Abstract
Adenovirus vectors based on human serotype 5 can induce potent CD8 T cell responses to vector-encoded transgenic antigens. However, the individual contribution of different cell types expressing antigen upon adenovirus vector injection to the generation of antigen-directed adaptive immune responses is poorly understood so far. We investigated the role of hepatocytes, skeletal muscle, and hematopoietic cells for the induction of cellular and humoral immune responses by miRNA-mediated tissue-specific silencing of antigen expression. Using hepatitis B small surface antigen (HBsAg) as the vector-encoded transgene we show that adenovirus vector dissemination from an intramuscular (i.m.) injection site into the liver followed by HBsAg expression in hepatocytes can limit early priming of CD8 T cells and the generation of anti-HBsAg antibody responses. However, hepatocyte-specific miRNA122a-mediated silencing of HBsAg expression overcame these limitations. Early clonal expansion of Kb/S190–197-specific CD8 T cells was significantly enhanced and improved polyfunctionality of CD8 T cells was found. Furthermore, miRNA122a-mediated antigen silencing induced significantly higher anti-HBsAg antibody titers allowing an up to 100-fold vector dose reduction. These results indicate that miRNA-mediated regulation of antigen expression in the context of adenovirus vectors can significantly improve transgene product-directed immune responses. This finding could be of interest for future adenovirus vaccine vector development.
Introduction
Adenovirus (Ad) vectors based on human adenovirus serotype 5 belong to the most immunogenic genetic vaccine vectors available to date. They are considered highly valuable tools to generate efficacious preventive or therapeutic vaccines against severe life-threatening diseases including HIV/AIDS, malaria, and tuberculosis.1 However, data from the recently failed STEP study2 strongly suggest that comprehensive understanding of both transgene product-directed and, importantly, vector-directed immunity is paramount for the endeavor to develop efficacious genetic vaccines based on adenovirus.3 It is generally agreed upon that transgene product-directed and vector-directed immunity can only be understood and beneficially modulated when basic questions addressing in vivo vaccine vector biology are thoroughly investigated.
Adenovirus-based vectors can transduce a wide variety of different cell types both in vitro and in vivo. In vitro transduction is well investigated and mainly depends on interactions of the vectors with the coxsackie and adenovirus receptor and αvβ3/5 integrins.4,5 However, the mechanisms underlying transduction upon vector injection in vivo are much more complex. For example, it has been shown that the strong hepatocyte tropism of Ad5-based vectors depends on a blood coagulation factor-mediated bridging mechanism that tethers the Ad5 capsids to cell surface receptors on hepatocytes.6 Importantly, vector dissemination to hepatocytes has been observed after local intramuscular (i.m.) vector injection7,8—the most common route for vaccine vector delivery.
Presumably owed to the complexity of the transduction patterns in vivo, only very little is known about the individual contribution of different Ad-transduced cell types for induction and maintenance of transgene product-directed cellular and humoral immune responses. Even the role of a potential direct transduction of professional hematopoietic antigen-presenting cells by Ad5-based vectors has been a matter of controversy.9,10,11 Only very recently, Bassett et al. demonstrated that both hematopoietic and nonhematopoietic antigen-presenting cells are required for the maintenance of the typical Ad vector-induced CD8+ T cell memory phenotype.12 Finally, approaches to improve humoral transgene product-directed responses have so far been based on the use of different vaccination routes,13,14,15 but the precise role of different vector-transduced nonhematopoietic cells in this process has hardly been elucidated.
The aim of this study was to investigate the influence of different cell types on the induction of transgene product-directed humoral and cellular immune responses after Ad vector vaccination employing cell-type specific silencing of transgene expression. We focused on cells of hematopoietic origin, skeletal muscle cells, and hepatocytes since these are the predominantly transduced cell types after i.m. Ad vaccine vector delivery. In order to selectively silence transgene expression in skeletal muscle, hepatocytes, and cells of hematopoietic origin we relied on miRNA-mediated tissue-specific silencing, a technology that has been described in the context of lentivirus vectors. Brown et al. demonstrated that the incorporation of target sequences with perfect complementarity to tissue-specific endogenous miRNAs into the 3′-untranslated region (3′UTR) of a transgene leads to tissue-specific translational repression and degradation of the transgene messenger RNA.16 In the context of Ad vectors only hepatocyte-specific mir-122a has been used to control liver toxicity of a suicide gene17 or pathology of wild-type adenovirus18,19 and so far this technology has not been explored for Ad vector silencing in skeletal muscle cells or cells of hematopoietic origin or ultimately for the modulation of transgene product-directed immune responses.
Our data show that antigen silencing in hepatocytes could significantly enhance early priming of CD8 T cells specific for an epitope generated only by the endogenous processing pathway. Independent of the antigen processing pathways cytokine polyfunctionality of specific CD8 T cells was generally improved by hepatocyte-specific antigen silencing. In contrast to published studies with lentivirus vectors,20 silencing in cells of hematopoietic origin did not induce immunological tolerance towards the transgene products, presumably due to the strong adjuvant effect of first-generation Ad vectors or cross-priming mechanisms. Silencing of transgene expression in hepatocytes also induced significantly increased anti-transgene product-directed antibody levels in two different mouse strains. This silencing allowed for an up to 100-fold reduction of the vector dose compared to a nonregulated Ad vector to achieve the same specific antibody titer. Our findings thus exemplify the need for precise tissue control of Ad vaccine vectors, contribute to a better understanding of Ad vaccine vector immunobiology and may have direct impact on the development of safe and efficacious Ad vector-based vaccines.
Results
miRNA-regulated Ad vectors are specifically and efficiently silenced in various cell types in vitro and in vivo
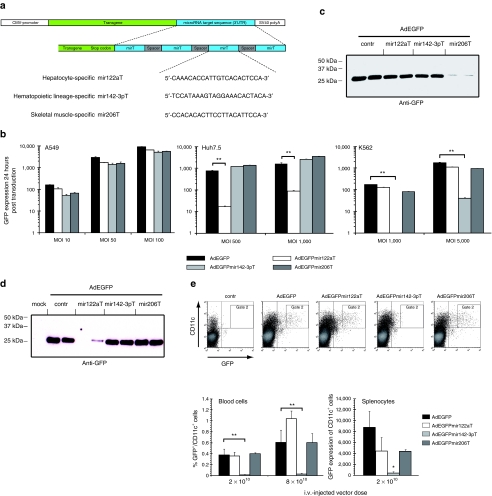
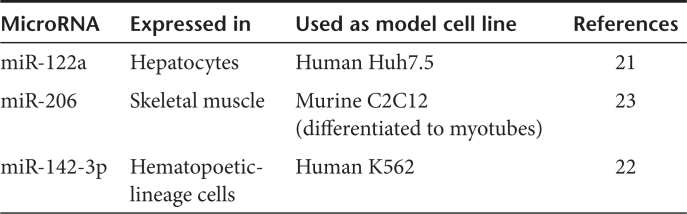
To characterize miRNA-mediated tissue-specific silencing of transgene expression in vitro and in vivo we constructed a set of miRNA-regulated Ad vectors bearing expression cassettes for enhanced green fluorescent protein (AdEGFPmirTs) with insertions in their 3′UTRs of none (control) or four tandem repeats of tissue-specific miRNA target sequences with perfect complementarity (Figure 1a and Table 1).21,22,23
Figure 1.
