Abstract
Purpose
To compare conventional laser peripheral iridotomy (LPI) and LPI combined with laser peripheral iridoplasty in eyes with primary angle closure suspect (PACS) by assessment of anterior chamber dimensional changes using a Pentacam.
Methods
Forty-eight eyes of 24 subjects with bilateral PACS were recruited consecutively. Each eye was randomly allocated to treatment with conventional LPI, argon LPI only, or LPI plus iridoplasty, which consisted of simultaneous argon LPI and peripheral iridoplasty. Anterior chamber measurements were performed on each eye using a Pentacam, both before and after treatment. Mean anterior chamber depth (ACD), anterior chamber volume (ACV), and anterior chamber angle were measured, and topographic ACD analysis was performed. Results were compared between the two treatment groups.
Results
After treatment with either conventional LPI or LPI plus iridoplasty, the mean ACD and ACV increased significantly. Topographic ACD analysis revealed that the mid-to-peripheral ACD increase was significantly greater in the LPI plus iridoplasty group than in eyes treated with conventional LPI. Intraocular pressure changes and post-LPI complications did not differ between the groups.
Conclusions
Compared with conventional LPI, our study showed that LPI plus iridoplasty improved the mid-to-peripheral ACD increase. This procedure may have a role as an adjunct for reducing angle closure by simultaneously eliminating pupillary and non-pupillary block components.
Keywords: Laser peripheral iridoplasty, Laser peripheral iridotomy, Pentacam, Primary angle closure suspect
The guidelines of the European Glaucoma Society (EGS) [1] state that if gonioscopic measurements indicate the anterior chamber angle (ACA) is in appositional contact between the iris and the posterior trabecular meshwork over at least 180°, the eye should be designated primary angle closure suspect (PACS). If peripheral anterior synechiae (PAS) are present, primary angle closure (PAC) is diagnosed. Further, if glaucomatous optic neuropathy (GON) and a corresponding visual field defect are evident in eyes with PAC, the condition is termed primary angle closure glaucoma (PACG). Acute PACG, if left untreated, can cause devastating blindness in a very short time. In some Asian countries, PACG is the most prevalent form of glaucoma, ranging from 0.12% in Singapore [2] to 2.5% in Myanmar [3]. In a population-based study [4,5], 22% of PACS patients progressed to PAC, and 28.5% of PAC patients developed PACG within 5 years if no treatment was given.
Laser peripheral iridotomy (LPI) has been the standard therapeutic modality for treatment of PACS. LPI can eliminate the pupillary block component and may widen the ACA by equilibrating the pressure between the anterior and posterior chambers. In a questionnaire-based survey conducted in Singapore [6], 84.9% of responding ophthalmologists reported that they routinely perform LPI on asymptomatic PACS patients to prevent acute angle closure. Another study found that only 9.3% of PAC patients developed PACG in the 5 years after LPI treatment and suggested that LPI appeared to alter the natural course of angle closure [7].
LPI can increase the limbal anterior chamber depth (ACD) in PACS eyes. Nevertheless, some eyes show residual angle closure even after LPI [8]. In other words, the etiology of PACS eye development may be a combination of both pupillary and non-pupillary block components. Laser peripheral iridoplasty applies a thermal energy that contracts the peripheral iris away from the trabecular meshwork, thus providing amelioration of any appositional angle closure caused by a mechanism other than pupillary block. Thus, we devised a modified LPI technique in which standard LPI was combined with laser peripheral iridoplasty to prevent pupillary block and to reduce PAS formation by widening the closure angle. The Pentacam (Oculus Inc., Wetzlar, Germany) is a rotating Scheimpflug camera that provides 50 cross-sectional images within a few seconds and has the advantage of generating both a three-dimensional model of the ACD as well as two-dimensional single scans. In the present study, we compared conventional LPI with LPI plus iridoplasty in PACS eyes with respect to the resulting anterior chamber dimensional changes visualized using a Pentacam.
