Universal chest radiography in a large pre-employment tuberculosis (TB) screening program was of low yield in the detection of either active TB or increased latent TB infection (LTBI) reactivation risk, and it provided no assistance in deciding which individuals to prioritize for LTBI treatment.
Abstract
Purpose:
To assess the frequency and spectrum of abnormalities on routine screening chest radiographs in the pre-employment evaluation of health care workers with positive tuberculin skin test (TST) results.
Materials and Methods:
The institutional review board approved this HIPAA-compliant retrospective study and waived the need for written informed patient consent. Chest radiographic reports of all 2586 asymptomatic individuals with positive TST results who underwent pre-employment evaluation between January 1, 2003, and December 31, 2007, were evaluated to determine the frequency of detection of evidence of active tuberculosis (TB) or latent TB infection (LTBI) and the spectrum of imaging findings. All chest radiographs interpreted as positive were reviewed by an experienced board-certified radiologist. If there was a discrepancy between the two readings, a second experienced radiologist served as an independent and final arbiter. Any follow-up chest radiographs or computed tomographic images that had been acquired by employee health services or by the employee’s private physician as a result of a suspected abnormality detected at initial screening were also evaluated.
Results:
Of the 159 (6.1%) chest radiographic examinations that yielded abnormal results, there were no findings that were consistent with active TB. There were 92 cases of calcified granulomas, calcified lymph nodes, or both; 25 cases of apical pleural thickening; 16 cases of fibrous scarring; and 31 cases of noncalcified nodules. All cases of fibrous scarring involved an area smaller than 2 cm2. All noncalcified nodules were 4 mm in diameter or smaller, with the exception of one primary lung malignancy and one necrotizing granuloma (negative for acid-fast bacilli) that grew Mycobacterium kansasii on culture.
Conclusion:
Universal chest radiography in a large pre-employment TB screening program was of low yield in the detection of active TB or increased LTBI reactivation risk, and it provided no assistance in deciding which individuals to prioritize for LTBI treatment.
© RSNA, 2010
Introduction
The majority of new cases of tuberculosis (TB) arise from reactivation of dormant foci of infection. Consequently, treatment of latent TB infection (LTBI) is a major component of the national strategy to eliminate this disease in the United States (1). The tuberculin skin test (TST) is the most common means of detecting prior TB infection. For subjects with positive test results, the American Thoracic Society and the Centers for Disease Control have recommended that routine screening chest radiography be performed to exclude clinically active TB (required prior to initiating treatment for LTBI) or to detect evidence of old healed disease to assess reactivation risk (2,3).
Health care workers (HCWs) constitute one of the largest populations of individuals in the United States for whom routine screening for LTBI is generally recommended (4). In a recent evaluation of the value of the lateral projection in screening chest radiography in 875 asymptomatic individuals with positive TST results in a pre-employment setting, we found no case of active TB or any radiographic findings that, per guidelines, would enable us to definitively classify an individual as being a high-priority candidate for LTBI therapy (5). This experience prompted a larger study to evaluate the spectrum of findings on screening chest radiographs in asymptomatic individuals with positive TST results in a pre-employment setting and to assess how these findings would be interpreted in the context of current treatment guidelines.
Materials and Methods
Our hospital’s institutional review board approved this retrospective study and waived the need for written informed patient consent. Furthermore, this study complied with the Health Insurance Portability and Accountability Act.
Our study included 2586 adults who were evaluated by the Employee and Occupational Health Service at our large tertiary care hospital and its affiliated outpatient sites. These subjects underwent routine chest radiography between January 1, 2003, and December 31, 2007, because they had positive TST results (defined as ≥10 mm induration). Our hospital does not perform annual radiography in employees known to have positive TST results, and there are few TST conversions; therefore, virtually all of these studies were performed as part of the pre-employment evaluation, with a small number of studies performed for follow-up. Per the Employee and Occupational Health Service protocol, a baseline TST was performed in all newly hired employees in accordance with published guidelines (2–4). TSTs (5 tuberculin units) were placed intradermally and read 48–72 hours later by experienced Employee and Occupational Health Service nurses. On occasion, a documented history of a positive TST result precluded repeat testing by the Employee and Occupational Health Service. Two-step testing was performed when indicated (4).
