Abstract
Successful drug delivery using implantable pumps may be found in over 12,500 published articles. Their versatility in delivering continuous infusion, intermittent or complex infusion protocols acutely or chronically has made them ubiquitous in drug discovery and basic research. The recent availability of iPRECIO®, a programmable, refillable, and implantable infusion pump has made it possible to carry out quantitative pharmacology (PKPD) in single animals. When combined with specialized catheters, specific administration sites have been selected. When combined with radiotelemetry, the physiologic gold standard, more sensitive and powerful means of detecting drug induced therapeutic, and/or adverse effects has been possible. Numerous application examples are cited from iPRECIO® use in Japan, United States, and Europe with iPRECIO® as an enabling drug delivery device where the refillable and programmability functionality were key benefits. The ability to start/stop drug delivery and to have control periods prior dosing made it possible to have equivalent effects at a much lower dose than previously studied. Five different iPRECIO® applications are described in detail with references to the original work where the implantable, refillable, and programmable benefits are demonstrated with their different end-points.
Keywords: drug delivery, mini pump, osmotic pumps, implantable pump, dose response, quantitative pharmacology, preclinical, telemetry
Introduction
Following a landmark report in 1990 that stated 40% of compounds failed clinical trials because of pharmacokinetics (PK), the pharmaceutical industry responded by integrating drug metabolism and pharmacokinetic (DMPK) scientists earlier in the drug discovery process with good success (Korfmacher, 2009). Today, the pharmacological industry continues to look for ways to reduce drug candidate attrition throughout the drug discovery and development process, where unforeseen efficacy and safety issues are currently cited as the cause of continually high attrition rates (Walker, 2004; Mass et al., 2007; Wehling, 2009; Wendler and Wehling, 2010; Wittenburg and Gustafson, 2011). In the past two decades, drug discovery has gone through significant changes with the advent of genomics, combinatorial chemistry, computational modeling, and proteomics. An unprecedented abundance of targets and compounds have also contributed to a fundamental shift in evaluation of pharmaceutical properties and impact of such properties on drug development (Neervannan, 2006).
More recently, quantitative pharmacology or pharmacokinetics–pharmacodynamics (PK/PD) has recently become a major theme in many pharmaceutical research organizations, and a key part of the drug discovery process (Van Der Graaf and Gabrielsson, 2009). The integration of PK and PD has made it possible to characterize the onset, intensity, and duration of a drug’s pharmacological effects and relate these effects to the drug’s mechanism of action (Gabrielsson et al., 2010).
Preclinical/non-clinical formulations and drug delivery are very important in order to support early in vivo studies efficiently and productively. As part of the discovery team, formulation scientists and medicinal chemists are required to ensure that new molecular entities (NME) are developed that optimize compound exposure with acceptable PK/PD and toxicologic results. Example successes resulting from this close collaboration between formulation scientists and discovery teams are cited in “strategies for bringing drug delivery tools into discovery” (Kwong et al., 2011). Obtaining meaningful data/information early in the discovery process elevates the best NME to the preclinical stage with the highest probability of successfully reaching the marketplace (Neervannan, 2006; Kwong et al., 2011).
With the plethora of new targets, optimized structures and good formulations in early discovery, drug discovery programs are also exploring new ways to validate the targets in vivo (Neervannan, 2006) especially since PD studies typically are longer in duration and often require chronic administration/repeat dose (Mass et al., 2007).
Drug Delivery
Implantable pumps have become a very convenient and cost effective method to deliver drugs for biochemical research in the pharmaceutical industry. They are also in their own right an important research tool for investigating continuous infusion versus the typical peak to trough exposure profiles of bolus injections. Further, this method has significant animal welfare benefits over repeat dosing by other routes where restraint and tethered infusion or injection may be required (Anonymous, 2001). With the use of specialized catheters, specific administration sites may be selected including ICV (by passing the blood brain barrier), intrathecal, IV, SC, and IP. Animal handling artifacts are also reduced to a minimum. A further advantage of using an implantable pump is that it allows different absorption profiles to be mimicked when infusing intravenously (Van Der Graaf and Gabrielsson, 2009). This characteristic is especially useful since the modeling of the profile of the plasma exposure achieved with intended therapeutic route often still requires the use of intravenous infusions.
Over 12,500 published studies demonstrate that implantable pumps reliably deliver a wide range of test agents (vehicles and active compounds) Anonymous (2011a). These implantable pumps are passive devices which deliver a continuous infusion flow based on the model of the pump and osmotic pressures. There are 12 pump models with 9 different infusion flow-rates with the lowest at 0.11 μl/h and the highest at 10 μl/h. Infusion durations start from 1 day to a maximum of 6 weeks. These implantable pumps are typically used in “early proof of concept studies,” in applications where the half-life of the molecule is very short, and in continuous infusion applications (Neervannan, 2006).
In addition to the 12,500 published studies, comprehensive formulation reviews and articles highlighting vehicle/solvent combinations have been published (Lee et al., 2003; Strickley, 2004, 2008; Gad et al., 2006; Li and Zhao, 2007; Mass et al., 2007; Palucki et al., 2010) and these will aid the user to formulate compatible and stable formulations for drug delivery. Further, there is an excellent review article (Gad et al., 2006) which lists many formulations used in preclinical toxicology studies. These publications, along with the commercially available kits for testing compatibility of active compounds and vehicles for implantable pumps, will make it easier for researchers to verify and utilize implantable pumps.
Pump Advantages Anonymous (2011a,b) Over Other Selected Methods of Administration
Ensure around-the-clock exposure to test agents at predictable levels.
Permit continuous administration of short half-life proteins and peptides.
Provide a convenient method for the chronic dosing of laboratory animals.
Minimize unwanted experimental variables and ensure reproducible, consistent results.
Eliminate the need for nighttime or weekend dosing.
Reduce handling and stress to laboratory animals.
Small enough for use in mice or very young rats.
Allow for targeted delivery of agents to virtually any tissue.
Serve as cost–effective research tools.