Materials and Methods
This study adhered to the tenets of the Declaration of Helsinki. Patients were consecutively and prospectively enrolled from the HanGil Eye Hospital, Incheon, Republic of Korea. After written informed consent was obtained, each patient underwent slit-lamp, optic disc, and fundus examinations; intraocular pressure (IOP) measurement by Goldmann applanation tonometry (GAT); and gonioscopy employing a Sussman four-mirror goniolens.
Only subjects in whom both eyes were PACS were eligible for inclusion in the study. Based on the EGS definition described above, a PACS eye showed an appositional contact between the iris and the posterior trabecular meshwork extending over at least 180° on gonioscopic examination and an IOP ≤ 21 mmHg by GAT. Patients were excluded if they met any of the following criteria: 1) IOP > 21 mmHg by GAT; 2) PAS, defined as abnormal adhesion of the iris at any angle that was at least half a clock-hour in width and was located in the anterior trabecular meshwork or higher; 3) GON such as neuroretinal rim notching and/or thinning and/or disc hemorrhage and an associated retinal nerve fiber layer defect; 4) visual field defects indicative of GON; 5) a previous episode of acute angle closure attack; or 6) secondary angle closure.
Conventional LPI was performed on one randomly selected eye by a single glaucoma specialist (JC) using an argon laser. One week later, the complementary eye was treated with LPI combined with laser peripheral iridoplasty. Prior to each laser procedure, one drop of pilocarpine 2% and one drop of brimonidine tartrate 0.15% were instilled into the eye.
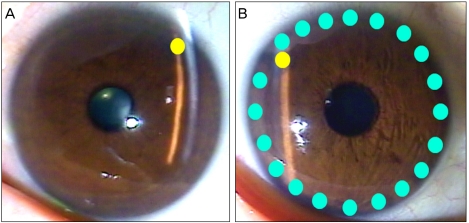
Conventional LPI used an argon laser to first create a contraction burn; the laser was activated at a setting of 200 to 250 mW with a spot size of 500 µm for 0.6 seconds. In addition, a penetrating burn was achieved using the argon laser at a setting of 1,000 mW with a spot size of 50 µm for 0.05 seconds. In LPI plus iridoplasty, the conventional procedure (described above) was followed by peripheral iridoplasty in the same session, during which the argon laser was used at a setting of 200 to 250 mW for 0.6 seconds, which created burn spots on the peripheral iris alongside the limbus. Each patient received about 20 burn spots over 360 degrees. After each procedure, prednisolone acetate 1% was instilled 4 times daily for 1 week (Fig. 1).
Fig. 1.
Conventional laser peripheral iridotomy (LPI) and the LPI combined with iridoplasty technique. (A) Conventional LPI refers to LPI alone. (B) The LPI combined with iridoplasty approach consists of LPI followed by laser peripheral iridoplasty administered in the same session. Yellow circle: LPI site, cyanine-blue circle: laser peripheral iridoplasty site; about 20 burn spots are placed alongside the limbus.
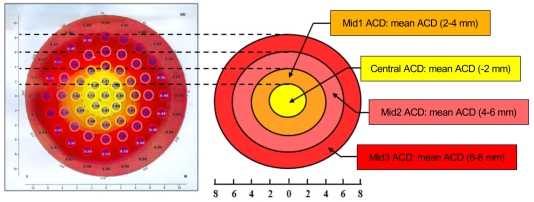
Before and 1 week after treatment with either the conventional or the LPI plus iridoplasty technique, anterior chamber volume (ACV), ACD, and ACA were analyzed using the Pentacam. To permit detailed data analysis, we devised novel advanced ACD topographic parameters based on the simple ACD output data from the Pentacam. Thus, 1) "Central ACD" was the average of ACD values taken at nine topographic points within 2 mm from the center of the eye; 2) "Mid1 ACD" was the average of ACD measurements at 12 topographic points within 2-4 mm from the center of the eye; 3) "Mid2 ACD" was the average of ACD values taken at 16 topographic points within 4 to 6 mm from the center of the eye; and 4) "Mid3 ACD" was the average of ACD measurements at 20 topographic points within 6 to 8 mm from the center of the eye (Fig. 2).
