Abstract
Generation of transgene-specific immune responses can constitute a major complication following gene therapy treatment. An in vivo approach to inducing selective expansion of Regulatory T (Treg) cells by injecting interleukin-2 (IL-2) mixed with a specific IL-2 monoclonal antibody (JES6-1) was adopted to modulate anti-factor VIII (anti-FVIII) immune responses. Three consecutive IL-2 complexes treatments combined with FVIII plasmid injection prevented anti-FVIII formation and achieved persistent, therapeutic-level of FVIII expression in hemophilia A (HemA) mice. The IL-2 complexes treatment expanded CD4+CD25+Foxp3+ Treg cells five- to sevenfold on peak day, and they gradually returned to normal levels within 7–14 days without changing other lymphocyte populations. The transiently expanded Treg cells are highly activated and display suppressive function in vitro. Adoptive transfer of the expanded Treg cells protected recipient mice from generation of high-titer antibodies following FVIII plasmid challenge. Repeated plasmid transfer is applicable in tolerized mice without eliciting immune responses. Mice treated with IL-2 complexes mounted immune responses against both T-dependent and T-independent neoantigens, indicating that IL-2 complexes did not hamper the immune system for long. These results demonstrate the important role of Treg cells in suppressing anti-FVIII immune responses and the potential of developing Treg cell expansion therapies that induce long-term tolerance to FVIII.
Introduction
Hemophilia A (HemA) is a congenital bleeding disorder caused by a deficiency of the blood clotting protein Factor VIII (FVIII);1 in its severe form, hemophilia is a life-threatening, crippling hemorrhagic disease. Currently, hemophilia patients are treated with protein-replacement therapy. However, a major ongoing problem is a very high frequency in the formation of inhibitory antibodies against FVIII following treatment.2 Recent studies in animal models suggest that continuous expression of FVIII following gene therapy would provide significant therapeutic benefits. Nevertheless, both viral and nonviral gene transfer of FVIII3,4,5,6 often result in transient gene expression in the absence of immunosuppression. Only in a few cases, sustained gene expression of FVIII was achieved in animals treated with gene therapy.7,8 It has been indicated in recent reviews9,10,11 that immune responses against transgenic products or vectors can become major obstacles to successful application of gene therapy. Therefore, it is essential to develop safe and effective methods to modulate immune responses against FVIII to ensure the success of hemophilia treatment.
Regulatory T (Treg) cells comprise a crucial T-cell compartment that maintains peripheral tolerance by suppressing autoreactive T-cell responses12 and orchestrating a balanced immune response to foreign antigens.13,14,15 It has an important role in the prevention of autoimmune12,16 and inflammatory17 diseases, regulation of immunity to viral and parasite infections,18,19 maintenance of maternal tolerance to fetus20 and inhibition of antitumor immunity.21 These findings have engendered enthusiasm for developing strategies utilizing Treg cells to regulate immune responses in clinically important settings including transplantation, autoimmunity, allergy, and cancer. Furthermore, many successful protocols for modulating FVIII-specific immune responses involve increases in either or both of the percentages and total number of CD4+Foxp3+ Treg cells in gene therapy settings.22 Recently, it was demonstrated that adoptively transferred Treg cells isolated from transgenic HemA/Foxp3 mice significantly reduced anti-FVIII inhibitory antibody titers following FVIII plasmid-mediated gene transfer into HemA mice23, indicating that Treg cells are important regulators for anti-FVIII immune responses.
Interleukin-2 (IL-2) is an important growth factor that drives T cells to proliferate and differentiate into functional effector cells. IL-2 is also essential in the development of Treg cells; in the absence of IL-2, Treg cells cannot survive or expand in the thymus or in the periphery.24,25 Recent studies demonstrated that IL-2 bound to a particular anti-IL-2 monoclonal antibody (mAb; JES6-1A12) can selectively and significantly expand CD4+CD25+ Treg cells.26 Treatment with these particular IL-2/anti-IL-2 mAb complexes can protect mice against experimental autoimmune encephalomyelitis and pancreatic islet allografts,27 and the Ab-mediated disease, experimental autoimmune myasthenia gravis.28
In this study, we investigated whether treatment with immune complexes consisting of IL-2 and anti-IL-2 mAb (JES6-1A12) (referred to as IL-2 complexes hereafter) to expand Treg cells can modulate anti-FVIII immune responses following gene therapy. Consistent with earlier reports in other model systems,27,29,30,31 we found that treatment with IL-2 complexes induced a five- to sevenfold expansion of Treg cells in the peripheral blood, lymph nodes (LNs) and spleen of the treated mice. Most significantly, this expansion of Treg cells prevented the formation of inhibitory antibodies against FVIII following plasmid-mediated gene therapy in HemA mice.
Results
IL-2 complexes induced tolerance to FVIII after FVIII plasmid-mediated gene therapy
Selective enrichment of Treg cells by the injection of IL-2 complexes (IL-2/ JES6-1mAb) has the potential to modulate transgene-specific immune responses following FVIII plasmid-mediated gene therapy. We treated HemA mice with IL-2 complexes at two different schedules: three daily intraperitoneal injections of IL-2 complexes at days −5, −4, and −3 in schedule 1 mice (n = 12/group) and at days −1, 0, and 1 in schedule 2 mice (n = 9/group), along with hydrodynamic injection of 50 µg FVIII plasmid at day 0. Plasma samples were collected from treated mice at scheduled time points and FVIII activities and inhibitory antibody titers were assessed. Groups of naive mice and mice treated with IL-2 complexes only, and FVIII plasmid only were included as controls.
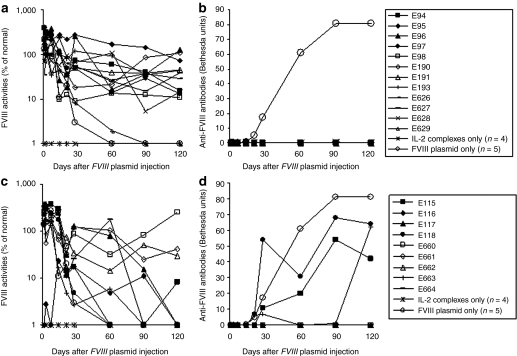
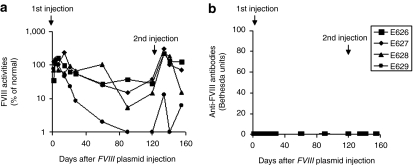
In a control experiment, injection of FVIII plasmid alone produced short-term (1–2 weeks) high levels of FVIII activity in HemA mice, followed by a gradual decrease to undetectable levels in 2–4 weeks due to the development of anti-FVIII inhibitory antibodies (Figure 1). In contrast, immune-modulation with IL-2 complexes successfully prevented anti-FVIII immune responses. Eleven out of the 12 schedule 1 mice produced persistent therapeutic levels of FVIII activities (10–100% of FVIII levels in normal human plasma) for 17 weeks (Figure 1a) and none of the treated mice developed anti-FVIII inhibitory antibodies (Figure 1b). Of the nine mice treated with IL-2 complexes using schedule 2, three mice produced persistent therapeutic FVIII levels without the generation of anti-FVIII antibodies (Figure 1c). For the remaining six mice, FVIII activity persisted at therapeutic levels for 4 weeks before dropping to undetectable levels (Figure 1c). Among these, three mice did not develop anti-FVIII inhibitory antibodies, whereas the other three developed antibodies starting at the 4th week post-treatment (Figure 1d). These data indicate that schedule 1 treatment is highly effective at preventing the formation of anti-FVIII antibodies, whereas schedule 2 treatment is only partially effective.