In vitro and in vivo expression profiles of miRNA-regulated adenovirus (Ad) vectors. (a) Schematic representation of miRNA-regulated expression cassettes inserted into E1-deleted adenoviral vectors. (b) miRNA-target sequences specifically downregulate Ad vector-delivered transgenes in vitro. A549, Huh7.5, and K562 cells were transduced with indicated pMOI of EGFP-expressing miRNA-regulated Ad vectors or control vector without mirT (AdEGFP) in a 24-well plate format. EGFP expression was quantified after 24 hours by flow cytometry as (GMeantransduced × %GFP+ cellstransduced) − (GMeanuntransduced × % GFP+ cellstransduced). The mean values (± SD) of one out of three independent experiments are shown. (c) Differentiated C2C12 myotubes were transduced with 2,000 pMOIs of EGFP-expressing miRNA-regulated Ad vectors or control vector (AdEGFP). 48 hours post transduction cell lysates were prepared and EGFP expression was analyzed by western transfer (20 µg protein per lane). (d,e) BALB/c mice (three mice per group) were injected i.v. with reporter gene expressing AdEGFPmirTs. Injected mice were sacrificed 48 hours postinjection and tissues were analyzed for EGFP expression. (d) EGFP expression of the different reporter gene expression vectors in livers was analyzed by western transfer of lysates of isolated hepatocytes 48 hours after i.v. delivery of 2 × 1010 Ad VP. EGFP expression was strongly reduced in hepatocytes of mice injected with AdEGFPmir122aT. Lysates of two out of three injected mice per vector are shown. (e) EGFP expression in CD11c+ cells of hematopoietic lineage is downregulated by mir-142-3p. Isolated blood cells and splenocytes of mice injected i.v. with indicated doses of AdEGFPmirTs were stained for CD11c and analyzed for EGFP expression by flow cytometry. Representative dot plots of blood cells are shown (upper panels). The mean percentages (± SD) of GFP+/CD11c+ blood cells in gate 2 for two different vector doses are presented in the lower left panel. The mean EGFP expression (± SD) of splenocytes (lower right panel) was calculated as (events GFP+CD11c+injected × Gmean of GFP+CD11c+injected) − (events GFP+CD11c+injected × Gmean of GFP+CD11c+uninjected). Significance levels of data were calculated by unpaired Student's t-test. *P < 0,05; **P < 0,01. CMV, cytomegalovirus; contr, control; EGFP, enhanced green fluorescence protein; i.v., intravenously; mirT, microRNA target sequence; pMOI, particle multiplicity of infection; SV, simian virus; UTR, untranslated region; VP, vector particles.
Table 1. Tissue-specific miRNAs and in vitro model cell lines.
Flow cytometric quantification of EGFP expression after transduction of lung-derived A549 cells, which do not express any miRNA with complementarity to the employed target sequences revealed equal expression levels for all vectors. In contrast, AdEGFPmir122aT was specifically silenced in hepatoma-derived Huh7.5 cells (up to 80-fold) and AdEGFPmir142-3pT was specifically silenced in myelogenous leukemia-derived K562 cells (up to 200-fold) (Figure 1b). In addition, specific silencing of AdEGFPmir142-3pT was corroborated in primary human macrophages differentiated from peripheral blood mononuclear cells (Supplementary Figure S1a,b). Western blot analysis and fluorescence microscopy revealed specific and nearly quantitative silencing of AdEGFPmir206T in differentiated C2C12 myotubes (Figure 1c, Supplementary Figure S1c) but not in undifferentiated myoblasts (data not shown).
To demonstrate silencing in vivo, Ad vectors were intravenously (i.v.) injected into BALB/c mice and 48 hours later EGFP expression was quantified in liver (Figure 1d), blood cells, (Figure 1e, upper panels and lower left panel) and spleen (Figure 1e, lower right panel). Injection of AdEGFP (control without target sequences), AdEGFPmir142-3pT, and AdEGFPmir206T resulted in strong EGFP expression in hepatocytes. In contrast, AdEGFPmir122aT showed no or very little EGFP content in isolated hepatocytes (Figure 1d), indicating efficient hepatocyte-specific silencing of this vector in vivo. In agreement with the results obtained in primary human macrophages (Supplementary Figure S1b) analysis of blood cells revealed that injection of AdEGFPmir142-3pT did not result in EGFP expression in CD11c+ blood cells (Figure 1e, upper panels and lower left panel), even not at a fourfold higher dose of 8 × 1010 VP. This vector was also silenced in splenocytes (Figure 1e, lower right panel).
Analysis of muscle cryosections after i.m. vector injection demonstrated silencing only for AdEGFPmir206T whereas the other vectors induced strong EGFP expression in muscle fibers around the injection site (Supplementary Figure S1d).
Taken together, these results demonstrated the feasibility of miRNA-mediated cell-type specific silencing of Ad vectors in hepatocytes, splenocytes and CD11c+ cells, and skeletal muscle in vivo.
Secreted systemically circulating serum HBsAg is mainly derived from hepatocytes after i.v. Ad vector administration
To assess the effects of miRNA-mediated tissue-specific silencing on the induction of humoral and cellular immune responses against Ad-encoded transgene products in a vaccination-relevant context we constructed a set of miRNA-regulated Ad vectors expressing the hepatitis B small surface antigen (HBsAg). This antigen which is secreted by transduced cells24 provides epitopes with well-characterized immunogenicity for T and B cells.25 These epitopes differ from each other in their MHC class I haplotype restriction and their intracellular processing pathway that generates the antigenic peptide.
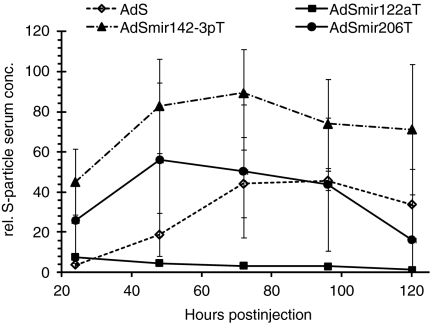
HBsAg-encoding Ad vectors bearing no or hepatocyte-irrelevant miRNA target sequences resulted in significant and constant HBsAg serum levels for several days after i.v. injection, whereas the vector AdSmir122aT induced only minimal HBsAg serum levels close or equal to baseline (Figure 2). These data exemplarily demonstrate that miRNA-regulation is functional in the context of HBsAg and identify hepatocytes as the main source of secreted HBsAg after blood-borne Ad vector-mediated hepatocyte transduction.
Figure 2.
Hepatocytes are the main source of transgenic HBsAg particles after i.v. delivery of adenovirus (Ad) vectors. BALB/c mice were injected intravenously with 2 × 1010 VP of AdS or AdSmirTs. Blood samples were taken from the tail vein of Ad vector-injected mice at the indicated time-points and S-particle concentrations in sera were measured. The mean serum concentrations (± SD) of three mice per group and time-point are shown. conc., concentration; HBsAg (or: S), hepatits B small surface antigen; rel., relative; VP, vector particles.
