Abstract
The European REACH Regulation (Registration, Evaluation, Authorization of CHemical substances) implies, among other things, the evaluation of the biodegradability of chemical substances produced by industry. A large set of test methods is available including detailed information on the appropriate conditions for testing. However, the inoculum used for these tests constitutes a “black box.” If biodegradation is achievable from the growth of a small group of specific microbial species with the substance as the only carbon source, the result of the test depends largely on the cell density of this group at “time zero.” If these species are relatively rare in an inoculum that is normally used, the likelihood of inoculating a test with sufficient specific cells becomes a matter of probability. Normally this probability increases with total cell density and with the diversity of species in the inoculum. Furthermore the history of the inoculum, e.g., a possible pre-exposure to the test substance or similar substances will have a significant influence on the probability. A high probability can be expected for substances that are widely used and regularly released into the environment, whereas a low probability can be expected for new xenobiotic substances that have not yet been released into the environment. Be that as it may, once the inoculum sample contains sufficient specific degraders, the performance of the biodegradation will follow a typical S shaped growth curve which depends on the specific growth rate under laboratory conditions, the so called F/M ratio (ratio between food and biomass) and the more or less toxic recalcitrant, but possible, metabolites. Normally regulators require the evaluation of the growth curve using a simple approach such as half-time. Unfortunately probability and biodegradation half-time are very often confused. As the half-time values reflect laboratory conditions which are quite different from environmental conditions (after a substance is released), these values should not be used to quantify and predict environmental behavior. The probability value could be of much greater benefit for predictions under realistic conditions. The main issue in the evaluation of probability is that the result is not based on a single inoculum from an environmental sample, but on a variety of samples. These samples can be representative of regional or local areas, climate regions, water types, and history, e.g., pristine or polluted. The above concept has provided us with a new approach, namely “Probabio.” With this approach, persistence is not only regarded as a simple intrinsic property of a substance, but also as the capability of various environmental samples to degrade a substance under realistic exposure conditions and F/M ratio.
Keywords: biodegradation, evaluation, inoculum, persistence, probability
Introduction
Biodegradation could eliminate man-made chemical substances (xenobiotic) and prevent accumulation in environmental compartments (soil, river, ocean, sediment, and activated sludge). Although studies on biodegradation and biodeterioration started for more than a century, the first study on biodegradation registered in databases was published in 1960 (Kagawa et al., 1960) and since then 16,657 works have been referenced in scientific databases (ISI Web of Knowledge, 2011). Three years after this study, Borstlap and Kooijman (1963) addressed the problem of testing biodegradation with reference to anionic synthetic surfactants. This pioneering study could be considered as the first of about 600 references dealing with biodegradation testing, i.e., the focus of this article.
The evaluation of the persistence or biodegradability of a chemical in the environment is one of the main issues in environmental risk assessment. Basically, biodegradability assays are designed to test in batch condition a chemical substance as the sole carbon source with inoculum from different origins (river water, sea water, activated sludges, soil). Microorganisms and the tested substance are usually in a buffered pH 7 medium containing N, P, and trace elements. The biodegradation kinetic is monitored during at least 28 days with simple parameters such as oxygen consumption, carbon dioxide production, or dissolved organic carbon consumption. The European REACH Regulation (Registration, Evaluation, Authorization of CHemical substances) envisions a tiered approach for persistence evaluation. The first tier (or screening level) includes the use of a cheap and simple test of ready biodegradability corresponding to the OECD guidelines (Organisation for Economic Co-operation and Development) no 301 A to F (OECD, 1992a). This yields a screening for those chemicals that are of no great concern in terms of environmental persistence. A non-readily biodegradable substance is considered persistent unless its environmental degradability is proven in more expensive and complex simulation tests, as described in the OECD guidelines no 303, 306, 307, 308, and 309 (OECD, 1992c,d,f,g,h). The type of simulation test (or tests) to be performed depends on the potential receptor environments that are causing concern (wastewater treatment plant, surface water, sediment, soil).