Figure 1.
Long-term factor VIII (FVIII) expression in hemophilia A mice after FVIII plasmid-mediated gene therapy and immunomodulation with interleukin-2 (IL-2) complexes. Two groups of hemophilia A mice were treated separately with three daily intraperitoneal (i.p.) injections of IL-2 complexes at days −5, −4, and −3 followed by intravenous (i.v.) injection of 50−ro;g of FVIII plasmid at day 0 (n = 12, schedule 1), or with three daily i.p. injections of IL-2 complexes at days −1, 0, and 1 concomitant by i.v. injection of 50 µg of FVIII plasmid at day 0 (n = 9, schedule 2). FVIII activities were assessed by a modified activated partial thromboplastin time assay and the anti-FVIII antibody titers by Bethesda assay over time. For schedule 1 mice, (a) FVIII activity, (b) anti-FVIII antibody titers. For schedule 2 mice, (c) FVIII activity, (d) anti-FVIII antibody titers. Each symbol represents data obtained from an individual mouse treated with FVIII plasmid and IL-2 complexes using either schedule 1 or 2. Mice treated with IL-2 complexes only (n = 4) and FVIII plasmid only (n = 5) were used as controls.
IL-2 complexes treatment selectively increased the percentages and activities of CD4+CD25+Foxp3+ Treg cells in peripheral blood, LNs and Spleen
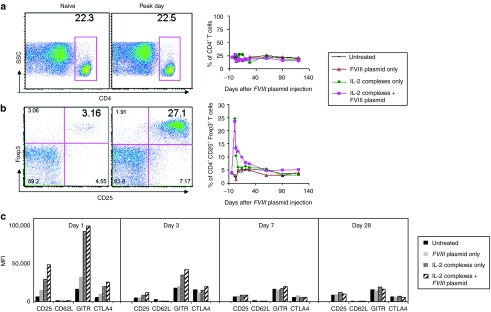
Next, we investigated if changes in cell populations of the T-cell compartments of HemA mice especially Treg cells correlated with tolerance induction by IL-2 complexes treatment. We analyzed peripheral blood in selected mice treated with IL-2 complexes + FVIII plasmid (schedule 1 mice, n = 4). Groups of naive mice and mice treated with IL-2 complexes only and FVIII plasmid only were used as controls. A detailed flow cytometry analysis showed that the percentage of CD4+ T cells did not change over time (Figure 2a; P < 0.05); however, the percentage of CD4+CD25+Foxp3+ T cells within the CD4+ T cell compartment transiently and significantly increased in the treated mice compared to the naive mice (Figure 2b). The expansion of Treg cells after three injections of IL-2 complexes was rapid but brief, reaching a peak on day 1 (peak day; Figure 2b, left panel), and then declining gradually to background levels by day 21 (Figure 2b, right panel). In addition, we found that the IL-2 complexes expanded Treg cells at 1 day after the FVIII plasmid injection showed considerable higher expression levels of molecules crucial for the suppressive function of Treg cells, including CD25, glucocorticoid-induced tumor necrosis factor receptor (GITR), and cytotoxic T-lymphocyte antigen 4 (CTLA-4) (Figure 2c); in contrast, injection of FVIII plasmid alone had little or no effect on Treg cell phenotype. This highly activated state of in vivo expanded Treg cells was brief, with the majority of these molecules returning to near-normal levels of expression by day 7. Similar results were obtained in schedule 2 mice treated with FVIII plasmid + IL-2 complexes including both tolerized and untolerized mice (Supplementary Figure S1). On average, 25% (fivefold increase) of CD4+CD25+Foxp3+ T cells in the CD4+ T cell compartment was detected on peak day in schedule 1 mice treated with IL-2 complexes + FVIII plasmid (Figure 2b), whereas 20% (fourfold increase) was found in schedule 2 mice (Supplementary Figure S1b), compared to untreated mice.
Figure 2.
Effect of interleukin-2 (IL-2) complexes treatment on CD4+ T cells and CD4+CD25+Foxp3+ regulatory T (Treg) cells in periphery blood using schedule 1 treatment. Blood cells were isolated from untreated mice and mice treated with IL-2 complexes only, factor VIII (FVIII) plasmid only and FVIII plasmid + IL-2 complexes (n = 4/group, schedule 1) at serial time points. Cells were stained and analyzed for CD4+ and CD4+CD25+Foxp3+ T cells by flow cytometry. For CD4+ T cells, (a) left panel, representative dot plot from naive and mice treated with FVIII plasmid + IL-2 complexes; right panel, summary plot over time. For CD4+CD25+Foxp3+ T cells gated on CD4+ T cells, (b) left panel, representative dot plot from mice treated with FVIII plasmid + IL-2 complexes; right panel, summary plot over time, and (c) the expression levels of their activation markers (shown by median fluorescence intensity (MFI) values): CD25, CD62L, glucocorticoid-induced tumor necrosis factor receptor (GITR) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) at days 1, 3, 7, and 28 after FVIII plasmid transfer. Data shown are MFI value of the four activation markers of each group.