HBsAg-silencing in hepatocytes can enhance early priming of CD8 T cells and improves cytokine polyfunctionality
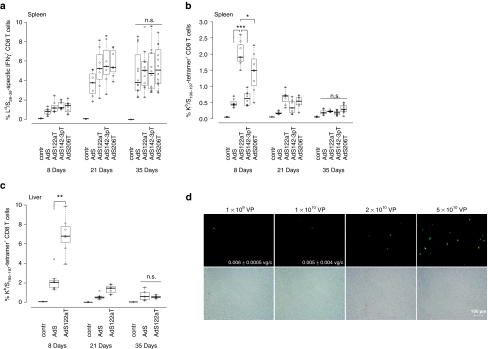
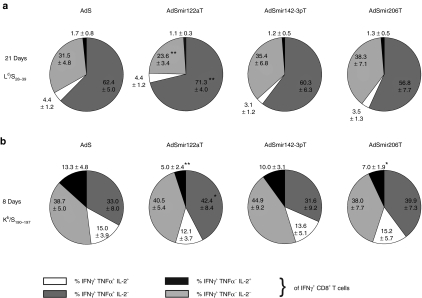
We vaccinated mice i.m. to investigate the effects of miRNA-mediated tissue-specific silencing on strength and kinetics of HBsAg-specific CD8 T cell responses. In H-2d-restricted BALB/c mice the frequencies of Ld/S28-39-specific CD8 T cells were determined by intracellular IFNγ staining and, confirming published data,26 were found to be at about 1% 8 days after vaccination, rising to 4–5% at day 21 without major contraction after 35 days (Figure 3a). Importantly, magnitudes of frequencies were independent of the miRNA target sequence incorporated.
Figure 3.
miRNA-mediated antigen silencing in hepatocytes can increase early priming of HBsAg-specific CD8 T cells. (a) Kinetics of Ld/S28–39-specific CD8 T cell frequencies. H-2d-restricted BALB/c mice were immunized intramuscularly with 108 VP of AdSmirTs. At the indicated time points (8d n = 8, 21d n = 7, 35d n = 12) frequencies of specific IFNγ+ CD8 T cells were determined after ex vivo stimulation of splenocytes with Ld/S28-39 peptide in the presence of brefeldin A. Combined data of two independent experiments at time-point day 8/35 days are shown. Boxplots show the median percentages of IFNγ+ CD8 T cells. (b) Kinetics of Kb/S190–197-specific CD8 T cell frequencies. H-2b-restricted C57BL/6 mice were immunized intramuscularly with 109 VP of AdSmirTs. At the indicated time points (8d n = 8, 21d/35d n = 7) Kb/S190–197-specific CD8 T cell numbers in spleen of vaccinated C57BL/6 mice were determined by tetramer staining. Combined data of two out of three independent experiments at time point day 8 are shown. Boxplots show the median percentage of tetramer+ CD8 T cells. (c) Kb/S190–197-specific CD8 T cell frequencies in liver. C57BL/6 mice were immunized intramuscularly with 109 vector particles of AdS or AdSmir122aT. At the indicated time points nonparenchymal liver cells were analyzed for specific CD8 T cells by tetramer staining (seven mice per vector and time point). (d) Liver transduction after intramuscular vector injection. BALB/c mice were injected intramuscularly with 109, 1010, 2 × 1010, or 5 × 1010 VP of AdEGFP in a total volume of 100 µl with 50 µl per limb. 48–72 hours postinjection liver cryosections were analyzed by fluorescence microscopy. Annotated numbers indicate vector genome copies per cell (vg/cell) determined by quantitative PCR. P values are based on two-sided Wilcoxon test. *P < 0.05; **P < 0.01; ***P < 0.001. HBsAg (or: S), hepatitis B small surface antigen; IFN, interferon; n.s., not significant; VP, vector particles.
To analyze responses independent of potential immunodominance effects of the Ld/S28–39 epitope25 we vaccinated H-2b-restricted C57BL/6 mice with the same set of vectors to determine the frequencies of tetramer+ CD8 T cells specific for the subdominant Kb-restricted epitope. In contrast to the Ld/S28-39 epitope, this epitope can only be generated by the endogenous processing pathway. Interestingly, when HBsAg expression was silenced in hepatocytes we detected fourfold higher Kb/S190–197-specific CD8 T cell frequencies 8 days after vaccination. Silencing in skeletal muscle showed similar but less pronounced effects. This early clonal expansion completely contracted to equal frequencies between the individual vectors at day 35 (Figure 3b). Subsequent intracellular IFNγ staining further corroborated this finding (data not shown). Of note, we detected the same CD8 T cell profiles and differences between AdS and AdSmir122aT locally in the liver (Figure 3c) but observed no relevant differences in Ld/S28-39-specific CD8 T cells in livers of BALB/c mice (Supplementary Figure S2a). This peak response of AdSmir122aT-induced Kb/S190–197-specific CD8 T cells differs markedly from the course of Ld/S28–39-specific CD8 T cells which is characterized by a delayed clonal expansion and a protracted contraction phase. This kinetic was observed for all vectors independent of their miRNA-regulation.
The surprising effect of hepatocyte-specific silencing on priming of Kb/S190–197-specific CD8 T cells suggested significant spreading of the vaccine vector from the i.m. injection site to the liver. Vector spreading was corroborated by fluorescence microscopic analysis of liver cryosections 48–72 hours post i.m. injection of AdEGFP (Figure 3d). Dose-dependent hepatocyte transduction was found for all vector doses tested. Detection of Ad vector genomes by quantitative PCR (qPCR) confirmed presence of vector in the liver after injection of low doses.
We further analyzed polyfunctionality and cytokine phenotype of specific CD8 T cells in spleen after ex vivo peptide restimulation. In BALB/c mice we detected an increase in the proportion of IFNγ+TNFα+IL-2− CD8 T cells with a concomitant decrease in monofunctional IFNγ-secreting cells after 21 days in AdSmir122aT-immunized mice (Figure 4a) suggesting a mature, polyfunctional effector phenotype of these T cells. The same increase was seen in AdSmir122aT-immunized C57BL/6 mice during the peak response after 8 days (Figure 4b). In addition, there were less IFNγ+TNFα−IL-2+ CD8 T cells detectable which represent a rather immature state. Interestingly, these differences were not yet present after 8 days in BALB/c mice and already disappeared after 21 days in C57BL/6 mice (Supplementary Figure S2b) suggesting the importance of this effect only during the phase of maximal clonal expansion of the CD8 T cells.
Figure 4.
