Abstract
Dendritic cell (DC)-based vaccination is a promising strategy for cancer immunotherapy. However, clinical trials have indicated that immunosuppressive microenvironments induced by tumors profoundly suppress antitumor immunity and inhibit vaccine efficacy, resulting in insufficient reduction of tumor burdens. To overcome these obstacles and enhance the efficiency of DC vaccination, we generated interleukin (IL)-12- and granulocyte-macrophage colony-stimulating factor (GM-CSF)-coexpressing oncolytic adenovirus (Ad-ΔB7/IL12/GMCSF) as suitable therapeutic adjuvant to eliminate immune suppression and promote DC function. By treating tumors with Ad-ΔB7/IL12/GMCSF prior to DC vaccination, DCs elicited greater antitumor effects than in response to either treatment alone. DC migration to draining lymph nodes (DLNs) dramatically increased in mice treated with the combination therapy. This result was associated with upregulation of CC-chemokine ligand 21 (CCL21+) lymphatics in tumors treated with Ad-ΔB7/IL12/GMCSF. Moreover, the proportion of CD4+CD25+ T-cells and vascular endothelial growth factor (VEGF) expression was decreased in mice treated with the combination therapy. Furthermore, combination therapy using immature DCs also showed effective antitumor effects when combined with Ad-ΔB7/IL12/GMCSF. The combination therapy had a remarkable therapeutic efficacy on large tumors. Taken together, oncolytic adenovirus coexpressing IL-12 and GM-CSF in combination with DC vaccination has synergistic antitumor effects and can act as a potent adjuvant for promoting and optimizing DC vaccination.
Introduction
Dendritic cells (DC) are potent antigen-presenting cells (APC) with a unique capability to induce primary immune responses to self or foreign antigens. DC-based vaccines are a promising strategy in cancer immunotherapy.1 DCs pulsed with tumor-associated antigen can induce protective and therapeutic tumor-specific immune responses to various tumor types.2,3 Recently, DC-based vaccine clinical trials have been performed with patients with prostate cancer, melanoma, lymphoma, and renal carcinoma.4,5,6,7 However, beneficial patient responses were fewer than predicted. Although tumor-specific immune responses were seen in most of the patients, objective clinical responses were only observed in a few patients.
DCs have been reported to have significantly impaired biological functions in advanced cancer patients, which might be associated with the failure of the DC vaccination in clinical trials.8,9 Emerging evidence indicates that this effect might be mediated by various factors produced by tumor cells. Tumor tissues can produce immunosuppressive molecules, such as vascular endothelial growth factor (VEGF), transforming growth factor-β, and interleukin (IL)-10. These molecules induce an immunosuppressive microenvironment within the tumors. The function of tumor-associated DCs may also be suppressed under these conditions.10 Therefore, DC vaccination efficacy may be improved by the addition of an adjuvant to reduce or eliminate tumor-induced immunosuppression.
Cytokine therapy has been reported to be an effective strategy for cancer therapy.11 Ectopic cytokine expression in tumor tissues can induce strong antitumor immune responses, and can also convert the immunosuppressive tumor microenvironment to one with more antitumor characteristics. IL-12 is an important cytokine in innate and adaptive immunity that is able to enhance T-helper 1 cells (Th1) immunity, increase cytotoxic T-lymphocytes cytotoxicity, and inhibit angiogenesis.12 Preclinical and clinical trials of IL-12-based immunotherapy have demonstrated strong antitumor immune responses.13,14,15 Previous studies reported that immunosuppressive conditions can be reversed by induction of intratumoral IL-12 expression. These results were associated with effector T-cell activation, Treg cell apoptosis induction, and cytotoxic T-lymphocytes infiltration.16 In addition, IL-12-induced IFN-γ-inducible protein 10 (IP-10) and Mig expression are strong inhibitors of tumor angiogenesis.17 Granulocyte-macrophage colony-stimulating factor (GM-CSF) is produced by a wide range of cell types and functions to stimulate proliferation, maturation, and function of APCs.18 Previous studies have demonstrated an antitumor effect of GM-CSF in cancer immunotherapy, indicating that GM-CSF is a useful therapeutic agent in cancer therapy.19,20
Recently, adenovirus (Ad) vectors as efficient gene delivery systems have been widely used in cancer gene therapy. Cytokine gene therapy can induce sustained antitumor effect via sustained expression of therapeutic dose of cytokine in the local tumor tissue and that can prolong the antitumor effect and reduce toxicity compared with treatment of recombinant cytokine protein.21,22,23 In an effort to overcome systemic cytokine toxicity, local injection of recombinant cytokine protein has been tried. But it was still not easily amenable in the clinics because it forced the continuous administration of cytokine.24 Moreover, Ad vectors can be modified to allow for replication in only cancer cells.25 Our previous studies have demonstrated that an oncolytic Ad can greatly amplify gene delivery efficacy in a cancer cell-restricted manner while they replicate.26,27 Furthermore, circulating Ad-specific neutralizing Ab may not significantly impact on the efficacy of local treatment of oncolytic Ad expressing cytokine, since there was no evident correlation between the therapeutic efficacy and Ad-specific antibody (Ab) titer have been reported in clinical setting.28,29
In this report, we investigated the antitumor effects of an IL-12- and GM-CSF-coexpressing oncolytic Ad (Ad-ΔB7/IL12/GMCSF) in combination with DC vaccination in a murine melanoma model. This combination therapy elicited strong and synergistic antitumor effects, correlated with the elimination of immunosuppression in tumor tissues, and facilitated DC migration to draining lymph nodes (DLNs). These preclinical results indicate that an oncolytic Ad coexpressing IL-12 and GM-CSF can act as a potent adjuvant for optimizing DC vaccination.
Results
Oncolytic Ad-mediated IL-12 and GM-CSF expression
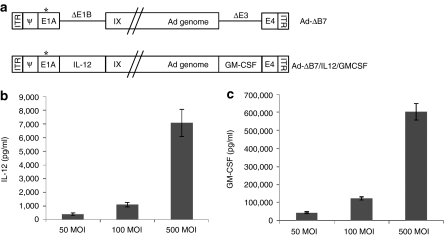
Ad-ΔB7, an oncolytic Ad, was mutated in the retinoblastoma binding sites of E1A and had the E1B region deleted, as shown in Figure 1a. The cancer-specific viral replication and cytotoxicity of Ad-ΔB7 has been previously confirmed.25 To generate the Ad-ΔB7/IL12/GMCSF oncolytic Ad, murine IL-12 and GM-CSF genes were inserted into the E1 and E3 regions of the Ad-ΔB7 viral genome, respectively. Cultured murine melanoma B16-F10 cells were infected with Ad-ΔB7/IL12/GMCSF at multiplicity of infection (MOIs) of 50, 100, and 500. As shown in Figure 1b,c a dose-dependent increase in secreted IL-12 and GM-CSF was observed in cells infected with Ad-ΔB7/IL12/GMCSF after 48 hour.
Figure 1.
Characterization of oncolytic adenovirus coexpressing interleukin (IL)-12 and granulocyte-macrophage colony-stimulating factor (GM-CSF). (a) Schematic representation of the genomic structures of adenovirus (Ad) Ad-ΔB7 and Ad-ΔB7/IL12/GMCSF. Ad-ΔB7 contains mutated E1A (open star, mutation at Rb protein–binding site), but lacks E1B 19 and 55 kDa (ΔE1B), and E3 region (ΔE3); the murine IL-12 and murine GM-CSF were inserted into E1 and E3 region of Ad genome, respectively. The level of IL-12 (b) and GM-CSF (c) expression was confirmed in B16-F10 cells after infection with Ad-ΔB7/IL12/GMCSF at different MOIs. Cell culture supernatants were collected at 48 hour after infection, and the level of IL-12 and GM-CSF was quantified by conventional enzyme-linked immunosorbent assay kit. Data represent the mean ± SE of triplicate experiments, and similar results were obtained from at least three separate experiments. MOI, multiplicity of infection.