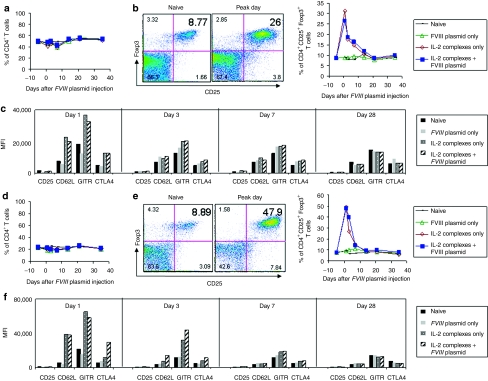
The substantial increase in Treg cells following injection of IL-2 complexes was widespread; it occurred not only in blood, but also appeared as a fourfold increase in LNs (Figure 3b, left panel), fivefold in spleen (Figure 3e, left panel), and threefold in thymus (data not shown) on day 1 post plasmid injection (peak day), respectively; these levels declined rapidly to normal by day14 (Figure 3b,e, right panels). In contrast, no significant change in the CD4+ T-cell population was observed in both LNs (Figure 3a) and spleen (Figure 3d). The expression levels of the activation markers of the Treg cells including CD25, GITR and CTLA-4 after IL-2 complexes treatment also reached their highest levels on the peak day in both LNs (Figure 3c) and spleen (Figure 3f), whereas neither Treg cell numbers nor expression of activation markers increased in mice treated only with FVIII plasmid. Similarly to what was observed in peripheral blood, the highly expanded and activated Treg cells were only transiently present; the majority of the activation markers returned to near-normal levels of expression in both LNs (Figure 3c) and spleen (Figur 3f) by day 7 following FVIII plasmid injection. In addition, treatment with isotype control antibodies + FVIII plasmid produced the same results as the plasmid only treatment in mice (data not shown).
Figure 3.
Effect of interleukin-2 (IL-2) complexes treatment on CD4+ T cells and CD4+CD25+Foxp3+ regulatory T (Treg) cells in lymph nodes (LNs) and spleen of mice using schedule 1 treatment. LNs and spleen cells were isolated from naive mice and mice treated with IL-2 complexes only, factor VIII (FVIII) plasmid only and IL-2 complexes + FVIII plasmid (n = 2/group, each time point) at serial time points. Cells were stained and analyzed for CD4+ and CD4+CD25+Foxp3+ T cells by flow cytometry. For (a–c) LNs and (d–f) spleen cells: (a,d) Summary plot for CD4+ T cells over time. (b,e) left panel, representative dot plot from naive and mice treated with FVIII plasmid + IL-2 complexes; right panel, summary plot for CD4+CD25+Foxp3+ T cells (gated on CD4+ T cells) over time, and (c,f) the expression levels of activation markers of CD4+CD25+Foxp3+ T cells (shown by median fluorescence intensity (MFI) values): CD25, CD62L, glucocorticoid-induced tumor necrosis factor receptor (GITR) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) at day 1, 3, 7, and 28 after FVIII plasmid transfer.
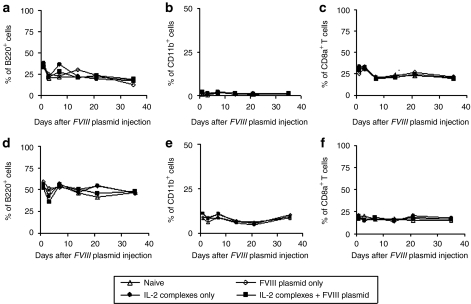
Furthermore, we tested if the specific IL-2 complexes induced only selective expansion of Treg cells without affecting the other cell populations in our HemA inhibitor mouse model. The percentage and total cell numbers were examined for several cell populations. As shown in Figure 4, there were no significant changes in the B cells (B220+ cells) (Figure 4a,d; P < 0.05), myeloid cells (CD11b+ cells) (Figure 4b,e; P < 0.05) and CD8+ T cells (Figure 4c,f) in both LNs (Figure 4a–c; P < 0.05) and spleen (Figure 4d–f; P < 0.05) over 35 days following IL-2 complexes treatment.
Figure 4.
Influence of interleukin-2 (IL-2) complexes on total B cells, myeloid cells and CD8+ T cells in lymph nodes (LNs) and spleen of mice using schedule 1 treatment. (a–c) LNs and (d–f) spleens cells were collected and isolated at serial time points from naive mice and mice treated with IL-2 complexes only, factor VIII (FVIII) plasmid only and IL-2 complexes + FVIII plasmid (n = 2/group, each time point). Cells were stained and analyzed for total (a,d) B cells (B220+ cells), (b,e) myeloid cells (CD11b+ cells) and (c,f) CD8+ T cells (CD8a+ cells).
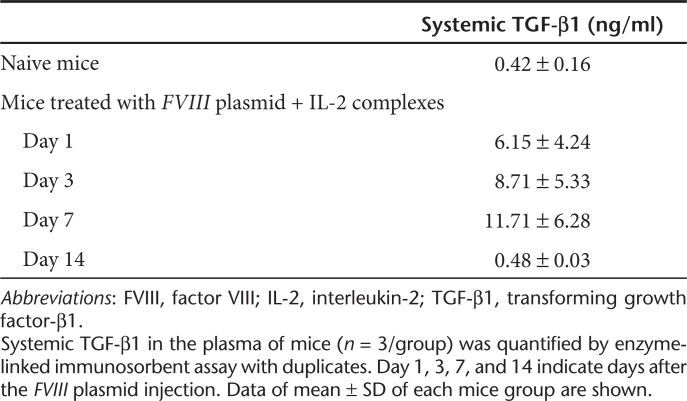
Since transforming growth factor-β1 (TGF-β1) is critical for Treg development, we also investigated the TGF-β1 levels in the plasma of naive mice and mice treated with FVIII plasmid + IL-2 complexes. Three FVIII plasmid + IL-2 complexes tolerized mice (n = 3/group, Table 1) had increased TGF-β1 levels at day 1, 3, and 7 after the FVIII plasmid injection, compared to those of naive mice (n = 3/group, Table 1). These data are consistent with the results reported by Liu et al., which showed that IL-2 complexes treatment induced a significant increase in TGF-β1 levels in an experimental mouse model for autoimmune myasthenia gravis.28
Table 1. IL-2 complexes treatment increases systemic TGF-β1.
Functional examination of effector T cells and CD4+CD25+Foxp3+ Treg cells in tolerance induction by IL-2 complexes treatment in vitro and in vivo
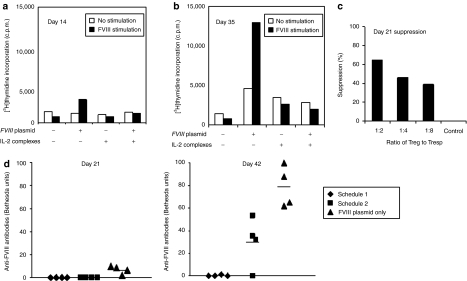
To assess the FVIII-specific proliferative activity of effector T cells post IL-2 complexes treatment, we isolated CD4+ T cells from the spleens of four groups of mice including naive mice, and mice treated with IL-2 complexes only, FVIII plasmid only and IL-2 complexes + FVIII plasmid 14 and 35 days after FVIII plasmid injection. These responder cells were cocultured with irradiated splenic CD4− cells serving as antigen presenting cells. When stimulated with 10 U/ml of FVIII protein which was the optimal dose response from our previous experiment,32 CD4+ T cells isolated from mice treated with plasmid only (with high-titer anti-FVIII inhibitory antibodies) proliferated robustly on both day 14 (Figure 5a) and day 35 (Figure 5b) after FVIII plasmid injection. The cells isolated at day 35 had higher FVIII-specific proliferative activity than the cells isolated at day 14. This is as expected, since the mice had higher inhibitory antibody titers at day 35 than at day 14. In contrast, CD4+ T cells isolated from mice treated with IL-2 complexes + FVIII plasmid on day 14 (Figure 5a) and day 35 (Figure 5b) after FVIII plasmid injection showed no FVIII-specific proliferation; comparable levels of nonspecific proliferation were observed from these cells with and without FVIII stimulation. In addition, no increase in proliferative responses to FVIII was observed from CD4+ T cells isolated from control naive and IL-2 complexes only treated mice.
