Abstract
Background
Endothelial dysfunction contributes to the development of atherosclerosis in patients with diabetes mellitus, but the mechanisms of endothelial dysfunction in this setting are incompletely understood. Recent studies have shown altered mitochondrial dynamics in diabetes mellitus with increased mitochondrial fission and production of reactive oxygen species (ROS). We investigated the contribution of altered dynamics to endothelial dysfunction in diabetes.
Methods and Results
We observed mitochondrial fragmentation (P=0.002) and increased expression of fission-1 protein (Fis1, P<0.0001) in venous endothelial cells freshly isolated from patients with diabetes mellitus (n=10) compared to healthy controls (n=9). In cultured human aortic endothelial cells exposed to 30 mM glucose, we observed a similar loss of mitochondrial networks and increased expression of Fis1 and dynamin-related protein-1 (Drp1), proteins required for mitochondrial fission. Altered mitochondrial dynamics was associated with increased mitochondrial ROS production and a marked impairment of agonist-stimulated activation of endothelial nitric oxide synthase (eNOS) and cGMP production. Silencing Fis1 or DRP1 expression with siRNA blunted high glucose-induced alterations in mitochondrial networks, ROS production, eNOS activation, and cGMP production. An intracellular ROS scavenger provided no additional benefit, suggesting that increased mitochondrial fission may impair endothelial function via increased ROS.
Conclusions
These findings implicate increased mitochondrial fission as a contributing mechanism for endothelial dysfunction in diabetic states.
Keywords: endothelium, mitochondria, mitochondrial dynamics, reactive oxygen species
Type 2 diabetes mellitus is an increasingly prevalent risk factor for atherosclerotic cardiovascular disease.1 A key mechanism in atherogenesis is endothelial dysfunction, which is characterized by decreased nitric oxide bioavailability and development of an inflammatory phenotype that promotes atherosclerosis.2 An improved understanding of the mechanisms of endothelial dysfunction could stimulate new approaches for the prevention and management of diabetic cardiovascular disease.
Prior studies implicate increased oxidative stress as a primary mechanism of endothelial dysfunction in diabetes mellitus.3 Exposure of cultured endothelial cells or isolated arterial tissue to high glucose concentrations increases production of reactive oxygen species (ROS) that decrease nitric oxide bioavailability and increase expression of pro-inflammatory genes.2,3 Several enzymatic sources of ROS have been implicated in diabetes mellitus, including NADPH oxidase, aldose reductase, and components of the mitochondrial electron transport chain.4–7
There is growing appreciation of the importance of altered mitochondrial dynamics in diabetes mellitus.8 Mitochondria undergo cycles of fusion to form networks and fission to form smaller individual mitochondria.8,9 Proteins controlling fusion include mitofusin-1 and -2 (Mfn-1, 2) and optic atropy-1 (Opa1). Fission is regulated by dynamin-related protein-1 (Drp1) and fission-1 (Fis-1). Fusion may be beneficial by allowing for distribution of metabolites, proteins, and DNA throughout the network. At the end of their life cycle, dysfunctional mitochondria and damaged mitochondrial components are eliminated by fission and subsequent autophagy.10 Under pathological conditions, including diabetes, fission is increased and autophagy is impaired, which leads to a loss of mitochondrial networks, accumulation of small dysfunctional mitochondria, and increased mitochondrial ROS.11
Prior studies have demonstrated a loss of mitochondrial networks under hyperglycemic conditions in a variety of cell types, including islet cells,12,13 hepatocytes,11 skeletal muscle cells,14,15 circulating blood mononuclear cells,16 and endothelial cells.17–19 The functional consequences of altered mitochondrial dynamics in diabetes mellitus, however, remains incompletely understood in the human vasculature. The present study was designed to investigate the contribution of altered mitochondrial dynamics to increased ROS production and impaired nitric oxide bioavailability under diabetic conditions.
Methods
Study Subjects
Adult patients with Type 2 diabetes mellitus and healthy volunteers were recruited at Boston Medical Center by advertisement. Diabetes mellitus was defined as fasting glucose ≥126 mg/dl or ongoing treatment for Type 2 diabetes mellitus. Healthy volunteers were taking no medications and had blood pressure <140/90 mmHg, fasting LDL cholesterol <160 mg/dl, fasting glucose <100 mg/dl, and had never smoked or had stopped smoking for more than a year prior to enrollment. Fasting glucose and lipids were measured in the Boston Medical Center Clinical Laboratory. The study protocol was approved by the Boston Medical Center Institutional Review Board and all participants provided written informed consent.
Non-Invasive Vascular Function Testing
Brachial artery flow-mediated dilation was measured in each patient as previously described.20,21 Briefly, Doppler flow signals and two-dimensional images were recorded from the brachial artery before and one minute after induction of reactive hyperemia by 5-minute cuff occlusion of the upper arm. We simultaneously measured endothelial vasodilator function in fingertip vessels using digital pulse amplitude tonometry (Endo-PAT, Itamar Medical Ltd., Caesarea, Israel).22
Materials and Reagents
Please see supplementary materials for a list of the sources for materials and reagents for the endothelial cell experiments.
Fresh Isolation of Endothelial Cells from Study Subjects
Endothelial collection was performed as previously described.23 Briefly, local anesthesia was administered and an 18 or 20 gauge catheter was placed in an arm vein using sterile technique. Endothelial cells were collected by gently abrading the luminal surface of the vein with a 0.018” J-wire. For immunofluorescence experiments, endothelial cells were recovered from the wire using a dissociation buffer, fixed with 4% paraformaldehyde, and plated on poly-lysine coated slides (Sigma). Fixed slides were stored at −80°C until further processing. For live cell studies, recovered endothelial cells were allowed to adhere to poly-lysine coated glass bottom dishes (MatTek, Ashland, MA, USA) for six hours in EGM-2 medium (Lonza Inc., Walkersville, MD, USA) prior to imaging.