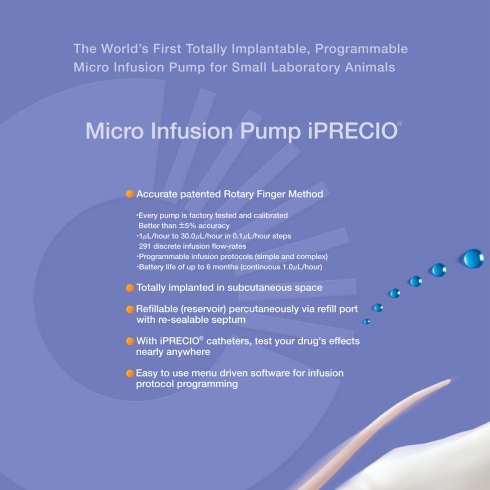
Since March 2007, iPRECIO® infusion pumps have been an alternative choice to the Alzet® Osmotic Pumps (Durect®, Cupertino, CA, USA). iPRECIO® is an infusion pump based on a miniature peristaltic action. It is a self-contained intelligent device consisting of a microcontroller with associated memory, embedded software, inputs/outputs, a micro pulse stepping motor, crystal oscillators, electronic circuitry, and a battery power source. Once programmed and activated, the infusion pump operates independently following its protocol. The embedded software in the microcontroller is programmed via a PC-based software platform (Abe et al., 2009; Tan and Tsuru, 2009). See Figure 1 for a picture of iPRECIO® Micro Infusion Pump.
Figure 1.
iPRECIO® SMP-200, 2nd generation implantable, refillable and programmable pump for small laboratory animals based on a mini-peristalsis flow infusion mechanism.
iPRECIO® brings a whole new level of sophistication to implantable lab animal infusion pump where it is both programmable and refillable. Key features of iPRECIO® pumps are briefly summarized in Figure 2 where circadian rhythm, dose response, bolus infusion protocols are programmable. Full description of specifications can be found on www.iprecio.com and in the references (Abe et al., 2009; Tan and Tsuru, 2009).
Figure 2.
Summary of the key features of the 2nd generation iPRECIO® Pumps reproduced from the current SMP-200 product brochure.
To date (Anonymous, 2011c), the majority of applications in Japan have been the use of iPRECIO® with a constant infusion rate with ±5% accuracy (specification of the pump) following a recovery period (after implantation) with constant dose infusion. For example, Yamato et al. (2010) used iPRECIO® as an enabling technology to allow baseline activity to be monitored after implantation of pump with brain infusion cannula in place while it only infused vehicle/solvent. (Yamato et al., 2010) The effect of agent/drug on animal activity compared to its baseline could then be analyzed without the effect of handling and anesthesia. These types of applications use only the most basic capabilities of the pump; specifically start, stop, duration, infusion flow-rate, and the possibility to refill/exchange agents without additional surgery. Even so, the possibility to allow the animal to recover fully to normal physiological conditions prior to accurate infusion of drug at programmed time points have been a compelling refinement for the researchers to adopt iPRECIO®. Table 1 summarizes selected example use of iPRECIO® in Japan. These pumps have mainly been used in rats but have also been used in goat, non-human primates, rabbits, and guinea pigs. Due to iPRECIO® pumps small size, good accuracy and functionality, they have also been used with mice with a tether system where they replaced the syringe pump. With such a compact system; animal, cage, and pump may be isolated very easily, for example isolated and metabolic cages.
Table 1.
Selected examples of iPRECIO® applications in Japan with details of species, agent/drug, administration site, and infusion protocol.
| Customer | Animal | Drug | Administration route | Mode | Flow-rate (μl/h) |
|---|---|---|---|---|---|
| Pharmaceutical Company | Rat | The toxicity test of a circulation related medicine | External jugular vein | Confidential | Confidential |
| Pharmaceutical Company | Rat | Reproductive hormone | External jugular vein | RCV/constant | 5–10 |
| Pharmaceutical Company | Rat | Diabetes related medicine | External jugular vein | RCV/variable | 3–15 |
| Pharmaceutical Company | Rat | Epilepsy related medicine | Injection in the skull | RCV/constant | 1–10 |
| Pharmaceutical Company | Rat | Antiphlogistic–sedative–drug relation | Subcutaneous | RCV/Constant | 1–20 |
| Pharmaceutical Company | Rat | Ophthalmology region medicine | Fundus oculi | RCV/constant | 3–15 |
| Pharmaceutical Company | Rat | Metabolism related | External jugular vein | RCV/constant | 5–10 |
| Pharmaceutical Company | Rat | pH5 strong acidity solution | Subcutaneous | Confidential | Confidential |
| Fukuyama University | Rat | Pain | Subcutaneous | Confidential | Confidential |
| Gifu university | Rat | Angiotensin 2 losartan | External jugular vein | Instant/variable | 3–30 |
| Hoshi University | Hamster | Confidential | External jugular vein and Subcutaneous | RCV/constant | 1–15 |
| Hoshi University | Rat | Confidential | External jugular vein | RCV/constant | 5–30 |
| Hoshi University | Rat | Pain clinic related | Hypodermoclysis | RCV/constant | 1–30 |
| Jichi Medical University | Mouse | Confidential | Subcutaneous | Confidential | Confidential |
| Nagoya City University | Rat | Angiotensin II | External jugular vein | Confidential | Confidential |
| National Institute of Agro biological Sciences | Goat | Confidential | Vein | RCV/constant | 5–30 |
| National Center of Neurology and Psychiatry (NCNP) | Mouse | Confidential | Subcutaneous | RCV/constant | 1–15 |
| Nihon University | Rat | Confidential | External jugular vein | RCV/constant | 3–10 |
| Osaka City University | Rat | Neurotransmitter | Injection in the skull | RCV/constant | 1–15 |
| Osaka University of Pharmaceutical Sciences | Rat | Vitamin B12 | External jugular vein | Confidential | Confidential |
| Shiga University of Medical Science | Confidential | Reproduction related | Subcutaneous | Instant/variable | 1–7 |
| Shiga University of Medical Science | Rat | Reproductive hormone | External jugular vein | RCV/constant | 5–10 |
| Tokyo Women’s Medical University (TWMU) | Hamster | Confidential | External jugular vein and Subcutaneous | RCV/constant | 1–15 |
| The University of Tokyo | Rat | Nicotine | External jugular vein | Confidential | Confidential |
| The University of Tokyo | Rat | Confidential | External jugular vein | RCV/constant | 5–15 |
RCV Mode is a function of iPRECIO® pumps which allow saline to be infused initially during the recovery period prior to agent administration. At the appropriate time, saline is replaced with agent to allow the programmed start time taking into account dead volumes within the infusion system.
More sophisticated and advanced infusion protocols have been evaluated by using the programmable nature of the iPRECIO® pumps (Abe et al., 2009) where dose response of Ang II was investigated along with effect of Losartan infusion in a second pump.