Characterization of cultured DCs
To evaluate DC quality and phenotype, in vitro cultures of bone marrow-derived mature and immature DCs were assayed by flow cytometry. After the 8-day culture regimen, the cultures consisted of >80% DCs (Supplementary Figure S1a). After antigen-pulsing and activation by lipopolysaccharide, the mature DCs showed markedly increased cell-surface expression of the costimulatory molecules CD40, CD80, CD86, and CC-chemokine receptor 7 (CCR7), as compared with immature DCs (Supplementary Figure S1b,c).
Therapeutic efficacy of Ad-ΔB7/IL12/GMCSF combined with DCs in an established murine melanoma model
To evaluate the therapeutic efficacy of Ad-ΔB7/IL12/GMCSF in combination with DCs in vivo, established B16-F10 melanoma tumors in C57BL/6 mice were intratumorally injected with three doses of Ad-ΔB7/IL12/GMCSF on days 0–2, followed by three injections of DCs on days 3–5. Treatment groups included phosphate-buffered saline (PBS)-only control, Ad-ΔB7/IL12/GMCSF only (5 × 109 viral particle (VP)/injection), DCs only (1 × 106/injection), or a combination of DCs and Ad-ΔB7/IL12/GMCSF, referred to as the combination therapy. In parallel, one group of mice was given a treatment regimen of a high dose of Ad-ΔB7/IL12/GMCSF (5 × 1010 VP/injection) in combination with DCs (1 × 106/injection), referred to as the high-dose combination therapy.
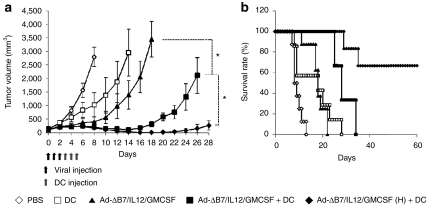
Control mice treated with PBS alone showed aggressive tumor growth and rapidly formed large tumors (over 2,500 mm3) by day 8 (Figure 2a). In contrast, mice treated with Ad-ΔB7/IL12/GMCSF or DCs alone showed significant inhibition of tumor growth. On day 8, the mean tumor volumes in mice treated with DCs alone, Ad-ΔB7/IL12/GMCSF alone, the combination therapy, or the high-dose combination therapy were 1,031 ± 443, 410 ± 170, 210 ± 24, and 177 ± 19 mm3, respectively. These data correlate to 63, 85, 92, and 93% tumor growth inhibition, respectively, as compared to PBS alone controls. Tumor growth inhibition was statistically significant in mice treated with the combination therapy as compared with individual treatment groups (P < 0.05 versus DCs or Ad-ΔB7/IL12/GMCSF). The high-dose combination therapy showed an even greater antitumor effect than combination therapy (on day 26, P < 0.05 versus combination therapy). In addition, the median survival of mice treated with Ad-ΔB7/IL12/GMCSF alone, DCs alone, the combination therapy, or high-dose combination therapy was 18, 18, 28, and over 90 days, respectively. All treatment groups showed significantly prolonged survival as compared to control mice (P < 0.01, Figure 2b). Importantly, four out of six mice treated with the high-dose combination therapy achieved complete remission and survived over 90 days. These results suggest that Ad-ΔB7/IL12/GMCSF in combination with DCs can induce a potent antitumor effect in the B16-F10 melanoma model.
Figure 2.
Antitumor effect of Ad-ΔB7/IL12/GMCSF in combination with dendritic cell (DCs). (a) Pre-established B16-F10 tumor was injected with phosphate-buffered saline (PBS) (open diamonds), DCs (open squares), Ad-ΔB7/IL12/GMCSF (filled triangles), Ad-ΔB7/IL12/GMCSF plus DCs (filled squares), or Ad-ΔB7/IL12/GMCSF (H; high-dose of Ad) plus DCs (filled diamonds). Tumor growth was monitored every day. (b) Survival percentage of tumor-bearing mice following treatment with Ad-ΔB7/IL12/GMCSF and/or DCs. Tumor size over 3,000 mm3 was regarded as death. Data points represent the mean ± SE. All results in this figure represent results of at least seven mice per group. *P < 0.05. GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin.
IL-12 and GM-CSF expression and immune cell infiltration in treated tumor tissues
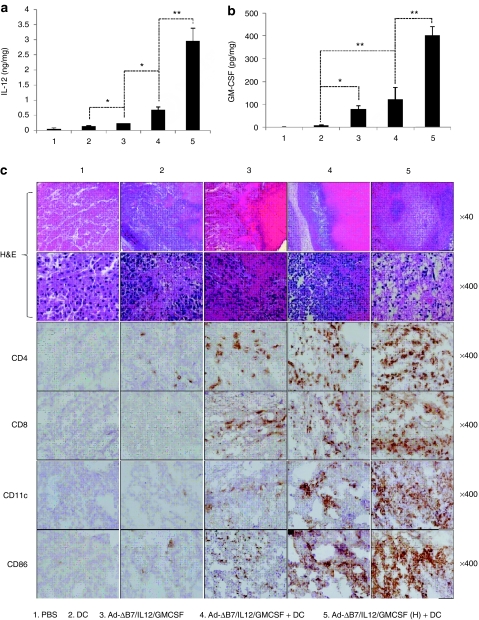
To examine the mechanisms of the observed antitumor effects, IL-12 and GM-CSF expression and immune cell infiltration in the tumor tissues were examined. Expression of IL-12 and GM-CSF was very minimal in tumors treated with PBS, but was present in tumors treated with Ad-ΔB7/IL12/GMCSF or DCs (Figure 3a,b). Tumors treated with the combination therapy showed markedly enhanced IL-12 and GM-CSF expression relative to tumors treated with Ad-ΔB7/IL12/GMCSF or DCs alone. This finding was even more dramatic in tumors treated with the high-dose combination therapy.
Figure 3.
Generation of antitumor immune response by Ad-ΔB7/IL12/GMCSF in combination with dendritic cell (DCs). The level of interleukin (IL)-12 (a) and granulocyte-macrophage colony-stimulating factor (GM-CSF) (b) expression in tumor tissues treated with phosphate-buffered saline (PBS), DCs, Ad-ΔB7/IL12/GMCSF, Ad-ΔB7/IL12/GMCSF plus DCs, or Ad-ΔB7/IL12/GMCSF (H; high-dose of Ad) plus DCs. (c) Histological and immunohistochemical analysis in tumor tissues treated with Ad-ΔB7/IL12/GMCSF and/or DCs. Tumor tissues were collected from mice at 3 days after final treatment, and paraffin section of tumor tissue was stained with hematoxylin and eosin (H&E) (top two rows, original magnification: ×40 and ×400). Immune cells infiltrated in tumor tissues were examined by anti-CD4 antibody (third row), anti-CD8 antibody (fourth row), anti-CD11c antibody (fifth row) and anti-CD86 antibody (bottom row). Original magnification: ×400. Data points represent the mean ± SE of at least three mice per group, and similar results were obtained from at least three separate experiments. *P < 0.05; **P < 0.01.
Histological analyses revealed enhanced tumor necrosis in tumor tissues from mice treated with the combination therapy as compared with individual treatments. Significantly, nearly all tumor cells were eliminated from mice treated with the high-dose combination therapy. Immunohistochemical analyses using CD4-, CD8-, CD11c-, and CD86-specific Abs were performed to identify the immune cells that infiltrated the tumor tissues. Significantly greater numbers of CD4+ T-cells, CD8+ T-cells, and CD11c+ DCs were observed in tumors treated with the combination therapy than in tumors receiving individual treatments (Figure 3c). Correlating with the data in Figure 2, tumors treated with the high-dose combination therapy showed increased levels of tumor-infiltrating T-cells and DCs. In addition, the expression of CD86 as a marker of activated APCs was significantly increased in tumors treated with the combination therapies as compared to the individual treatments.