In accordance with the Employee and Occupational Health Service protocol, all individuals with positive TST results routinely underwent posteroanterior and lateral chest radiography. Written documentation of the results of chest radiography is required as a condition of employment. A radiologist (R.L.E.) retrospectively reviewed the written radiographic reports on these individuals to determine whether they were considered normal or positive in that they demonstrated evidence of active or prior tuberculosis by using established radiographic criteria previously published in the literature (3,6). Indications of active TB included cavitation, consolidation, and pleural effusion. Evidence of prior TB infection included apical or basal pleural thickening; fibrous scarring; calcified granulomas, calcified lymph nodes, or both; and noncalcified nodules. All chest radiographs interpreted as positive were reviewed by a board-certified radiologist (R.L.E., more than 30 years of experience) who knew that the chest radiographs had been read as abnormal but was blinded to the specific interpretations. If there was a discrepancy between the two readings, a second experienced radiologist with 15 years of experience served as an independent and final arbiter. Any follow-up chest radiographs or computed tomographic (CT) scans that had been obtained by employee health services or by the employee’s private physician as a result of a suspected abnormality detected at initial screening were also evaluated.
In late 2006, the routine (nonresearch) Employee and Occupational Health Service TB screening algorithm was adjusted to include testing of all individuals who had positive TST results with an in vitro diagnostic laboratory test (QuantiFERON-TB Gold test; Cellistis, Victoria, Australia) to aid in the diagnosis of TB infection. All new employees with positive TST results (≥10 mm induration) underwent this test, typically on the day of TST reading. Those with a documented history of positive TST results (approximately 5% of the individuals with positive TST results; the majority of this small group had a measured reading in millimeters from an outside institution) underwent in vitro diagnostic laboratory testing without undergoing TST. Employees who had previously received at least 1 month of therapy for active TB or LTBI were excluded from in vitro diagnostic laboratory testing.
In vitro diagnostic laboratory tests were performed in accordance with the recommendations of the manufacturer, with whole blood samples incubated with stimulation antigens within 12 hours after collection. As per the package insert, an interferon gamma concentration of 0.35 IU/mL in response to either early secretory antigenic target 6 or culture filtrate protein 10, relative to the negative control, was considered positive. Chest radiographic results for all individuals who had undergone both in vitro diagnostic laboratory testing and chest radiography between October 2006 and November 2008 (n = 660) were reviewed to determine whether positive findings at chest radiography, as defined previously, would be found more frequently in individuals with positive TST and positive in vitro diagnostic laboratory test results than in those with positive TST and negative in vitro diagnostic laboratory test results. All radiographic results that were interpreted as positive were reviewed by an experienced board-certified radiologist (R.L.E.). In cases of discrepancy, a second experienced radiologist reviewed the results, as described previously.
Data were collected and analyzed with an Excel spreadsheet (Microsoft, Redmond, Wash). We used the χ2 test to compare the proportion of subjects with positive chest radiographic findings between the positive and negative in vitro diagnostic laboratory test result groups. A P value of less than .05 indicated a significant difference.
Results
Of the 2586 individuals with positive TST results, 1422 were women (mean age, 36 years ± 12 [standard deviation]; age range, 18–65 years), and 1164 were men (mean age, 37 years ± 11; age range, 18–65 years). We used the findings of a study in a smaller but otherwise representative group of newly hired HCWs at our institution (7) and estimated that approximately 95% of these individuals were not born in the United States and approximately 93% had a history of bacille Calmette-Guérin vaccination. Approximately 70% of the HCWs with positive TST results at our institution have multiple risk factors for latent TB infection (7), the most common of which are induration of at least 15 mm at TST, history of direct daily patient care for at least 1 year, and long-term residence (≥5 years) in an area in which TB is highly endemic (incidence rate ≥40 cases per 100 000 persons according to World Health Organization data from 2004 to 2006, as summarized by the Public Health Agency of Canada [http://www.phac-aspc.gc.ca/tbpc-latb/itir_e.html]). These HCWs also have varying degrees of patient contact: Some (doctors, nurses, physical therapists, nurses’ aides, interpreters, and patient transporters) have daily contact with patients, while others (food service workers and custodians) have less contact. Still others (researchers) have essentially no contact with patients.