Figure 5.
Functional examination of CD4+ T cells and CD4+CD25+Foxp3+ regulatory T (Treg) cells from mice treated with interleukin-2 (IL-2) complexes in vitro and in vivo. (a,b) Proliferation assay. CD4+ T cells were isolated by magnetic activated cells sorting from spleens of naive mice and mice treated with IL-2 complexes only, factor VIII (FVIII) plasmid only and IL-2 complexes + FVIII plasmid (n = 2/group, each time point) (a) 14 and (b) 35 days after plasmid injection. 1.0 × 105 CD4+ T cells were cocultured with irradiated 1.0 × 105 CD4− cells (antigen presenting cell) with or without the presence of FVIII at 10 U/ml for 72 hours, followed by adding 1 µCi[3H] thymidine for 18 hours. (c) FVIII suppressive assay. T-responder cells were isolated from the spleen of an inhibitor mouse treated with FVIII plasmid only. CD4+CD25+ T cells from pooled splenic cells of naive mice or mice at day 21 following FVIII plasmid + IL-2 complexes treatment were used as suppressive cells. The final coculture system consisted of 0.8 × 105 CD4+ Tresp cells, 1.5 × 105 irradiated CD4+ cells, and CD4+CD25+ Treg cells at the indicated Treg:Tresp ratio. Data shown are mean ± SD of counts per minute (c.p.m.) of [3H] thymidine incorporation in triplicate wells. (d) Adoptive transfer experiments. CD4+CD25+ Treg cells were isolated from spleen of hemophilia A (HemA) mice using schedule 1 and 2 treatments 5 days post FVIII plasmid injection, and adoptively transferred into naive HemA mice. Schedule 1 recipient mice (n = 4) and schedule 2 recipient mice (n = 4) as well as a control group of naive untreated HemA mice (n = 4) were subsequently challenged with FVIII plasmid injection one day following adoptive transfer. Anti-FVIII antibody titers on day 21 (left panel) and day 42 (right panel) were evaluated. Each symbol represents an individual mouse.
Next, we tested the suppressive function of Treg cells in tolerized mice treated with FVIII plasmid + IL-2 complexes. The suppressive activity of CD4+CD25+ Treg cells isolated from tolerized mice at 3 weeks following FVIII plasmid injection were evaluated in a FVIII-specific suppression assay using CD4+ T cells from mice treated with FVIII plasmid only as responder T cells. As anticipated, we observed significant FVIII-specific suppression by CD4+CD25+ Treg cells isolated from FVIII plasmid + IL-2 complexes tolerized mice (Figure 5c). Additional experiments of evaluating the suppressive functions of expanded Treg cells by treatment with IL-2 complexes combined with either FVIII or nonspecific antigens will be performed to confirm the induction of antigen-specific tolerance to FVIII.
In addition, the in vivo function of expanded Treg cells were examined by adoptive transfer experiments. Treg cells were isolated from either schedule 1 or 2 mice 5 days after FVIII plasmid injection and adoptively transferred (1 × 106 cells/mouse) into naive HemA mice. The two groups of recipient mice (n = 4) and a group of control untreated HemA mice (n = 4) were challenged with 50 µg of FVIII plasmid 1 day after the cell transfer. On day 21 post plasmid challenge, control mice produced anti-hFVIII antibodies, whereas both groups receiving Treg cells from schedule 1 and schedule 2 mice generated no inhibitory antibodies (Figure 5d; left panel). Importantly, mice receiving Treg cells from schedule 1 mice were better protected from inhibitory antibodies formation than mice receiving Treg cells from schedule 2 and the control untreated mice on day 42 (Figure 5d; right panel).
Together, these data indicate that FVIII-specific Treg cells from tolerized mice treated with IL-2 complexes are functionally suppressive to anti-FVIII immune responses both in vitro and in vivo.
Long-term tolerance to FVIII by second challenge of FVIII plasmid
To further investigate whether immunomodulation by IL-2 complexes induced short-term unresponsiveness or long-term tolerance to FVIII, we challenged the FVIII plasmid + IL-2 complexes tolerized mice with FVIIII plasmid a second time at 18 weeks after the 1st FVIII plasmid injection. In this experiment, we chose 4 schedule 1 mice which did not produce antibody responses. The 2nd challenge with FVIII plasmid induced a short burst of high-level FVIII activities in all four mice (Figure 6a). Three mice with persistent FVIII expression after the 1st FVIII plasmid challenge continued to generate therapeutic levels of FVIII expression after the 2nd challenge (Figure 6a). Most significantly, none of the mice treated with IL-2 complexes developed detectable inhibitory antibodies against FVIII after the 2nd challenge (Figure 6b), indicating the establishment of long-term tolerance to FVIII.
Figure 6.
Maintenance of immune tolerance to factor VIII (FVIII) after a second challenge with FVIII plasmid. Mice treated with FVIII plasmid + interleukin-2 (IL-2) complexes were given a second plasmid challenge at 18 weeks after the first plasmid injection. Three mice which had persistent FVIII activity and one mouse which had short-term FVIII activity after first treatment were chosen for this experiment. (a) FVIII activity, (b) anti-FVIII antibody titers were examined after second challenge as described in Figure 1. Each line represents an individual mouse.