VEGF expression and tumor angiogenesis is inhibited by combination therapy
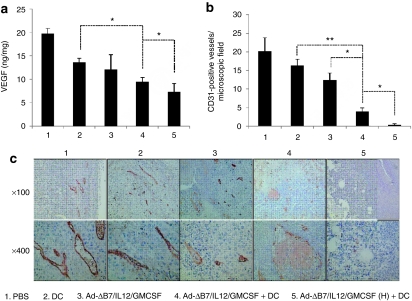
IL-12 and E1A protein have been reported to inhibit tumor angiogenesis and downregulate VEGF expression.30,31 As shown in Figure 4a, VEGF expression was decreased in tumors from all treatment groups as compared to the PBS control, with a more robust reduction in the combination therapy group (P < 0.05 versus DCs alone). As expected, VEGF expression was decreased to the lowest levels in tumors treated with the high-dose combination therapy (P < 0.05 versus the combination therapy).
Figure 4.
Decreased vascular endothelial growth factor (VEGF) expression and angiogenesis in tumors treated with combination therapy. Tumors were treated with phosphate-buffered saline (PBS), dendritic cells (DCs), Ad-ΔB7/IL12/GMCSF, Ad-ΔB7/IL12/GMCSF plus DCs, or Ad-ΔB7/IL12/GMCSF (H; high-dose of Ad) plus DCs, and harvested from mice at 3 days after final treatment. (a) The level of VEGF expression in tumor tissues was quantified by conventional enzyme-linked immunosorbent assay. *P < 0.05. (b) CD31+ microvessel immunohistochemistry in the tumor tissues treated with PBS, DC, Ad-ΔB7/IL12/GMCSF, Ad-ΔB7/IL12/GMCSF plus DCs, or Ad-ΔB7/IL12/GMCSF (H) plus DCs. Original magnification: ×100 and ×400. (c) The means of CD31+ vessel density in microscopic field for each treatment group (×200). Data points represent the mean ± SE of three mice per group, and similar results were obtained from at least two separate experiments. *P < 0.05; **P < 0.01. GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin.
Tumor angiogenesis was examined by immunohistochemical analysis using a CD31-specific Ab. A reduced number of CD31+ vessels were observed in mice treated with any of the individual or combination treatments as compared to the PBS controls (Figure 4b,c). Consistent with the data above, the decrease in CD31+ vessels was statistically greater in the combination-treated tumors than in tumors that received DCs or Ad-ΔB7/IL12/GMCSF alone (P < 0.05). Likewise, the high-dose combination therapy showed the greatest decrease (P < 0.05 versus the combination therapy) to nearly complete inhibition of angiogenesis.
Combination therapy promotes DC migration to draining lymphnodes
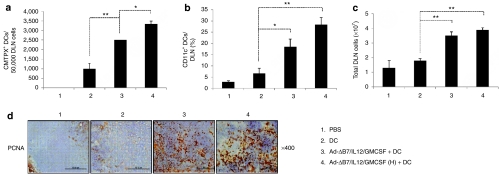
To explore whether DC migration to DLNs is promoted by combination therapy, DCs were labeled with a fluorescent probe, CMTPX (Supplementary Figure S2a). A significantly increased percentage of CMTPX+ DCs was observed in DLNs from mice treated with the combination therapy than from mice treated with DCs alone (P < 0.01). This finding was enhanced in mice treated with the high-dose combination therapy (P < 0.05 versus the combination therapy; Figure 5a, Supplementary Figure S2b). Moreover, combination therapy significantly augmented the proportion of CD11c+ DCs in DLNs, implying that combination therapy also promotes migration of endogenous DCs to DLNs (P < 0.05 versus Ad-ΔB7/IL12/GMCSF plus DCs; Figure 5b and Supplementary Figure S2c). Mice treated with the combination therapy showed significantly increased total cell numbers in DLNs as compared to mice treated with DCs alone (P < 0.01; Figure 5c). Consistent with augmented total cell numbers in DLN from mice treated with the combination therapies, the expression of proliferating cell nuclear antigen as a marker for cellular proliferation was significantly increased compared to the individual treatments (Figure 5d). These results suggest that the combination therapy significantly promotes DC migration to DLNs and induces proliferation of immune cells in the DLNs.
Figure 5.
Dendritic cells (DC) migration to the draining lymph nodes (DLNs). Ex vivo generated DCs were labeled with CMTPX. Two days after final treatment, single cells were collected from DLNs and the migration was quantified by fluorescence-activated cell sorting analysis. (a) The number of CMTPX+ DCs in DLNs from mice treated with phosphate-buffered saline (PBS), DC, Ad-ΔB7/IL12/GMCSF plus DCs, or Ad-ΔB7/IL12/GMCSF (H; high-dose of Ad) plus DCs were quantified on a fluorescence-activated cell sorter, and data from 50,000 events were represented. (b) CD11c+ DCs in the DLNs from mice treated with Ad-ΔB7/IL12/GMCSF and/or DCs. (c) Total cell number of DLNs from different groups of mice. Data points represent the mean ± SE of triplicate experiments. All results in this figure represent results of at least three mice per group, and similar results were obtained from at least two separate experiments. *P < 0.05; **P < 0.01. (d) Immunohistochemical staining of proliferating cell nuclear antigen in draining lymph node tissues. Original magnification: ×400. GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin.
Upregulation of CCL21+ lymphatic vessels in tumor tissues treated with the combination therapy
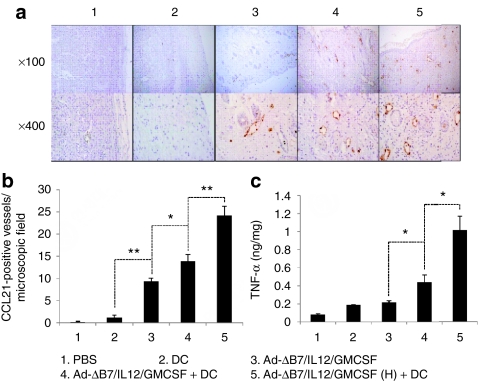
A previous study reported that DC migration to DLNs depends on CCR7/CC-chemokine ligand 21 (CCL21) interaction and tumor necrosis factor (TNF)-α regulation of CCL21+ lymphatics within the tissue.32 Tumors from mice treated with Ad-ΔB7/IL12/GMCSF alone showed a significantly increased number of CCL21+ lymphatic vessels around the tumor tissues (P < 0.01 versus PBS and DCs alone; Figure 6a,b). Combination therapy greatly enhanced the number of CCL21+ lymphatic vessels as compared with the virus alone-treatment group (P < 0.05). Moreover, the high-dose combination therapy showed the greatest level of CCL21+ lymphatics in the tumor tissues (P < 0.01 versus the combination therapy).
Figure 6.
Visualization and quantification of CCL21+ lymphatic vessels in tumors treated with Ad-ΔB7/IL12/GMCSF and/or dendritic cells (DCs). Tumors harvested from mice treated with phosphate-buffered saline (PBS), DC, Ad-ΔB7/IL12/GMCSF, Ad-ΔB7/IL12/GMCSF plus DCs, or Ad-ΔB7/IL12/GMCSF (H; high-dose of Ad) plus DCs were embedded in paraffin and sectioned. (a) Immunohistochemical staining of CCL21+ lymphatic vessels in tumor tissues. Original magnification: ×100 & ×400. (b) The means of CCL21+ lymphatic vessel in microscopic field (×200). (c) Tumor necrosis factor (TNF)-α expression in the tumor tissues was measured by conventional enzyme-linked immunosorbent assay. Data points represent the mean ± SE of triplicate experiments. All results in this figure represent results of at least three mice per group, and similar results were obtained from at least two separate experiments. *P < 0.05; **P < 0.01. GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin.