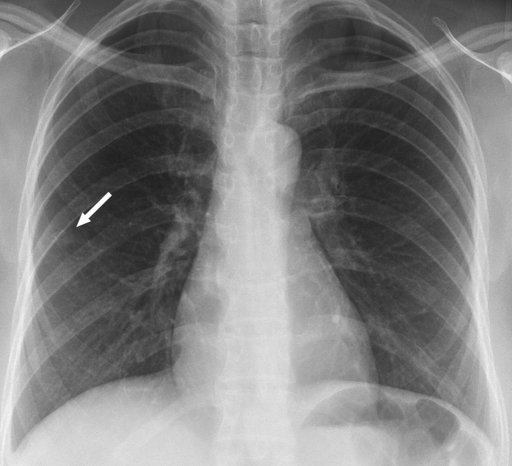
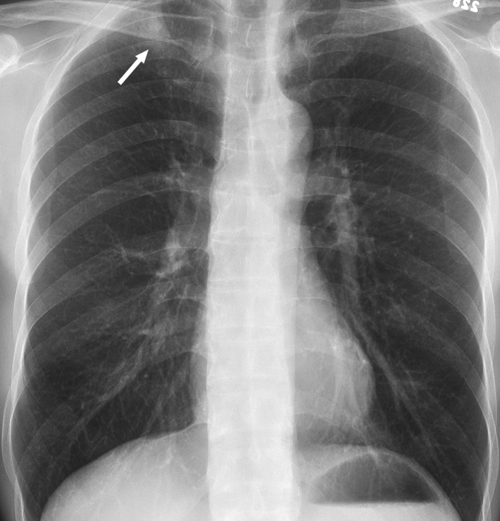
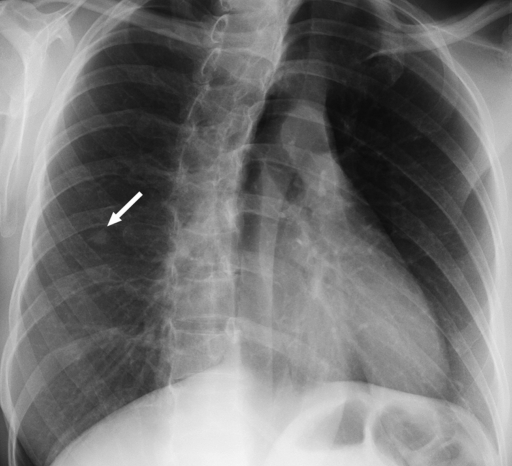
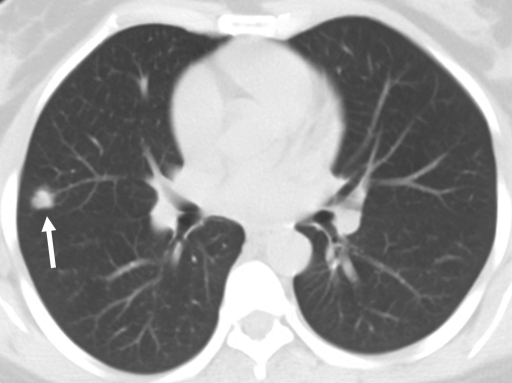
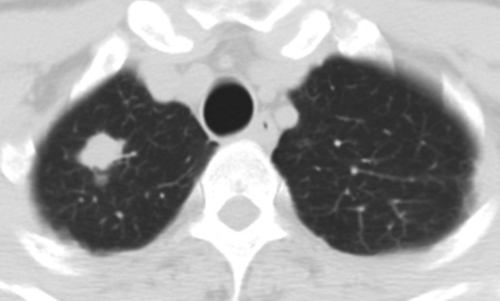
Review of the official interpretations of the initial screening chest radiographs revealed 164 positive findings in 159 (6.1%) of 2586 individuals. In these 159 individuals, we identified 92 (57.9%) cases of calcified granulomas, calcified lymph nodes, or both; 25 (15.7%) cases of apical pleural thickening; 16 (10.0%) cases of fibrous scarring; and 31 (19.5%) cases of noncalcified nodules. In all cases of fibrous scarring, the area involved was smaller than 2 cm2. Of the 31 noncalcified nodules, 16 (52%) were not confirmed at follow-up chest radiography or CT, whereas five (16%) were shown to be calcified at CT; no new findings were observed on any follow-up images. Of the remaining 10 cases of noncalcified nodules, five were 4 mm in diameter or smaller and showed no change on subsequent chest radiographs. Three nodules of similar size (≤4 mm in diameter) were found in individuals who were lost to follow-up. Of the remaining two cases of noncalcified nodules, one was confirmed to be a primary lung malignancy (Fig 1). In the other, the individual was asymptomatic, and a sputum smear was negative for acid-fast bacilli. CT-guided aspiration biopsy of the mass yielded tissue that was consistent with necrotizing granuloma (negative for acid-fast bacilli) at pathologic analysis and grew Mycobacterium kansasii on culture (Fig 2).
Figure 1a:
Carcinoma of the lung found in a 47-year-old woman at pre-employment screening. (a) Posteroanterior chest radiograph shows a suspected nodule in the right lower lobe (arrow). (b) Oblique chest radiograph shows the nodule (arrow) is parenchymal. (c) CT image shows the malignant 1-cm nodule (arrow).