Immunization with mir-122a-regulated adenovirus (Ad) vector results in a different cytokine phenotype of HBsAg-specific CD8 T cells. (a) Groups of BALB/c mice (n = 7) were vaccinated intramuscularly with 108 VP of AdSmirTs. Twenty- one days postvaccination splenocytes were restimulated ex vivo overnight with Ld/S28–39 peptide in the presence of brefeldin A with subsequent intracellular cytokine staining of CD8 T cells for IFNγ, TNFα, and IL-2. (b) Groups of C57BL/6 mice (n = 8, two independent experiments) were vaccinated intramuscularly with 109 VP of AdSmirTs. Eight days postvaccination splenocytes were stimulated ex vivo overnight with Kb/S190–197 peptide in the presence of brefeldin A with subsequent intracellular cytokine staining of CD8 T cells for IFNγ, TNFα, and IL-2. Pie charts show the mean percentages ± SD of multicytokine-secreting CD8 T cells of total HBsAg-specific CD8 T cells (see Figure 3a,b). Significance levels of data were calculated by unpaired Student's t-test. *P < 0.05; **P < 0.01. IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
Of note, tissue-specific silencing of HBsAg expression in hematopoietic cells by mir142-3p neither prevented the induction of specific CD8 T cells nor induced an increase in the number of regulatory Foxp3+/CD25+ CD4 T cells (Supplementary Figure S2c) as it has been described in the context of lentivirus vectors.27
miRNA-mediated suppression of HBsAg expression in hepatocytes induced higher antibody levels after i.m. vaccination
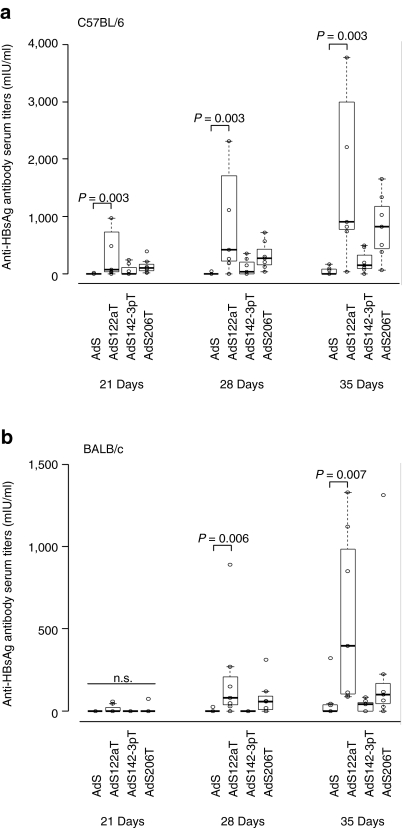
We further investigated humoral immune responses in AdHBsAgmirT-immunized mice and measured anti-HBsAg antibodies in the serum (Figure 5a). Starting from day 21 postvaccination AdSmir122aT induced higher HBsAg-specific antibody responses compared to AdS (control vector without target sequence) and AdSmir142-3pT with a significant increase of the titers at days 28 and 35. In contrast, the antibody titers after vaccination with AdS and AdSmir142-3pT stayed close to zero or increased only marginally at later time-points. Similar to the CD8 T cell responses in C57BL/6 mice we detected slightly enhanced antibody titers after vaccination also with the skeletal muscle-specific regulated vector AdSmir206T.
Figure 5.
Suppression of antigen expression in hepatocytes enhances anti-HBsAg antibody responses of adenovirus (Ad) vector-based genetic vaccines. Female C57BL/6 (a) or BALB/c (b) mice were immunized intramuscularly with 109 (a) or 108 (b) AdSmirT VP (seven mice per group). Blood samples were taken at days 21, 28, and 35 after vaccination. Serum was prepared and analyzed for anti-HBsAg antibody titers. Boxplots show the median of HBsAg-specific antibody titers. P values are based on two-sided Wilcoxon test. For day 35 one out of two (b) or three (a) independent experiments are shown. HBsAg (or: S), hepatitis B small surface antigen; VP, vector particles.
The same effect for AdSmir122aT was confirmed in vaccinated BALB/c mice (Figure 5b) excluding a mouse strain-dependent phenomenon. Of note, the induction of increased antibody titers by AdSmir206T was only observed at day 28 postvaccination in this mouse strain and titers did not increase further.
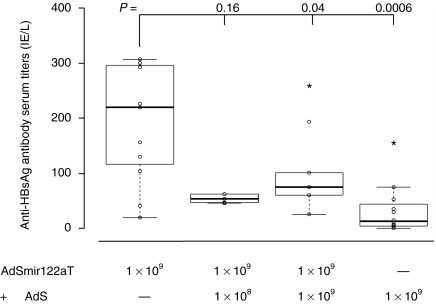
Based on our observations we speculated that antigen expression in hepatocytes as it is induced by dissemination of a nonregulated vector from the i.m. injection site (see Figure 3d) may suppress the generation of anti-HBsAg-directed antibody responses. To validate this hypothesis we performed a coinjection experiment in C57BL/6 mice. We injected 109 VP of AdSmir122aT into one limb and injected the other limb of the same mouse with 108 or 109 VP of AdS without any target sequence. Confirming our hypothesis anti-HBsAg antibody titers were significantly reduced after coinjection of both vectors AdS (109 particles) and AdSmir122aT (109 particles) when compared to those in mice that only received AdSmir122aT (Figure 6). We observed a similar picture after coinjection of only 108 AdS vector particles although differences in antibody titers were not statistically significant for this low dose. These data further suggest that the suppressive effects of nonregulated AdS on antibody responses act locally separated from the i.m. injection site and appear to be caused by systemic vector spreading.
Figure 6.
Coinjection of ubiquitously expressing adenovirus (Ad) vector partly counteracts the effects of hepatocyte-silenced Ad vector on antibody titers. Groups of C57BL/6 mice were vaccinated intramuscularly with 109 VP of AdSmir122aT in the right limb and coinjected with 108 VP (n = 5) or 109 VP (n = 5) of AdS in the left limb. Control groups received a single injection of 109 VP of AdSmir122aT (n = 11) or AdS (n = 12). Five weeks postvaccination serum was analyzed for anti-HBsAg antibody titers by enzyme linked immunosorbent assay. Boxplots show the median with 25% and 75% percentiles. P values are based on one-sided Wilcoxon test. HBsAg (or: S), hepatitis B small surface antigen; VP, vector particles.
Taken together our data indicate (i) that dose-dependent Ad vector dissemination from the injection site into liver and subsequent antigen expression in hepatocytes can counteract the generation of high level anti-HBsAg antibody immune responses, and (ii) that miRNA-mediated hepatocyte-specific silencing of transgene expression can overcome this effect.
Hepatocyte-specific silencing of transgenic antigen expression allowed for up to 100-fold vector dose reduction
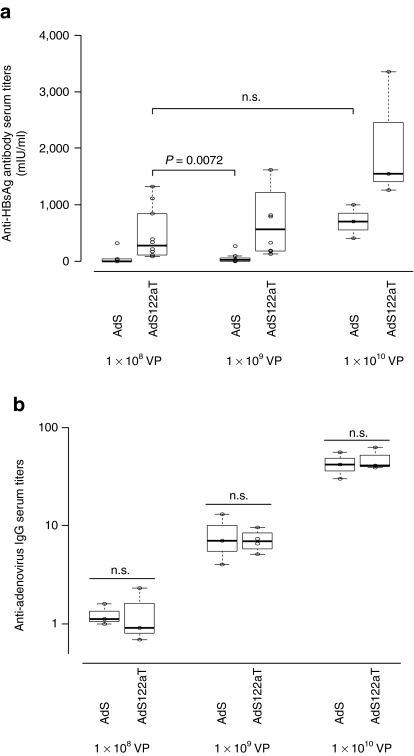
Transgene product-specific antibody responses exhibit a clear dose–response correlation.28 In order to assess whether the effects of silencing in hepatocytes allow for vector dose reduction we performed dose escalation experiments in BALB/c mice with 108, 109, and 1010 VP of AdS (control vector without target sequence) and AdSmir122aT and determined anti-HBsAg antibody levels 35 days postvaccination (Figure 7a). Injection of 108 VP of AdSmir122aT induced higher antibody titers than injection of 109 VP of AdS (control without target sequences). Furthermore, the antibody titers obtained by injection of 108 VP of AdSmir122aT were statistically not different from those obtained after injection of 1010 VP of AdS. As a control and to verify that the mice received equal amounts of vector particles for each group we measured the anti-adenovirus antibody levels by enzyme-linked immunosorbent assay (ELISA) (Figure 7b). Anti-adenovirus antibody titers were equal in all groups, showed a clear dose–response correlation and thus confirmed delivery of equal vector particle numbers. These observations revealed that miRNA-mediated, hepatocyte-specific silencing of transgene expression allows for an up to 100-fold reduction of the vector dose when transgene product-directed humoral immune responses are of interest.