To better understand the mechanism of CCL21+ lymphatic vessel upregulation in tumors treated with the combination therapy, expression of TNF-α in the tumors was measured by enzyme-linked immunosorbent assay (ELISA). TNF-α levels were augmented in the tumors treated with Ad-ΔB7/IL12/GMCSF or DCs alone and were significantly increased in the combination therapy group (P < 0.05 versus individual treatments; Figure 6c). Consistently, the high-dose combination therapy group had the highest levels of TNF-α expression (P < 0.05 versus combination therapy). These results indicate that increased TNF-α expression in tumor tissues treated with combination therapies might be the cause of the upregulation of CCL21+ lymphatics.
The CD4+CD25+ T-cell population decreased in DLNs of mice treated with the combination therapy
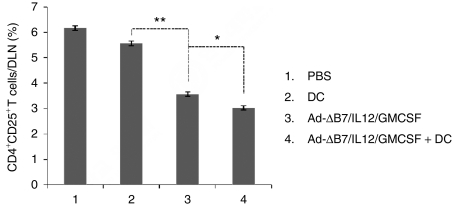
CD4+CD25+ T-cells are important immune suppressors whose development is partially regulated by VEGF. As described above, the expression of VEGF was markedly decreased in tumors treated with the combination therapy (Figure 4a). To evaluate changes in the CD4+CD25+ T-cell population within the DLNs, lymphocytes were collected from DLNs of mice treated with PBS, DCs, Ad-ΔB7/IL12/GMCSF, or the combination therapy. The CD4+CD25+ T-cell population from the DLNs was significantly decreased in mice treated with Ad-ΔB7/IL12/GMCSF as compared to mice treated with PBS or DCs (P < 0.01; Figure 7 and Supplementary Figure S3). The decrease in the CD4+CD25+ T-cell population was still greater in mice treated with the combination therapy (P < 0.05 versus Ad-ΔB7/IL12/GMCSF alone).
Figure 7.
CD4+CD25+ T-cells in draining lymph nodes (DLNs) from mice treated with Ad-ΔB7/IL12/GMCSF and/or dendritic cells (DCs). DLNs were collected from mice treated with phosphate-buffered saline (PBS), DC, Ad-ΔB7/IL12/GMCSF, Ad-ΔB7/IL12/GMCSF plus DCs, or Ad-ΔB7/IL12/GMCSF (H; high-dose of Ad) plus DCs. Proportion of CD4+CD25+ T-cells in the DLNs from different groups of mice were measured by fluorescence-activated cell sorting analysis. Data points represent the mean ± SE of three mice per group, and similar results were obtained from at least two separate experiments. *P < 0.05; **P < 0.01. GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin.
Antitumor effect induced by Ad-ΔB7/IL12/GMCSF in combination with immature DCs
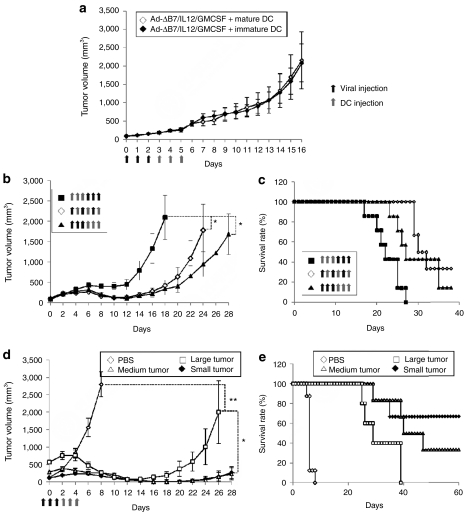
Immature DCs differentiated from progenitor cells can actively uptake and process exogenous antigen. Following maturation, DCs downregulate their antigen acquisition and processing abilities and display increased immunogenicity. Immature DCs differ from mature DCs pulsed with tumor antigen in vitro in that immature DCs can take up in situ tumor antigens. B16-F10 melanoma models were used to evaluate the therapeutic effects of combination therapy using immature DCs in comparison to mature DCs. Similar antitumor effects were observed in combination therapies using mature or immature DCs (Figure 8a). This finding indicates that the combinatorial strategy using the cytokine-expressing oncolytic Ad can promote maturation of immature DCs within the tumor tissue. Therefore, Ad-ΔB7/IL12/GMCSF-mediated expression of IL-12 and GM-CSF prior to DC injection might reverse the immunosuppressive microenvironment within the tumor, resulting in a microenvironment favorable to the maturation and function of immature DCs.
Figure 8.
Therapeutic efficacy of combination therapy based on the dendritic cells (DC) maturation, treatment schedule, and tumor size. (a) Tumor-bearing mice were treated with Ad-ΔB7/IL12/GMCSF in combination with mature or immature DCs. Similar antitumor effects were observed in combination therapies using mature or immature DCs. (b,c) Effect of the treatment schedule of oncolytic adenovirus and DCs on antitumor efficacy. Tumors were treated with Ad-ΔB7/IL12/GMCSF prior to DC vaccination (filled triangles), Ad-ΔB7/IL12/GMCSF and DCs on alternating days (open diamonds), or Ad-ΔB7/IL12/GMCSF after DC vaccination (filled squares). Tumor treatment with Ad-ΔB7/IL12/GMCSF prior to DC vaccination induced the most potent antitumor efficacy. (d,e) Antitumor effect of combination therapies on large tumors. High-dose combination therapy induced potent antitumor effect on large tumors. Data points represent the mean ± SE. All results in this figure represent results of at least six mice per group. *P < 0.05; **P < 0.01. GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin.
We further assessed the effect of the treatment schedule of oncolytic Ad and DCs on gross antitumor efficacy. The antitumor efficacy and survival rates were significantly improved in tumors treated with Ad-ΔB7/IL12/GMCSF prior to DC vaccination (Figure 8b,c; filled triangles) or in tumors treated with Ad-ΔB7/IL12/GMCSF and DCs on alternating days (open diamonds) as compared to tumors treated with Ad-ΔB7/IL12/GMCSF following DC vaccination (filled squares) (P < 0.05). Clearly, tumor treatment with Ad-ΔB7/IL12/GMCSF prior to DC vaccination has a higher antitumor efficacy.
The antitumor effect of combination therapies on large tumors which represents late-stage tumors was assessed. Mice were treated with high-dose combination therapy when tumor volumes reached three different sizes: 117.54 ± 14.29, 288.89 ± 39.96, and 573.66 ± 110.15 mm3. Potent antitumor effects were induced by high-dose combination therapy even when the initial tumor size reached over 500 mm3. However, the therapeutic efficacy was significantly improved when the initial tumor sizes were <300 mm3. These data suggest that combination therapy can elicit potent antitumor effects even when the tumor burden is large and in late-stage tumors.
Discussion
Tumor-induced immune suppression mediated by a variety of molecules, such as VEGF, IL-10, and transforming growth factor-β, is a major problem faced by cancer immunotherapy. These molecules produced by several tumor compartments not only impair the recruitment and maturation of DCs and T-cells in the tumor tissue, but also induce abnormal systemic DC differentiation and activation. This immune modulation is a major obstacle in DC-based cancer vaccination.33,34 Therefore, many recent studies have focused on optimizing DC vaccination protocols to overcome tumor-induced immune suppression.
IL-12 is a potent immune-stimulatory cytokine that can reverse tumor microenvironment immunosuppression.16 DCs activated in the presence of IL-12, IL-18, and anti-CD40 antibody have been shown to potently improve antitumor immunity.35 In addition, DCs transduced with the IL-12 gene and melanoma-associated antigen (gp100) were shown to induce more effective tumor-specific immune responses by upregulating costimulatory molecules and by improving T-cell stimulation.36 Therefore, IL-12 is a good candidate for combination with DC vaccinations to improve the therapeutic efficiency. GM-CSF is associated with the function of APCs, but GM-CSF also plays an important role in the development of DCs in vitro and in vivo.37 Recently, the therapeutic efficacy of DC vaccination for the treatment of murine renal cell carcinomas was improved when combined with GM-CSF-secreting tumor cells.38 To improve the efficacy of DC vaccination, IL-12 and GM-CSF were selected for use in combination therapies to activate and enhance host immune responses.