Endothelial Cell Culture
Human aortic endothelial cells (HAEC’s) were purchased from Lonza, Inc. and maintained using the EGM-2 Bullet Kit media (Lonza, Inc.) containing 5 mM glucose. Cells were cultured according to the manufacturer’s instructions at 37°C with 5% CO2. For immunofluorescence experiments, cells were grown in BD Falcon 4-well chambered slides (BD Biosciences). The effects of elevated glucose on endothelial cell function were investigated by incubating cells in EGM-2 media with a final glucose concentration of 30mM (540 mg/dL). This concentration is relevant to diabetic patients with severe hyperglycemia and has been used in many prior studies of the vascular effects of high glucose concentrations. To control for the osmotic effects of high glucose, we also completed studies using EGM-2 medium containing 5 mM glucose and 25 mM mannitol. Cells were stored at −80°C until immunofluorescence, protein, or gene expression measurements were performed.
Immunofluorescence Staining and Fluorescence Microscopy
Fixed samples were rehydrated with phosphate-buffered saline (PBS) containing 50mM glycine and permeablized using 0.1% Triton X-100. After blocking non-specific binding sites with 0.5% bovine serum albumin, slides were incubated with two of the following primary antibodies: anti-von Willebrand’s factor (vWF) 1:200, anti-cytochrome C 1:300, anti-cytochrome c oxidase-IV (COX IV) 1:400, anti-Fis1 (1:100) or anti-phosphorylated endothelial nitric oxide synthase (eNOS) 1:200. Slides were incubated with corresponding Alexa Fluor-488 and Alexa Fluor-594 secondary antibodies and mounted using Vectasheild containing the nuclear stain DAPI (Vector Laboratories). All staining was performed in one batch to avoid any day-today variability in staining sessions.
Slides were examined using a fluorescence microscope (Nikon Eclipse TE2000-E) at 100x magnification and cellular images were digitally captured by a Photometric CoolSnap HQ2 Camera (Photometrics, Tucson, AZ, USA). All images were captured at the same exposure time and presented values are corrected for local background fluorescence. For the freshly isolated patient samples, endothelial cells were distinguished from other cells by vWF staining. Images were captured and intensity was measured using NIS Elements AR Software (Nikon Instruments Inc, Melville, NY, USA).
Assessment of Mitochondrial Networks
We used a semi-quantitative scale to rate the extent of mitochondrial networks in patient cells and in HAEC’s under different experimental conditions. Cells were stained for cytochrome c and evaluated by two blinded observers. Mitochondrial network extent was graded on a scale from 0 to 3, where 0 = wholly fragmented or punctuated mitochondria and 3 = clearly defined mitochondrial networks. The average score from 20 cells from each patient or condition was determined by two people, read separately and the readings were averaged. The coefficient of variation was 7.5% (n=20 cells, measured 3 times) for repeated assessment of cells under the same experimental condition.
We also performed live cell imaging to further evaluate mitochondrial morphology. For these experiments, mitochondria were labeled with 100nM MitoTracker Green FM (Invitrogen) in HEPES-buffered physiologic salt solution (HB-PSS) in accordance with manufacturer’s recommendations. Images were captured at 100x magnification.
Mitochondrial ROS Production
Mitochondrial ROS production was measured using fluorescence microscopy in live cells. HAECs were incubated with 5μM MitoSox and 100nM MitoTracker Green FM (Invitrogen) for 30 minutes at 37°C and imaged according to the manufacturer’s instructions (excitation/emission 510/580 nm). Co-localization of MitoSox and MitoTracker Green signal verified that ROS signal was localized to the mitochondria. Thirty cells were analyzed per experiment to obtain an average intensity value. In some experiments the contribution of intracellular ROS to vascular dysfunction was evaluated by treating cells with a superoxide scavenger, tiron (5mM). The coefficient of variation for ROS measurement was 9.7%.
RNA Isolation and Quantitative Gene Expression
Total RNA was isolated from HAEC’s using the miRNeasy Mini kit (Qiagen, Inc.) according to the manufacturer’s instructions. RNA was reverse transcribed to complimentary DNA (cDNA) using TaqMan Reverse Transcription Reagents followed by cDNA preamplification using TaqMan PreAmp Master Mix (Applied Biosystems) according to the manufacturer’s instructions. Quantitative real-time PCR was performed using Taqman Gene Expression Assays (see supplementary materials) and the following protocol: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, 60°C for 1 min. Using GAPDH as a loading control, results were interpreted by the relative quantity method (ΔΔCt).
Protein Isolation
Collected cell pellets were resuspended and briefly sonicated in a cell lysis buffer (Cell Signaling) containing 1% protease inhibitor cocktail (Sigma). Cell lysates were spun at 10,000 rpm for 10 min at 4°C, and the resulting supernatants were stored at −80°C. Protein concentration was determined by the Pierce BCA protein assay (Thermo Fisher Scientific).
Western Blot Analysis
Proteins were subjected to 8–12% SDS-PAGE and transferred to PVDF membranes (GE Healthcare). Membranes were initially blocked (PBS, 0.1% Tween 20, 5% nonfat dry milk) for 1 hour. Membranes were probed in blocking buffer containing one of the following primary antibodies: anti-OPA1, anti-Drp1, anti-Mfn2 or anti-Fis1 antibody followed by the appropriate HRP-conjugated secondary antibody. Immunoreactions were visualized with Amersham ECL Plus Western Blotting Detection Reagents (GE Healthcare). Membranes were stripped (62.5 mM Tris-HCl (pH 6.8), 100 mM β-mercaptoethanol, 2% SDS) for 30 min at 50°C and reprobed using anti-actin antibodies to verify equal protein loading. Resulting bands were quantified by densitometry.