Figure 7.
mir-122a-regulated adenovirus (Ad) vector-induced humoral immune responses at low doses are superior to those of nonregulated Ad vector at higher doses. (a) anti-HBsAg antibody responses to increasing doses of Ad vector. BALB/c mice were immunized intramuscularly with 108 (n = 10), 109 (n = 7–8), or 1010 (n = 3) VP of AdS or AdSmir122aT, respectively. Thirty- five days postinjection mice were killed and sera were analyzed for anti-HBsAg antibody titers. Combined data of two independent experiments are shown for vector doses of 108 and 109. Boxplots show the median with 25% and 75% percentiles. (b) A subset of each group (n = 3) of the sera in a was analyzed for anti-adenovirus IgG by enzyme- linked immunosorbent assay. Boxplots show the median antibody titers calculated by the reciprocal dilution where a fixed threshold of absorbance is reached. P values are based on two-sided Wilcoxon test. HBsAg (or: S), hepatitis B small surface antigen; n.s., not significant; VP, vector particles.
Discussion
In this study we exploited a miRNA-mediated silencing strategy to investigate the role of different antigen-expressing tissues for the generation of HBsAg-directed humoral and cellular immune responses in the context of adenovirus gene transfer vectors. Our data suggest that antigen expression in hepatocytes after Ad vector spreading from an i.m. injection site can hinder effective early priming of antigen-specific CD8 T cells and antagonizes the generation of humoral immune responses to the antigen. Importantly, we demonstrated that miRNA-mediated hepatocyte-specific silencing of transgene expression could significantly improve the generation of antigen-specific antibody responses in two mouse strains. In addition, hepatocyte-specific silencing enhanced CD8 T cell priming with concomitant shifting of the cytokine profile to a matured, polyfunctional effector phenotype. Furthermore, our data revealed that miRNA-mediated silencing of transgene expression in cells of the hematopoietic origin did not significantly alter the frequencies of transgene product-specific CD8 T cells.
Previous studies with lentivirus vectors demonstrated that silencing of factor IX expression specifically in hematopoietic professional antigen-presenting cells by the mir142-3p target sequence effectively prevented immune-mediated transgene rejection and stably corrected hemophilia B mice.29 However, Ad vectors appear to induce immunity against transgene products to a significant degree by cross priming30,31 what may serve as an explanation for the ineffectiveness of mir-142-3p-mediated silencing to suppress adaptive immune responses. Dendritic cells are certainly key players for CD8 T cell responses after Ad vector immunization11,32 and various approaches employing transcriptional9 or transductional Ad vector targeting33,34,35 to dendritic cells can improve adaptive immune responses to the transgene product. However, none of these studies clearly show the absolute necessity of endogenous antigen production in pAPCs to generate an adaptive immune response. Our data rather suggest that endogenous antigen synthesis in pAPCs is not per se required. Instead, other cell types might be able to present antigen and prime CD8 T cells. It has been reported for Ad vectors that endothelial cells, myoblasts and even muscle stem cells can act as APCs.10,36 Regarding mir-142-3p-based silencing strategies studies based on plasmid-mediated gene transfer37 and our data show that the immunological outcome is highly dependent on the gene transfer vector system and other variables. Incorporation of mir-142-3p does therefore not provide an all-purpose-tool in the development of gene transfer vector systems to prevent immune responses e.g., when long term gene transfer is desired.
The hepatic environment as a site of antigen presentation has been associated with immunological tolerance in many reports38 and active targeting of transgene expression to hepatocytes has been used to induce immunological tolerance in the context of adeno-associated virus- and lentivirus vector-mediated gene transfer.39,40,41 Annoni et al. further showed that miRNA-mediated suppression of antigen expression in hematopoietic cells promotes tolerance to the transgene only with concomitant antigen expression in hepatocytes.27 Gene transfer approaches using Ad vector-based systems with reduced immunogenicity (E1/E3-deleted and helper-dependent vectors) revealed that active tolerance to human factor IX and human α1 antitrypsin can be achieved after i.v. delivery to immunocompetent mice suggesting a predominant role for hepatocyte transduction in this process.42,43
In line with this, our data indicate that also in a vaccination context hepatocyte transduction after i.m. delivery limits the generation of an adaptive immune response. It has been shown that i.v. immunization with Ad vectors results in a functional exhausted phenotype that directly correlated with persistent antigen expression in liver.44 This supports our finding that HBsAg-silencing in hepatocytes improves the polyfunctionality of CD8 T cells and favors a mature effector phenotype. In the same study peripherally expressed antigen was associated with specific deletion of CTLs. This fits to our observations that also muscle-specific antigen silencing conferred some beneficial effects for CD8 T cell priming and antibody responses. A detailed analysis of vaccination route and dose by Holst et al. further demonstrated that the reduced quality of Ad vector-induced CD8 T cells is directly connected to excessive Ad vector dissemination from a peripheral site to the liver or systemic vector administration.45 We show here that already minimal vector dissemination from muscle to the liver negatively impacts on both cellular and humoral immune responses and this can be overcome by miRNA-regulation. In fact, the peak-contraction kinetic of Kb/S190–197-specific CD8 T cells induced by AdSmir122aT resembles that of Holst et al. after footpad-vaccination which resulted in enhanced quality and functionality of these CD8 T cells. We have observed enhanced early CD8 T cell expansion with AdSmir122aT for an epitope that can exclusively be generated by the endogenous processing pathway, but not for an epitope from the same antigen that can be generated by both the endogenous and exogenous processing pathway.25 Due to the need for the endogenous processing pathway this finding indicates that direct MHC class I presentation of this epitope by hepatocytes is responsible for repression of CD8 T cell expansion. However, the underlying mechanisms remain unclear. Hepatocyte-specific silencing might not have an impact on CD8 T cell expansion in case of an epitope predominantly processed by the exogenous pathway after uptake of secreted antigen. Blocking this pathway or using an intracellular version of HBsAg could answer this question. However, improved polyfunctionality was also observed in the CD8 T cell population specific for this epitope.
The absolute vector dose applied for an effective vaccination with Ad vectors has to be considered as an especially critical parameter as it is directly associated with the extent of vector dissemination and hepatocyte transduction. Data from our experiments demonstrate the effectiveness of mir-122a to control hepatic antigen expression over a dose range from 108 to 1010 VP resulting in higher antibody levels compared to a nonregulated vector. However, a coinjection of 108 VP of this nonregulated vector already started to partially counteract this effect. As vector toxicity is a major concern of Ad vector-based vaccines maximal effectiveness at low doses is a desirable feature of future developments. Our observation that vector dose of AdSmir122aT could be lowered 100-fold without decreasing antibody titers might therefore be promising.