Matsuoka et al. (2007) used iPRECIO® pumps to administer vitamin B12 at onset of the light cycle and at the onset of the dark cycle to study the effect B12 on the arousal and body temperature rhythm.
Shobo et al. (2010, 2011) used iPRECIO® as an enabling technology to continuously administer olanzapine® chronically to maintain higher exposure levels and with more stability than any other published reports (five cited) to induce adiposity in male rats.
In July 2009, a second generation iPRECIO® pump was released in smaller package (30% smaller by volume), infrared communications replaced metallic contact pins for download/activation, improved septum port design to facilitate filling/refilling in vivo for up to 50 operations and new repeat function to allow the 10 flow-steps to be increased to 2,500 steps. These new devices are now being used extensively both domestically in Japan and internationally worldwide. Recent selected research publications using these devices are:
-
(1)
“Vestibular-mediated increase in central serotonin plays an important role in hyper gravity-induced hypophagia in rats” (Abe et al., 2010).
-
(2)
“GPR54 agonists a way forward for down regulation of the GnRH pathway?” (Douglas et al., 2011).
-
(3)
“Extracellular protein phosphorylation promotes functional recovery from experimental spinal cord injury” (Yoshinori, 2011).
-
(4)
“Use of novel programmable pump for intra cranial administration in orthotopic glioblastoma tumor models in rats” (Schnell and Ferrat, 2011).
-
(5)
“Refined rat pharmacology studies with infusion using implantable pumps” (Martel, 2011).
-
(6)
“Implantable infusion pumps for chronic rodent studies” (Osborn et al., 2011).
Advantages cited re-emphasized our Japanese use experience where iPRECIO® allowed a control response to be obtained before a long infusion of substances of interest begins. Further advantages cited were that it was possible to have a single surgery for both tumor cell injection and pump implantation (saline infusion initially). The flexibility then to go from saline infusion to infusion of therapeutic at a researcher/operator defined time which is related to clinical signs and/or tumor size, by refilling the reservoir with the substance was also cited as an advantage.
More recently, intermittent infusions and bolus injection(s) per day (24 h cycles) have been of strong of interest to iPRECIO® users. An example for the former, Davis et al. (2010) used 0.2 μl/h for 48 h to simulate stopping cycles which acted as control periods for the dose response study of 5-HT infusion. Good results were obtained with a clear dose response dependency with control periods of mean arterial pressure (MAP). For more details see example 3 later within this article. For an example of the latter, iPRECIO® pumps have been referenced as a great potential/promise of dramatic changes of the microinjection paradigm for neuroscience where 50–100 nl are most common in current rat studies (Zaretskya et al., 2011).
Internally, we have been able to confirm that the accuracy of iPRECIO® pumps remained within ±5% for total volume infused (bolus). Tests are continuing, with results obtained for 60 continuous days. Test parameters were to infuse a single bolus of 15 μl/day with different infusion/bolus rates; 5 μl/h for 3 h, 10 μl/h for 1.5 h, 20 μl/h for 0.75 h, and 30 μl/h for 0.5 h. For the rest of the 24 h period, the iPRECIO® pumps were programmed to infuse 0 μl/h. Total of 12 pumps were used, 3 per bolus flow-rate.
The most recent and additional iPRECIO® references may be found at http://www.iprecio.com/support/index.html
Paired Data Sets
The refillable and programmable nature of iPRECIO® allows each rat to be its own control especially if terminal end-points are not required for the treatments. This would be a more powerful test of difference because the variance between subsamples is lower than the variance between independent samples (Kenakin, 2009).
After recovery from the implantation of the pump, baseline physiological and behavior information may be obtained prior to drug administration and then followed by escalation study comprising vehicle, low dose, medium dose, and high dose. Therefore, instead of having groups of five animals per treatment, it could be possible to have one group of animals subjected to a sequence of treatments (with washout period if necessary) without additional surgery and with minimum handling. This would significantly reduce the required number of animals while giving a more powerful test of difference as the same animals are subjected to the different treatments.
Superior Telemetric Studies When Combined with Use of iPRECIO® Pump
Radiotelemetry (telemetry) has become the gold standard for monitoring physiological data in unrestrained, conscious laboratory animals. The physiological parameters measured through telemetry include mean arterial blood pressure, heart rate activity, and temperature, to name a few. The use of stress-free laboratory animals leads to higher sensitivity for drug induced effects and lower variability of parameters measured. In combination with this gold standard, sophisticated implantable infusion pumps will lead to ever more sensitive and powerful means of detecting drug induced therapeutic and/or adverse effects.
Example Uses of iPRECIO®
The next section of this technology article summarizes both published data and data provided by personal communication with iPRECIO® users. These applications will be referenced accordingly.
Dependence and withdrawal in the same cohort.
Rapid and long term delivery of drug with radiotelemetry (blood pressure endpoint).
Dose response curve within an individual animal with radiotelemetry (blood pressure endpoint).
Dose response of multiple drugs, within a single animal with radiotelemetry (systemic and ventricular pressure endpoints).
Drug exposure levels in blood for three dose profiles (PK).
-
1
Dependence and withdrawal in the same cohort
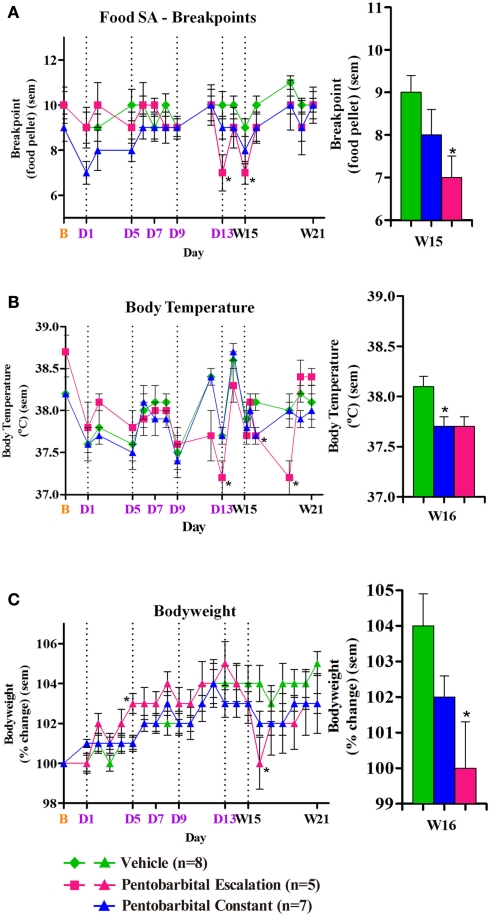
For the first time, a physical dependence and withdrawal (PDW) study could be carried out with unrestrained freely moving animals (as opposed to tethering) for a vehicle, constant dose and escalation dose group of pentobarbital. Constant dose is where the pump was programmed to infuse at fixed dose and escalation dose is where the pump was programmed to infuse an increasing dose. Functional Observation Battery, food self administration (SA), body temperature and bodyweight were monitored on baseline day 1, throughout the infusion period (days 1–14) and withdrawal (days 15–21). This was made possible with the use of the implantable, programmable and refillable iPRECIO® pumps. Summary of results are shown in Table 2 and Figure 3.