Assessment of eNOS Activation and Endothelial cyclic GMP Production
eNOS activation was assessed by measuring phosphorylation of eNOS at serine 1177. After exposure of HAEC’s to 5 mM or 30 mM glucose for 24 hours, cells were incubated with the calcium ionophore A23187 (1 μM), acetylcholine (1 μM), insulin (10 nM), or vehicle for five minutes. Cells were then fixed and stained for phosphorylated-eNOS as described above.
Bioactivity of endothelium-derived nitric oxide was assayed as change in cyclic-guanosine monophosphate (cGMP) concentration as previously described.24 Briefly, cells were equilibrated for 30 min with 200 μM 3-isobutyl-1-methylxanthine (IBMX) to inhibit phosphodiesterases. Cells were then exposed to A23187 or vehicle for 5 min. Cells were lysed and cell supernatants and protein pellets were stored at −80°C prior to processing. Supernatant cGMP was determined using a commercially available EIA kit (Cayman Chemical) and corrected for total cell protein.
Suppression of Gene Expression using siRNA
HAEC’s were transfected with double-stranded siRNA targeting Fis1 or Drp1 using siPORT NeoFX Transfection Agent (Applied Biosystems) according to the manufacturer’s instructions. Forty-eight hours after transfection, cells were incubated with medium containing 5mM or 30mM glucose for 24 hours. Cells were then processed as described above for assessment of mitochondrial network extent, protein expression, gene expression, or cGMP production. Non-specific scrambled siRNA and GAPDH siRNA were used as negative and positive transfection controls, respectively.
Statistical Analyses
Statistical analyses were completed using Sigma Stat Version 3.1 (Systat Software, Inc.). Clinical characteristics and vascular function in the diabetic and healthy control groups were compared using the unpaired t-test or chi-square test for continuous and categorical variables respectfully. For cell experiments, we used the Student’s T test for two-group comparisons. For experiments involving more than two groups, we used analysis of variance (ANOVA) with Student-Newman-Keuls (SNK) multiple pairwise comparisons. P<0.05 was considered to be statistically significant. Data are expressed as mean ± SEM.
Results
Study Subjects and Vascular Function
We enrolled ten patients with diabetes mellitus and nine controls. The clinical characteristics and measures of vascular function are shown in Table 1. As expected, the patients with diabetes mellitus had higher fasting glucose levels, higher body mass index, and lower HDL levels. Endothelium-dependent flow-mediated dilation of the brachial artery, and flow-induced changes in pulse amplitude in the fingertip were lower in the diabetics compared to the controls. Baseline diameter, baseline flow, and extent of reactive hyperemic flow volume were similar in the two groups, suggesting impaired flow-mediated dilation was not attributable to differences in arterial geometry or the stimulus for dilation.
Table 1.
Clinical Characteristics and Vascular Function
| Non-Diabetic(n=9) | Diabetic(n=10) | P-value | |
|---|---|---|---|
| Clinical Characteristics | |||
| Age (years) | 51 ± 12 | 53 ± 8 | 0.78 |
| Gender, Female, n (%) | 3 (33%) | 4 (40%) | 0.66 |
| Body Mass Index | 27 ± 7 | 36 ± 10 | 0.03 |
| Total Cholesterol (mg/dl) | 206 ± 43 | 179 ± 27 | 0.12 |
| LDL (mg/dl) | 113 ± 26 | 101 ± 23 | 0.28 |
| HDL (mg/dl) | 68 ± 31 | 45 ± 17 | 0.03 |
| Triglycerides | 117 ± 72 | 164 ± 74 | 0.19 |
| Glucose (mg/dl) | 89 ± 15 | 171 ± 101 | 0.001 |
| SBP (mmHg) | 121 ± 12 | 126 ± 22 | 0.74 |
| DBP (mmHg) | 71 ± 9 | 76 ± 10 | 0.25 |
| Vascular Function | |||
| Baseline Diameter (mm) | 4.55 ± 1.01 | 4.47 ± 0.81 | 0.86 |
| Baseline Flow (ml/min) | 102 ± 80 | 137 ± 74 | 0.33 |
| Hyperemic Flow (ml/min) | 1009 ± 357 | 887 ± 397 | 0.53 |
| FMD (%) | 8.7 ± 3.3 | 6.0 ± 2.3 | 0.046 |
| ln PAT Ratio | 0.69 ± 0.29 | 0.34 ± 0.40 | 0.03 |
FMD=brachial artery flow-mediated dilation expressed as percentage change from baseline and PAT ratio = pulse amplitude tonometry as described in Methods (Mean ± SD)
Mitochondrial Network Extent in Freshly Isolated Endothelial Cells
Figure 1A shows mitochondrial morphology in freshly isolated endothelial cells. Cells from the healthy controls displayed elongated, thread-like mitochondria in complex networks while cells from diabetics displayed smaller punctate mitochondria. Group data show that patients with diabetes mellitus had lower mitochondrial network extent compared to controls (0.77±0.09 vs. 1.2±0.06, P=0.002, respectively) as judged by blinded assessment of cytochrome c-stained cells (arbitrary scale 0 to 3). Figure 1B shows Fis1 protein levels in the freshly isolated endothelial cells. Diabetic individuals have significantly higher Fis1 expression compared to healthy controls (1154±60 vs. 568±68, P<0.0001, respectively). These results suggest altered mitochondrial dynamics in endothelial cells in patients with diabetes mellitus.