Although Blok and Booy (1984) pointed out that inoculum characteristics are of crucial importance for the outcome of a biodegradability test, and in spite of the harmonization of other test conditions and the strict application of test protocols in accordance with Good Laboratory Practice rules, the microbial inoculum is still very poorly defined (as emphasized in only 72 articles).
The first part of this paper deals with the main parameters governing a biodegradability test and the second part puts forward a new concept which focuses on inoculum.
Microbial Inoculum in Biodegradation Tests Remains a “Black Box”
Currently there is little guidance with respect to the preparation and standardization of the inoculum that is derived from samples of unexposed soil, river water, activated sludge, marine water, or sediment. Before being used for biodegradability testing, inoculum sources are either washed (to limit carbon contamination other than that from the tested substance) or acclimated in a peptone/glucose medium for the 301C test (ECETOC, 2003).
Test results are variable, particularly for the category of substances that are neither readily biodegradable nor persistent. This variability is mainly caused by the variability of the used inoculums (Thouand et al., 1995; Blok, 2001), even if Boethling and Lynch (2007) observed more consistent data for US premanufacture notice chemicals. In general the test result may be influenced by (1) the total cell density, (2) the diversity of species, (3) the origin and history of the sample, and (4) the ratio between food and biomass.
Cell density
According to recent studies an inoculum sample may be characterized by a total cell density which can be determined using several quantification methods, e.g., epifluorescence, most probable number (MPN), etc. In practice the organic fraction of solids for a sample is often used as a rough indication of the cell density. Total bacterial cell densities vary from 1012.L−1 for activated sludge to 105.L−1 for sea water. However, the total cell density alone has little relevance because by definition biodegradation of not readily degradable substances often requires relatively rare species. Thus only a very low specific fraction (Degrader versus Total, XD0/XT0) of the total biomass is relevant for the test result and this fraction is not specified by nor even included in the total cell counts. Based on kinetic growth data and length of log and lag phases in Zahn/Wellens tests (OECD 302 B, OECD, 1992b) with activated sludge, Blok (2001) estimated the specific fractions for 137 aromatic substances varying between 1.7 × 10−2 and 0.5 × 10−7.
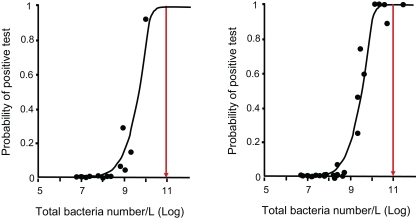
In theory it can be understood that if this specific fraction is lower than the reciprocal of the total amount of cells in the inoculated medium [(XD0/XT0) × XT0 < 1] the specific degraders are absent and the test fails to give a positive result. This can be overcome by adding a larger absolute amount of biomass. Therefore, the probability of obtaining a positive result is determined by both the total cell number and by the volume of an inoculum, which still has an indirect influence on the test result. Such a probability can be demonstrated by the classical dilution method. With a series of replicate tests conducted at various total cell densities it appears that some test vials fail and others pass when the density of specific degraders is critical, whereas at a lower density most or all vials fail and at higher cell density all test vials pass (Thouand et al., 1995, 1996; Blok, 2001; Figure 1).
Figure 1.
Relationship between cell density (direct count of bacterial cell number with epifluorescence) and results of para-nitrophenol biodegradability test (shown by the points) and probability model (solid line) for tests conducting with river waters inocula (A) or activated sludge inoculums (B) (according to Thouand et al., 1996). Each point is the probability of para-nitrophenol tests (or the percentage of tests) obtained for a specific cell density. The red arrows represent the cell density (1011 bacteria/L) leading to 99.9% of positive tests. (i.e., biodegradation of para-nitrophenol). At this cell level, the origin of the inoculum did not influence the issue of the test.