This study demonstrates that the combination of DC vaccination and oncolytic Ad coexpressing both IL-12 and GM-CSF greatly enhanced the antitumor effects and survival rates in a murine melanoma model, as compared with individual treatments (Figure 2a,b). Moreover, IL-12 levels were also markedly enhanced in tumor tissues that received the combination therapy (Figure 3a). IL-12 not only enhances Th1 immunity and cytotoxic T-lymphocytes cytotoxicity, but at high concentrations, IL-12 can also activate natural killer cells. Activated natural killer cells promote DC maturation, which induces a positive feedback loop in that the mature DCs secrete additional IL-12.39 In addition, GM-CSF secreted from Ad-ΔB7/IL12/GMCSF-infected tumor cells can also recruit and induce maturation of DCs within tumor tissues (Figure 3c). Taken together, these findings suggest that IL-12 and GM-CSF are potent stimuli that can activate and boost the host immune system, and can also synergistically promote DC vaccination efficacy.
VEGF not only plays a critical role in the development of tumor angiogenesis, but also inhibits the differentiation and maturation of DCs.40 In vivo administration of neutralizing VEGF-specific antibody can improve the function of DCs and increase the number of mature DCs in tumor-bearing mice.41 IL-12 downregulated VEGF expression in a murine breast cancer model,42 and inhibited tumor angiogenesis.31 Here, VEGF expression was inversely related to IL-12 expression. Specifically, VEGF expression was significantly reduced in tumors treated with the combination therapy as compared with control tumors or tumors that received individual treatments (Figure 4a). Likewise, enhanced antiangiogenesis was observed in tumors that received the combination treatments (Figure 4b,c). These data suggest that IL-12-mediated antiangiogenesis played a role in the combination therapy-induced antitumor effect. Importantly, reduction of VEGF expression due to the combination therapy might contribute to the reduction of the tumor microenvironment immunosuppression, therefore enhancing DC vaccination efficacy.
Following maturation, DCs upregulate costimulatory molecules and acquire the ability to migrate to regional lymph nodes. Migration of DCs to regional lymph nodes is an essential step in the induction of immune responses and, therefore, is an important aspect of DC vaccination.33 However, only a small proportion of injected DCs migrated to DLNs in cancer patients during clinical trials of DC vaccination.43,44 To increase the efficiency of DC vaccination, DC migration to DLNs must be enhanced. In this study, mice treated with the combination therapy showed significantly increased numbers of endogenous as well as exogenously injected DCs that migrated to the DLNs (Figure 5a,b). This migration likely played a significant role in enhancing DC vaccination efficacy in tumor-bearing mice. This finding is in line with our previous study that indicated that DC migration can be promoted when DCs are combined with oncolytic Ad coexpressing IL-12 and 4-1BB ligand.45
Migration of mature DCs to draining lymph nodes is driven by the interaction of high levels of CCR7 on mature DCs with its ligand, CCL21 in the lymphatics.46 Mice treated with Ad-ΔB7/IL12/GMCSF alone or in combination with DCs showed a markedly increased number of CCL21-positive lymphatic vessels surrounding tumor tissues as compared with mice treated with PBS or DCs alone (Figure 6a,b). This suggests that the increased DC migration in the combination therapy group was due to upregulation of CCL21+ lymphatic vessels in the tumor tissue. Martin-Fontecha et al. reported that TNF-α-induced tissue inflammation greatly increases DC migration to DLNs due to upregulation of CCL21 expression on lymphatic vessels in situ.32 Therefore, TNF-α may be an inflammatory stimuli associated with the regulation of CCL21 expression on the endothelial cells of lymphatics. Interestingly, combination therapy significantly increased the level of TNF-α in tumors (Figure 6c). TNF-α was likely secreted by tumor-infiltrating T-cells, monocytes, and macrophages recruited to the CCL21+ lymphatic vessels in the tumor tissues by IL-12 and GM-CSF expressed by Ad-ΔB7/IL12/GMCSF. However, it remains unclear whether IL-12 or GM-CSF directly regulates CCL21 expression on endothelial cells of the lymphatics.
Emerging evidence suggests that CD4+CD25+ T cell-mediated immune suppression is a crucial tumor immune evasion mechanism and is often a barrier for successful tumor immunotherapy. A previous study demonstrated that local IL-12 expression by tumor tissues can reverse the tumor immune suppression by inducing apoptosis of CD4+CD25+Foxp3+ T-cells.16 In our study, the CD4+CD25+ T-cell population was reduced in the DLNs of tumor-bearing mice treated with Ad-ΔB7/IL12/GMCSF alone or in combination with DCs as compared with the PBS and DCs-alone treatment groups. It is likely that the high level of IL-12 expressed in the tumor tissue contributed to CD4+CD25+ T-cell elimination (Figure 7). In addition, VEGF is associated with the development of CD4+CD25+ T-cells in tumor-bearing mice and cancer patients.47,48 Therefore, the decreased VEGF expression in the tumors from mice that received the combination therapy likely contributed to the CD4+CD25+ T-cell population reduction in the DLNs.
Interestingly, combination therapy using immature DCs had similar antitumor effects as the combination therapy using mature DCs (Figure 8a). IL-12 and GM-CSF secreted from Ad-ΔB7/IL12/GMCSF likely directly activated the immature DCs or induced an immune response that produced a number of immune-stimulating factors to circumvent the immunosuppressive microenvironment. Moreover, injected immature DCs can efficiently uptake tumor antigens in situ that were generated by oncolytic Ad-mediated oncolysis of cancer cells.
The order of virus and DC injection also affected the antitumor effects of the combination therapies (Figure 8b,c). These results indicate that injection of the cytokine-expressing oncolytic Ad prior to DC vaccination can reduce immune suppression and induce favorable immune response conditions within the tumor tissue, resulting in improved efficacy of DC vaccination. In addition, combination therapy also elicited notable antitumor effects in large tumors (Figure 8d,e), suggesting that combination therapy can overcome the limitation of DC vaccinations to reduce tumor burden in established solid tumors.49
This study demonstrates synergistic antitumor effects between Ad-ΔB7/IL12/GMCSF and DCs in a murine melanoma model. The mechanism is associated with the elimination of immune suppression in the tumor and promotion of DC maturation and migration to DLNs. These findings provide the basis for a feasible oncolytic Ad-based cytokine gene therapy in combination with DC vaccination for cancer treatment in the clinic.
Materials and Methods
Cell lines and cell culture. B16-F10 (murine melanoma) cells were maintained in Dulbecco's modified Eagle's medium (Gibco BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (Gibco BRL), 100 IU/ml penicillin, and 100 µg/ml streptomycin.
Mice. C57BL/6 mice were obtained from SLC (Tokyo, Japan). Mice were used at 6–7 week of age, and all animal studies were performed according to institutionally approved protocols at Yonsei University College of Medicine.
Adenoviral vectors. To generate an Ad expressing IL-12 and GM-CSF at the E1 and E3 region, respectively, we first constructed an E3 shuttle vector expressing GM-CSF. The murine GM-CSF gene was excised from pCDNA3.1/GMCSF using NheI and XhoI, and sub-cloned into the Ad E3 shuttle vector, pSP72-E3, generating a pSP72/E3/GMCSF E3 shuttle vector. The newly constructed pSP72/E3/GMCSF was then cotransformed with an Ad total vector (pvmdl324BstBI) into Escherichia coli BJ5183, yielding a pdl324ΔE3/GMCSF Ad plasmid. Structure of the resultant recombinant vector was confirmed by restriction enzyme digestion and PCR analysis. To construct an Ad E1 shuttle vector expressing IL-12, murine IL-12 gene excised from pCA14/IL1249 was sub-cloned into pXC1ΔB7 E1 shuttle vector,25 generating a pXC1ΔB7/IL12 E1 shuttle vector. The newly constructed pXC1ΔB7/IL12 E1 shuttle vector was then cotransformed with pdl324ΔE3/GMCSF into E. coli BJ5183 for homologous recombination, generating a pAd-ΔB7/IL12/GMCSF Ad vector (Figure 1a). All viruses were propagated in 293 cells and purified by CsCl density purification, dissolved in storage buffer (10 mmol/l Tris, 4% sucrose, 2 mmol/l MgCl2), and stored at −80 °C. Viral particle numbers were calculated from measurements of absorbance at 260 nm (A260), where one absorbency unit is equivalent to 1012 viral particles/ml. The infectious titers (plaque-forming units per milliliter) were determined by limiting dilution assay on 293 cells. The particle/plaque-forming unit ratio for Ad-ΔB7 and Ad-ΔB7/IL12/GMCSF were 23.7:1 and 20.9:1, respectively. The MOI was calculated from infectious titers.
