Abstract
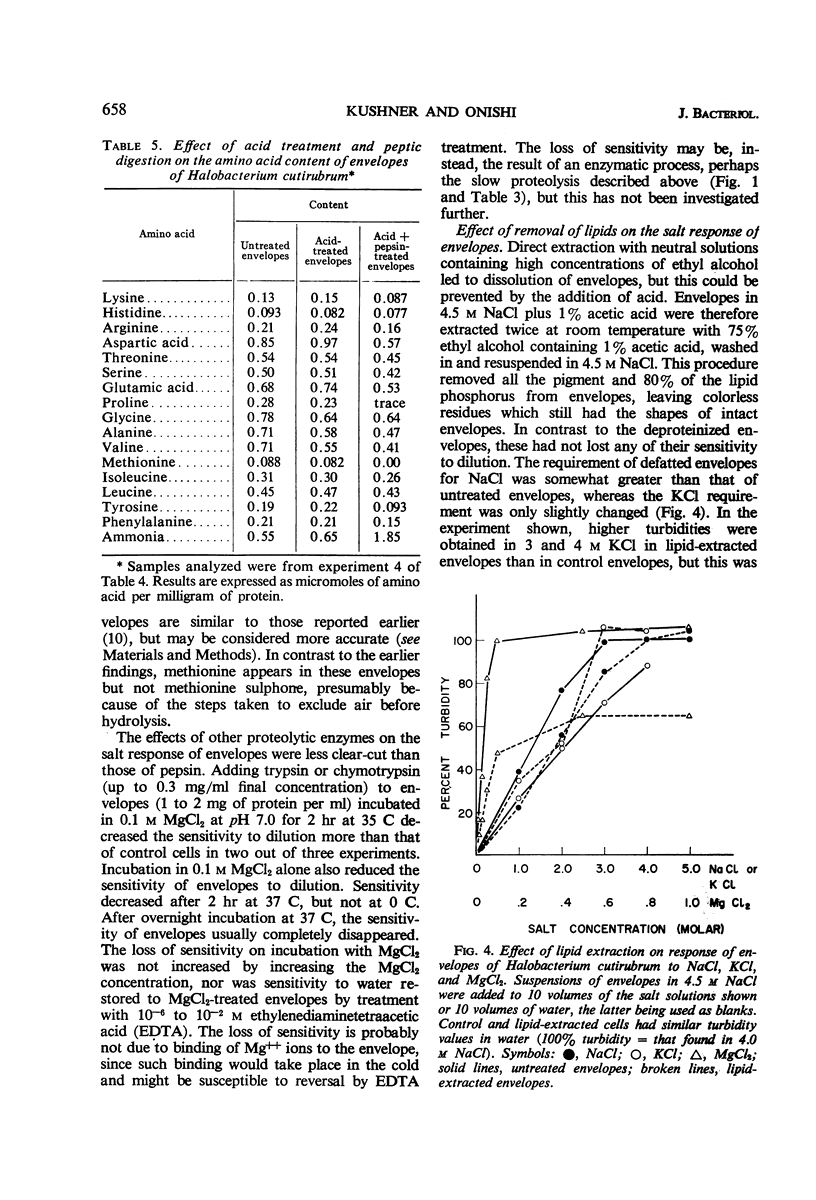
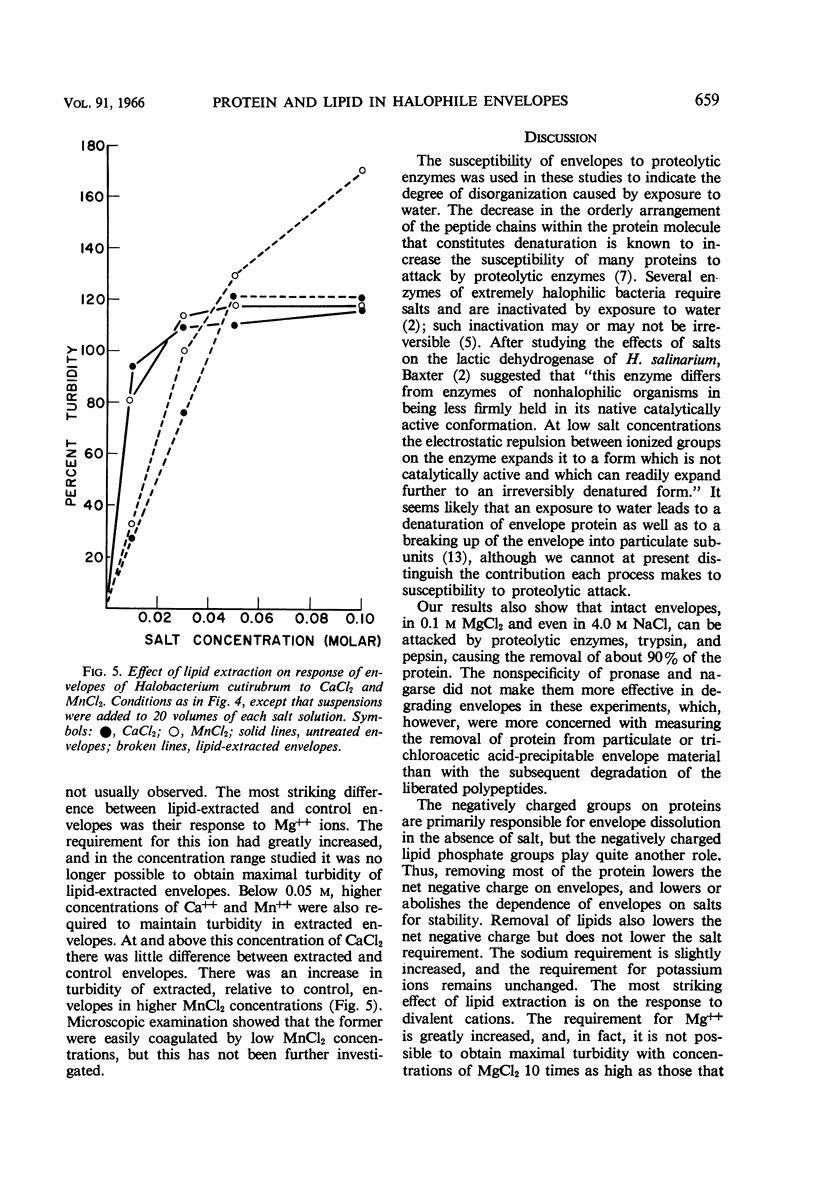
Kushner, D. J. (National Research Council, Ottawa, Ontario, Canada), and H. Onishi. Contribution of protein and lipid components to the salt response of envelopes of an extremely halophilic bacterium. J. Bacteriol. 91:653–660. 1966.—Removal of protein from envelopes of Halobacterium cutirubrum by peptic digestion left residues that required little or no salt for stability. The salt requirement of envelopes was also lowered by incubation in 0.1 m MgCl2, and could be lowered even further by digestion with trypsin or chymotrypsin in 0.1 m MgCl2. Dissolution of envelopes in low salt concentrations made their protein more susceptible to attack by these and other proteolytic enzymes. Removal of lipids raised the requirement for divalent cations, particularly for Mg++; it slightly increased the Na+ requirement and did not affect the requirement for K+. It was concluded that the requirement for high salt concentrations in extreme halophiles is due to mutual repulsion between negatively charged groups on proteins rather than to repulsion between negatively charged phosphate groups on the lipids. The latter act primarily as sites on which divalent cations, especially Mg++ which is required in high concentrations by growing cells, are bound. In this manner, the phosphate groups support envelope structure.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAM D., GIBBONS N. E. Turbidity of suspensions and morphology of red halophilic bacteria as influenced by sodium chloride concentration. Can J Microbiol. 1960 Oct;6:535–543. doi: 10.1139/m60-062. [DOI] [PubMed] [Google Scholar]