Species diversity
As not readily degradable substances often require relatively rare species, a higher, or lower diversity of species has often been postulated as the reason respectively for a pass or a fail in a test. Forney et al. (2001), demonstrated that the diversity of several activated sludges differed not only worldwide but also changed in the same wastewater treatment plant during the course of one working day. Moreover it depends on the loading rate. Diversity is often related to bacterial genera but it is sometimes more complicated because activated sludge contains on average: bacteria (1012/L), bacteriophages (1013/L), and protozoa (106/L). Bacteriophages and protozoa have a huge impact on bacterial density due to grazing and lysis in the lag phase at the beginning of a test (Ramadan et al., 1990; Hennes and Simon, 1995; Sandaa et al., 2009).
It is obvious that an inoculum with many different species offers a greater chance for those rare species to be included than an inoculum with only a few generally occurring species. However, this theoretical consideration has presently no practical impact because as things currently stand there exists no practical tool to measure diversity in the industrial laboratories. The current DGGE method (Denaturing Gradient Gel Electrophoresis) is difficult to operate for those not in the possession of the required skills. In future we could see specific DNA microarray techniques being used to quickly check overall diversity before running a test (Huyghe et al., 2008; Auffret, 2009). Alternatively, high throughput methods such as the PhyloChips probes designed in the Lawrence Berkeley National Laboratory (DeSantis et al., 2003) or the denaturing high performance liquid chromatography may be applied (Barlaan et al., 2005; Maukonen and Saarela, 2009). Nevertheless, this diversity remains both a very difficult subject in terms of the microbial ecology of the samples, and poorly understood, as pointed out in recent publications (Chouari et al., 2010; Fierer et al., 2010).
Origin and history of the sample
The presence and density of a relatively rare fraction in an inoculum is greatly influenced by the origin and history of that sample. This is especially evident for samples from an area that is already pre-exposed to the test substance. With xenobiotic pollutants, similar substances may occur in nature, generating a specific microbial fraction which is also capable of transforming the xenobiotic substance.
On the one hand the presence of pollution resulting from a mixture of many readily degradable substances will create a sample dominated by a few generic species that degrade these readily degradable substances and such a sample may contain only low specific fractions of rare species. On the other hand, in areas with important hydraulic residence time, the more recalcitrant substances are left over and hence a microbial sample may contain a broader spectrum of species capable of transforming these substances. Substances that are to be tested for persistence also often tend to be adsorbed to suspended solids and sediments. Thus specific degraders for not readily degradable substances are more likely to be found in the sediment of rivers and coastal areas than in the water, whereas samples from pristine areas not only have low total cell numbers but may also contain very small fractions of specific degraders.
These aspects regarding the quality of the inoculum may cause (1) the (well known) extreme variability in the test results, (2) incomparability between various methods and, in spite of simulation, (3) a low predictive value for the real environment (Blok, 2001).
When referring to the evaluation of persistency within a legal framework, the most important criterion should be the probability of a fraction of relatively rare specific degraders being present in a variety of environmental samples. If present, this fraction should be large enough: firstly, to be included in a sample with a certain probability and secondly, to enable the transformation of the test substance if present at low concentrations (micro–nanograms per liter) during the test duration.