Fig. 2.
Advanced anterior chamber depth (ACD) topographic parameters derived from routine ACD data from the Pentacam output. Central ACD is the average of ACD values at nine topographic points within 2 mm from the center of the eye; Mid1 ACD is the average of ACD measurements at 12 topographic points within 2 to 4 mm from the eye center; Mid2 ACD is the average of ACD values at 16 topographic points within 4 to 6 mm from the center of the eye; Mid3 ACD is the average of ACD measurements at 20 topographic points within 6 to 8 mm from the eye center.
Changes in the basic anterior chamber parameters and advanced topographic ACD parameters, and IOP after LPI, were compared in each group using the paired t-test. Additionally, any increases in the values of the advanced topographic ACD parameters and complications after LPI were compared between the two groups employing the unpaired t-test. Transient elevation of IOP (an IOP spike) was defined as an IOP increase above 5 mmHg from baseline within 1 week of a laser procedure. In all analyses, a difference with a probability p-value < 0.05 was considered to be statistically significant.
Results
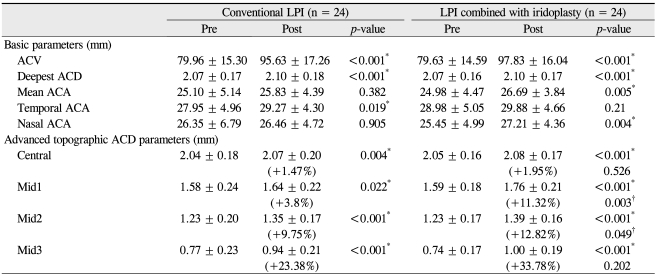
Forty-eight eyes of 24 patients were enrolled in the study; mean patient age was 59.1 ± 9.0 years (range, 40 to 74 years). Four patients were male and 20 were female. The basic parameters of ACV and deepest ACD and all of the advanced topographic ACD parameters (Central ACD, Mid1 ACD, Mid2 ACD, and Mid3 ACD) increased significantly in both the conventional and LPI plus iridoplasty groups. The extent of increase in the advanced topographic ACD parameters was compared between the groups, and an increase in the Central ACD of was found approximately 2.0% for eyes treated by LPI plus iridoplasty and approximately 1.5% for eyes that received conventional LPI; these values were not significantly different (p = 0.526). However, the increase in the Mid1 ACD was approximately 11.3% for eyes treated with LPI plus iridoplasty and 3.8% for eyes that received conventional LPI (p = 0.003). The increase in the Mid2 ACD was approximately 12.8% for eyes treated with LPI plus iridoplasty and 9.8% for eyes that received conventional LPI (p = 0.049). Although the increase in the Mid3 ACD was approximately 33.8% for eyes that received LPI plus iridoplasty and 23.4% for eyes treated with conventional LPI, the difference was not statistically significant (p = 0.202). In brief, changes in the Mid1 ACD and Mid2 ACD, namely the mid-peripheral ACD, were significantly greater for eyes treated with LPI plus iridoplasty than in those treated by conventional LPI, whereas the central and far-peripheral mid3 ACD values did not differ significantly between the two groups (Table 1).
Table 1.
Descriptive statistics for basic and advanced topographic anterior chamber depth parameters measured in the two study groups
Data are means ± standard deviations.
LPI = laser peripheral iridotomy; ACV = anterior chamber volume; ACD = anterior chamber depth; ACA = anterior chamber angle.
*p<0.05, paired t-test comparing pre- and post-LPI parameters in the conventional LPI and LPI combined with iridoplasty groups, respectively.
