Abstract
In this issue of Molecular Cell, Echtenkamp et al. (2011) show that the molecular chaperone Sba1/p23, thought to function primarily as a key modulator of the HSP90 chaperone complex, also operates in its own sphere of influence outside of its obligations to HSP90.
Preview:
In order for a cell to function effectively, the proteins within it must be properly folded and assembled. Molecular chaperones are a unique set of proteins that facilitate protein folding and assembly of protein complexes, and they are essential for cellular homeostasis, differentiation, proliferation, and protection from proteotoxic stress. Many molecular chaperones accomplish their task by utilizing a network of ancillary components, often referred to as co-chaperones. The Heat Shock Protein 90 (HSP90) chaperone complex interacts with an array of signal transducers and is comprised of two dimerized HSP90 protomers surrounded by an ever-changing cloud of co-chaperones. These co-chaperones vary in task from recruiting client proteins to modulating the ATPase cycle that drives HSP90’s protein folding ability. Because of its identification as both an anti-cancer and anti-infection molecular target, HSP90 and its interactome have received intense scrutiny in recent years (Trepel et al., 2010; Zhao et al., 2005). However, full characterization of its many co-chaperones, in particular whether they may have activities that are independent of HSP90, has been less well developed. In this issue of Molecular Cell, Echtenkamp et al provide the first extensive interactome analysis of the HSP90 co-chaperone Sba1 in yeast (a homolog of the mammalian protein prostaglandin E synthase [PGES]/p23) (Echtenkamp et al., 2011). The data presented provide evidence that Sba1/p23 possesses its own unique set of interacting proteins, independent of HSP90, and suggests that the biological role of Sba1/p23 is significantly more complex than previously appreciated.
Originally discovered to be an essential component of the minimal in vitro system required to chaperone steroid hormone receptors, Sba1/p23 is now understood to help couple HSP90 ATPase activity to client protein folding. This is accomplished by its binding to the ATP-engaged N-terminal domain of HSP90 to stabilize a high-affinity client binding conformation, at the same time slowing the hydrolysis of ATP to increase the dwell time of the client protein in the HSP90 chaperone complex. While bound to HSP90, Sba1/p23 also antagonizes the binding of HSP90 inhibitors. The smallest of all known HSP90 co-chaperones, Sba1/p23 is composed of a simple molecular structure consisting of a compact 8 beta-strand anti-parallel sandwich followed by an acidic C-terminal tail (Felts et al., 2003; Ali et al., 2006). This Sba1/p23 structure is conserved from yeast to humans with orthologs in both plants and protozoa.
Existing evidence also suggests that Sba1/p23 functions as a molecular chaperone independent of HSP90. It can suppress the aggregation of experimentally denatured proteins, and has been shown to remain associated with client proteins after they have been released from the HSP90 chaperone complex (Felts et al., 2003). One of the more interesting functions being studied for mammalian p23 is its role in prostaglandin synthesis. In this capacity, p23 (with the aid of HSP90) catalyzes the isomerization of the cyclo-oxygenase metabolite PGH2 to PGE2 (Tanioka et al., 2003). PGE2 production has a variety of physiological effects, but when present in the blood in sufficient amounts it stimulates systemic fever via the hypothalamus. Currently it is not known if this isomerase activity is conserved in species other than mammals, and to what extent it is necessary for other aspects of Sba1/p23 chaperone activity.
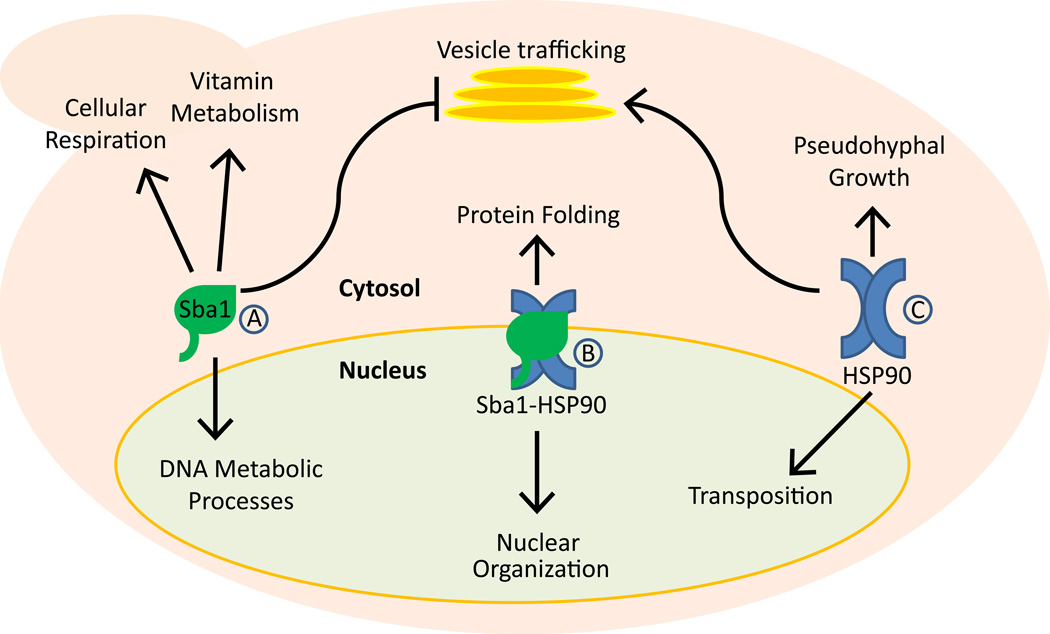
Echtenkamp et al have identified the cellular interactome for yeast Sba1 using a combination of genetic and proteomic high-throughput techniques along with bioinformatic gene ontology analysis (Echtenkamp et al., 2011). The function of Sba1/p23 in newly identified cellular pathways was then assessed using classical bench techniques in both yeast and mammalian cell culture models. Results indicate that Sba1/p23 not only functions independently of HSP90 in many cases, but even opposes HSP90 in certain signaling pathways, such as vesicle trafficking, for example. These observations are supported by a recent bioinformatic study of gene expression patterns in yeast in which HSP90 and Sba1/p23 were individually perturbed either pharmacologically or genetically (Echeverria et al., 2011). Echtenkamp et al also found that Sba1 strongly associated with certain cellular processes, such as vitamin metabolism and respiration, whereas HSP90 did not. In contrast, HSP90 strongly associated with psuedohyphal growth and DNA transposition, and Sba1 did not. In addition, this work provides further evidence that Sba1/p23 and HSP90 are major effectors in the nucleus (Freeman & Yamamoto, 2002), although they frequently interact with different components of the same signaling pathway or process (see Figure 1).