The ratio between food and biomass
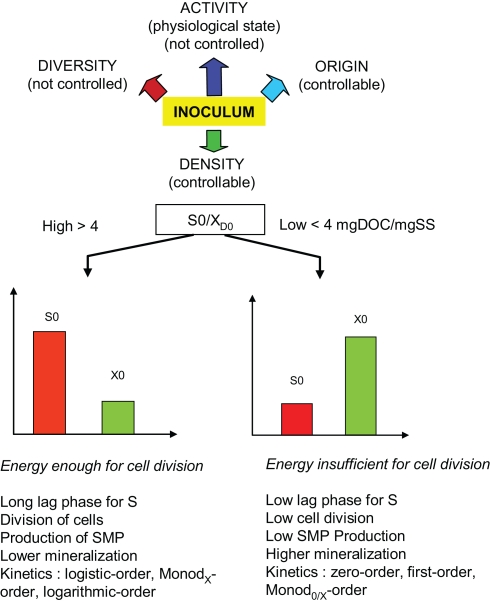
Fujimoto (1963) first described the F/M ratio as the amount of test substance (S) versus the amount of specific degraders (X) at “time zero” of a test so: (S0/XD0). At a high F/M ratio selective proliferation of the specific degraders occurs at the maximum growth rate of these species (Figure 2). This condition is present in a readily biodegradable test (RBT) during lag and log phase. At a low F/M ratio selective proliferation is insignificant and only the already existing cell number is responsible for the transformation. This situation will most likely prevail during degradation of not readily degradable substances after their dilution to low concentrations in the environment. Therefore persistence evaluation requires not only the enumeration of specific degraders but also the ratio S0/XD0. Figure 2 shows some of the consequences at a high or low F/M ratio, as highlighted by Vazquez-Rodriguez et al. (1999).
Figure 2.
The four inoculum parameters (controllable or not) that govern the issue of a biodegradability test. The S0/XD0 is the concentration of test substance versus the concentration of specific degraders at “time zero” in a test which plays a major role in the kinetics of biodegradation. According to Chudoba et al. (1992) if the ratio is over 4 mg DOC/mg SS, the carbon source offers enough energy for the cell division. Conversely, if the ratio is under this average value, no cell division will occur due to lack of energy provided by the carbon source in the test. SMP, soluble microbial polymer; DOC, dissolved organic carbon; SS, suspended solid.
The ProbaBio Concept: “Probability of Biodegradation”
Probabio: Evaluating the biodegradation of a substance in the laboratory to assess its probability of persisting in the environment
Two strategies are possible regarding the variability of the inoculum. The first approach is “standardization.” To this end several authors have suggested an increase in the cell density to control the F/M ratio or to acclimate the inocula, but unfortunately without creating sufficient interest amongst regulators (Thouand et al., 1995, 1996, 1999; Ingerslev et al., 2000; Vasquez-Rodriguez et al., 2007). Other ways have been attempted, especially the use of commercial seeds, but the undefined inocula were only of interest in relation to readily biodegradable substances (Tabka et al., 1993; Thouand et al., 1999; Sharma et al., 2000; Paixão et al., 2006).
The alternative approach is the “probability approach” or “Probabio.” With this concept, persistence is not only regarded as a simple intrinsic substance property, but also as the potential for various environmental samples to degrade a substance under realistic exposure and F/M ratio. This potential first has to be quantified in a relative way as a specific fraction of degraders (XD0/XT0) and secondly, the absolute value has to be evaluated in relation to the substance concentration (S0/XDO) at realistic exposure. To apply this concept the probability of pass/fail with various types of inocula and at various dilutions is determined in a matrix of replicate tests.
The Probabio concept differs considerably from the screening methods for ready biodegradable substances assayed in RBT tests. RBT methods all use a relatively high substance concentration (10–100 mg/L) and a limited density of inoculum (<30 mg SS/L, SS: Suspended Solid). The high F/M ratio creates a selective proliferation of degraders at maximum growth rate. Because of the limited incubation time (<28 days) and the 10-day window criterion in the REACH regulations, the test procedure is selective for degraders that can grow relatively fast (maximum growth rates > 1 Day−1) and are present without pre-exposure in relatively large fractions in the original inoculum (XD0/XT0) > 10−6 (Blok, 2001). For the testing of environmental persistency of not readily degradable substances the growth rate criterion is not relevant because under realistic environmental conditions the substance concentrations will be in the nanogram/Liter range where a fast growth rate is very unlikely.