Table 2.
Clinical signs observed during dosing and withdrawal following pentobarbital and vehicle.
| Day | Hours | Vehicle | Pentobarbital constant | Pentobarbital escalation |
|---|---|---|---|---|
| D2 | 26 | – | ↓ Body tone | – |
| D13 | 290 | – | – | ↓ Handling reactivity |
| ↓ body tone* | ||||
| ↓ ataxia | ||||
| D14 | 314 | – | – | ↓ Body tone |
| W15 | 338 | – | – | Straub tail* |
*p ≤ 0.05 compared to vehicle.
Figure 3.
(A–C). Food self administration break point reached, body temperature, and body weight following chronic pentobarbital or vehicle B=baseline, D=dosing, W=withdrawal, * = p ≤ 0.05 compared to vehicle, dotted line = anesthesia for pump loading. Points represent mean ± SEM.
Coles et al. (2010) successfully characterized an escalating sodium pentobarbital regime as a positive control in this PDW model using iPRECIO®.
-
2
Rapid and long term delivery of drug with radiotelemetry (blood pressure endpoint).
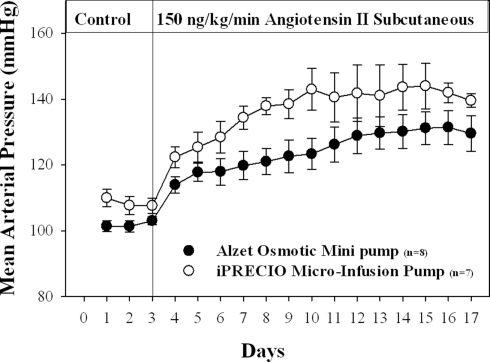
Seitz et al. (personal communication)1 recently evaluated iPRECIO® pumps as an Ang II drug delivery system respect to existing technology, namely the Alzet® osmotic pump (Durect®, Cupertino, CA, USA). The authors, have extensive experience in infusion of angiotensin II to produce chronic hypertension (assessed by DSI radiotelemetric measurements).
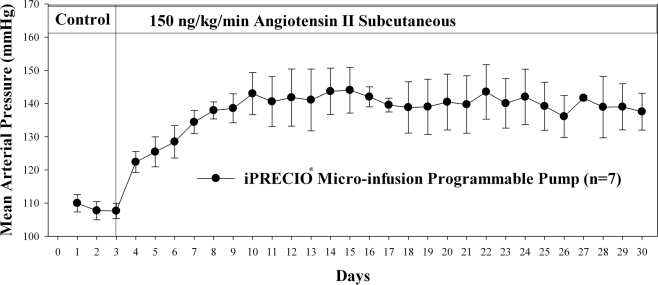
iPRECIO® pumps were found to be reliable and easy to use. Their reliability appears to exceed that of osmotic pumps, based on the level of blood pressure produced during angiotensin II infusion. The pumps were well tolerated for long periods. Results for Ang II infusion with Alzet® osmotic pumps and iPRECIO® are shown in Figures 4 and 5 respectively.
Figure 4.
iPRECIO® appeared to initiate hypertension more rapidly, and maintained a higher level of arterial pressure than the Alzet® pump. Points represent means ± SEM for number of animals indicated in parentheses (n). Vertical line indicates beginning of Angiotensin II infusion.
Figure 5.
While the Alzet® pump (Figure 4) could only be used for 14 days, the iPRECIO® pump (same infusion flow-rate as Alzet®), after transcutaneous refills, maintained an elevated level of arterial pressure. There are longer duration Alzet® pumps but they operate with a lower infusion rate. iPRECIO® pumps will infuse for up to 6 months at 1 μl/h. The longest duration Alzet® pump would only infuse for 6 weeks.
-
3
Dose response curve within an individual animal with radiotelemetry (blood pressure endpoint).
(Davis et al. (2010) previously established, using both the iPRECIO® micro infusion pump and radiotelemetry devices, that 5-HT administration produced repeated falls in blood pressure in the sham (normotensive) and deoxycorticosterone acetate (DOCA)–salt (hypertensive) rat when the pump was turned on. When the pump was turned off (0.2 μl/h), blood pressure returned to normal. This study demonstrated that an equivalent effect was possible at a much lower dose than was previously studied (25 μg/serotonin hydrochloride/kg/min) in the sham and DOCA–salt rat.
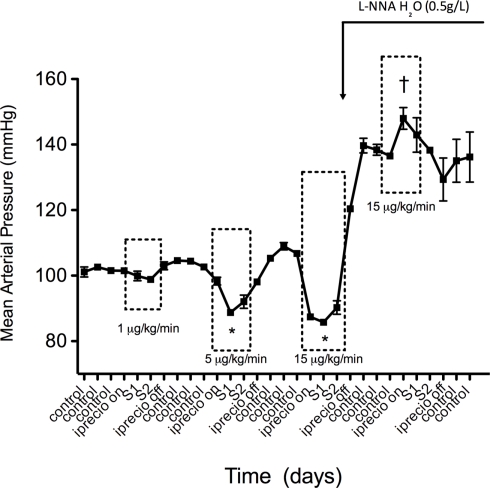
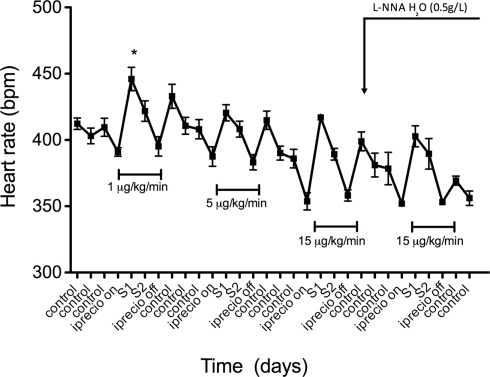
Additionally, for the first time, a 5-HT dose response study was carried out in a single animal with unrestrained movement using radiotelemetry devices and the iPRECIO® micro infusion pump. This study demonstrated that increasing concentrations of 5-HT (1, 5, 15 μg/kg/min) produced correspondingly greater falls in blood pressure in the normotensive Sprague Dawley rat (Figure 6). Heart rate (HR) was also elevated following each administration of 5-HT (1, 5, 15 μg/kg/min; Figure 7). The programmable feature of iPRECIO® pump also allowed baseline measurements to be made after the implantation but prior to 5-HT infusion. Additionally, L-NNA administered in the drinking water when the iPRECIO® pump was turned off raised blood pressure in these rats and prevented a subsequent fall in blood pressure when the pump was turned on (5-HT – 15 μg/kg/min).