The beneficial effects for the induction of cellular and humoral immune responses by mir-122a-mediated antigen suppression in this study have been shown for a defined model antigen with clinical relevance in hepatitis B virus vaccination. However, it has to be evaluated if these effects are HBsAg-specific or also hold true for other secreted or intracellular antigens. Taken together, the results presented here provide evidence that minimizing antigen expression in hepatocytes through miRNA-regulation might be a useful strategy to enhance immune responses to Ad vector-delivered antigens. Combining other approaches to reduce liver transduction might further include physical detargeting of Ad vectors from liver46 or the use of serotypes with decreased liver tropism and will contribute to a more rational design for effective Ad vector-based genetic vaccines.
Materials and Methods
Plasmid construction. Coding sequences of EGFP (from pEGFP-N1; Clontech, Heidelberg, Germany) or HBsAg (from pCI/S small47) were cloned into a cytomegalovirus (CMV) promoter-driven expression cassette derived from pCMVβ (Clontech) that was subcloned into pGS70 plasmid backbone.48 To insert miRNA target sequences into the 3′UTR of EGFP or HBsAg plasmid constructs were digested by NotI and ligated with annealed oligonucleotides. The following oligonucleotides were used.
mir122aT: 5′-GGCCGCAGTCGACCAAACACCATTGTCACACT CCATTCGAAACAAACACCATTGTCACACTCCAACGCGTACA AACACCATTGTCACACTCCAATGCATACAAACACCATTGT CACACTCCACGCGCGCAC-3′, 5′-GGCCGTGCGCGCGTGGAGTG TGACAATGGTGTTTGTATGCATTGGAGTGTGACAATG GTGTTTGTACGCGTTGGAGTGTGACAATGGTGTTTGTTT CGAATGGAGTGTGACAATGGTGTTTGGTCGACTGC-3′, mir142-3pT: 5′-GGCCGCAGTCGACTCCATAAAGTAGGAAACACTACATCA CTTCCATAAAGTAGGAAACACTACAACCGGTTCCATAAA GTAGGAAACACTACACGATTCCATAAAGTAGGAAACA CTACACGCGCGCAC-3′, 5′-GGCCGTGCGCGCGTGTAGTGTTTCC TACTTTATGGAATCGTGTAGTGTTTCCTACTTTATGGAA CCGGTTGTAGTGTTTCCTACTTTATGGAAGTGATGTAGTGTTT CCTACTTTATGGAGTCGACTGC-3′, mir206T: 5′-GGCCGCAGTCG ACCCACACACTTCCTTACATTCCATCACTCCACACACT TCCTTACATTCCAACCGGTCCACACACTTCCTTACATTC CACGATCCACACACTTCCTTACATTCCACGCGCGCAC-3′, 5′GGCCGTGCGCGCGTGGAATGTAAGGAAGTGTGTGGA TCGTGGAATGTAAGGAAGTGTGTGGACCGGTTGGAA TGTAAGGAAGTGTGTGGAGTGATGGAATGTAAGGAAG TGTGTGGGTCGACTGC-3′. The four tandem repeats of perfectly complementary miRNA target sequences in the sense oligonucleotides are underlined. CMV-EGFPmirTs and CMV-HBsAgmirTs expression cassettes were excised by PacI and cloned in place of the E1-deleted region of infectious plasmid pGS66.48
Ad vectors. Ad5-based E1-deleted first generation vectors were generated by transfection of SwaI-linearized infectious plasmids into E1-transcomplementing cell line N52.E6 followed by subsequent vector amplification. Vector purification was performed by CsCl density gradient and vector titers were determined by a DNA-based slot-blot procedure as described previously.49
Cell lines. N52.E6 cell line48 was cultured in α-minimal essential medium (Gibco, Eggenstein, Germany) with passaging twice weekly. Hepatocarcinoma cell line Huh7.5 was obtained from Apath, Brooklyn, NY and maintained in Dulbecco's modified Eagle's medium (Gibco) and subcultured twice weekly. A549 cell line (ATCC #CCL-185; ATCC, Manassas, VA) was kept in minimal essential medium (Gibco) and passaged twice weekly. Myelogenous leukemia cell line K562 (ATCC #CCL 243; ATCC) was cultured in RPMI-1640 (Gibco) and subcultured twice weekly. C2C12 myoblasts (ATCC #CRL 1772; ATCC) were cultured in Dulbecco's modified Eagle's medium and subcultured three times a week. All media were supplemented with 10% fetal calf serum (FCS) and 1% penicillin/streptomycin/glutamine. For differentiation of C2C12 myoblasts into myotubes 8 × 104 cells were seeded on a 24 well plate and kept in skeletal muscle differentiation medium (PromoCell, Heidelberg, Germany) supplemented with 3% FCS and Supplement-Mix (C-39366; PromoCell) for 48 hours. Medium was then replaced by growth medium (Dulbecco's modified Eagle's medium, 10% FCS, 1% P/S/G) and cells were cultured to complete differentiation without passaging for 30 days.
Mice. Female C57BL/6 and BALB/c mice were obtained from Charles River (Sulzfeld, Germany) and kept in standard pathogen-free environment in the animal facility of the University of Ulm. For all immunization experiments mice were used at an age of 6–8 weeks. All animal experiments were approved by the Animal Care Commission of the Government of Baden-Württemberg and were in accordance with institutional guidelines.
Vector injection and immunization. For transgene expression analysis indicated amounts of Ad vector particles (2 × 1010–8 × 1010) were diluted in a total volume of 200 µl phosphate-buffered saline (PBS) and injected into the tail vein. For i.m. injection mice were anaesthetized by intraperitoneal injection with ketamine/xylazine and 5 × 1010 vector particles in a total volume of 100 µl were injected into the gastrocnemius muscle at two sites on each limb. Immunizations were performed by injection of either 1 × 108 or 1 × 109 vector particles in 50 µl of PBS (25 µl per gastrocnemius muscle) with an Omnican 20 syringe (Braun, Melsungen, Germany).
Autopsy and cell preparation. Mice were killed by inhalation of isoflurane (Forene; Abbott, Ludwigshafen, Germany). Blood samples were taken by puncture of the heart. For isolation of blood cells 10 µl of heparin was immediately added and erythrocytes were lysed with a 0.8% NH4Cl 0.1% KHCO3-containing buffer. For isolation of hepatocytes and nonparenchymal liver cells livers were perfused through the portal vein with 10 ml of liver perfusion medium (Gibco) and subsequently perfused with 5 ml of liver digestion medium (Gibco). Livers were excised, cut into small pieces, and digested for 30 minutes at 37 °C. Crude homogenates were passaged through a nylon mesh and the cell suspension was washed with PBS. Hepatocytes were separated by low-speed centrifugation at 50g with subsequent washing in PBS and subjected to further analysis. The supernatant containing the nonparenchymal liver cells fraction was subjected to Percoll (EasyColl; Biochrom, Berlin, Germany) density gradient centrifugation (70% Percoll in 1% FCS/PBS and 45% Percoll in 1% FCS/PBS) at 2,000 rpm for 20 minutes. Cells were collected at the interface, washed in PBS, and erythrocytes were lysed as described above. Splenocytes were isolated as previously described.46 For subsequent culturing cells were resuspended in BioWhittaker-UltraCulture medium (Cambrex Bioscience, Verviers, Belgium) supplemented with 1% penicillin/streptomycin/glutamine.
Histology and fluorescence microscopy. Forty-eight hours post vector injection gastrocnemius muscle or perfused liver were excised and fixed in 2% paraformaldehyde in PBS at 4 °C over night. Organs were incubated in 30% sucrose and embedded in Tissue-Tek (Sakura, Zoeterwoude, NL) for preparing cryosections of 8 µm. Cryosections were mounted with fluorescence mounting medium (Dako, Copenhagen, Denmark) and analyzed for GFP fluorescence on a Zeiss Axioskop2 plus fluorescence microscope.