For the evaluation of environmental persistence a proper understanding of the mechanism is required. Persistency may be observed either because (1) the chemical structure is inert for any enzymatic attack (known as intrinsic persistence) as a sole carbon source but may be gratuitously transformed thanks to co-metabolism, (2) the specific degraders do exist but are relatively rare and occur at low density in the environment (known as environmental persistence) or incubation conditions are unsuitable or (3) the substance is degradable but is not available to the existing degraders (known as physical persistence).
It has been shown that screening tests for ready degradability fail if the fraction of specific degraders is lower than 10−6 (Blok, 2001). Conversely it has been shown that many of these not readily degradable substances are still degraded in tests where the total cell density is above 1010 cells.L−1 (Thouand et al., 1996). Thus the environmental persistence depends on the number of specific degraders in environmental samples. Nevertheless, if a substance repeatedly fails in tests with such high total cell densities and in samples of widely different origin, it may be concluded that the substance is intrinsically persistent.
Hence, we propose an approach that can evaluate the first two types of persistence of the tested substance as the only carbon source. Since co-metabolism is relevant in the environment for xenobiotic transformation, the approach could be duplicated with a second biodegradable carbon source that could promote co-metabolism of the tested substance.
Proposal of a practical approach
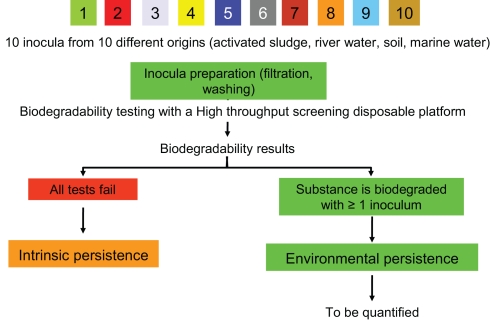
For the first tier the probability of the presence of specific degraders is maximized because of a high total cell density and a wide variety of inoculum sources to yield maximum biodiversity (Figure 3). At this stage, the probability of biodegradation can be estimated according to the number of passes and fails. If a substance fails in all of these tests the substance is considered intrinsically persistent (i.e., the probability of persistence is estimated at 100%). If a substance passes one or more of these tests, it means that the persistence depends on the environment. This environmental persistence requires a further quantification.
Figure 3.
Overall scheme of the strategy used in the “Probabio” concept. I, inoculum (see explanation in the text).
For the second tier the environmental persistence could be assessed using a series of dilutions (to reach a cell density between 105 and 1011.L−1) of the effective inocula in order to find the critical dilution level. Beyond the critical dilution all tests will fail. Based on that critical dilution and the total cell density the number of specific degraders could be calculated from the original environmental samples. The environmental persistence is then evaluated as the probability that sufficient specific degraders are present in environmental samples to transform the substance if the environment is exposed to the substance at an environmentally realistic F/M ratio.
This concept requires a large number of replicates in a matrix to be tested. In the first stage inocula are prepared from various origins and these inocula are concentrated at a cell density > 1011.L−1 (X0opt, Optimal initial cell density). The inocula should be chosen in order to be representative of an environmental compartment, e.g., river sediment, marine sediment, soil, activated sludge. These samples may or may not be pre-exposed at realistic concentrations of the test substance and under realistic conditions (e.g., temperature) in order to be representative of a situation after the release of the substance into the environment (Table 1).
Table 1.
Experimental design for biodegradability testing (Dil, dilution).
| S0 | Temperature (°C) | Cell density | Inoculum |
|---|---|---|---|
| Low S0 conc. (μg/L) | 10 | X0opt 6xdilX0 | 3 Activated sludges |
| 25 | X0opt 6xdilX0 | 2 Soils | |
| High S0 conc. (mg/L) | 10 | X0opt 6xdilX0 | 2 River waters |
| 25 | X0opt 6xdilX0 | 3 Marine waters |
X0, initial total cell density; X0opt, optimal cell density; 6xdilX0, series of 6 dilutions of the inoculum.