Figure 1.
Diabetes mellitus is associated with increased mitochondrial fission in endothelial cells. Venous endothelial cells were collected from healthy volunteers (n=9) and patients with diabetes mellitus (n=10) as described in Methods. A: Mitochondria were labeled in live endothelial cells with Mitotracker Green FM (Invitrogen) and images were captured at 100X. A representative endothelial cell from a healthy control (left) shows a complex network of threadlike mitochondria, while a representative cell from a diabetic patient (right) shows smaller, punctate mitochondria. Fixed cells were stained for cytochrome c and mitochondrial network extent was rated in a blinded manner using a semi-quantitative scale. Network extent was lower in the diabetics (*P=0.002). B: Freshly isolated human venous endothelial cells were fixed and stained for Fis1 protein expression. A representative cell from a diabetic patient (right) shows markedly higher Fis1 expression compared to a cell from a healthy control (left). Pooled data show that Fis1 levels were significantly higher in the diabetics (n=6 per group, *P<0.0001).
Effect of High Glucose on Mitochondrial Networks and Dynamic Proteins in HAEC’s
To investigate potential mechanisms and functional consequences of altered mitochondrial dynamics in the endothelium, we exposed cultured HAEC’s to 30 mM glucose as a model of hyperglycemic conditions. As shown in Figure 2A, exposure to high glucose induced a marked and sustained loss of mitochondrial networks over 24 hours. In separate experiments, exposure to 5mM glucose plus 25 mM mannitol for 24 hours had no effect on network extent, suggesting that the effect of glucose exposure is not attributable to an osmotic effect.
Figure 2.
High glucose concentration induces mitochondrial fission in endothelial cells. As described in Methods, human aortic endothelial cells were incubated with 5 mM, 30 mM glucose, or 5 mM glucose + 25 mM mannitol (as an osmotic control) for 24 hours. A. To assess network extent, cells were fixed at the indicated time points, stained for cytochrome c, and rated on a semi-quantitative scale. Left panel: High glucose was associated with a rapid and sustained loss of mitochondrial networks (interaction P<0.001 by repeated measures ANOVA). Right panel: Mannitol had no effect on network extent, arguing against an osmotic effect. B. Gene expression was assessed by quantitative real time PCR after 24 hours of glucose incubation, and there were significant increases in Fis1 and Mfn2. C. Protein expression was measured by Western blot analysis after 24 hours of glucose incubation. Expression of Fis1 and Drp1 increased significantly. There was no significant change in Mfn2 or Opa1. AU = arbitrary units. Data are shown as mean ± SEM for 3 experiments (*P<0.05 versus 5 mM glucose).
The observed change in mitochondrial morphology was accompanied by a shift in mitochondrial dynamics gene and protein expression favoring fission. Exposure to 30 mM glucose increased message (Figure 2B) and protein levels (Figure 2C) of the fission protein Fis-1. Drp1 protein expression was increased (P<0.05) and there was a trend for increased Drp1 message (P=0.07). There was a modest increase in message level (P<0.05), but no significant change in protein level of the fusion protein Mfn2 (P=0.11). There were no significant changes in message or protein expression for the fusion proteins OPA1 and MFN1.
Effect of High Glucose on Mitochondrial ROS Production, eNOS Activation and NO Production
We examined the effects of high glucose concentration on mitochondrial ROS production in HAEC’s. As shown in Figure 3, high glucose concentrations induced a marked increase in mitosox fluorescence that co-localized with Mitotracker Green fluorescence, consistent with a mitochondrial source of ROS.
Figure 3.
High glucose concentration induces mitochondrial ROS production. As described in Methods, HAEC’s were incubated with 5 mM or 30 mM glucose for 24 hours. Mitochondrial ROS production was assessed using 5 μM Mitosox (red fluorescence) and mitochondria were localized using 100 nM Mitotracker Green (green fluorescence). A. Representative fluorescence images showing increased ROS production that co-localizes with mitochondria in endothelial cells incubated with high glucose. High glucose is also associated with mitochondrial fragmentation. B. Pooled data showing that ROS production is higher in cells exposed to 30 mM glucose. Data are shown as mean ± SEM for 3 experiments (*P=0.01 versus 5 mM glucose).
We next examined the functional consequences of high glucose concentrations on eNOS activation and production of bioactive NO (Figure 4A-4C). In cells exposed to 5 mM glucose, acetylcholine, insulin, and calcium ionophore (A23187) individually activated eNOS, as reflected by an increase in eNOS phosphorylation at serine 1177. Exposure to 30 mM glucose markedly impaired eNOS phosphorylation in response to all three agonists (P<0.001 by ANOVA), consistent with impaired eNOS activation vasodilator function.
Figure 4.
Elevated glucose level impairs eNOS activation. HAEC’s were incubated with 5 mM or 30 mM glucose for 24 hours and eNOS activation was evaluated as eNOS phosphorylation in response to acetylcholine (A), insulin (B), or A23187 (C), as described in Methods. As shown, 30 mM glucose blunted the eNOS phosphorylation to each agonist (interaction P<0.001 by ANOVA). Data are mean ± SEM for 3 experiments. D. Production of bioactive nitric oxide was assessed as the relative increase in cGMP in response to A23187. As shown, 30 mM glucose blunted cGMP production (interaction P<0.001 by ANOVA; *P<0.01 versus control and versus 30 mM glucose by SNK pairwise comparison). Data are mean ± SEM for 12 experiments.