Figure 6.
Effects of increasing doses of 5-HT on mean arterial pressure (MAP) in male Sprague Dawley rats. Points represent mean ± SEM for five (5) animals. Time is represented by days on the x-axis. S represents days of 5-HT infusion. *Statistically significant (p < 0.05) difference compared to control BP. †Statistically significant (p < 0.05) difference compared to blood pressure following L-NNA administration. Boxes represent window of time during which 5-HT infusion was on.
Figure 7.
Effects of increasing doses of 5-HT on mean heart rate (HR) in male Sprague Dawley. Points represent mean ± SEM for five (5) animals. Time is represented by days on the x-axis. S represents days of 5-HT infusion. *Statistically significant (p < 0.05) difference from control heart rate. Horizontal bracketed bars indicate the time window over which 5-HT infusion was on.
In summary, the observation of a lower threshold for inducing a 5-HT-induced fall in blood pressure, and the creation of a dose response curve in a single experiment would not have been possible using a traditional osmotic drug delivery device. Thus, the iPRECIO® micro infusion pump may offer significant benefits to investigators.
Gold Standard Cardiovascular Application Using iPRECIO® and Radiotelemetry to have Maximum Sensitivity
-
4
Dose response of multiple drugs, within a single animal with radiotelemetry (systemic and ventricular pressure endpoints).
For the first time, both systemic and ventricular pressure in the rat could be evaluated in a free moving animal model using radiotelemetry and iPRECIO® pumps (Gizzi et al., 2011a,b). Four (4) animals were instrumented with transmitters for left ventricular pressure monitoring (DSI-TA11PA-C10) and aortic blood pressure monitoring (DSI-TA11PA-C40). Then after a recovery period, Baird et al. (personal communication) administered each animal sequentially with vehicle (0.9% sodium chloride for injection, USP), dobutamine, and verapamil using the iPRECIO® pumps. Dobutamine is a synthetic catecholamine with positive ionotropic activity which is used therapeutically to increase cardiac output, via an ionotropic mechanism. Paradoxically, it produces relatively little change (increase) in HR rate and exhibits lusitropic activity. Verapamil is an antiarrhythmic phenylalkylamine which blocks ionic current across L-type voltage gated calcium channels causing variable effects on HR (depending on the chronicity of exposure), decreased conduction through SA and AV node tissues, decreased contractility, peripheral vasodilation, increased coronary blood flow, and decreases in myocardial oxygen demand.
Details of the infusion protocol are shown in Table 3. Treatments 1, 2, and 3 were administered to the same four animals with the appropriate washout periods. Each treatment was administered stepwise (low to high dose) at four infusion rates, and the duration of each individual rate/exposure was approximately 1.5 h. The vehicle was administered at rates equivalent to those programmed during the verapamil exposures. Doses of dobutamine was prepared individually for each animal based on that animal’s body weight taken on the day prior to dose preparation. Within each designated treatment condition, temporally contiguous ventricular and aortic pressures (in addition to HR and other derived parameters as indicated below), were recorded at least once every 15 min during the course of the infusions, for a total of six segments of data collected for each transmitter per dose.
Table 3.
Treatments and dose details used in the study for saline, dobutamine, and verapamil HCl.
| Treatment | Vehicle/positive control article | μg/kg/min | Cardiovascular parameters (left ventricular and aortic pressure) |
|---|---|---|---|
| Number of animals (male) | |||
| 1 | Saline | 0 | 4 |
| 2 | Dobutamine | 1, 3, 10, 30 | 4 |
| 3 | Verapamil HCl | 100, 180, 320, 560 | 4 |
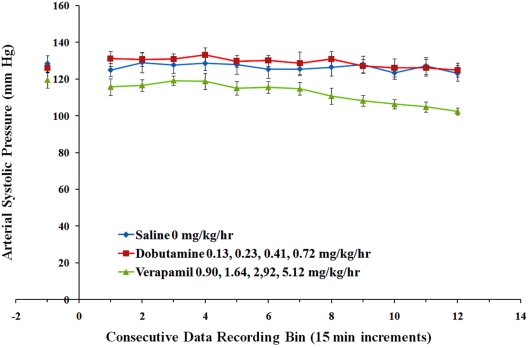
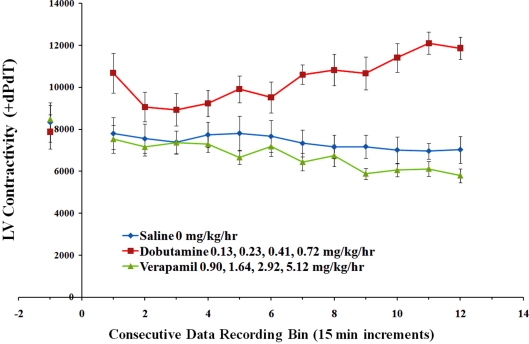
Results are shown in Figures 8 and 9. iPRECIO® pumps successfully infused successive doses of the three treatments. The effect of dobutamine and verapamil were consistent with their pharmacology across their selected dose ranges for continuous infusion because they caused an:
Figure 8.
Shows results for arterial systolic pressure for the three different treatments. Arterial systolic pressure was decreased by administration of successive doses of verapamil; arterial systolic pressure was unaffected by successive doses of dobutamine; points represent means ± SEM.
Figure 9.
Shows results for LV contractility respectively for the three different treatments. LV contractility was increased by successive doses of dobutamine. LV contractility was decreased by administration of successive doses of verapamil. Points represent means ± SEM.
increase in LV contractility estimate, slight increase in HR (data not shown), no change in systemic arterial pressure for dobutamine.
decrease in LV systolic and developed pressures, decreased LV contractility estimate and systolic arterial pressure, and lack of change in HR (data not shown) for verapamil.