Generation of bone marrow-derived DC. Bone marrow cells were harvested from flushed marrow cavities of femurs and tibias of C57BL6 mice under aseptic conditions. The cells were depleted of erythrocytes using RBC lysis buffer (Sigma, St Louis, MO) and were cultured in complete RPMI 1640 media (Gibco BRL) supplemented with 10% fetal bovine serum, GM-CSF (10 ng/ml, ENDOGEN, Woburn, MA), and IL-4 (10 ng/ml, ENDOGEN), 2-mercaptoethanol 50 µmol, 100 IU/ml penicillin, and 100 µg/ml streptomycin. On day 2, the nonadherent cells were removed and the plates were replenished with fresh complete media containing GM-CSF and IL-4. On day 4, culture supernatant was collected and centrifuged, and the cell pellet was resuspended in fresh media containing cytokines and returned to the plate. On day 6, the DCs were incubated with the tumor lysate (50 µg/ml) for 24 hour lipopolysaccharide (1 µg/ml, Sigma) were added at day 7 for DCs maturation. After incubation for 24 hours, mature DCs were harvested and used in following studies. Immature DCs were cultured at same condition for 6 days as described above. But, the cell was not exposed to the tumor lysate and lipopolysaccharide.
Fluorescence-activated cell sorting (FACS) analysis. For the phenotypic analysis, the DCs were stained with surface molecules using immunofluorescence and analyzed by FACS analysis. Cells were stained with anti-mouse CD11c, CCR7, CD40, CD80, CD86, or MHC I/II (Pharmingen, San Diego, CA) Ab at 4 °C for 45 minutes. After twice of PBS washing, the cells were incubated with fluorescein isothiocyanate-conjugated goat anti-rat IgG secondary Ab at 4 °C for 15 minutes. For the assessment of CD4+CD25+ T-cell population in the DLN, single cells were obtained from DLN after mechanical dissociation. The cells were stained with anti-mouse CD4 (phycoerythrin) and anti-mouse CD25 (fluorescein isothiocyanate) Ab at 4 °C for 45 minutes. All samples were analyzed on a BD Biosciences BD-LSR II Analytic Flow Cytometer, using FACSDiva software (BD Biosciences, San Jose, CA).
In vivo antitumor effect. B16-F10 cells (5 × 105) were injected subcutaneously into the right abdomen of 6–7 week-old male C57BL/6 mice. When the tumor volumes reached of around 120–130 mm3, mice were sorted into groups with similar means of tumor volumes. Treatment groups included PBS-only control, Ad-ΔB7/IL12/GMCSF only (5 × 109 VP/injection), DCs only (1 × 106 cells/injection), or a combination of DCs and Ad-ΔB7/IL12/GMCSF). In parallel, one group of mice was given a treatment regimen of a high dose of Ad-ΔB7/IL12/GMCSF (5 × 1010 VP/injection) in combination with DCs (1 × 106/injection). Tumor-bearing mice were intratumorally injected with three doses of Ad on days 0–2, followed by three injections of DCs on days 3–5. Tumor growth was monitored every other day using a caliper, and tumor volume was calculated by the following formula: volume = 0.523 LW2, where L is length and W is width. Animals with tumors that were >3,000 mm3 were killed for ethical reasons.
Expression of IL-12 and GM-CSF. IL-12 and GM-CSF expression were determined using an ELISA according to the manufacturer's instructions. B16-F10 melanoma cells were plated onto six-well plates at 1 × 104 per well and then infected with Ad-ΔB7/IL12/GMCSF at MOIs of 50, 100, and 500. At 48 hour after infection, supernatants were harvested and the level of IL-12 and GM-CSF was determined with conventional IL-12 ELISA kit (ENDOGEN) and GM-CSF ELISA kit (R&D systems, Minneapolis, MN), respectively. For the assessment of cytokine expression in tumor tissue, tumor tissues were removed from mice treated with Ad and/or DCs at 3 days after final treatment. Tissues were homogenized and liquefied in PBS containing protease inhibitor cocktail (Sigma). IL-12, GM-CSF, VEGF, and TNF-α level were measured by conventional ELISA kits (ENDOGEN and R&D systems). Each experiment was carried out three to four times with three replicates in each group.
Histology and immunohistochemistry. Tumor tissues were harvested from mice after 3 days of final treatment, and embedded in paraffin and sectioned at a thickness of 4 µm for hematoxylin and eosin (H&E) staining. For immunohistochemical staining, tumor tissues were snap-frozen and sectioned at a thickness of 7 µm. Tumor sections were blocked with 4% PBS–bovine serum albumin (Sigma) for 1 hour and incubated over night with appropriate dilution of anti-CD4 (purified rat anti-mouse CD4 monoclonal Ab; Pharmingen), anti-CD8 (purified rat anti-mouse CD8 monoclonal Ab; Pharmingen), anti-CD86 (purified rat anti-mouse CD86 monoclonal Ab; Pharmingen), anti-CD11c (purified hamster anti-mouse CD11c monoclonal Ab; Pharmingen), or anti-CCL21 (purified rat anti-mouse CCL21 monoclonal Ab; R&D systems) in Ab diluents (DAKO, Glostrup, Denmark). After overnight incubation, the sections were washed twice in PBS and incubated with horseradish peroxidase-conjugated goat anti-rat or mouse anti-hamster Ab (Southern Biotechnology, Birmingham, AL) for 1 hour Diaminobenzidine/hydrogen peroxidase (DAKO) was used as the chromogen substrate. All slides were counterstained with Meyer's hematoxylin.
Evaluation of DC migration in vivo. DCs were labeled with CellTracker Red CMTPX (Invitrogen, Carlsbad, CA) on day 6 of DC culture and were harvested on day 8. The tumor-bearing mice were intratumorally injected with DCs (1 × 106/time) alone three times every day or intratumorally injected with Ad-ΔB7/IL12/GMCSF (5 × 109 VP/time or 5 × 1010 VP/time) three times every day prior to DC injection. At 48 hour after final treatment, the DLNs were harvested and dissociated into single cells for FACS analysis. The number of CMTPX+ DCs and CD11c+ DCs was quantified on a fluorescence-activated cell sorter (Becton Dickinson, Sunnyvale, CA) and data from 50,000 events were collected for further analysis.
Statistical analysis. The data was expressed as mean ± SE. Statistical analyses of the data were performed using the two-tailed Student's t-test (SPSS 13.0 software; SPSS, Chicago, IL). P values of <0.05 were considered statistically significant (*P < 0.05; **P < 0.01). Analysis of variance was used for multiple group comparison on antitumor effect examination.