Calcium ionophore increased cGMP levels in endothelial cells exposed to 5 mM glucose (Figure 4D), consistent with production of bioactive nitric oxide that is capable of activating guanylyl cyclase. High glucose exposure blunted the cGMP response to A23187, consistent with impaired bioactivity of endothelium-derived nitric oxide (P<0.001 by ANOVA).
Silencing Fis1 or Drp1 Protects Mitochondrial Networks and Blunts ROS Production
We next sought to determine whether increased mitochondrial fission contributes to altered mitochondrial morphology and ROS production in the presence of high glucose. To test this hypothesis, we used siRNA to silence the expression of the fission proteins Fis1 or Drp1 in cultured endothelial cells. As shown in Figure 5, message levels were markedly reduced 48 hours after transfection with siRNA targeting Fis1 or Drp1, and there were corresponding decreases in protein expression.
Figure 5.
siRNA transfection effectively reduces expression of Fis1 and Drp1. HAEC’s were transfected with Fis1 or Drp1 siRNA for 48 hours as described in Methods. siRNA transfection reduced message (A and B) and protein (C and D) in HAEC’s exposed to 5 mM or 30 mM glucose (interaction P<0.01 by ANOVA; *P<0.05 for 5 mM versus 30 mM glucose and #P<0.05 for siRNA versus control by SNK pairwise comparison). Data are mean ± SEM for 3 experiments
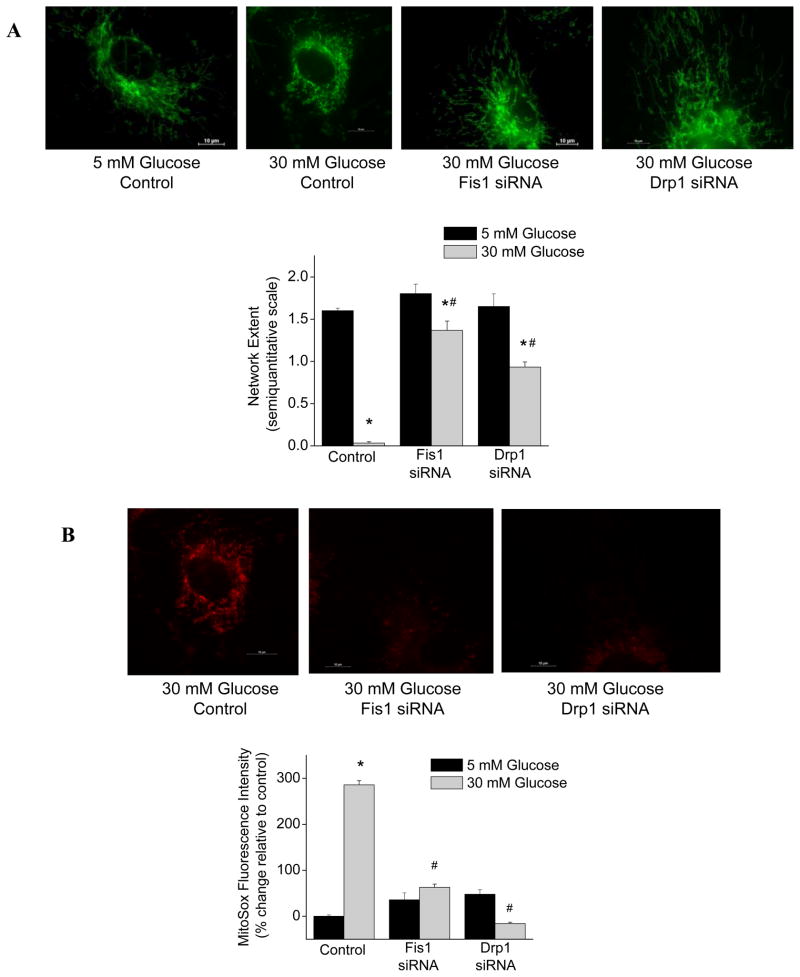
As shown in Figure 6A, silencing Fis1 or Drp1 prevented the loss of mitochondrial networks following exposure of endothelial cells to 30 mM glucose. Fis1 or Drp1 siRNA treatment did not alter mitochondrial network extent in cells exposed to 5 mM glucose. As shown in Figure 6A, silencing either of these fission proteins markedly reduced ROS production in endothelial cells exposed to 30 mM glucose. There was no effect of Fis1 or Drp1 siRNA treatment on ROS production in cells exposed to 5 mM glucose.
Figure 6.
Silencing Fis1 or Drp1 expression prevents glucose-induced loss of mitochondrial networks and glucose-induced ROS production. A. Mitochondria were imaged in HAEC’s with Mitotracker Green and representative images show that silencing either fission protein maintained mitochondrial networks under high glucose conditions. Pooled data show a protective effect on network extent (interaction P<0.001 by ANOVA). B. Mitochondrial ROS production was imaged in HAEC’s with Mitosox and representative images show that silencing either fission protein produces a marked decrease in ROS production. Pooled data confirm that Fis1 and Drp1 siRNA prevent glucose-induced increases in ROS (interaction P<0.001 by ANOVA; *P<0.001 versus 5 mM glucose and #P<0.01 versus control by SNK pairwise comparison). Data are mean ± SEM for 3 experiments.
Silencing Fis1 or Drp1 Prevents Glucose-Induced Impairment of eNOS Activation
The effects of Fis1 or Drp1 siRNA on eNOS activation are displayed in Figure 7. As shown, silencing these fission proteins did not alter the basal expression of phosphorylated eNOS. However, silencing Fis1 or Drp1 expression prevented glucose-induced impairment of eNOS phosphorylation in response to calcium ionophore, acetylcholine, and insulin. As shown in Figure 8, silencing Fis1 expression also prevented glucose-induced impairment of agonist-induced cGMP production in endothelial cells. Collectively, these results suggest that glucose-induced alterations in mitochondrial dynamics have broad effects on endothelial function.