In vitro transduced cell lines were overlayed with PBS and directly analyzed on a Leica DMIL fluorescence microscope without fixing.
SDS-PAGE and western blot. Isolated hepatocytes of Ad vector-injected mice were lysed with 1% TritonX-100, insoluble material was cleared by centrifugation and protein concentrations of the supernatants were determined by BCA protein assay reagent (Pierce, Bonn, Germany). 10 µg of protein per lane was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred on a nitrocellulose membrane by western blot for detection of EGFP protein with a monoclonal anti-GFP antibody (Invitrogen, Darmstadt, Germany) and ECL detection kit (Pierce, Bonn, Germany).
Quantification of HBsAg-particles. HBsAg-particle serum concentrations were measured by a microparticle enzyme immunoassay (HBsAg (V2); Abbott Diagnostics, Wiesbaden, Germany) on an IMx system according to manufacturer's instruction. Relative S-particle serum concentrations are based on the S/N ratio = (value of sample/MODE 1 calibrator value). MODE 1 calibrator is nonreactive for HBsAg. Samples with a S/N value ≥2 are considered as reactive. Each reagent pack of a different batch is calibrated by a negative and a positive control containing 4–15 ng/ml of HBsAg.
Quantification of Ad vector genomes by qPCR. Total DNA was isolated from liver tissue and analyzed for Ad vector genomes by qPCR with Brilliant II SYBR Green QRT-PCR Master Mix Kit from Stratagene as described earlier.50 Details can be obtained upon request.
Determination of antibody titers. Serum was obtained by centrifugation of blood samples at 14,000 rpm in a microcentrifuge. HBsAg-specific antibody titers were determined by microparticle enzyme immunoassay (AxSYM AUSAB; Abbott Diagnostics) on an IMx system or alternatively by a noncompetitive sandwich ELISA Kit (ETI-AB-AUK-3; Diasorin, Dietzenbach, Germany) according to manufacturer's instructions. Anti-adenovirus-specific IgG titers were quantified by ELISA. In brief, 96-well plates (NUNC-Maxisorp, #267245; NUNC, Rochester, NY) were coated with heat-inactivated (10 minutes, 60 °C) adenovirus vector particles (6 × 108 VP/well) at 4 °C overnight. Plates were blocked with PBS/3% bovine serum albumin for 1 hour, washed five times with PBS/0.05% Tween20 and incubated with serial dilutions of serum for 2 hours at room temperature. Plates were washed and incubated with horseradish peroxidase-labeled anti-mouse IgG (BD Biosciences, Heidelberg, Germany) for 1 hour at 37 °C and developed with o-phenyldiamine dihydrochloride (Sigma, Taufkirchen, Germany) /H2O2 substrate and stopped with 1 mol/l sulfuric acid. Absorbance was measured at 492 nm/620 nm on an ELISA reader.
Specific CD8+ T cell frequencies. IFNγ+/CD8+ T cells were determined on a FACSCalibur as previously described.46 In brief, spleen cells or nonparenchymal liver cells were stimulated overnight with 2.5 µg/ml of antigenic peptide or control peptide in the presence of 5 µg/ml of brefeldin A. After stimulation Fc-receptors were blocked by anti-CD16/32 mAb (clone 2.4G2) and cells were surface-stained with phycoerythrin-conjugated anti-CD8 mAb (BD Pharmingen, Heidelberg, Germany) or biotin-labeled anti-CD8 mAb (BD Pharmingen) and peridinin-chlorophyll protein-conjugated streptavidin (BD Pharmingen). Cells were fixed with 2% paraformaldehyde and permeabilized with 0.5% saponin containing buffer. Permeabilized cells were stained for intracellular cytokines for 30 minutes at room temperature with fluorescein isothiocyanate-conjugated anti-IFNγ mAb (BD Pharmingen) and when indicated with phycoerythrin-conjugated anti-IL-2 mAb (BD Pharmingen) and allophycocyanin-conjugated anti-TNFα mAb (BD Pharmingen). For direct detection of specific T cell receptors cells were stained with allophycocyanin-conjugated anti-CD8 mAb (eBiosciences, Frankfurt, Germany) and phycoerythrin-labeled specific Kb/S190–197 MHC/peptide tetramers (#T04001; Beckman-Coulter, Marseille, France) for 30 minutes at 4 °C. Cells were washed and analyzed by flow cytometry.
Regulatory T cells. The total pool of regulatory T cells was determined by surface staining with allophycocyanin-conjugated anti-CD4 mAb (eBiosciences) and phycoerythrin-conjugated anti-CD25 mAb (eBiosciences). Cells were fixed, permeabilized, and stained for Foxp3 by using the Foxp3 Staining Buffer Set (eBiosciences) and fluorescein isothiocyanate-conjugated anti-Foxp3 mAb (eBiosciences) according to the manufacturer's one-step protocol.
Synthetic peptides. Peptides were purchased from JPT Peptide Technologies (Berlin, Germany). Ld-restricted HBsAg-specific peptide corresponds to residues 28–39 (Ld/S, IPQSLDSWWTSL). Kb-restricted HBsAg-specific peptide is corresponding to antigen residues 190–197 (Kb/S, VWLSVIWM). As controls Kd-restricted CSP-specific peptide from plasmodium berghei (Kd/CSP252-260, SYIPSAEKI) or Kb-restricted ovalbumin-specific peptide (OVA257-264, SIINFEKL) were used. Peptide purity was greater than 70%. Peptide stocks were prepared at 10 mg/ml in DMSO and stored at −20 °C. Immediately prior to use peptides were diluted with culture medium.
Statistics. The significance of data was determined by unpaired Student's t-test. For CD8 T cell frequencies and antibody titers boxplots show the median (horizontal thick band) with 25% and 75% percentiles (bottom and top of box). P values are based on two-sided Wilcoxon test calculated with R software.
SUPPLEMENTARY MATERIAL Figure S1. Transgene silencing of miRNA-regulated Ad vectors in skeletal muscle (mir-206) and primary human macrophages (mir-142-3p). Figure S2. Ld/S28-39-specific CD8 T cells in liver, multifunctionality of HBsAg-specific CD8 T cells and total regulatory CD4 T cells.
Acknowledgments
We thank Jörg Reimann for his support and helpful discussions. This work was supported by the EU-GIANT network (FP6) and the German Federal Ministry for Education and Research (BMBF, GO-Bio 0315562).
Supplementary Material
Transgene silencing of miRNA-regulated Ad vectors in skeletal muscle (mir-206) and primary human macrophages (mir-142-3p).
Ld/S28-39-specific CD8 T cells in liver, multifunctionality of HBsAg-specific CD8 T cells and total regulatory CD4 T cells.