SUPPLEMENTARY MATERIAL Figure S1. Characterization of bone marrow-derived DCs. DCs were cultured in the presence of IL-4 and GM-CSF, and were matured by tumor lysate pulsing and lipopolysaccharide stimulation. (a) The purity of DCs culture was evaluated by fluorescence-activated cell sorting analysis using PE-conjugated CD11c-specific antibody. (b, c) Co-stimulator molecules were significantly upregulated in mature DCs. Similar results were obtained in three independent experiments. Figure S2. DC migration assay in vivo. (a) DCs were labeled with CMTPX in vitro. The CMTPX labeled DCs in fluorescence microscopic field (× 200 & × 400). The percentage of CMTPX+ DCs (b) and CD11c+ DCs (c) in the draining lymph nodes (DLNs) from mice treated with Ad-ΔB7/IL12/GMCSF and/or DC. Figure S3. Fluorescence-activated cell sorting analysis of CD4+CD25+ T-cells in draining lymph nodes (DLNs) from mice treated with Ad-ΔB7/IL12/GMCSF and/or DCs. The tumor-bearing mice were intra-tumorally injected with DCs (1 × 106/time) alone three times on days 0-2 (DC alone group) or intra-tumorally injected with Ad-ΔB7/IL12/GMCSF (5 × 109 VP/time) three times on days 0-2 prior to DC injection on days 3-5 (Ad-ΔB7/IL12/GMCSF and/or DC group). At 48 hr after final treatment, the DLNs were harvested and dissociated into single cells for FACS analysis.
Acknowledgments
This work was supported by grants from the Ministry of Knowledge Economy (10030051, C.-O.Y.), the Korea Science and Engineering Foundation (R15-2004-024-02001-0, 2009K001644, 2010-0029220, C.-O.Y.), the Korea Food and Drug Administration (KFDA-10172-332 to C.-O.Y.), and a Faculty Research Grant from Yonsei University College of Medicine (6-2010-0052, C.-O.Y.). S.-N.Z., J.-H.H., J.-Y.Y., and K.-J.C. are graduate students sponsored by the Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, South Korea. I.-K.C. is a graduate student sponsored by KOSEF through National Core Research Center for Nanomedical Technology. The authors declared no conflict of interest.
Supplementary Material
Characterization of bone marrow-derived DCs. DCs were cultured in the presence of IL-4 and GM-CSF, and were matured by tumor lysate pulsing and lipopolysaccharide stimulation. (a) The purity of DCs culture was evaluated by fluorescence-activated cell sorting analysis using PE-conjugated CD11c-specific antibody. (b, c) Co-stimulator molecules were significantly upregulated in mature DCs. Similar results were obtained in three independent experiments.
DC migration assay in vivo. (a) DCs were labeled with CMTPX in vitro. The CMTPX labeled DCs in fluorescence microscopic field (× 200 & × 400). The percentage of CMTPX+ DCs (b) and CD11c+ DCs (c) in the draining lymph nodes (DLNs) from mice treated with Ad-ΔB7/IL12/GMCSF and/or DC.
Fluorescence-activated cell sorting analysis of CD4+CD25+ T-cells in draining lymph nodes (DLNs) from mice treated with Ad-ΔB7/IL12/GMCSF and/or DCs. The tumor-bearing mice were intra-tumorally injected with DCs (1 × 106/time) alone three times on days 0-2 (DC alone group) or intra-tumorally injected with Ad-ΔB7/IL12/GMCSF (5 × 109 VP/time) three times on days 0-2 prior to DC injection on days 3-5 (Ad-ΔB7/IL12/GMCSF and/or DC group). At 48 hr after final treatment, the DLNs were harvested and dissociated into single cells for FACS analysis.
REFERENCES
- Fong L., and, Engleman EG. Dendritic cells in cancer immunotherapy. Annu Rev Immunol. 2000;18:245–273. doi: 10.1146/annurev.immunol.18.1.245. [DOI] [PubMed] [Google Scholar]
- Proudfoot O, Pouniotis D, Sheng KC, Loveland BE., and, Pietersz GA. Dendritic cell vaccination. Expert Rev Vaccines. 2007;6:617–633. doi: 10.1586/14760584.6.4.617. [DOI] [PubMed] [Google Scholar]
- Asavaroengchai W, Kotera Y., and, Mulé JJ. Tumor lysate-pulsed dendritic cells can elicit an effective antitumor immune response during early lymphoid recovery. Proc Natl Acad Sci USA. 2002;99:931–936. doi: 10.1073/pnas.022634999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R.et al. (1998Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells Nat Med 4328–332. [DOI] [PubMed] [Google Scholar]
- Tjoa BA, Erickson SJ, Bowes VA, Ragde H, Kenny GM, Cobb OE.et al. (1997Follow-up evaluation of prostate cancer patients infused with autologous dendritic cells pulsed with PSMA peptides Prostate 32272–278. [DOI] [PubMed] [Google Scholar]
- Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B.et al. (1996Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells Nat Med 252–58. [DOI] [PubMed] [Google Scholar]
- Wierecky J, Müller MR, Wirths S, Halder-Oehler E, Dörfel D, Schmidt SM.et al. (2006Immunologic and clinical responses after vaccinations with peptide-pulsed dendritic cells in metastatic renal cancer patients Cancer Res 665910–5918. [DOI] [PubMed] [Google Scholar]
- Gervais A, Levêque J, Bouet-Toussaint F, Burtin F, Lesimple T, Sulpice L.et al. (2005Dendritic cells are defective in breast cancer patients: a potential role for polyamine in this immunodeficiency Breast Cancer Res 7R326–R335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratta M, Fagnoni F, Curti A, Vescovini R, Sansoni P, Oliviero B.et al. (2002Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6 Blood 100230–237. [DOI] [PubMed] [Google Scholar]
- Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- Podhajcer OL, Lopez MV., and, Mazzolini G. Cytokine gene transfer for cancer therapy. Cytokine Growth Factor Rev. 2007;18:183–194. doi: 10.1016/j.cytogfr.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- Torrero MN, Xia X, Henk W, Yu S., and, Li S. Stat1 deficiency in the host enhances interleukin-12-mediated tumor regression. Cancer Res. 2006;66:4461–4467. doi: 10.1158/0008-5472.CAN-05-3554. [DOI] [PubMed] [Google Scholar]
- Adris S, Chuluyan E, Bravo A, Berenstein M, Klein S, Jasnis M.et al. (2000Mice vaccination with interleukin 12-transduced colon cancer cells potentiates rejection of syngeneic non-organ-related tumor cells Cancer Res 606696–6703. [PubMed] [Google Scholar]
- Bramson JL, Hitt M, Addison CL, Muller WJ, Gauldie J., and, Graham FL. Direct intratumoral injection of an adenovirus expressing interleukin-12 induces regression and long-lasting immunity that is associated with highly localized expression of interleukin-12. Hum Gene Ther. 1996;7:1995–2002. doi: 10.1089/hum.1996.7.16-1995. [DOI] [PubMed] [Google Scholar]
- Kilinc MO, Aulakh KS, Nair RE, Jones SA, Alard P, Kosiewicz MM.et al. (2006Reversing tumor immune suppression with intratumoral IL-12: activation of tumor-associated T effector/memory cells, induction of T suppressor apoptosis, and infiltration of CD8+ T effectors J Immunol 1776962–6973. [DOI] [PubMed] [Google Scholar]
- Kanegane C, Sgadari C, Kanegane H, Teruya-Feldstein J, Yao L, Gupta G.et al. (1998Contribution of the CXC chemokines IP-10 and Mig to the antitumor effects of IL-12 J Leukoc Biol 64384–392. [DOI] [PubMed] [Google Scholar]
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP., and, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol. 2007;18:226–232. doi: 10.1093/annonc/mdl158. [DOI] [PubMed] [Google Scholar]
- Curiel-Lewandrowski C, Mahnke K, Labeur M, Roters B, Schmidt W, Granstein RD.et al. (1999Transfection of immature murine bone marrow-derived dendritic cells with the granulocyte-macrophage colony-stimulating factor gene potently enhances their in vivo antigen-presenting capacity J Immunol 163174–183. [PubMed] [Google Scholar]