-
5
Drug exposure levels in blood for three dose profiles (PK)
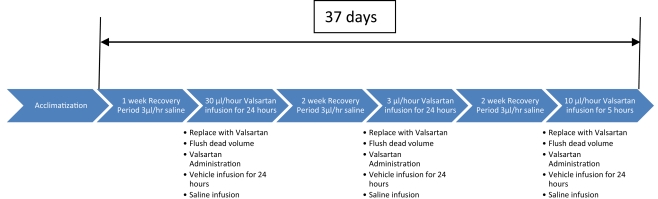
For the first time, the advantages and relevance of iPRECIO® micro infusion pumps to perform pharmacokinetic experiments in rats requiring intravenous infusion at low infusion rates, low dose volume with repeated infusion session in the same animal over several weeks and using a pharmacological substance (i.e., Valsartan®) is demonstrated in detail. The study involved one (1) group of five (5) male Wistar rats weighing between 404.6 and 459.9 g on the day of the implantation. Blood samples were collected from fed animals. Figure 10 and Tables 4 and 5 describe the study protocol.
Figure 10.
Brief description of study protocol for Valsartan® infusion and blood sampling. More detailed descriptions are shown in Tables 4 and 5.
Table 4.
Brief summary of study protocol.
| Recovery period | Dosing | Recovery and washout | Dosing | Recovery and washout | Dosing | Total |
|---|---|---|---|---|---|---|
| 1 week | 30 μl/h for 24 h | 2 weeks | 3 μl/h for 24 h | 2 weeks | 10 μl/h for 5 h | 37 days |
Table 5.
iPRECIO® pump study protocol.
| Recovery duration (approx) | Exchange and flush | Dosing | Washout and recovery | Exchange and flush | Dosing | Washout and recovery | Exchange and flush | Dosing | Total in vivo |
|---|---|---|---|---|---|---|---|---|---|
| 7 Days | 0 μl for 30 min for exchange to Valsartan® | 30 μl/h for 24 h | 3 μl/h for 2 weeks | 0 μl for 18 min for exchange to Valsartan® | 3μl/h for 24 h | 3μl/h for 2 weeks | 0 μl for 18 min for exchange to Valsartan®. | 10μl/h for 5 h | 37 days |
| 30 μl for 1.2 h for flushing | Blood sampling: predose, 1, 2, 4, 6, and 23 h postdose | Initial 24 h with vehicle for flushing | 30 μl for 1.2 h for flushing | Blood sampling: predose, 1, 2, 4, 6, and 23 h postdose | Initial 24 h with vehicle for flushing | 30 μl for 1.2 h for flushing | Blood sampling: predose, 1, 2, 4, 6, and 23 h postdose |
Agent concentration: 100 mg/ml Valsartan® in 50% DMSO 50% PEG 400. Total study duration: 37 days, n = 5. Post-dosing at 30 and 3 μl/h, iPRECIO® pumps and catheter were flushed with vehicle only (50% DMSO, 50% PEG 400) after extracting remaining volume of agent for 24 h before being replaced with saline for the remainder of the washout and recovery period.
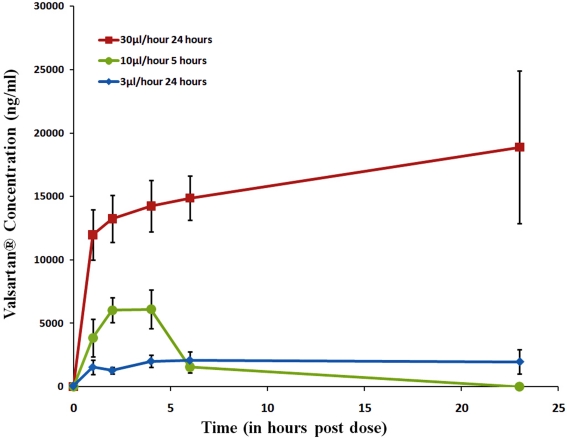
The Valsartan® exposure levels are shown in Figures 11 and 12. Figure 11 shows the mean plasma values for the three doses. These results showed that the plasma exposure were dose-dependent and less than dose proportional, especially at 1 and 4 h after the start of the infusion (around ×2.5 and ×7.8 the exposure reached at 3 μl/h for 24 h, and around ×3, and ×7.1 at 4 h). Valsartan® plasma exposure for the 10 μl/h for 5 h infusion showed that levels dropped rapidly after the stopping of the infusion, i.e., 5 h after the start. At 23 h post-dosing, the plasma exposure was at the low limit of quantification (LLQ) or at the low limit of detection (LLD) in all animals.
Figure 11.
Valsartan® plasma exposure for the three doses.
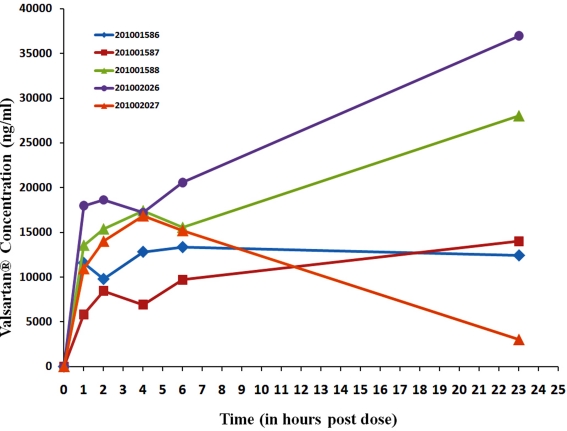
Figure 12.
Individual data for 30 μI/h Valsartan® exposure.
At the 30 μl/h infusion rate as shown in Figure 12, plasma concentrations achieved in the different animals were quite reproducible, except in Animal No. 201002027 which displayed a very low plasma level at the 23 h time point. This discrepancy was not attributable to an infusion abnormality because the actual volumes administered (measured) in all the animals were consistent and close to the theoretical infusion volumes expected as shown in Table 6 below.
Table 6.
Individual data for infusion verification and validation for the 30 μl/h Valsartan® infusion.
| Animal number | Filled | Programmed | Remaining (theoretical) | Remaining (extracted) | Volume administered (measured) | Difference (volume administered) | #x00025; Difference (volume administered) | |
|---|---|---|---|---|---|---|---|---|
| 201001586 | 900 | 755.4 | 144.6 | 170 | 730 | −25.4 | −3.36 | |
| 201001587 | 900 | 755.4 | 144.6 | 140 | 760 | 4.6 | 0.61 | |
| 201001588 | 900 | 755 | 145 | 150 | 750 | −5 | −0.66 | |
| 201002026 | 900 | 756 | 144 | 120 | 780 | 24 | 3.17 | |
| 201002027 | 900 | 756 | 144 | 180 | 720 | −36 | −4.76 |
The results showed that all the animals were exposed to Valsartan® in a dose-dependent manner with respect to infusion flow-rates and filling/extracting/refilling procedures with saline, Valsartan®, and vehicle. Valsartan® was successfully administered with iPRECIO® pumps over a study lasting 37 days with high dose (24 h 30 μl/h), low dose (24 h 3 μl/h) and mid-dose with a stop (5 h 10 μl/h). These results are consistent with published Valsartan® exposure data.