†p<0.05, unpaired t-test between the conventional LPI and LPI combined with iridoplasty groups with respect to increases in the levels of advanced topographic parameters after LPI.
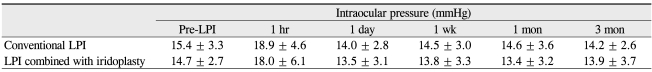
The baseline IOP did not significantly differ between eyes treated by the conventional and LPI plus iridoplasty techniques. Post-procedure IOP elevation was not significant in either group, and also did not significantly differ between the two groups during the 3 months after treatment (Table 2).
Table 2.
Intraocular pressure changes following conventional LPI and LPI combined with iridoplasty
No between-group statistical significance was apparent at any intraocular pressure measurement time point.
LPI = laser peripheral iridotomy.
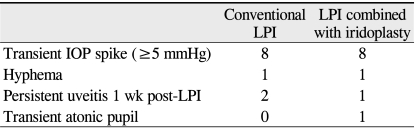
Post-procedural complications were similar in frequency and severity in the two groups. IOP spikes occurred in eight eyes from both groups. Hyphema occurred in one eye treated with conventional LPI, whereas post-laser uveitis persisted in two eyes for 1 week after conventional LPI treatment. In eyes receiving the LPI plus iridoplasty procedure, hyphema was evident in one eye, persistent post-laser uveitis was evident in one eye, and a transient atonic pupil occurred in one eye over the 3-month follow-up period (Table 3).
Table 3.
Complications after intervention in the conventional LPI and LPI combined with iridoplasty groups
Numbers refer to the number of eyes.
No statistically significant difference in complication rate or nature was found between the conventional LPI and LPI combined with iridoplasty groups.
LPI = laser peripheral iridotomy; IOP = intraocular pressure.
Discussion
Previously, Kumar et al. [9] reported that approximately 30% of PACS eyes had plateau iris prior to LPI, and this condition persisted in 75% of such eyes after LPI. Another study, in an Asian cohort, found that 30% of PACG eyes previously treated with LPI had plateau iris [10]. This implies that a mechanism other than pupillary block may play a significant role in the occurrence and progression of angle closure. One non-pupillary block mechanism seen in PACS, PAC, and PACG eyes with plateau iris is bunching of the peripheral iris resulting in closure of the anterior chamber angle despite a patient iridotomy. The suggested mechanism of this effect is an anteriorly dislocated and/or large ciliary body serves as a contact between the iris and the angle [11,12]. In such eyes, laser peripheral iridoplasty may help to eliminate appositional angle closure and widen the ACA, because iridoplasty pulls the peripheral iris away from the angle [13,14]. General indications for laser peripheral iridoplasty include the following: acute and chronic angle closure, phacomorphic glaucoma, and nanophthalmos. Further, the technique may be used as an adjunctive method to deepen the anterior chamber, facilitating selective laser trabeculoplasty [13]. One histopathologic study suggested that laser peripheral iridoplasty not only induced short-term thermal shrinkage of stromal collagen, but the contraction effect may persist long-term mediated by fibroblast-like cells [15].
In the present work, we investigated the efficacy and safety of LPI combined with iridoplasty, which consisted of simultaneous argon LPI and peripheral iridoplasty. We measured anterior chamber dimensional changes and IOP and assessed post-laser complications after application of two different laser procedures to PACS eyes. We found that the mid-peripheral ACD was significantly increased in eyes treated with LPI plus iridoplasty, without any additional complications, compared to eyes that received conventional LPI. As previously mentioned by van Herick et al. [16], far-peripheral ACD is widely regarded as one of the important risk factors for angle closure. However, increases in the far-peripheral ACD and ACA did not differ significantly between the two groups. This may be attributable to the rotating Scheimpflug camera, Pentacam. Boker et al. [17] previously reported that the Pentacam was unable to directly visualize the ACA, i.e., on Pentacam images, it is difficult to identify the most peripheral part of the iris and the base of the ACA. Because the Pentacam assumes and automatically localizes the apex of the ACA, the ACA and adjacent far-peripheral ACD measurements can be incorrect and unreliable.