Figure 2a:
Necrotizing granuloma found in a 51-year-old man at pre-employment screening. (a) Posteroanterior chest radiograph shows an ill-defined right apical mass (arrow) obscured by overlying body structures. (b) CT image shows the irregular mass in the right upper lobe. The diagnosis was made on the basis of tissue obtained with CT-guided aspiration biopsy. Pathologic analysis revealed the mass was consistent with necrotizing granulomas (negative for acid-fast bacilli), and Mycobacterium kansasii grew on culture.
Figure 1b:
Carcinoma of the lung found in a 47-year-old woman at pre-employment screening. (a) Posteroanterior chest radiograph shows a suspected nodule in the right lower lobe (arrow). (b) Oblique chest radiograph shows the nodule (arrow) is parenchymal. (c) CT image shows the malignant 1-cm nodule (arrow).
Figure 1c:
Carcinoma of the lung found in a 47-year-old woman at pre-employment screening. (a) Posteroanterior chest radiograph shows a suspected nodule in the right lower lobe (arrow). (b) Oblique chest radiograph shows the nodule (arrow) is parenchymal. (c) CT image shows the malignant 1-cm nodule (arrow).
Figure 2b:
Necrotizing granuloma found in a 51-year-old man at pre-employment screening. (a) Posteroanterior chest radiograph shows an ill-defined right apical mass (arrow) obscured by overlying body structures. (b) CT image shows the irregular mass in the right upper lobe. The diagnosis was made on the basis of tissue obtained with CT-guided aspiration biopsy. Pathologic analysis revealed the mass was consistent with necrotizing granulomas (negative for acid-fast bacilli), and Mycobacterium kansasii grew on culture.