Technical repercussions for research and development
The practical application of this testing approach in a matrix may require a high throughput of replicate tests performed within the framework of a simple but rigorous procedure (Table 1). Therefore an experimental design that enables such a high throughput has to be constructed.
The practical application of the concept requires several technical requirements in order to:
• Overcome organic carbon contamination. The concentration of the biomass entails DOC (Dissolved Organic Carbon) contaminations that disturb the detection of transformation reactions. Washing followed by centrifugations could reduce the carbon contamination.
• Overcome a reduction in the cell number during the lag phase through the presence of grazing protozoa. A pre-filtration of the inoculum on a bolting cloth (5 μm mesh size) would resolve the problem.
• Produce a method for a high throughput of replicates to be carried out within the framework of a factorial experimental design. Table 1 presents such a design that would need approximately 28 Sturm tests (OECD 301 B, OECD, 1992a) for only one substance and one inoculum. This method is conceivable in a research project but it is not practical for industry under REACH. Fortunately, new systems are now available, namely a platform using an array of optrodes for either oxygen or CO2 detection (Malins and MacCraith, 1998), but they remain quite expensive.
• Quantify the total phage population. In fact, no method exists to decrease the phage population, but an initial quantification would act as a “tool” to explain some test failures (for example a long lag time period).
In conclusion, evaluating the environmental persistence of a chemical substance is not a simple task. Testing still remains a research subject almost three decades after the first conclusions were reached by fellow scientists. Here, we wish to reassess the testing of the biodegradation of chemical substances with an emphasis on the biological part of the test, i.e., the microbial inoculum. If the substance is tested with a large number of inocula that originate from a representative environment, the intrinsic persistence, and the environmental persistence of the substance can be evaluated. In this “Probabio” approach, persistence will not only be regarded as a simple intrinsic substance property, but also as the capability of various environmental samples to degrade a substance under realistic exposure conditions and F/M ratio.
The main drawback of this approach is the large number of replicate tests. Nevertheless, we believe that the advent of a new miniaturized sensing platform for a high throughput screening will stimulate the development of the technical tools required for this new approach.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Auffret M. (2009). New Tools to Evaluate the Biodegradability of Petroleum Compounds. Ph.D. thesis, Nantes University, France [Google Scholar]
- Barlaan E., Sugimori M., Furukawa S., Takeuchi K. (2005). Profiling and monitoring of microbial populations by denaturing high-performance liquid chromatography. J. Microbiol. Methods 61, 399–412 10.1016/j.mimet.2005.01.002 [DOI] [PubMed] [Google Scholar]
- Blok J. (2001). A Quest for the Right Order, Biodegradation Rates in the Scope of Environmental Risk Assessment of Chemicals. Thesis, University Utrecht, Netherlands [Google Scholar]
- Blok J., Booy M. (1984). Biodegradability test-results related to quality and quantity of the inoculums. Ecotoxicol. Environ. Saf. 8, 410–422 10.1016/0147-6513(84)90063-0 [DOI] [PubMed] [Google Scholar]
- Boethling R. S., Lynch D. G. (2007). Biodegradation of US premanufacture notice chemicals in OECD tests. Chemosphere 66, 715–722 10.1016/j.chemosphere.2006.07.063 [DOI] [PubMed] [Google Scholar]
- Borstlap C., Kooijman P. L. (1963). A study of biodegradation of anionic synthetic detergents a new laboratory test. J. Am. Oil Chem. Soc. 40, 78–80 10.1007/BF02654749 [DOI] [Google Scholar]
- Chouari R., Le Paslier D., Daegelen P., Dauga C., Weissenbach J., Sghir A. (2010). Molecular analyses of the microbial community composition of an anoxic basin of a municipal wastewater treatment plant reveal a novel lineage of proteobacteria. Microb. Ecol. 60, 272–281 10.1007/s00248-009-9632-7 [DOI] [PubMed] [Google Scholar]
- Chudoba P., Capdeville B., Chudoba J. (1992). Explanation of the biological meaning of the S0/X0 ratio in batch cultivation. Water Sci. Technol. 26, 743–752 [Google Scholar]
- DeSantis T. Z., Dubosarskiy I., Murray S. R., Andersen G. L. (2003). Comprehensive aligned sequence construction for automated design of effective probes (CASCADE-P) using 16S rDNA. Bioinformatics 19, 1461–1468 10.1093/bioinformatics/btg200 [DOI] [PubMed] [Google Scholar]
- ECETOC (2003). Persistence of Chemicals in the Environment. Technical Report No. 90, Brussels, 195.