Gonioscopy is the gold standard modality for assessment of ACA. However, this method is relatively subjective, and accuracy increases with the experience of the ophthalmologist. Further, it is difficult to quantitatively describe the AC morphology. Previous studies have found that ultrasound biomicroscopy may offer a detailed view of anatomical structures in the ACA [11,18]. However, this technique requires that patients are maintained in a supine position, with an ocular cup, saline bath, and probe contacting the eyeball. We analyzed anterior chamber dimensions in PACS eyes using a noncontact rotating Scheimpflug camera, the Pentacam, and estimated novel parameters representing the ACD at different locations within the eye. Kurita et al. found that the Pentacam was potentially useful for examining both PACS and PAC eyes, except for those with plateau iris, to calculate the ACV and ACD, but not ACA [19]. In addition, they found the Pentacam measurement of anterior chamber dimensions was reliable [20].
The present study has some limitations. First, the data reflect only changes in ACD, and not the detailed variations in angle structure, thus, the number of subjects in this study with plateau iris was not determined. Second, we have not shown that the LPI plus iridoplasty technique is better than conventional LPI in preventing the progression of PACS eyes to PACG. A long-term follow-up study is needed to explore whether the LPI plus iridoplasty technique is superior to the conventional LPI approach in this regard. Third, our relatively small sample size may limit data interpretation. Fourth, no study has optimized either the laser beam width or spot number when laser peripheral iridoplasty is used to treat PACS eyes. Although we found no significant difference in the extent of complications between the two groups during the short-term follow-up, laser peripheral iridoplasty can cause permanent iris burn scars which can affect pupil size or pupil constriction.
The present study is the first to explore ACD topographic changes after use of different laser techniques. Both of the methods increased the anterior chamber volume and depth in PACS eyes. However, the LPI plus iridoplasty technique was more effective in elevating the mid-peripheral ACD than was conventional LPI, while the complication rate did not differ between the two groups. In conclusion, the LPI plus iridoplasty technique may be effective and safe for inhibiting the development of unwanted anterior chamber dimensional changes in PACS eyes. Further work is needed to determine if this combined laser technique prevents the progression of PACS eyes to eyes with PAC or PACG.
Footnotes
This article was presented at the 100th Korean Ophthalmological Society Annual Meeting, November 1, 2008, Goyang, Korea.
No potential conflict of interest relevant to this article was reported.
References
- 1.European Glaucoma Society. Terminology and guidelines for glaucoma. 3rd ed. Savona: Dogma; 2008. [Google Scholar]
- 2.Shen SY, Wong TY, Foster PJ, et al. The prevalence and types of glaucoma in malay people: the Singapore Malay Eye Study. Invest Ophthalmol Vis Sci. 2008;49:3846–3851. doi: 10.1167/iovs.08-1759. [DOI] [PubMed] [Google Scholar]