Unrelated T-dependent and T-independent antigen challenge
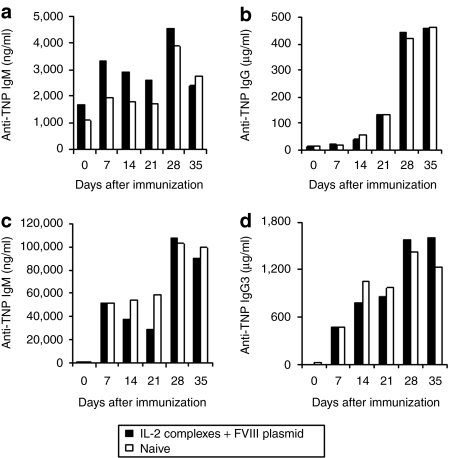
Since IL-2 complexes treatment transiently increased a large number of Treg cells, the long-term effect on the immune system was investigated. Two FVIII plasmid + IL-2 complexes tolerized mice were inoculated with the T-dependent antigen, TNP-KLH, and another two tolerized mice with the T-independent antigen, TNP-Ficoll 18 weeks after plasmid delivery. Untreated naive HemA mice (n = 2/group) were used as controls in both experiments. Peak antibody titers were observed at 2 weeks post primary immunization and 1 week post secondary TNP-KLH and TNP-Ficoll immunization. The levels of specific immunoglobulin (Ig) M (Figure 7a) and total IgG (Figure 7b) were equivalent between tolerized and untreated naive mice following challenges with TNP-KLH. Similarly, no significant differences in serum levels of anti-TNP IgM (Figure 7c), and IgG3 (Figure 7d) were found between tolerized and untreated naive mice following challenges with TNP-Ficoll. Together, these data indicated that transient immunomodulation with IL-2 complexes did not adversely affect immune responses to T-dependent or T-independent neoantigens in the long term.
Figure 7.
Challenge of tolerized mice with unrelated T-dependent and T-independent antigens. Tolerized mice (mice treated with interleukin-2 (IL-2) complexes + factor VIII (FVIII) plasmid, n = 2/group) and naive mice (n = 2/group) were challenged with the T-dependent antigen (TNP-KLH, 100 µg/mouse) and T-independent antigen (TNP-Ficoll, 25 µg/mouse) starting at week 18 after plasmid injection, respectively. (a) Anti-TNP immunoglobulin (Ig) M and (b) anti-TNP IgG levels were assessed over time following primary (day 0) and secondary (day 21) TNP-KLH immunization. (c) Anti-TNP IgM and (d) anti-TNP IgG3 levels were assessed over time following primary and secondary TNP-Ficoll immunization. No significant differences were found between the groups for anti-TNP IgM, anti-TNP IgG, and anti-TNP IgG3 levels.
Discussion
Immune response against FVIII is a major obstacle for successful gene therapy for HemA. Many approaches developed toward induction of tolerance to FVIII22 and other antigens33 involve suppressive function of Treg cells. Our lab has recently demonstrated that adoptive transfer of transgenic CD4+Foxp3+ Treg cells protected recipient mice from high-titer anti-FVIII inhibitory immune responses for prolonged periods of time following plasmid-mediated gene therapy.23 However, some potential challenges and risks may be involved in adoptive Treg cell therapy, including inefficient isolation and expansion of Treg cells with or without defined allospecificities and the possibility of expanded Treg cells reverting to proinflammatory effector cells. Here, we report a promising strategy to induce selective expansion of functional Treg cells in vivo by injection of a particular IL-2 complex to modulate anti-FVIII responses in HemA mice.
Three consecutive injections of the optimal 1:2 ratio of IL-2 complexes delivered with specific schedule 1 together with one injection of plasmid DNA encoding the FVIII gene prevent the formation of inhibitory antibodies, resulting in long-term, stable FVIII expression in treated HemA mice. Among mice with specific schedule 2 treatment, only small portion of the mice developed the anti-FVIII inhibitory antibody starting on day 30. We tested two different injection schedules of IL-2 complexes in an effort to induce higher number of FVIII-specific Treg cells to modulate anti-FVIII immune responses. For treatment using schedule 1, IL-2 complexes were administered at days −5, −4, and −3, to first induce a robust expansion of Treg cells and FVIII plasmid was injected at the peak of Treg expansion (day 0), whereas for treatment using schedule 2, IL-2 complexes treatment was performed at days −1, 0, and 1, with FVIII plasmid injection at day 0 so that antigen was present during the initial phase of Treg expansion. As shown in the results, schedule 1 treatment appeared to be much more effective in modulating FVIII-specific responses than schedule 2 treatment, although both treatments induced significant expansion of Treg cells in mice including those mice that developed inhibitory antibodies. This evidence was also supported by the following adoptive transfer data: the mice receiving the FVIII-specific Treg cells from the mice treated by schedule 1 showed more protection form inhibitory antibody formation than those receiving cells from the schedule 2 mice. The difference in the effectiveness of the two protocols may be due to a requirement that highly expanded Treg cell populations are present upon first antigen exposure. At the time of FVIII plasmid injection, Treg cell expansion is at its peak levels in the schedule 1 mice, whereas in schedule 2 mice, Treg expansion was just initiated with Treg cells at low levels. Thus, schedule 1 treatment can not only more effectively suppress the initial responses, but also induce antigen-specific Treg cells upon antigen exposure at a later phase. In comparison, FVIII expression in mice treated with FVIII plasmid only declined to background levels in 2–4 weeks and FVIII inhibitors could be detected as early as 3–4 weeks.32,34 Splenic T cells isolated from mice treated with FVIII plasmid + IL-2 complexes showed no indication of recall proliferation to FVIII stimulation in vitro. Furthermore, following a 2nd plasmid challenge at week 18 when mice had long recovered from the immune suppression of IL-2 complexes, the tolerized mice failed to elicit an immune response. Moreover, challenges with either T-dependent antigen (TNP-KLH) under a stringent condition (emulsified in Freund's complete adjuvant) or T-independent antigen (TNP-Ficoll) promoted similar levels of immune responses in FVIII plasmid + IL-2 complexes tolerized mice and untreated naive mice, indicating that IL-2 complexes treatment did not affect the host's ability to mount immune responses to other T-dependent and T-independent neoantigens. Taken together, these data indicate that immunomodulation with IL-2 complexes following FVIII plasmid-mediated gene therapy induced long-term protection against FVIII-specific immune responses in HemA mice.
In addition to treating autoimmune disease28 and graft rejection in transplantation27 with selective and efficient expansion of Treg cells, our study demonstrated a new novel application of IL-2 complexes, which was to modulate antigen-specific immune responses following gene therapy. In our study, we found that IL-2 complexes treatment resulted in expansion of approximately five- to sevenfold Treg cells in periphery blood, LNs and spleen at day 5 (peak day) after IL-2 complexes treatment, and these levels gradually returned to normal within the next 7–14 days. Similarly to naturally occurring Treg cells, the expanded Treg cells achieved stable Foxp3 expression. The increase of Treg cell numbers was also accompanied by a significant increase in the expression levels of the activation markers CD25, CTLA-4 and GITR on these cell populations. These short-lived expanded Treg cells are highly activated and display in vitro suppressive function. In addition, adoptive transfer of the expanded Treg cells protected recipient mice from generation of high-titer antibodies following FVIII plasmid challenge. However, very little changes were observed for other lymphocyte populations, including CD4+ T cells, CD8+ T cells, myeloid cells, and total B cells, confirming that IL-2 complexes induced selective expansion of functional Treg cells.