Of the 660 individuals with positive TST results who had undergone both chest radiography and in vitro diagnostic laboratory testing, 135 (20.5%) had positive in vitro diagnostic test results, and 517 (78.3%) had negative in vitro diagnostic test results. The remaining eight individuals had indeterminate in vitro diagnostic test results. Of the 135 individuals with positive in vitro diagnostic test results, eight (5.9%) had positive findings at chest radiography that were potentially consistent with LTBI, as defined previously. The abnormal results included six cases of calcified granulomas, calcified lymph nodes, or both; one case of apical pleural thickening; two cases of fibrous scarring; and one case of noncalcified nodules. For the cases of fibrous scarring, both were smaller than 2 cm2 in area. One subject had a history of prior treated TB. The other had a scar that was partially calcified, and the physician decided to treat for LTBI without performing a sputum culture. In the patient with noncalcified nodules, nodules were numerous and tiny. Sputum and bronchoalveolar lavage cultures were performed, and all specimens were culture negative for acid-fast bacilli. Of the 517 individuals with negative in vitro diagnostic laboratory test results, 40 (7.7%) had positive radiographic findings, a proportion that was not significantly different from that in the group of patients with positive in vitro diagnostic laboratory test results (P = .58). The abnormal examinations in the group with negative in vitro diagnostic laboratory test results included 15 cases of calcified granulomas, calcified lymph nodes, or both; seven cases of apical pleural thickening; eight cases of fibrous scarring (all ≤2 cm2); and 11 cases of noncalcified nodules. Of the 11 cases of noncalcified nodules, one case was not confirmed at follow-up CT, and one case was shown to be calcified at CT. Of the nine remaining cases of noncalcified nodules, nodules were smaller than 4 mm in diameter in four cases and between 6 and 10 mm in diameter in the remaining five cases. In all cases, serial radiologic follow-up was recommended but had not been performed during the time frame of this study.
Discussion
It can be argued that there are three basic reasons to obtain screening chest radiographs in asymptomatic individuals with positive TST results. The first is to exclude active disease. The second is to assess for evidence of prior TB (and associated relative reactivation risk) to help guide treatment decisions. The third is to provide a baseline for future comparisons. It is critical to exclude active TB prior to treatment for LTBI, given the difference in treatment regimens for the two conditions (multidrug therapy vs single-agent therapy) and the potential for the development of antibiotic resistance with inadequate therapy of active disease. In our study of 2586 asymptomatic individuals in a pre-employment setting in a large American hospital over 5 years, no case of active TB was found.
Given the low yield of screening for active disease in these asymptomatic individuals with positive TST results, we must then evaluate the second reason for radiographic screening: to assess for any evidence of past TB and, more specifically, to assess LTBI reactivation risk to allow the clinician and patient to make informed decisions about LTBI treatment. The American Thoracic Society and the Centers for Disease Control (3) suggest that individuals with “abnormal chest radiographs consistent with prior TB” have a higher risk of reactivation disease. In accordance with this statement, they recommend that induration of 5 mm or more (rather than 10 mm) at TST should be considered a positive result in these individuals. However, it appears that there is a good deal of confusion among radiologists and clinicians regarding the definition of an “abnormal chest radiograph consistent with prior TB” and particularly regarding the relative significance of noncalcified fibrotic lesions of varying sizes. Jasmer et al (8) suggested that individuals whose radiographs show “opacities occupying more than 2 cm2 of the upper lobe” should actually be evaluated for active TB before LTBI treatment is started; however, they did not comment on the relative reactivation risk in those with parenchymal opacities smaller than 2 cm2. In fact, they implied that such smaller lesions would not be particularly consistent with past TB. Jasmer et al did explicitly state that “radiographs showing pleural thickening or isolated calcified granulomas are not considered to be suggestive of previous tuberculosis.” In contrast, the American Thoracic Society and the Centers for Disease Control (2,3) stated that both “nodules and fibrotic lesions” (with no comment on their size) suggest increased TB reactivation risk; however, calcified nodular lesions and apical or basal pleural thickening—while consistent with prior TB—suggest a “lower risk for future progression to active TB.” In these guidelines, the authors cited three references that showed that “persons with fibrotic lesions on chest radiographs consistent with prior, healed TB have a risk for progression to active TB of 2.0–13.6 per 1000 person-years of observation.” One of these studies (9) showed that subjects with fibrotic pulmonary lesions larger than 2 cm2 had an incidence of active disease that was almost twice as high as the incidence of active disease in subjects with smaller fibrotic lesions; however, the latter group still had individuals who developed progressive TB disease. Moreover, in both groups, LTBI therapy decreased the proportion of individuals who developed progressive disease.