Figure 7.
Silencing Fis1 or Drp1 expression prevents glucose-induced impairment of eNOS activation. As described in Methods, eNOS phosphorylation was measured after 5 minute exposure to acetylcholine (A), insulin (B), or A23187 (C) in the presence of 5 or 30 mM (high) glucose. Silencing either fission protein restored the eNOS phosphorylation response to each agonist with 30 mM glucose (interaction P<0.001 by ANOVA; *P<0.01 compared to control by SNK pairwise comparison). Addition of tiron (5mM) also restored the response to A23187 with 30 mM glucose, and the combination of siRNA and tiron had no additional effect. Data are shown as mean ± SEM for 3 experiments.
Figure 8.
Silencing Fis1 expression or scavenging ROS prevents glucose-induced impairment of cGMP production. As described in Methods, cGMP was measured after 5 minute exposure to A23187 in the presence of 5 or 30 mM glucose. Silencing Fis1 expression or addition of tiron (5mM) prevented endothelial dysfunction in the setting of high glucose (interaction P<0.001 by ANOVA; *P<0.01 compared to control and #P<0.05 compared to Fis1 siRNA, tiron, and siRNA + tiron by SNK pairwise comparison). The combination of Fis1 siRNA and tiron produced no additional effect compared to Fis1 siRNA alone. Data are shown as mean ± SEM for 9 to 12 experiments.
Decreased ROS May Account for the Protective Effects of Fis1 and Drp1 siRNA
We tested the hypothesis that reversal of endothelial dysfunction by inhibition of mitochondrial fission under high glucose conditions might be explained by the observed decreased in ROS. eNOS phosphorylation (Figure 7) and cGMP production (Figure 8) in response to calcium ionophore was preserved in the presence of 30 mM glucose when cells are incubated with the cell permeable ROS scavenger, tiron. A similar effect was produced by Fis1 or Drp1 siRNA. When combined with siRNA treatment, tiron had no additional benefit beyond the effect of Fis1 or Drp1 siRNA. These results are consistent with the possibility that glucose-induced alterations in mitochondrial dynamics affect eNOS activation and nitric oxide bioavailability via excess ROS.
Discussion
In this study, we observed altered mitochondrial morphology, reduced network extent, and increased Fis1 protein expression in endothelial cells from patients with diabetes mellitus with endothelial dysfunction compared to healthy volunteers. In cultured cells exposed to high glucose, we observed a similar loss of mitochondrial networks accompanied by increased expression of fission proteins, and the effect were not attributable to an osmotic effect of glucose. The observed alteration in mitochondrial dynamics was associated with increased mitochondrial ROS production and a generalized impairment in agonist-stimulated eNOS activation and nitric oxide bioavailability. Silencing Fis1 or Drp1 expression blunted glucose-induced alterations in mitochondrial networks, ROS production, eNOS activation, and nitric oxide bioavailability. An intracellular ROS scavenger provided no additional benefit, suggesting that increased mitochondrial fission may impair endothelial function via increased ROS. These findings implicate altered mitochondrial dynamics as a contributing mechanism for endothelial dysfunction in diabetic states.
Several prior studies have demonstrated altered mitochondrial dynamics in non-vascular tissue from experimental models of diabetes and patients with diabetes mellitus or insulin resistance. For example, exposure of rat hepatocytes to high glucose concentrations induces mitochondrial fragmentation and increases ROS production, and the effect can be inhibited with dominant-negative Drp1.11 Similar findings are observed in pancreatic islet cells exposed to high glucose or free fatty acids that can be prevented by Fis1 siRNA.12 Mitochondrial fragmentation and decreased expression of Mfn2 is also observed in the skeletal muscle of obese, insulin resistant rats and diabetic humans.14,25 Recently, we observed fragmented mitochondria and increased ROS production in peripheral blood mononuclear cells from patients with type 2 diabetes mellitus.16
Recent studies have shown that diabetic conditions alter mitochondrial dynamics and morphology in endothelial cells. Mitochondrial fragmentation occurs when cells from an immortalized endothelial cell line (EAhy926) are exposed to high glucose.17 Incubation of rat retinal endothelial cells with high glucose concentrations induces a loss of mitochondrial networks and increases apoptosis, which could contribute to diabetic retinopathy.18 In a recent study by Makino and colleagues, endothelial cells isolated from the coronary arteries of diabetic mice displayed fragmented mitochondria and increased ROS production.19 Those changes were associated with increased expression of Drp1 and decreased expression of OPA1, consistent with a shift toward mitochondrial fission. Drp1 siRNA prevented mitochondrial fragmentation under high glucose conditions, implicating increased fission as a cause of mitochondrial fragmentation.
The present study extends our understanding of the functional importance and clinical relevance of altered mitochondrial dynamics in the diabetic endothelium. We observed increased expression of Fis1 and a protective effect of Fis1 siRNA on mitochondrial networks in cultured human cells exposed to high glucose. We gained information about the clinical relevance of these mechanisms by showing a loss of mitochondrial networks in endothelial cells freshly isolated from diabetic patients that displayed impaired endothelium-dependent dilation. Most importantly, our study provides information about the functional consequences of altered mitochondrial dynamics in the diabetic endothelium by showing that increased mitochondrial ROS, impaired eNOS activation, and loss of nitric oxide bioavailability can be prevented by inhibiting mitochondrial fission.