The common γ-chain cytokine IL-2 predominantly activates cells expressing high-affinity receptors composed of three chains [IL-2Rα (CD25), IL-2Rβ (CD122), and γc (CD132)], such as activated CD4+ and CD8+ T cells.35,36,37 It also plays a critical role in regulating the homeostasis of CD4+Foxp3+ Treg cells.38,39,40 Studies in mice engineered to express a Foxp3 reporter demonstrated that Foxp3 expression is confined to a subset of αβ T-cells and correlates with their suppressive activity.41,42 Biological function of most αβ T-cells is to respond to diverse foreign antigens presented as peptides on the major histocompatibility complex molecules of antigen presenting cell, and they make a major contribution to host defense. As described earlier, biological activity of IL-2 in vivo can be greatly enhanced by association with particular anti-IL-2 mAbs. To induce expansion of Treg cells, we took advantage of IL-2/IL-2mAb immune complexes that were initially described by Boyman et al.26 and Kamimura et al.31 IL-2/JES6-1 complexes interact almost exclusively with T cells expressing high levels of CD25, thus inducing selective expansion of CD4+CD25+ Treg cells. This is because JES6-1 mAb binds to an IL-2 site that is crucial for interaction with CD122 but less crucial for binding to CD25. The other anti-IL-2 mAbs, S4B6 (specific to murine IL-2) and MAB201 (specific to human IL-2) formed IL-2/IL-2 mAb complexes preferentially interact with CD122, leading to the expansion of CD8+ T cells and natural killer cells. The mechanism by which IL-2 complexes exhibit superior capacity to treatment compared with IL-2 or anti-IL-2 mAb alone in different applications remains elusive. It was suggested that immune complexes containing anti-IL-2 mAb may increase the half-life of IL-2 in vivo26,43 or their function may depend on the presence of neonatal FcR.44 The precise mechanisms remain to be investigated.
It has been shown that IL-2 mRNA expression was significantly induced by TGF-β1, which led to the induction of IL-2 secretion. Furthermore, TGF-β1 supports the maintenance of Foxp3 expression, regulatory function, and homeostasis in peripheral CD4+CD25+ regulatory T cells,45 indicating the importance of TGF-β1 in maintaining tolerance. In our study, mice treated with IL-2 complexes exhibited increased TGF-β1 levels in blood at days 1, 3, and 7 post plasmid injection, which correlated with the expansion and activation of Treg cells. We hypothesize that FVIII plasmid + IL-2 complexes treatment induced TGF-β1 dependent maturation of CD4+CD25− cells into CD4+CD25+Foxp3+ Treg cells, which maintain long-term FVIII-specific tolerance.
It was demonstrated in our lab that several immunosuppressive regimens including CTLA4-Ig/anti-CD40L combination32 and anti-ICOS34 targeting B- and T-cell costimulatory pathways, or anti-CD346 targeting T-helper cells successfully prevented induction of anti-FVIII immune responses in HemA mice treated with FVIII plasmid-mediated gene therapy. These regimens induced increases in either or both of the percentages and total numbers of CD4+Foxp3+ Treg cells, indicating the involvement of Treg cells in modulation of anti-FVIII immune responses. Furthermore, the current study of selective in vivo expansion of Treg cells by IL-2 complexes treatment provides direct evidence for the important role of Treg cells in suppressing anti-FVIII immune responses. Most importantly, short-term treatment that promotes transient expansion of Treg cells without depletion or alteration of other cell populations may be beneficial in clinical applications compared to other immunomodulation therapies. This was demonstrated in the results that the host immune responses to other T-dependent or T-independent neoantigens were not perturbed in mice treated with IL-2 complexes several months after treatment. IL-2 complexes immunomodulation has the potential to be a safe and effective strategy for modulating FVIII-specific immune responses following gene therapy.
Materials and Methods
Mice. All mice were kept according to the National Institutes of Health guidelines for animal care and the guidelines of Seattle Children's Research Institute, and maintained at a specific pathogen-free facility. HemA mice in a 129/SV × C57BL/6 mixed genetic background were generated by targeted disruption of exon 16 of FVIII gene47 and were used at the age of 10–16 weeks.
Gene transfer of FVIII into HemA mice with immunomodulation by IL-2 complexes injection. HemA mice were intravenously injected with 50 µg of FVIII plasmid (pBS-HCRHPI-FVIIIA48) in 2 ml phosphate-buffered saline via tail vein in 8–10 seconds. IL-2/anti-IL-2 mAb (JES6-1A12) complexes were prepared as previously described.27 One microgram recombinant mouse IL-2 (PeproTech, Rocky Hill, NJ) was mixed with 5 µg anti-IL-2 mAb (JES6-1A12) (eBioscience, San Diego, CA), incubated at 37 °C for 30 minutes, and injected intraperitoneally into mice according to schedules specified in Results. Groups of mice treated with IL-2 complexes only, FVIII plasmid only and naive mice were included as controls. Selected immunomodulated mice received a second plasmid challenge at 18 weeks after the first FVIII plasmid injection. Blood samples were taken from the retro-orbital plexus at serial time points and assessed for FVIII activity and anti-FVIII antibody levels.
Flow cytometry and antibodies. Cell suspensions of peripheral blood, LNs (from superficial cervical) and spleens of each treated mouse group were prepared according to standard protocols. Cell suspensions were stained for fluorescence-activated cell sorting analysis using the following antibodies (obtained from eBioscience unless otherwise stated): PE-Cy5- anti-mouse CD25; FITC-anti-mouse CD62L (L-selectin); Alexa Fluor647- anti-mouse/rat Foxp3; PE-anti-mouse CD152 (CTLA-4); Alexa Fluor 700- anti-mouse CD4 (BD Pharmingen, San Jose, CA), and PE-Cy7- anti-mouse GITR (BD Pharmingen). Cells were first stained for surface markers CD4, CD25, CD62L, and GITR, and subsequently stained intracellularly for Foxp3 and CTLA-4 following the company protocol (eBioscience). Samples were analyzed on an LSRII flow cytometer (Becton Dickinson, Palo Alto, CA) and data were analyzed using FlowJo software (Tree Star, Ashland, OR).
