt.2009.263. [DOI] [PubMed] [Google Scholar]
- 9.Kumar D, Morris MI, Kotton CN, Fischer SA, Michaels MG, Allen U, et al. Guidance on novel influenza A/H1N1 in solid organ transplant recipients. Am J Transplant. 2010;10(1):18–25. doi: 10.1111/j.1600-6143.2009.02960.x. [DOI] [PubMed] [Google Scholar]
- 10.Engelhard D, Nagler A, Hardan I, Morag A, Aker M, Baciu H, et al. Antibody response to a two-dose regimen of influenza vaccine in allogeneic T cell-depleted and autologous BMT recipients. Bone Marrow Transplant. 1993;11(1):1–5. [PubMed] [Google Scholar]
- 11.Pauksen K, Linde A, Hammarstrom V, Sjolin J, Carneskog J, Jonsson G, et al. Granulocyte-macrophage colony-stimulating factor as immunomodulating factor together with influenza vaccination in stem cell transplant patients. Clin Infect Dis. 2000;30(2):342–8. doi: 10.1086/313663. [DOI] [PubMed] [Google Scholar]
- 12.Yalcin SS, Kondolot M, Albayrak N, Altas AB, Karacan Y, Kuskonmaz B, et al. Serological response to influenza vaccine after hematopoetic stem cell transplantation. Ann Hematol. 2010;89(9):913–8. doi: 10.1007/s00277-009-0897-1. [DOI] [PubMed] [Google Scholar]
- 13.Engelhard D, Zakay-Rones Z, Shapira MY, Resnick I, Averbuch D, Grisariu S, et al. The humoral immune response of hematopoietic stem cell transplantation recipients to AS03-adjuvanted A/California/7/2009 (H1N1)v-like virus vaccine during the 2009 pandemic. Vaccine. 2011;29(9):1777–82. doi: 10.1016/j.vaccine.2010.12.113. [DOI] [PubMed] [Google Scholar]
- 14.Denholm JT, Gordon CL, Johnson PD, Hewagama SS, Stuart RL, Aboltins C, et al. Hospitalised adult patients with pandemic (H1N1) 2009 influenza in Melbourne, Australia. Med J Aust. 2010;192(2):84–6. doi: 10.5694/j.1326-5377.2010.tb03424.x. [DOI] [PubMed] [Google Scholar]
- 15.Machado CM, Boas LS, Mendes AV, da Rocha IF, Sturaro D, Dulley FL, et al. Use of Oseltamivir to control influenza complications after bone marrow transplantation. Bone Marrow Transplant. 2004;34(2):111–4. doi: 10.1038/sj.bmt.1704534. [DOI] [PubMed] [Google Scholar]
- 16.Besselaar TG, Naidoo D, Buys A, Gregory V, McAnerney J, Manamela JM, et al. Widespread oseltamivir resistance in influenza A viruses (H1N1), South Africa. Emerg Infect Dis. 2008;14(11):1809–10. doi: 10.3201/eid1411.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gratwohl A, Brand R, Apperley J, Crawley C, Ruutu T, Corradini P, et al. Allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia in Europe 2006: transplant activity, long-term data and current results. An analysis by the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Haematologica. 2006;91(4):513–21. [PubMed] [Google Scholar]
- 18.Launes C, Rives S, Catala A, Berrueco R, Toll T, Camos M, et al. Pandemic influenza A (2009 H1N1) in children with acute lymphoblastic leukaemia. British J Haematol. 2010;149(6):874–8. doi: 10.1111/j.1365-2141.2010.08178.x. [DOI] [PubMed] [Google Scholar]
- 19.Caselli D, Carraro F, Castagnola E, Ziino O, Frenos S, Milano GM, et al. Morbidity of pandemic H1N1 influenza in children with cancer. Pediatr Blood Cancer. 2010;55(2):226–8. doi: 10.1002/pbc.22619. [DOI] [PubMed] [Google Scholar]
- 20.Janies DA, Voronkin IO, Studer J, Hardman J, Alexandrov BB, Treseder TW, et al. Selection for resistance to oseltamivir in seasonal and pandemic H1N1 influenza and widespread co-circulation of the lineages. Int J Health Geogr. 2010;9:13. doi: 10.1186/1476-072X-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tramontana AR, George B, Hurt AC, Doyle JS, Langan K, Reid AB, et al. Oseltamivir resistance in adult oncology and hematology patients infected with pandemic (H1N1) 2009 virus, Australia. Emerg Infect Dis. 2010;16(7):1068–75. doi: 10.3201/eid1607.091691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1–62. [PubMed] [Google Scholar]
- 23.Garcia-Garcia L, Valdespino-Gomez JL, Lazcano-Ponce E, Jimenez-Corona A, Higuera-Iglesias A, Cruz-Hervert P, et al. Partial protection of seasonal trivalent inactivated vaccine against novel pandemic influenza A/H1N1 2009: case-control study in Mexico City. BMJ. 2009;339:b3928. doi: 10.1136/bmj.b3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, et al. Cross reactive anti-body responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361(20):1945–52. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]