The full study report is available from Primetech.
Overall Discussions and Conclusion
The “fail early; fail economically” model seems to be a continuing trend for Big Pharma. It requires the front loading of drug discovery with safety and efficacy studies to select and prioritize the most promising agents from the abundance of NMEs to enter the preclinical development stage. This in turn requires better formulations and cost effective drug delivery solutions to maximize exposure for PKPD and ADME studies. More recently, even innovative drug delivery systems primarily used with approved drugs, have been used in the preclinical environment for difficult to deliver agents and challenging half-life molecules (Rosen and Abribat, 2005).
Implantable pumps and especially sophisticated implantable pumps like iPRECIO® will allow cost effective PD studies and repeat dose studies to be carried out and will allow for new ways to validate the target in vivo. Over 12,500 studies demonstrating the reliable use of implantable pumps to deliver a wide range of test agents have been published. The various summaries and detailed examples highlighted within this technology article demonstrated the clear advantages of an active programmable, refillable, and implantable device versus a passive device. With a reliable, convenient, and cost effective method to carry out PKPD studies in vivo in non-clinical studies, the reality of extrapolation of the essentials in PKPD will be possible. These extrapolations will facilitate the important translation from animal to human testing (Neervannan, 2006). A holistic understanding of NME, formulation vehicle, dose, exposures, and concomitant efficacy enables scientists to better correlate study outcomes with provided test articles and thus, carve a development path forward with significant implications to downstream success of the product (Kwong et al., 2011).
The industry is also focusing on science based understanding of the mechanism of action. We believe that the iPRECIO® infusion pump with its programmable and refillable features would allow quantitative pharmacology, PKPD in a free moving animal model which would allow greater understanding of the mechanisms in a cost effective manner. In addition, facilitate the investigation and commercialization of new chronopharmaceuticals to add to those currently in the market. “Drug delivery technologies for chronotherapeutic applications” reviewed circadian rhythm in various disease states including cancer and the cardiovascular system (Khan et al., 2009).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Table 2 and Figure 3 were reproduced with permission from Elsevier. Reprinted from Journal of Pharmacological and Toxicological Methods, Vol. 62:2, Darryl I. Coles, Amy Broad, Andy Mead, Pg. e9, Copyright (2010), with permission from Elsevier. The authors gratefully acknowledge Darryl Coles, Greg Fink, Ted Baird for the examples and data included in this paper. The authors would also like to thank Jay Gizzi and Ayumu Takeuchi for their comments and thorough reading of this manuscript.
Footnotes
References
- Abe C., Tanaka K., Iwata C., Morita H. (2010). Vestibular-mediated increase in central serotonin plays an important role in hyper gravity-induced hypophagia in rats. J. Appl. Physiol. 109, 1635–1643 10.1152/japplphysiol.00515.2010 [DOI] [PubMed] [Google Scholar]
- Abe C., Tashiro T., Tanaka K., Ogihara R., Morita H. (2009). A novel type of implantable and programmable infusion pump for small laboratory animals. J. Pharmacol. Toxicol. Methods 59, 7–12 10.1016/j.vascn.2008.09.002 [DOI] [PubMed] [Google Scholar]
- Anonymous (2001). Refining procedures for the administration of substances. Lab. Anim. 35, 1–41 10.1258/0023677011911967 [DOI] [PubMed] [Google Scholar]
- Anonymous (2011a). Alzet®. Available at: http://www.alzet.com/ May 9th 2011 12:58
- Anonymous (2011b). iPRECIO®. Available at: http://www.iprecio.com/ May 9th 13:33
- Anonymous (2011c). To our knowledge (at Primetech). Data and application information have not always been shared freely by users of iPRECIO® pumps due to confidentiality. Available at: http://www.iprecio.com/application/index.html
- Coles D. I., Warr A., Mead A. (2010). Characterization of pentobarbital physical dependence and withdrawal in rats. J. Pharmacol. Toxicol. Methods 62, e9. 10.1016/j.vascn.2010.11.029 [DOI] [Google Scholar]
- Davis P., Fink G., Seitz B., Watts W. (2010). Serotonin infusion via the iPrecio® micro infusion pump results in repeated reductions in blood pressure in the normotensive Sprague Dawley rat. FASEB J. 24, lb551 [Google Scholar]
- Douglas G., Jones H., Edgington A., Saunders K., Neil Morgan S., Pullen N., Parenty G. (2011) GPR54 Agonists a Way Forward for Downregulation of the GnRH Pathway? 10th International Symposium on GnRH, Salzburg. Geneva: Kenes International [Google Scholar]
- Gabrielsson J., Green A. R., Van der Graaf P. H. (2010). Optimizing in vivo pharmacology studies-practical PKPD considerations. J. Pharmacol. Toxicol. Methods 61, 146–156 10.1016/j.vascn.2010.02.002 [DOI] [PubMed] [Google Scholar]
- Gad S. C., Cassidy C. D., Aubert N., Spainhour B., Robbe H. (2006). Non clinical vehicle use in studies by multiple routes in multiple species. Int. J. Toxicol. 25, 499–521 10.1080/10915810600609080 [DOI] [PubMed] [Google Scholar]
- Gizzi J., Baird T., O’Donohue K., Yoder J., Grenwis J., Bogie H. (2011a). Optimization of a fully implantable small animal infusion model involving multi-pressure data collection. FASEB J. 25, 11264 [Google Scholar]
- Gizzi J., Baird T., O’Donohue K., Yoder J., Grenwis J., Bogie H. (2011b). Michigan State University, Personal communication with the authors.