In addition to the uncertainty in the relative reactivation risk conferred by particular radiologic findings, there is also the caveat that all of the radiologic findings must be interpreted in the context of the individual’s exposure and medical history. This makes the overall assessment of the realistic reactivation risk of the individual being studied a substantial challenge. As mentioned, at our institution, approximately 95% of newly hired employees with positive TST results were born outside of the United States(7), often in areas of high TB prevalence, and the timing of immigration relative to pre-employment screening varies greatly (7). Medical reactivation risk factors—such as diabetes, human immunodeficiency virus, and other relevant medical conditions (2,3)—are also important variables for some of the employees; however, these types of data are not consistently collected at pre-employment screening. Thus, our HCW population represents a heterogeneous group with varying baseline likelihood of having acquired TB and varying baseline reactivation risk.
In our study, 94% of those with positive TST results had a negative chest radiograph. The others had calcified granulomas, apical pleural thickening, minimal scarring (<1 cm2), or small noncalcified nodules that, if we use the cutoff of 2 cm2, were not suggestive of significant reactivation risk per the references discussed previously. Indeed, in our cohort of 2586 individuals, there was no case in which fibrotic lesions were 2 cm2 or larger. Furthermore, in the cohort of subjects who underwent the in vitro diagnostic laboratory test, those with positive in vitro test results (who arguably definitively have TB infection) were no more likely to have positive chest radiographs than were those with negative in vitro test results, and no large fibrotic lesions were observed in either group. Thus, chest radiography did not clarify whom we should prioritize for LTBI treatment. This finding is supported by the results of a smaller study of asymptomatic individuals with positive TST results. Gottridge et al (10) reported that “in no case [of 247 patients] did the physicians alter their recommendations concerning isoniazid chemoprophylaxis based on the finding on the chest roentgenograms.” In contrast, a study of asymptomatic Indian HCWs with presumed LTBI (11) reported a higher percentage of abnormal chest radiographs, some with large fibrotic lesions, indicating that utility does vary with the HCW population being studied. Nonetheless, we would argue that the results of our study would apply to most, if not all, HCW screening programs in hospitals that have relatively low TB case rates but diverse employee populations with varying likelihood of pre-employment TB exposure and varying reactivation risk factors.
We recognize two limitations of our study. First, we were unable to retrospectively obtain personal data (country of origin, TB exposure history) for the individuals whose chest radiographs were evaluated because of limited access to pre-employment data. However, as described, we recently evaluated LTBI risk factors and test results (TST and in vitro diagnostic laboratory test) in a group of newly hired HCWs at our institution (7). We believe that the smaller group in that study provides a valid representation of the larger group evaluated here. In terms of results that might be observed in employee populations with a lower bacille Calmette-Guérin vaccination rate, we note that the bacille Calmette-Guérin vaccine is primarily given in countries in which TB is endemic and does not prevent pulmonary TB infection. Thus, we would expect that a population that did not receive the bacille Calmette-Guérin vaccine would have fewer findings on chest radiographs than would the individuals in our study. Second, we did not validate all the negative radiologic results; however, we do not expect that any large lesions (≥2 cm2) that would have changed our overall conclusions were missed. Moreover, no discrepancies were identified on review of cases with positive radiologic results.
If the goal of chest radiography in TB screening programs in the pre-employment setting is to search for active TB or evidence of significant LTBI reactivation risk as defined by fibrotic noncalcified lesions of 2 cm2 or larger, our findings suggest that chest radiography is of extremely low yield in this setting, as we found neither. Given the fact that even completely asymptomatic individuals can occasionally have active TB and that missing active TB in a HCW could be disastrous, we are not arguing that chest radiography should not be performed routinely in the pre-employment setting. Rather, we suggest that we take a hard look at what we actually learn from chest radiography. Review of the published guidelines regarding the relative reactivation risk conferred by specific radiographic findings showed that, in our population, these guidelines would provide no assistance in deciding which individuals to prioritize for LTBI treatment. In our hospital, treatment is offered to all HCWs with positive test results (formerly TST; more recently, in vitro diagnostic laboratory test); however, many individuals refuse treatment because they are wary of its side effects, and others have characteristics that may make treatment somewhat more challenging (increased age or baseline mild liver function abnormalities).