- 3.Casson RJ, Newland HS, Muecke J, et al. Prevalence of glaucoma in rural Myanmar: the Meiktila Eye Study. Br J Ophthalmol. 2007;91:710–714. doi: 10.1136/bjo.2006.107573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas R, George R, Parikh R, et al. Five year risk of progression of primary angle closure suspects to primary angle closure: a population based study. Br J Ophthalmol. 2003;87:450–454. doi: 10.1136/bjo.87.4.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas R, Parikh R, Muliyil J, Kumar RS. Five-year risk of progression of primary angle closure to primary angle closure glaucoma: a population-based study. Acta Ophthalmol Scand. 2003;81:480–485. doi: 10.1034/j.1600-0420.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 6.Ang MH, Baskaran M, Kumar RS, et al. National survey of ophthalmologists in Singapore for the assessment and management of asymptomatic angle closure. J Glaucoma. 2008;17:1–4. doi: 10.1097/IJG.0b013e318133a81c. [DOI] [PubMed] [Google Scholar]
- 7.Pandav SS, Kaushik S, Jain R, et al. Laser peripheral iridotomy across the spectrum of primary angle closure. Can J Ophthalmol. 2007;42:233–237. [PubMed] [Google Scholar]
- 8.He M, Friedman DS, Ge J, et al. Laser peripheral iridotomy in primary angle-closure suspects: biometric and gonioscopic outcomes: the Liwan Eye Study. Ophthalmology. 2007;114:494–500. doi: 10.1016/j.ophtha.2006.06.053. [DOI] [PubMed] [Google Scholar]
- 9.Kumar RS, Baskaran M, Chew PT, et al. Prevalence of plateau iris in primary angle closure suspects an ultrasound biomicroscopy study. Ophthalmology. 2008;115:430–434. doi: 10.1016/j.ophtha.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 10.Kumar RS, Tantisevi V, Wong MH, et al. Plateau iris in Asian subjects with primary angle closure glaucoma. Arch Ophthalmol. 2009;127:1269–1272. doi: 10.1001/archophthalmol.2009.241. [DOI] [PubMed] [Google Scholar]
- 11.Pavlin CJ, Ritch R, Foster FS. Ultrasound biomicroscopy in plateau iris syndrome. Am J Ophthalmol. 1992;113:390–395. doi: 10.1016/s0002-9394(14)76160-4. [DOI] [PubMed] [Google Scholar]
- 12.Ritch R. Plateau iris is caused by abnormally positioned ciliary processes. J Glaucoma. 1992;1:23–26. [Google Scholar]
- 13.Ritch R, Tham CC, Lam DS. Argon laser peripheral iridoplasty (ALPI): an update. Surv Ophthalmol. 2007;52:279–288. doi: 10.1016/j.survophthal.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Ritch R, Tham CC, Lam DS. Long-term success of argon laser peripheral iridoplasty in the management of plateau iris syndrome. Ophthalmology. 2004;111:104–108. doi: 10.1016/j.ophtha.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Sassani JW, Ritch R, McCormick S, et al. Histopathology of argon laser peripheral iridoplasty. Ophthalmic Surg. 1993;24:740–745. [PubMed] [Google Scholar]
- 16.Van Herick W, Shaffer RN, Schwartz A. Estimation of width of angle of anterior chamber. Incidence and significance of the narrow angle. Am J Ophthalmol. 1969;68:626–629. doi: 10.1016/0002-9394(69)91241-0. [DOI] [PubMed] [Google Scholar]
- 17.Boker T, Sheqem J, Rauwolf M, Wegener A. Anterior chamber angle biometry: a comparison of Scheimpflug photography and ultrasound biomicroscopy. Ophthalmic Res. 1995;27(Suppl 1):104–109. doi: 10.1159/000267854. [DOI] [PubMed] [Google Scholar]
- 18.Pavlin CJ, Harasiewicz K, Foster FS. Ultrasound biomicroscopy of anterior segment structures in normal and glaucomatous eyes. Am J Ophthalmol. 1992;113:381–389. doi: 10.1016/s0002-9394(14)76159-8. [DOI] [PubMed] [Google Scholar]
- 19.Kurita N, Mayama C, Tomidokoro A, et al. Potential of the pentacam in screening for primary angle closure and primary angle closure suspect. J Glaucoma. 2009;18:506–512. doi: 10.1097/IJG.0b013e318193c141. [DOI] [PubMed] [Google Scholar]
- 20.Rabsilber TM, Khoramnia R, Auffarth GU. Anterior chamber measurements using Pentacam rotating Scheimpflug camera. J Cataract Refract Surg. 2006;32:456–459. doi: 10.1016/j.jcrs.2005.12.103. [DOI] [PubMed] [Google Scholar]