- Fierer N., Nemergut D., Knight R., Craine J. M. (2010). Changes through time: integrating microorganisms into the study of succession. Res. Microbiol. 161, 635–642 10.1016/j.resmic.2010.06.002 [DOI] [PubMed] [Google Scholar]
- Forney L. J., Liu W.-T., Guckert J. B., Kumagai Y., Namkung E., Nishihara T., Larson R. J. (2001). Structure of microbial communities in activated sludge: potential implications for assessing the biodegradability of chemicals. Ecotoxicol. Environ. Saf. 49, 40–53 10.1006/eesa.2001.2034 [DOI] [PubMed] [Google Scholar]
- Fujimoto Y. (1963). Kinetics of microbial growth and substrate consumption. J. Theor. Biol. 5, 171–191 10.1016/0022-5193(63)90058-4 [DOI] [PubMed] [Google Scholar]
- Hennes K. P., Simon M. (1995). Significance of bacteriophages for controlling bacterioplankton growth in a mesotrophic lake. Appl. Environ. Microbiol. 61, 333–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyghe A., Francois P., Charbonnier Y., Tangomo-Bento M., Bonetti E.-J., Paster B. J., Bolivar I., Baratti-Mayer D., Pittet D., Schrenzel J., Geneva Study Group on Noma (GESNOMA) (2008). A novel microarray design strategy to study complex bacterial communities. Appl. Environ. Microbiol. 74, 1876–1885 10.1128/AEM.01722-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingerslev F., Torang L., Nyholm N. (2000). Importance of the test volume on the lag phase in biodegradation studies. Environ. Toxicol. Chem. 19, 2443–2447 10.1002/etc.5620191011 [DOI] [Google Scholar]
- Kagawa Y., Mano Y., Shimazono N. (1960). Biodegradation of dehydro-L-ascorbic acid-2,3-diketo-aldonic acid decarboxylase from rat liver. Biochim. Biophys. Acta 43, 348–349 10.1016/0006-3002(60)90451-0 [DOI] [PubMed] [Google Scholar]
- Malins C., MacCraith B. D. (1998). Dye-doped organically modified silica glass for fluorescence based carbon dioxide gas detection. Analyst 123, 2373–2376 10.1039/a805803b [DOI] [Google Scholar]
- Maukonen J., Saarela M. (2009). Microbial communities in industrial environment. Curr. Opin. Microbiol. 12, 238–243 10.1016/j.mib.2009.04.002 [DOI] [PubMed] [Google Scholar]
- OECD (1992a). OECD guidelines for testing of chemicals. 301 series. Ready biodegradability (301A: DOC die away test; 301B: CO2 evolution test; 301C: modified MITI Test (I); 301D: closed bottle test; 301E: modified OECD screening test; 301F: Manometric respirometry test). 12.05.1981, last updated 17.07.1992. Organisation for Economic Co-operation and Development, Paris.
- OECD (1992b). OECD Guideline 302 B, Inherent Biodegradability: Zahn-Wellens/EVPA Test, Organisation for Economic Co-operation and Development, Paris.
- OECD (1992c). OECD Guideline 303 A, Simulation Test – Aerobic Sewage Treatment. A: Activated Sludge Units, Organisation for Economic Co-operation and Development, Paris.