If future large-scale studies were to be performed to clarify the relative reactivation risk conferred by specific imaging findings within the spectrum we observed, the contribution of chest radiography to patient care in the pre-employment setting would be greatly increased.
In conclusion, universal chest radiography in a large pre-employment TB screening program was of extremely low yield in the detection of either active TB or increased LTBI reactivation risk, and it provided no assistance in deciding which individuals to prioritize for LTBI treatment.
Advances in Knowledge.
Universal chest radiography in a large preemployment tuberculosis (TB) screening program was of low yield in the detection of either active TB or increased reactivation risk of latent TB infection (LTBI).
Published guidelines for interpretation of relative reactivation risk conferred by specific findings on screening chest radiographs provided no assistance in deciding which individuals to prioritize for LTBI treatment.
Implication for Patient Care.
The contribution of chest radiography to case management in the pre-employment setting would be greatly increased by future large-scale studies to clarify the relative reactivation risk conferred by specific abnormal imaging findings.
Received March 4, 2010; revision requested April 2; revision received April 6; accepted April 10; final version accepted April 21.
From the 2009 RSNA Annual Meeting.
Authors stated no financial relationship to disclose.
Supported by the National Institutes of Health (grant 1 K23 AI074638-01A2).
Funding: This research was supported by the National Institutes of Health (grant 1 K23 AI074638-01A2).
Abbreviations:
- HCW
- health care worker
- LTBI
- latent TB infection
- TB
- tuberculosis
- TST
- tuberculin skin test
References
- 1.Horsburgh CR., Jr Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med 2004;350(20):2060–2067 [DOI] [PubMed] [Google Scholar]
- 2.Targeted tuberculin testing and treatment for latent tuberculosis infection: American Thoracic Society. MMWR Recomm Rep 2000;49(RR-6):1–51 [PubMed] [Google Scholar]
- 3.Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med 2000;161(4 pt 2):S221–S247 [DOI] [PubMed] [Google Scholar]
- 4.Jensen PA, Lambert LA, Iademarco MF, Ridzon R. CDC Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005;54(RR-17):1–141 [PubMed] [Google Scholar]
- 5.Eisenberg RL, Romero J, Litmanovich D, Boiselle PM, Bankier AA. Tuberculosis: value of lateral chest radiography in pre-employment screening of patients with positive purified protein derivative skin test results. Radiology 2009;252(3):882–887 [DOI] [PubMed] [Google Scholar]
- 6.Jeong YJ, Lee KS. Pulmonary tuberculosis: up-to-date imaging and management. AJR Am J Roentgenol 2008;191(3):834–844 [DOI] [PubMed] [Google Scholar]
- 7.Pollock NR, Campos-Neto A, Kashino S, et al. Discordant QuantiFERON-TB Gold test results among US healthcare workers with increased risk of latent tuberculosis infection: a problem or solution? Infect Control Hosp Epidemiol 2008;29(9):878–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jasmer RM, Nahid P, Hopewell PC. Clinical practice: latent tuberculosis infection. N Engl J Med 2002;347(23):1860–1866 [DOI] [PubMed] [Google Scholar]
- 9.Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial—International Union Against Tuberculosis Committee on Prophylaxis. Bull World Health Organ 1982;60(4):555–564 [PMC free article] [PubMed] [Google Scholar]
- 10.Gottridge JA, Meyer BR, Schwartz NS, Lesser RS. The nonutility of chest roentgenographic examination in asymptomatic patients with positive tuberculin test results. Arch Intern Med 1989;149(7):1660–1662 [PubMed] [Google Scholar]
- 11.Joshi R, Patil S, Kalantri S, Schwartzman K, Menzies D, Pai M. Prevalence of abnormal radiological findings in health care workers with latent tuberculosis infection and correlations with T cell immune response. PLoS One 2007;2(8):e805. [DOI] [PMC free article] [PubMed] [Google Scholar]