Our study provides insight into the importance of mitochondrial network formation as a regulator of mitochondrial ROS generation in the endothelium. High glucose concentrations drive the electron transport chain to hyperpolarize the mitochondrial membrane and increase ROS production at complexes I and III via uncoupled respiration.7 In hepatocytes, mild membrane depolarization prevents ROS generation, but does not prevent glucose-induced mitochondrial fragmentation, suggesting that increased mitochondrial ROS is a consequence of mitochondrial fragmentation and not the cause.11 Consistent with this prior work, we observed that inhibiting Fis1 or Drp1 expression was sufficient to completely inhibit glucose-induced ROS production and network fragmentation in endothelial cells. Collectively, these findings support the idea that network formation limits mitochondrial ROS production under conditions of increased fuel, possibly by allowing for appropriate distribution of mitochondrial components, including uncoupling proteins and antioxidant enzymes.8 It also has been argued the fragmentation alters the spatial orientation of the electron transport chain enzymes in a manner that promotes uncoupled respiration and ROS production.11 On the other hand, Makino and colleagues showed that scavenging ROS prevents glucose-induced mitochondrial fragmentation in mouse endothelial cells, suggesting that ROS is a trigger for fission under these conditions.19 Further studies are needed to elucidate how high glucose concentrations stimulate mitochondrial fission and whether ROS is a cause, consequence, or exacerbating mechanism.
The present study suggests that mitochondrial fission is a major cause of endothelial dysfunction in the setting of hyperglycemia, likely via increased mitochondrial ROS. In addition to directly reacting with nitric oxide, ROS may lead to the uncoupling of eNOS, oxidation of cofactors, and oxidative modification of target enzymes.2,3 Another consequence of increased oxidative stress in the setting of high glucose may be O-linked N-acetylglycosylation of serine 1177 on eNOS, which blocks phosphorylation at this site and attenuates nitric oxide production.26 In our study, inhibiting the expression of fission proteins was sufficient to normalize agonist-induced eNOS phosphorylation and production of bioactive nitric oxide. The beneficial effects were observed when eNOS was activated by insulin, acetylcholine or calcium ionophore, suggesting that the effect did not depend on the signaling mechanism for eNOS activation. Further studies will be needed to determine whether there is a relation between mitochondrial dynamics and other enzymatic sources of ROS such as NADPH oxidase, which also has been implicated in endothelial dysfunction in diabetes5–7 and has been shown to be localized to mitochondria.27
Our study has a number of limitations. First, the sample size for the clinical study was relatively modest, which precluded multivariable analysis to adjust for confounding factors. The analyses were completed in venous, rather than arterial cells, reducing the relevance to atherosclerosis, although diabetes mellitus is a systemic disease that affects the endothelium in both arteries and veins. In addition, a prior study demonstrated significant correlations between findings in venous and arterial endothelial cells.23 Secondly, we may have overestimated the effects of high glucose on absolute mitochondrial ROS production by using an excitation wavelength of 510 nm as recommended by the manufacturer rather than 395 nm for the MitoSox experiments as has been suggested by Robinson and colleagues, although our studies focused on the relative changes in ROS according to glucose concentration.28 Further studies are merited to define interactions between mitochondrial and non-mitochondrial sources of ROS under diabetic conditions. Finally, 24-hour glucose exposure does not fully mimic the endothelial pathology observed in patients who have had diabetes mellitus for many years, although our model is well accepted for study of mechanisms of diabetic endothelial dysfunction. These limitations are balanced by the novel information about mitochondrial dynamics in endothelial cells from human subjects and evidence for a mechanistic link between mitochondrial fission and impaired eNOS activation.
Our study may have important clinical implications. A large body of work indicates that endothelial dysfunction contributes to the pathogenesis of atherosclerotic disease in diabetes, but the mechanisms of impaired nitric oxide bioavailabity in this setting remain incompletely understood. Pharmacological inhibitors of mitochondrial fission are under development.29 Identification of increased mitochondrial fission as a mechanism of endothelial dysfunction in diabetes mellitus suggests that such drugs might have utility as therapy to prevent and manage diabetic vascular disease.
Supplementary Material
Clinical Summary.
Type 2 diabetes mellitus is an increasingly prevalent risk factor for atherosclerotic cardiovascular disease, and dysfunction of the vascular endothelium contributes to the development of diabetic vascular disease. We studied a previously unrecognized mechanism of endothelial dysfunction in human diabetes mellitus, and show that an alteration in mitochondrial homeostasis is important. In addition to serving as the primary source of ATP in the cell, mitochondria also participate in many other cellular functions. In the past, mitochondrial were viewed as discrete oval shaped organelles, but recent studies have shown that mitochondrial fuse to form complex networks within the cell and that these networks are important for normal mitochondrial function. We observed that endothelial cells collected from patients with diabetes mellitus show a loss of normal networks and marked fragmentation of mitochondria. These changes were accompanied by increased levels of Fis1, a protein that controls mitochondrial fission. When commercially available endothelial cells were exposed to high glucose concentrations in tissue culture, we observed a similar loss of mitochondrial networks, increased Fis1, and impaired endothelial function. When we prevent mitochondrial fragmentation by blocking expression of Fis1, we maintain normal mitochondrial networks and prevent endothelial dysfunction. This study suggests a new target for therapy in diabetes mellitus and raises the possibility that a drug that prevents mitochondrial fission might protect against the development of vascular disease in diabetic patients. Such drugs are currently under development.
Acknowledgments
The authors gratefully acknowledge the intellectual support of the Mitochondrial Affinity Research Collaborative at the Boston University Medical Campus Evans Center for Interdisciplinary Biomedical Research.