- Kenakin T. (2009). “Statistics and experimental design,” in Pharmacology Primer, 2nd Edn (San Diego, CA: Academic Press; ). [Google Scholar]
- Khan Z., Pillay V., Choonara Y. E., du Toit L. C. (2009). Drug delivery technologies for chronotherapeutic applications. Pharm. Dev. Technol. 14, 602–612 10.3109/10837450902922736 [DOI] [PubMed] [Google Scholar]
- Korfmacher W. A. (2009). Advances in the integration of drug metabolism into the lead optimization paradigm. Mini Rev. Med. Chem. 9, 703–716 10.2174/138955709788452694 [DOI] [PubMed] [Google Scholar]
- Kwong E., Higgins J., Templeton A. C. (2011). Strategies for bringing drug delivery tools into discovery. Int. J. Pharm. 412, 1–7 10.1016/j.ijpharm.2011.03.024 [DOI] [PubMed] [Google Scholar]
- Lee Y. C., Zocharski P. D., Samas B. (2003). An intravenous formulation decision tree for discovery compound formulation development. Int. J. Pharm. 252, 111–119 10.1016/S0378-5173(02)00704-4 [DOI] [PubMed] [Google Scholar]
- Li P., Zhao L. (2007). Developing early formulations: practice and perspective. Int. J. Pharm. 341, 1–19 10.1016/j.ijpharm.2007.05.049 [DOI] [PubMed] [Google Scholar]
- Martel E. (2011). “Refined rat pharmacology studies with infusion using implantable pumps,” in DSI User Group Meeting, Paris [Google Scholar]
- Mass J., Kamm W., Hauck G. (2007). An integrated early formulation strategy-from hit evaluation to preclinical candidate profiling. Eur. J. Pharm. Biopharm. 66, 1–10 10.1016/S0939-6411(07)00180-4 [DOI] [PubMed] [Google Scholar]
- Matsuoka E., Miyazaki M., Iwanaga K., Kakemi M. (2007) “Assessment of pharmacokinetic–pharmacodynamic relationship of vitamin B12 in the treatment of somnipathy in rats,” in 14th Annual Meeting of Japanese Society for Chronobiology, Tokyo [Google Scholar]
- Neervannan S. (2006). Preclinical formulations for discovery and toxicology: physiochemical challenges. Expert Opin. Drug Metab. Toxicol. 2, 715–731 10.1517/17425255.2.5.715 [DOI] [PubMed] [Google Scholar]
- Osborn J. presenter Fink G. discussant. (2011). “Implantable infusion pumps for chronic rodent studies,” in Workshop: Rodent Instrumentation Workshop M. Knuepfer, Experimental Biology 2011, Washington, DC [Google Scholar]
- Palucki M., Higgins J., Kwong E., Templeton A. (2010). Strategies at the interface of drug discovery and development: early optimization of the solid state phase and preclinical toxicology formulation for potential drug candidates. J. Med. Chem. 53, 5897–5905 10.1021/jm1002638 [DOI] [PubMed] [Google Scholar]
- Rosen H., Abribat T. (2005). The rise and rise of drug delivery. Nat. Rev. Drug Discov. 4, 381–385 10.1038/nrd1721 [DOI] [PubMed] [Google Scholar]
- Schnell C., Ferrat T. (2011) “Use of novel programmable pump for intracranial administration in an orthotopic glioblastoma tumor model in rats,” in DSI User Group Meeting, Paris [Google Scholar]
- Shobo M., Yamada H., Koakutsu A., Hamada N., Fujii M., Harada K., Ni K., Matsuoka N. (2011). Chronic treatment with olanzapine via a novel infusion pump induces adiposity in male rats. Life Sci. 88, 761–765 10.1016/j.lfs.2011.02.014 [DOI] [PubMed] [Google Scholar]
- Shobo M., Yamada H., Koakutsu A., Harada K., Ni K., Matsuoka N. (2010). “Effect of long-term electrical infusion of olanzapine on fat weight in rats: a novel method for administration of biologically and/or chemically fragile compounds,” in The 83rd Annual Meeting of the Japanese Pharmacological Society, Osaka [Google Scholar]
- Strickley R. G. (2004). Solubilizing excipients in oral and injectable formulations. Pharm. Res. 21, 201–230 10.1023/B:PHAM.0000016235.32639.23 [DOI] [PubMed] [Google Scholar]
- Strickley R.G. (2008). “Formulation in drug discovery,” in Annual Reports in Medicinal Chemistry, Vol. 43, ed Macor J. E. (London: Academic Press; ), 419–451 [Google Scholar]
- Tan T., Tsuru T. (2009) Infusion Pumps for Small Laboratory Animals. Available at: ALN Magazine May/June http://www.alnmag.com/article/infusion-pumps-small-laboratory-animals
- Van Der Graaf P. H., Gabrielsson J. (2009). Pharmacokinetic-pharmacodynamic reasoning in drug discovery and early development. Future Med. Chem. 1, 1371–1374 10.4155/fmc.09.124 [DOI] [PubMed] [Google Scholar]
- Walker D. K. (2004). The use of pharmacokinetic and pharmacodynamic data in the assessment of drug safety in early drug development. Br. J. Clin. Pharmacol. 58, 601–608 10.1111/j.1365-2125.2004.02194.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehling M. (2009). Assessing the translatability of drug projects: what needs to be scored to predict success? Nat. Rev. Drug Discov. 8, 541–546 10.1038/nrd2898 [DOI] [PubMed] [Google Scholar]
- Wendler A., Wehling M. (2010). The translatability of animal models for clinical development: biomarkers and disease models. Curr. Opin. Pharmacol. 10, 601–606 10.1016/j.coph.2010.05.009 [DOI] [PubMed] [Google Scholar]
- Wittenburg L. A., Gustafson D. L. (2011). Optimizing preclinical study design in oncology research. Chem. Biol. Interact. 190, 73–78 10.1016/j.cbi.2011.01.029 [DOI] [PubMed] [Google Scholar]
- Yamato M., Okuyama K., Jin G. H., Eguchi A., Watanabe Y., Kataoka Y. (2010). “Endogenous IL-1β and IL-1 receptor antagonist in the brain are involved in poly I: c-induced immunological fatigue,” in The 33rd Annual Meeting of the Japan Neuroscience Society, Kobe [Google Scholar]
- Yoshinori T. (2011) “Extracellular protein phosphorylation promotes functional recovery from experimental spinal cord injury,” in The 6th International Symposium on Receptor Mechanisms, Signal Transduction and Drug Effects-Development of Novel Therapy to Specific Disease in organ, Kyoto [Google Scholar]
- Zaretskya D. V., Zaretskaiaa M. V., Rusyniaka D. E., DiMiccob J. A. (2011). Stress-free microinjections in conscious rats. J. Neurosci. Methods 199, 199–207 10.1016/j.jneumeth.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]