Figure 1.
A brief overview of the Sba1/p23 chaperone network in budding yeast. A schematic representation of the biological processes in yeast that are associated by either genetic or proteomic methods with Sba1 alone (A), HSP90 alone (C), or with the HSP90-Sba1 complex (B). In some cases (e.g., vesicle trafficking), the two chaperones functionally oppose each other’s activities. The small and large pink ovals together represent a single budding yeast cell (the small oval represents a budding daughter cell).
The discovery that a large portion (approximately 69–75%) of the Sba1/p23 interactome in yeast is not shared with HSP90 raises some interesting questions. Specifically, how is p23 distributed among its various interacting partners in the cell and does it biophysically interact with these partners (including HSP90) in a uniform manner? Further, calculations by D. Picard (University of Geneva) indicate that approximately 90% of p23 in HeLa cells colocalizes with HSP90. In yeast, the ratio of Sba1 to Hsp82 (yeast HSP90) is approximately 1:17 (Picard, 2006). Taken at face value, these calculations have difficulty conforming to the current finding that only 25–31% of the Sba1 interactome overlaps either genetically or physically with the yeast HSP90 interactome. However, Echetenkamp et al provide several examples showing that Sba1 and yeast HSP90 might simultaneously but separately interact with adjacent components of multi-protein complexes, suggesting that a simple comparison of their overlapping interactomes may underestimate the degree of their co-localization in the cell (Echtenkamp et al., 2011).
Nonetheless, it seems likely that the relationship and interaction frequency of Sba1/p23 with HSP90 in complex metazoans may differ significantly from what is seen in yeast, and perhaps in other single-celled eukaryotes also. Certainly, the client protein sets for p23 and Sba1 differ in mammals and yeast. For example, in mammals p23 interaction with HSP90 is required for steroid hormone receptor activity, while yeast do not naturally express these proteins (although Sba1 enhances the activity of exogenously expressed steroid hormone receptors in yeast) (Felts et al., 2003). Further, Sba1 is a non-essential gene in yeast while p23 gene knock-out is perinatally lethal in mice (Grad et al., 2006), suggesting that the Sba1/p23 clientele in mammals, whether involving HSP90 or not, has evolved beyond that of yeast.
If Sba1/p23 chaperoning activity emerged independently from HSP90 in single-celled eukaryotes and then followed a parallel evolutionary path, it is possible that an expanded requirement for control of numerous complex and overlapping signaling networks in metazoans, necessary for the ordered development of tissues with specialized functions, resulted in an increased need for a more coordinated chaperone machinery, and thus favored increased utilization of p23 as a cochaperone modifier of the HSP90 multi-chaperone complex. Whether this hypothesis proves to have validity and also pertains to other co-chaperones, such as kinase-specific Cdc37/p50, will require further investigation. However, the study by Echetenkamp et al provides a strong basis for a more detailed understanding of the role of Sba1/p23 in maintaining cellular homeostasis in yeast, and other single-celled and multicellular eukaryotes (Echtenkamp et al., 2011).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ali MM, Roe SM, Vaughan CK, Meyer P, Panaretou B, Piper PW, et al. Nature. 2006;440:1013–1017. doi: 10.1038/nature04716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria PC, Forafonov F, Pandey DP, Muehlebach G, Picard D. BioData Min. 2011;4:15. doi: 10.1186/1756-0381-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtenkamp FJ, Zelin E, Oxelmark E, Woo JI, Andrews BJ, Garabedian M, et al. Mol. Cell …. 2011 doi: 10.1016/j.molcel.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felts SJ, Toft DO. p23, a simple protein with complex activities. Cell Stress Chaperones. 2003;8:108–113. doi: 10.1379/1466-1268(2003)008<0108:paspwc>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman BC, Yamamoto KR. Science. 2002;296:2232–2235. doi: 10.1126/science.1073051. [DOI] [PubMed] [Google Scholar]
- Grad I, McKee TA, Ludwig SM, Hoyle GW, Ruiz P, Wurst W, et al. Mol. Cell Biol. 2006;26:8976–8983. doi: 10.1128/MCB.00734-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D. Exp. Cell Res. 2006;312:198–204. doi: 10.1016/j.yexcr.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Tanioka T, Nakatani Y, Kobayashi T, Tsujimoto M, Oh-ishi S, Murakami M, et al. Biochem. Biophys. Res. Commun. 2003;303:1018–1023. doi: 10.1016/s0006-291x(03)00470-4. [DOI] [PubMed] [Google Scholar]
- Trepel J, Mollapour M, Giaccone G, Neckers L. Nat. Rev. Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Davey M, Hsu YC, Kaplanek P, Tong A, Parsons AB, et al. Cell. 2005;120:715–727. doi: 10.1016/j.cell.2004.12.024. [DOI] [PubMed] [Google Scholar]