Funding Sources
Drs. Tabit, Hamburg, and Vita receive support from the NIH-sponsored Boston University Medical Center Leadership Program in Vascular Medicine (K12 HL083781). Dr. Widlansky is supported by an NIH training grant (K23HL089326). Dr. Shirihai is supported by NIH grants DK035914 and DK074778. Dr. Vita is supported by NIH grants HL083801, HL081587, HL083269, and HL75795 and HL102299. Itamar Medical, Ltd provided the laboratory with an EndoPAT device and probes for measuring endothelial function by digital pulse amplitude tonometry.
Footnotes
Disclosures
None.
References
- 1.Center for Disease Control and Prevention. 2007 National Diabetes Fact Sheet. 2007. [Google Scholar]
- 2.Tabit CE, Chung WB, Hamburg NM, Vita JA. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord. 2010;11:61–74. doi: 10.1007/s11154-010-9134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen RA, Tong X. Vascular oxidative stress: the common link in hypertensive and diabetic vascular disease. J Cardiovasc Pharmacol. 2010;55:308–316. doi: 10.1097/fjc.0b013e3181d89670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tesfamariam B, Cohen RA. Free radicals mediate endothelial cell dysfunction caused by elevated glucose. Am J Physiol. 1992;263:H321–H326. doi: 10.1152/ajpheart.1992.263.2.H321. [DOI] [PubMed] [Google Scholar]
- 5.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 6.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–E22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- 7.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 8.Twig G, Hyde B, Shirihai OS. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta. 2008;1777:1092–1097. doi: 10.1016/j.bbabio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liesa M, Palacin M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89:799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 10.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molina AJ, Wikstrom JD, Stiles L, Las G, Mohamed H, Elorza A, Walzer G, Twig G, Katz S, Corkey BE, Shirihai OS. Mitochondrial networking protects beta cells from nutrient induced apoptosis. Diabetes. 2009;58:2303–2315. doi: 10.2337/db07-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 14.Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J, Daugaard JR, Lloberas J, Camps M, Zierath JR, Rabasa-Lhoret R, Wallberg-Henriksson H, Laville M, Palacin M, Vidal H, Rivera F, Brand M, Zorzano A. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem. 2003;278:17190–17197. doi: 10.1074/jbc.M212754200. [DOI] [PubMed] [Google Scholar]
- 15.Zorzano A, Liesa M, Palacin M. Role of mitochondrial dynamics proteins in the pathophysiology of obesity and type 2 diabetes. Int J Biochem Cell Biol. 2009;41:1846–1854. doi: 10.1016/j.biocel.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Widlansky ME, Wang J, Shenouda SM, Hagen TM, Smith AR, Kizhakekuttu TJ, Kluge MA, Gutterman DD, Vita JA. Altered mitochondrial membrane potential, mass, and morphology in the mononuclear cells of humans with type 2 diabetes. Transl Res. 2010;156:15–25. doi: 10.1016/j.trsl.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paltauf-Doburzynska J, Malli R, Graier WF. Hyperglycemic conditions affect shape and Ca2+ homeostasis of mitochondria in endothelial cells. J Cardiovasc Pharmacol. 2004;44:423–436. doi: 10.1097/01.fjc.0000139449.64337.1b. [DOI] [PubMed] [Google Scholar]
- 18.Trudeau K, Molina AJ, Guo W, Roy S. High glucose disrupts mitochondrial morphology in retinal endothelial cells: implications for diabetic retinopathy. Am J Pathol. 2010;177:447–455. doi: 10.2353/ajpath.2010.091029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makino A, Scott BT, Dillmann WH. Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologia. 2010 doi: 10.1007/s00125-010-1770-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vita JA. Nitric oxide-dependent vasodilation in human subjects. Methods Enzymol. 2002;359:186–200. doi: 10.1016/s0076-6879(02)59183-7. [DOI] [PubMed] [Google Scholar]
- 21.McMackin CJ, Vita JA. Update on nitric oxide-dependent vasodilation in human subjects. Methods Enzymol. 2005;396:541–553. doi: 10.1016/S0076-6879(05)960-16-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamburg NM, Benjamin EJ. Assessment of endothelial function using digital pulse amplitude tonometry. Trends Cardiovasc Med. 2009;19:6–11. doi: 10.1016/j.tcm.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colombo PC, Ashton AW, Celaj S, Talreja A, Banchs JE, Dubois NB, Marinaccio M, Malla S, Lachmann J, Ware JA, Le Jemtel TH. Biopsy coupled to quantitative immunofluorescence: a new method to study the human vascular endothelium. J Appl Physiol. 2002;92:1331–1338. doi: 10.1152/japplphysiol.00680.2001. [DOI] [PubMed] [Google Scholar]
- 24.Huang A, Vita JA, Venema RC, Keaney JF., Jr Ascorbic acid enhances endothelial nitric oxide synthase activity by increasing intracellular tetrahydrobiopterin. J Biol Chem. 2000;275:17399–17406. doi: 10.1074/jbc.M002248200. [DOI] [PubMed] [Google Scholar]
- 25.Bach D, Naon D, Pich S, Soriano FX, Vega N, Rieusset J, Laville M, Guillet C, Boirie Y, Wallberg-Henriksson H, Manco M, Calvani M, Castagneto M, Palacin M, Mingrone G, Zierath JR, Vidal H, Zorzano A. Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes. 2005;54:2685–2693. doi: 10.2337/diabetes.54.9.2685. [DOI] [PubMed] [Google Scholar]
- 26.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108:1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A. 2009;106:14385–14390. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, Murphy MP, Beckman JS. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci U S A. 2006;103:15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lackner LL, Nunnari J. Small molecule inhibitors of mitochondrial division: tools that translate basic biological research into medicine. Chem Biol. 2010;17:578–583. doi: 10.1016/j.chembiol.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.