- BAXTER R. M. An interpretation of the effects of salts on the lactic dehydrogenase of Halobacterium salinarium. Can J Microbiol. 1959 Feb;5(1):47–57. doi: 10.1139/m59-006. [DOI] [PubMed] [Google Scholar]
- BROWN A. D. THE PERIPHERAL STRUCTURES OF GRAM-NEGATIVE BACTERIA.IV. THE CATION-SENSITIVE DISSOLUTION OF THE CELL MEMBRANE OF THE HALOPHILIC BACTERIUM, HALOBACTERIUM HALOBIUM. Biochim Biophys Acta. 1963 Nov 29;75:425–435. doi: 10.1016/0006-3002(63)90630-9. [DOI] [PubMed] [Google Scholar]
- Hill R. L. Hydrolysis of proteins. Adv Protein Chem. 1965;20:37–107. doi: 10.1016/s0065-3233(08)60388-5. [DOI] [PubMed] [Google Scholar]
- KATES M., YENGOYAN L. S., SASTRY P. S. A DIETHER ANALOG OF PHOSPHATIDYL GLYCEROPHOSPHATE IN HALOBACTERIUM CUTIRUBRUM. Biochim Biophys Acta. 1965 Apr 5;98:252–268. doi: 10.1016/0005-2760(65)90119-0. [DOI] [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- KUSHNER D. J., BAYLEY S. T., BORING J., KATES M., GIBBONS N. E. MORPHOLOGICAL AND CHEMICAL PROPERTIES OF CELL ENVELOPES OF THE EXTREME HALOPHILE, HALOBACTERIUM CUTIRUBRUM. Can J Microbiol. 1964 Jun;10:483–497. doi: 10.1139/m64-058. [DOI] [PubMed] [Google Scholar]
- Kushner D. J. Lysis and dissolution of cells and envelopes of an extremely halophilic bacterium. J Bacteriol. 1964 May;87(5):1147–1156. doi: 10.1128/jb.87.5.1147-1156.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Onishi H., Kushner D. J. Mechanism of dissolution of envelopes of the extreme halophile Halobacterium cutirubrum. J Bacteriol. 1966 Feb;91(2):646–652. doi: 10.1128/jb.91.2.646-652.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]