FVIII activities and inhibitor titer assays. Peripheral blood samples were taken from the experimental mice and collected in a 3.8% sodium citrate solution. FVIII activities were measured by a modified clotting assay using FVIII deficient plasma and reagents to measure activated partial thromboplastin time and FVIII deficient plasma.5,48 FVIII activities were calculated from a standard curve generated by using serially diluted normal human pooled plasma. Anti-FVIII antibodies were measured by Bethesda assay as previously described.49
Proliferation and suppressive assay. CD4+ T cells were isolated from spleens of mice by magnetic activated cells sorting (Miltenyi Biotec, Auburn, CA). The CD4+CD25−, CD4+CD25+ subsets were further purified from the CD4+ T cells using a CD25+ Treg MACS isolation kit (Miltenyi Biotec). For proliferation assay, 1.0 × 105 CD4+ T cells were cocultured with 1.0 × 105 CD4− cells (irradiated, used as antigen presenting cells) per well in 96-well round-bottom plate with the presence of FVIII at 10 U/ml (1 U = 100 ng FVIII protein)32 for 72 hours, followed by adding 1 µCi [3H]thymidine per well for the final 18 hours. [3H]thymidine incorporation was measured as counts per minute (c.p.m.) in a Betaplate scintillation counter (Perkin-Elmer, Shelton, CT). For suppressive assay, CD4+ T cells from spleens of mice treated with FVIII plasmid only were used as responders and CD4+CD25+ T cells from spleens of mice treated with FVIII plasmid + IL-2 complexes at different time points after plasmid injection or from spleens of age matched naive mice were added as suppressor cells. To the coculture of 0.8 × 105 CD4+ T cells and 1.5 × 105 antigen presenting cells, we added CD4+CD25+ T cells at indicated ratios. The cultures were stimulated with 10 U/ml of FVIII for 72 hours, followed by adding 1 µCi [3H] thymidine per well for the final 18 hours incubation. All cultures were done in triplicates. Suppression was calculated as:50
% suppression = [1 – (c.p.m. (CD4+CD25− T cells + CD4+CD25+ T cells)/c.p.m. CD4+CD25− T cells alone)] × 100%
Adoptive transfer of hFVIII-specific Treg cells. Spleens were removed aseptically from schedule 1 or 2 or naïve untreated control HemA mice at 5 days post FVIII plasmid injection. CD4+CD25+ splenic T cells were isolated and injected into the tail vein of HemA recipients (1 × 106 cells/mouse). Twenty-four hours post-transfer, recipient mice were challenged with hydrodynamic tail-vien injection of 50 µg of pBS-HCRHPI-hFVIIIA plasmid in 2 ml phosphate-buffered saline. Plasma samples collected at various time points were analyzed for FVIII content and anti-FVIII antibody titers by activated partial thromboplastin time and Bethesda assays, respectively.
Immunization with the T-independent antigen TNP-Ficoll and T-dependent antigen TNP-KLH. Groups of tolerized mice (n = 2) and naive mice (n = 2) were immunized with 25 µg TNP-Ficoll and 100 µg TNP-KLH emulsified in Freund's complete adjuvant intraperitoneally each 24 weeks after plasmid injection. A second boost was administered intraperitoneally 3 weeks later with the same amount of TNP-Ficoll and TNP-KLH. Mice were bled every 7 days and serum samples were analyzed for anti-TNP Ab by enzyme-linked immunosorbent assay in the plate coated with trinitrophenyl-bovine serum albumin (TNP-BSA). The TNP-specific IgM, IgG, and/or IgG3 Abs were detected by incubating with biotinylated anti-mouse Ab, followed by the addition of horseradish peroxidase-labeled streptavidin, and finally the wells were developed with substrate.
Quantitation of TGF-β1 levels. CD4+ T cells were prepared from the spleens of tolerized mice, mice treated with IL-2 complexes only, hFVIII plasmids only, or untreated control mice as described in “proliferation assays.” TGF-β1 concentrations were quantified in plasma pretreated with 1 N HCL, then assayed using the ELISA kit (BD Biosciences, San Jose, CA) according to the manufacturer's recommendation, and the data were interpolated against the linear range on the standard curves.
Statistical analysis. Results are presented as means ± SD The statistical significance of the difference between means was determined using the two-tailed Student's t test. Differences were considered significant at P < 0.05.
SUPPLEMENTARY MATERIAL Figure S1. Treg cells and activation markers in periphery blood using schedule 2 treatment.
Acknowledgments
This work was supported by R01 grants (R01 HL69049 and R01 HL82600) from NIH-NHLBI. We would like to thank Dr Peng for his help.
Supplementary Material
Treg cells and activation markers in periphery blood using schedule 2 treatment.
REFERENCES
- Antonarakis SE, Youssoufian H., and, Kazazian HH., Jr Molecular genetics of hemophilia A in man (factor VIII deficiency) Mol Biol Med. 1987;4:81–94. [PubMed] [Google Scholar]
- Zhang AH, Skupsky J., and, Scott DW. Factor VIII inhibitors: risk factors and methods for prevention and immune modulation. Clin Rev Allergy Immunol. 2009;37:114–124. doi: 10.1007/s12016-009-8122-5. [DOI] [PubMed] [Google Scholar]
- VandenDriessche T, Vanslembrouck V, Goovaerts I, Zwinnen H, Vanderhaeghen ML, Collen D.et al. (1999Long-term expression of human coagulation factor VIII and correction of hemophilia A after in vivo retroviral gene transfer in factor VIII-deficient mice Proc Natl Acad Sci USA 9610379–10384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell JS, Ragni MV, White GC, 2nd, Lusher JM, Hillman-Wiseman C, Moon TE.et al. (2003Phase 1 trial of FVIII gene transfer for severe hemophilia A using a retroviral construct administered by peripheral intravenous infusion Blood 1022038–2045. [DOI] [PubMed] [Google Scholar]
- Ye P, Thompson AR, Sarkar R, Shen Z, Lillicrap DP, Kaufman RJ.et al. (2004Naked DNA transfer of Factor VIII induced transgene-specific, species-independent immune response in hemophilia A mice Mol Ther 10117–126. [DOI] [PubMed] [Google Scholar]
- Moayeri M, Hawley TS., and, Hawley RG. Correction of murine hemophilia A by hematopoietic stem cell gene therapy. Mol Ther. 2005;12:1034–1042. doi: 10.1016/j.ymthe.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Lillicrap D, Patarroyo-White S, Liu T, Qian X, Scallan CD.et al. (2006Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs Blood 108107–115. [DOI] [PubMed] [Google Scholar]
- McCormack WM, Jr, Seiler MP, Bertin TK, Ubhayakar K, Palmer DJ, Ng P.et al. (2006Helper-dependent adenoviral gene therapy mediates long-term correction of the clotting defect in the canine hemophilia A model J Thromb Haemost 41218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters B., and, Lillicrap D. The molecular mechanisms of immunomodulation and tolerance induction to factor VIII. J Thromb Haemost. 2009;7:1446–1456. doi: 10.1111/j.1538-7836.2009.03538.x. [DOI] [PubMed] [Google Scholar]
- Miao CH. Recent advances in immune modulation. Curr Gene Ther. 2007;7:391–402. doi: 10.2174/156652307782151524. [DOI] [PubMed] [Google Scholar]
- Herzog RW., and, Dobrzynski E. Immune implications of gene therapy for hemophilia. Semin Thromb Hemost. 2004;30:215–226. doi: 10.1055/s-2004-825635. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z.et al. (2006Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease Immunol Rev 2128–27. [DOI] [PubMed] [Google Scholar]
- Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol. 2007;7:875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- Hawrylowicz CM., and, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5:271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- Wu K, Bi Y, Sun K., and, Wang C. IL-10-producing type 1 regulatory T cells and allergy. Cell Mol Immunol. 2007;4:269–275. [PubMed] [Google Scholar]
- Shevach EM, DiPaolo RA, Andersson J, Zhao DM, Stephens GL., and, Thornton AM. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol Rev. 2006;212:60–73. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- Izcue A, Coombes JL., and, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Blank RB., and, Suffia I. Natural regulatory T cells and parasites: a common quest for host homeostasis. Immunol Rev. 2006;212:287–300. doi: 10.1111/j.0105-2896.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- Rouse BT, Sarangi PP., and, Suvas S. Regulatory T cells in virus infections. Immunol Rev. 2006;212:272–286. doi: 10.1111/j.0105-2896.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- Aluvihare VR, Kallikourdis M., and, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- Beyer M., and, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–811. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- Miao CH. Immunomodulation for inhibitors in hemophilia A: the important role of Treg cells. Expert Rev Hematol. 2010;3:469–483. doi: 10.1586/ehm.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao CH, Harmeling BR, Ziegler SF, Yen BC, Torgerson T, Chen L.et al. (2009CD4+FOXP3+ regulatory T cells confer long-term regulation of factor VIII-specific immune responses in plasmid-mediated gene therapy-treated hemophilia mice Blood 1144034–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida AR, Borghans JA., and, Freitas AA. T cell homeostasis: thymus regeneration and peripheral T cell restoration in mice with a reduced fraction of competent precursors. J Exp Med. 2001;194:591–599. doi: 10.1084/jem.194.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curotto de Lafaille MA, Lino AC, Kutchukhidze N., and, Lafaille JJ. CD25- T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J Immunol. 2004;173:7259–7268.. doi: 10.4049/jimmunol.173.12.7259. [DOI] [PubMed] [Google Scholar]
- Boyman O, Kovar M, Rubinstein MP, Surh CD., and, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD.et al. (2009In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression J Exp Med 206751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Zhou Q, La Cava A, Campagnolo DI, Van Kaer L., and, Shi FD. Expansion of regulatory T cells via IL-2/anti-IL-2 mAb complexes suppresses experimental myasthenia. Eur J Immunol. 2010;40:1577–1589. doi: 10.1002/eji.200939792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyman O, Surh CD., and, Sprent J. Potential use of IL-2/anti-IL-2 antibody immune complexes for the treatment of cancer and autoimmune disease. Expert Opin Biol Ther. 2006;6:1323–1331. doi: 10.1517/14712598.6.12.1323. [DOI] [PubMed] [Google Scholar]
- Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E.et al. (2008Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction Immunity 28687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura D, Sawa Y, Sato M, Agung E, Hirano T., and, Murakami M. IL-2 in vivo activities and antitumor efficacy enhanced by an anti-IL-2 mAb. J Immunol. 2006;177:306–314. doi: 10.4049/jimmunol.177.1.306. [DOI] [PubMed] [Google Scholar]
- Miao CH, Ye P, Thompson AR, Rawlings DJ., and, Ochs HD. Immunomodulation of transgene responses following naked DNA transfer of human factor VIII into hemophilia A mice. Blood. 2006;108:19–27. doi: 10.1182/blood-2005-11-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao O, Loduca PA., and, Herzog RW. Role of regulatory T cells in tolerance to coagulation factors. J Thromb Haemost. 2009;7 Suppl 1:88–91. doi: 10.1111/j.1538-7836.2009.03417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng B, Ye P, Blazar BR, Freeman GJ, Rawlings DJ, Ochs HD.et al. (2008Transient blockade of the inducible costimulator pathway generates long-term tolerance to factor VIII after nonviral gene transfer into hemophilia A mice Blood 1121662–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell DA., and, Smith KA. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983;158:1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell DA., and, Smith KA. The interleukin-2 T-cell system: a new cell growth model. Science. 1984;224:1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., and, Minami Y. The IL-2/IL-2 receptor system: a current overview. Cell. 1993;73:5–8. doi: 10.1016/0092-8674(93)90152-g. [DOI] [PubMed] [Google Scholar]
- Setoguchi R, Hori S, Takahashi T., and, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Cruz LM., and, Klein L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol. 2005;6:1152–1159. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Gavin MA., and, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- Ponpuak M, Davis AS, Roberts EA, Delgado MA, Dinkins C, Zhao Z.et al. (2010Delivery of cytosolic components by autophagic adaptor protein p62 endows autophagosomes with unique antimicrobial properties Immunity 32329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan YY., and, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci USA. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman FD, Madden KB, Morris SC, Holmes JM, Boiani N, Katona IM.et al. (1993Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes J Immunol 1511235–1244. [PubMed] [Google Scholar]
- Létourneau S, van Leeuwen EM, Krieg C, Martin C, Pantaleo G, Sprent J.et al. (2010IL-2/anti-IL-2 antibody complexes show strong biological activity by avoiding interaction with IL-2 receptor alpha subunit CD25 Proc Natl Acad Sci USA 1072171–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie JC, Letterio JJ, Gavin M., and, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng B, Ye P, Rawlings DJ, Ochs HD., and, Miao CH. Anti-CD3 antibodies modulate anti-factor VIII immune responses in hemophilia A mice after factor VIII plasmid-mediated gene therapy. Blood. 2009;114:4373–4382. doi: 10.1182/blood-2009-05-217315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi L, Sarkar R, Naas T, Lawler AM, Pain J, Shumaker SL.et al. (1996Further characterization of factor VIII-deficient mice created by gene targeting: RNA and protein studies Blood 883446–3450. [PubMed] [Google Scholar]
- Miao CH, Ye X., and, Thompson AR. High-level factor VIII gene expression in vivo achieved by nonviral liver-specific gene therapy vectors. Hum Gene Ther. 2003;14:1297–1305. doi: 10.1089/104303403322319381. [DOI] [PubMed] [Google Scholar]
- Kasper CK, Aledort L, Aronson D, Counts R, Edson JR, van Eys J.et al. (1975Proceedings: A more uniform measurement of factor VIII inhibitors Thromb Diath Haemorrh 34612. [PubMed] [Google Scholar]
- Belghith M, Bluestone JA, Barriot S, Mégret J, Bach JF., and, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Treg cells and activation markers in periphery blood using schedule 2 treatment.