- Chada S, Ramesh R., and, Mhashilkar AM. Cytokine- and chemokine-based gene therapy for cancer. Curr Opin Mol Ther. 2003;5:463–474. [PubMed] [Google Scholar]
- Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB.et al. (1997Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production Blood 902541–2548. [PubMed] [Google Scholar]
- Sangro B, Melero I, Qian C., and, Prieto J. Gene therapy of cancer based on interleukin 12. Curr Gene Ther. 2005;5:573–581. doi: 10.2174/156652305774964712. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Ohnami S., and, Aoki K. Development of gene therapy to target pancreatic cancer. Cancer Sci. 2004;95:283–289. doi: 10.1111/j.1349-7006.2004.tb03204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim JH, Choi KJ, Kim PH., and, Yun CO. E1A- and E1B-Double mutant replicating adenovirus elicits enhanced oncolytic and antitumor effects. Hum Gene Ther. 2007;18:773–786. doi: 10.1089/hum.2006.167. [DOI] [PubMed] [Google Scholar]
- Yoo JY, Kim JH, Kwon YG, Kim EC, Kim NK, Choi HJ.et al. (2007VEGF-specific short hairpin RNA-expressing oncolytic adenovirus elicits potent inhibition of angiogenesis and tumor growth Mol Ther 15295–302. [DOI] [PubMed] [Google Scholar]
- Choi KJ, Kim JH, Lee YS, Kim J, Suh BS, Kim H.et al. (2006Concurrent delivery of GM-CSF and B7-1 using an oncolytic adenovirus elicits potent antitumor effect Gene Ther 131010–1020. [DOI] [PubMed] [Google Scholar]
- DeWeese TL, van der Poel H, Li S, Mikhak B, Drew R, Goemann M.et al. (2001A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy Cancer Res 617464–7472. [PubMed] [Google Scholar]
- Freytag SO, Khil M, Stricker H, Peabody J, Menon M, DePeralta-Venturina M.et al. (2002Phase I study of replication-competent adenovirus-mediated double suicide gene therapy for the treatment of locally recurrent prostate cancer Cancer Res 624968–4976. [PubMed] [Google Scholar]
- Saito Y, Sunamura M, Motoi F, Abe H, Egawa S, Duda DG.et al. (2006Oncolytic replication-competent adenovirus suppresses tumor angiogenesis through preserved E1A region Cancer Gene Ther 13242–252. [DOI] [PubMed] [Google Scholar]
- Morini M, Albini A, Lorusso G, Moelling K, Lu B, Cilli M.et al. (2004Prevention of angiogenesis by naked DNA IL-12 gene transfer: angioprevention by immunogene therapy Gene Ther 11284–291. [DOI] [PubMed] [Google Scholar]
- MartIn-Fontecha A, Sebastiani S, Höpken UE, Uguccioni M, Lipp M, Lanzavecchia A.et al. (2003Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming J Exp Med 198615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J., and, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- Lim DS, Kim JH, Lee DS, Yoon CH., and, Bae YS. DC immunotherapy is highly effective for the inhibition of tumor metastasis or recurrence, although it is not efficient for the eradication of established solid tumors. Cancer Immunol Immunother. 2007;56:1817–1829. doi: 10.1007/s00262-007-0325-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkow S, Loser K, Krummen M, Higuchi T, Rothoeft T, Apelt J.et al. (2009Dendritic cell activation by combined exposure to anti-CD40 plus interleukin (IL)-12 and IL-18 efficiently stimulates anti-tumor immunity Exp Dermatol 1878–87. [DOI] [PubMed] [Google Scholar]
- Okada N, Iiyama S, Okada Y, Mizuguchi H, Hayakawa T, Nakagawa S.et al. (2005Immunological properties and vaccine efficacy of murine dendritic cells simultaneously expressing melanoma-associated antigen and interleukin-12 Cancer Gene Ther 1272–83. [DOI] [PubMed] [Google Scholar]
- Kingston D, Schmid MA, Onai N, Obata-Onai A, Baumjohann D., and, Manz MG. The concerted action of GM-CSF and Flt3-ligand on in vivo dendritic cell homeostasis. Blood. 2009;114:835–843. doi: 10.1182/blood-2009-02-206318. [DOI] [PubMed] [Google Scholar]
- Driessens G, Hoffmann P, Pouwels M, Zlotta A, Schulman C, Velu T.et al. (2009Synergy between dendritic cells and GM-CSF-secreting tumor cells for the treatment of a murine renal cell carcinoma J Immunother 32140–144. [DOI] [PubMed] [Google Scholar]
- Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G., and, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S.et al. (1996Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells Nat Med 21096–1103. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Ishida T, Nadaf S, Ohm JE., and, Carbone DP. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin Cancer Res. 1999;5:2963–2970. [PubMed] [Google Scholar]
- Dias S, Boyd R., and, Balkwill F. IL-12 regulates VEGF and MMPs in a murine breast cancer model. Int J Cancer. 1998;78:361–365. doi: 10.1002/(SICI)1097-0215(19981029)78:3<361::AID-IJC17>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Morse MA, Coleman RE, Akabani G, Niehaus N, Coleman D., and, Lyerly HK. Migration of human dendritic cells after injection in patients with metastatic malignancies. Cancer Res. 1999;59:56–58. [PubMed] [Google Scholar]
- De Vries IJ, Krooshoop DJ, Scharenborg NM, Lesterhuis WJ, Diepstra JH, Van Muijen GN.et al. (2003Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state Cancer Res 6312–17. [PubMed] [Google Scholar]
- Huang JH, Zhang SN, Choi KJ, Choi IK, Kim JH, Lee MG.et al. (2010Therapeutic and tumor-specific immunity induced by combination of dendritic cells and oncolytic adenovirus expressing IL-12 and 4-1BBL Mol Ther 18264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster R, Davalos-Misslitz AC., and, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- Wada J, Suzuki H, Fuchino R, Yamasaki A, Nagai S, Yanai K.et al. (2009The contribution of vascular endothelial growth factor to the induction of regulatory T-cells in malignant effusions Anticancer Res 29881–888. [PubMed] [Google Scholar]
- Li B, Lalani AS, Harding TC, Luan B, Koprivnikar K, Huan Tu G.et al. (2006Vascular endothelial growth factor blockade reduces intratumoral regulatory T cells and enhances the efficacy of a GM-CSF-secreting cancer immunotherapy Clin Cancer Res 126808–6816. [DOI] [PubMed] [Google Scholar]
- Lee YS, Kim JH, Choi KJ, Choi IK, Kim H, Cho S.et al. (2006Enhanced antitumor effect of oncolytic adenovirus expressing interleukin-12 and B7-1 in an immunocompetent murine model Clin Cancer Res 125859–5868. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characterization of bone marrow-derived DCs. DCs were cultured in the presence of IL-4 and GM-CSF, and were matured by tumor lysate pulsing and lipopolysaccharide stimulation. (a) The purity of DCs culture was evaluated by fluorescence-activated cell sorting analysis using PE-conjugated CD11c-specific antibody. (b, c) Co-stimulator molecules were significantly upregulated in mature DCs. Similar results were obtained in three independent experiments.
DC migration assay in vivo. (a) DCs were labeled with CMTPX in vitro. The CMTPX labeled DCs in fluorescence microscopic field (× 200 & × 400). The percentage of CMTPX+ DCs (b) and CD11c+ DCs (c) in the draining lymph nodes (DLNs) from mice treated with Ad-ΔB7/IL12/GMCSF and/or DC.
Fluorescence-activated cell sorting analysis of CD4+CD25+ T-cells in draining lymph nodes (DLNs) from mice treated with Ad-ΔB7/IL12/GMCSF and/or DCs. The tumor-bearing mice were intra-tumorally injected with DCs (1 × 106/time) alone three times on days 0-2 (DC alone group) or intra-tumorally injected with Ad-ΔB7/IL12/GMCSF (5 × 109 VP/time) three times on days 0-2 prior to DC injection on days 3-5 (Ad-ΔB7/IL12/GMCSF and/or DC group). At 48 hr after final treatment, the DLNs were harvested and dissociated into single cells for FACS analysis.