- OECD (1992d). OECD Guideline 303 B, Simulation Test – Aerobic Sewage Treatment. B: Biofilms, Organisation for Economic Co-operation and Development, Paris.
- OECD (1992e). OECD Guideline 306, Biodegradability in Seawater, Organisation for Economic Co-operation and Development, Paris.
- OECD (1992f). OECD Guideline 307, Aerobic and Anaerobic Transformation in Soil, Organisation for Economic Co-operation and Development, Paris.
- OECD (1992g). OECD Guideline 308, Aerobic and Anaerobic Transformation in Aquatic Sediment Systems, Organisation for Economic Co-operation and Development, Paris.
- OECD (1992h). OECD Guideline 309, Aerobic Mineralisation in Surface Water – Simulation Biodegradation Test, Organisation for Economic Co-operation and Development, Paris.
- Paixão S. M., Sàágua M. C., Tenreiro R., Anselmo A. M. (2006). Biodegradability testing using standardized microbial communities as inoculum. Environ. Toxicol. 21, 131–140 10.1002/tox.20165 [DOI] [PubMed] [Google Scholar]
- Ramadan M. A., El-Tayeb O. M., Alexander M. (1990). Inoculum size as a factor limiting success of inoculation for biodegradation. Appl. Environ. Microbiol. 56, 1392–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandaa R.-A., Gomez-Consarnau L., Pinhassi J., Riemann L., Malits A., Weinbauer M., Gasol P. A., Thingstad T. F. (2009). Viruses control of bacterial biodiversity – linkages between viral and bacterial community structure in a nutrient enriched mesocosm experiment. Environ. Microbiol. 11, 2585–2595 10.1111/j.1462-2920.2009.01983.x [DOI] [PubMed] [Google Scholar]
- Sharma A., Kumar R., Kumar A., Gangal S. V. (2000). Application of defined co-immobilized microbial consortium as a ready-to-use seed inoculum in BOD analysis. Environ. Monit. Assess. 60, 251–260 10.1023/A:1006192616987 [DOI] [Google Scholar]
- Tabka H., Seignez C., Adler N., Pulgarin C., Péringer P. (1993). Inoculum standardization for biodegradability tests. Biotechnol. Tech. 7, 217–222 10.1007/BF02566151 [DOI] [Google Scholar]
- Thouand G., Bauda P., Oudot J., Kirsch G., Sutton C., Vidaly J. F. (1999). Laboratory evaluation of crude oil biodegradation with commercial or natural microbial inocula. Can. J. Microbiol. 45, 106–115 10.1139/w98-210 [DOI] [PubMed] [Google Scholar]
- Thouand G., Capdeville B., Block J. C. (1996). Preadapted inocula for limiting the risk of errors in biodegradability tests. Ecotoxicol. Environ. Saf. 33, 261–267 10.1006/eesa.1996.0033 [DOI] [PubMed] [Google Scholar]
- Thouand G., Friant P., Bois F., Cartier A., Maul A., Block J. C. (1995). Bacterial inoculum density and probability of para-nitrophenol biodegradability test response. Ecotoxicol. Environ. Saf. 30, 274–282 10.1006/eesa.1995.1031 [DOI] [PubMed] [Google Scholar]
- Vasquez-Rodriguez G., Garabetian F., Rols J. L. (2007). Inocula from activated sludge or ready biodegradability testing: homogenization by preconditioning. Chemosphere 68, 1447–1454 10.1016/j.chemosphere.2007.03.073 [DOI] [PubMed] [Google Scholar]
- Vazquez-Rodriguez G., Palluy F., Goma G., Rols J. L. (1999). Procedures in ready biodegradability testing: effects of the inoculation and the monitored parameter. Environ. Technol. 20, 301–308 10.1080/09593332008616821 [DOI] [Google Scholar]