REFERENCES
- Lasaro MO., and, Ertl HC. New insights on adenovirus as vaccine vectors. Mol Ther. 2009;17:1333–1339. doi: 10.1038/mt.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G, Buchbinder S., and, Duerr A. Overview of STEP and Phambili trial results: two phase IIb test-of-concept studies investigating the efficacy of MRK adenovirus type 5 gag/pol/nef subtype B HIV vaccine. Curr Opin HIV AIDS. 2010;5:357–361. doi: 10.1097/COH.0b013e32833d2d2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekaly RP. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development. J Exp Med. 2008;205:7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS.et al. (1997Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5 Science 2751320–1323. [DOI] [PubMed] [Google Scholar]
- Wickham TJ, Mathias P, Cheresh DA., and, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H.et al. (2008Adenovirus serotype 5 hexon mediates liver gene transfer Cell 132397–409. [DOI] [PubMed] [Google Scholar]
- Yang HS, Lee H, Kim SJ, Lee WW, Yang YJ, Moon DH.et al. (2004Imaging of human sodium-iodide symporter gene expression mediated by recombinant adenovirus in skeletal muscle of living rats Eur J Nucl Med Mol Imaging 311304–1311. [DOI] [PubMed] [Google Scholar]
- Vajanto I, Rissanen TT, Rutanen J, Hiltunen MO, Tuomisto TT, Arve K.et al. (2002Evaluation of angiogenesis and side effects in ischemic rabbit hindlimbs after intramuscular injection of adenoviral vectors encoding VEGF and LacZ J Gene Med 4371–380. [DOI] [PubMed] [Google Scholar]
- De Geest BR, Van Linthout SA., and, Collen D. Humoral immune response in mice against a circulating antigen induced by adenoviral transfer is strictly dependent on expression in antigen-presenting cells. Blood. 2003;101:2551–2556. doi: 10.1182/blood-2002-07-2146. [DOI] [PubMed] [Google Scholar]
- Mercier S, Gahéry-Segard H, Monteil M, Lengagne R, Guillet JG, Eloit M.et al. (2002Distinct roles of adenovirus vector-transduced dendritic cells, myoblasts, and endothelial cells in mediating an immune response against a transgene product J Virol 762899–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay RW, Darrah PA, Quinn KM, Wille-Reece U, Mattei LM, Iwasaki A.et al. (2010CD8+ T cell responses following replication-defective adenovirus serotype 5 immunization are dependent on CD11c+ dendritic cells but show redundancy in their requirement of TLR and nucleotide-binding oligomerization domain-like receptor signaling J Immunol 1851513–1521. [DOI] [PubMed] [Google Scholar]
- Bassett JD, Yang TC, Bernard D, Millar JB, Swift SL, McGray AJ.et al. CD8+ T cell expansion and maintenance following recombinant adenovirus immunization rely upon co-operation between hematopoietic and non-hematopoietic antigen-presenting cells Blood 117(4)1146–1155. [DOI] [PubMed] [Google Scholar]
- Steitz J, Wagner RA, Bristol T, Gao W, Donis RO., and, Gambotto A. Assessment of route of administration and dose escalation for an adenovirus-based influenza A Virus (H5N1) vaccine in chickens. Clin Vaccine Immunol. 2010;17:1467–1472. doi: 10.1128/CVI.00180-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe S, Fooks A, Lee J, Hayes K, Clegg C., and, Cranage M. Single oral immunization with replication deficient recombinant adenovirus elicits long-lived transgene-specific cellular and humoral immune responses. Virology. 2002;293:210–216. doi: 10.1006/viro.2001.1281. [DOI] [PubMed] [Google Scholar]
- Croyle MA, Patel A, Tran KN, Gray M, Zhang Y, Strong JE.et al. (2008Nasal delivery of an adenovirus-based vaccine bypasses pre-existing immunity to the vaccine carrier and improves the immune response in mice PLoS ONE 3e3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BD, Gentner B, Cantore A, Colleoni S, Amendola M, Zingale A.et al. (2007Endogenous microRNA can be broadly exploited to regulate transgene expression according to tissue, lineage and differentiation state Nat Biotechnol 251457–1467. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Sakurai F, Nakamura S, Kouyama E, Kawabata K, Kondoh M.et al. (2008miR-122a-regulated expression of a suicide gene prevents hepatotoxicity without altering antitumor effects in suicide gene therapy Mol Ther 161719–1726. [DOI] [PubMed] [Google Scholar]
- Cawood R, Chen HH, Carroll F, Bazan-Peregrino M, van Rooijen N., and, Seymour LW. Use of tissue-specific microRNA to control pathology of wild-type adenovirus without attenuation of its ability to kill cancer cells. PLoS Pathog. 2009;5:e1000440. doi: 10.1371/journal.ppat.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylösmäki E, Hakkarainen T, Hemminki A, Visakorpi T, Andino R., and, Saksela K. Generation of a conditionally replicating adenovirus based on targeted destruction of E1A mRNA by a cell type-specific MicroRNA. J Virol. 2008;82:11009–11015. doi: 10.1128/JVI.01608-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BD, Venneri MA, Zingale A, Sergi Sergi L., and, Naldini L. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat Med. 2006;12:585–591. doi: 10.1038/nm1398. [DOI] [PubMed] [Google Scholar]
- Chang J, Nicolas E, Marks D, Sander C, Lerro A, Buendia MA.et al. (2004miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1 RNA Biol 1106–113. [DOI] [PubMed] [Google Scholar]
- Chen CZ, Li L, Lodish HF., and, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- McCarthy JJ. MicroRNA-206: the skeletal muscle-specific myomiR. Biochim Biophys Acta. 2008;1779:682–691. doi: 10.1016/j.bbagrm.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen RG, Leclerc C, Dériaud E, Schirmbeck R, Reimann J., and, Davis HL. DNA-mediated immunization to the hepatitis B surface antigen. Activation and entrainment of the immune response. Ann N Y Acad Sci. 1995;772:64–76. doi: 10.1111/j.1749-6632.1995.tb44732.x. [DOI] [PubMed] [Google Scholar]
- Schirmbeck R, Stober D, El-Kholy S, Riedl P., and, Reimann J. The immunodominant, Ld-restricted T cell response to hepatitis B surface antigen (HBsAg) efficiently suppresses T cell priming to multiple Dd-, Kd-, and Kb-restricted HBsAg epitopes. J Immunol. 2002;168:6253–6262. doi: 10.4049/jimmunol.168.12.6253. [DOI] [PubMed] [Google Scholar]
- Schirmbeck R, Reimann J, Kochanek S., and, Kreppel F. The immunogenicity of adenovirus vectors limits the multispecificity of CD8 T-cell responses to vector-encoded transgenic antigens. Mol Ther. 2008;16:1609–1616. doi: 10.1038/mt.2008.141. [DOI] [PubMed] [Google Scholar]
- Annoni A, Brown BD, Cantore A, Sergi LS, Naldini L., and, Roncarolo MG. In vivo delivery of a microRNA-regulated transgene induces antigen-specific regulatory T cells and promotes immunologic tolerance. Blood. 2009;114:5152–5161. doi: 10.1182/blood-2009-04-214569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophorst OJ, Radosevic K, Havenga MJ, Pau MG, Holterman L, Berkhout B.et al. (2006Immunogenicity and protection of a recombinant human adenovirus serotype 35-based malaria vaccine against Plasmodium yoelii in mice Infect Immun 74313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BD, Cantore A, Annoni A, Sergi LS, Lombardo A, Della Valle P.et al. (2007A microRNA-regulated lentiviral vector mediates stable correction of hemophilia B mice Blood 1104144–4152. [DOI] [PubMed] [Google Scholar]
- Prasad SA, Norbury CC, Chen W, Bennink JR., and, Yewdell JW. Cutting edge: recombinant adenoviruses induce CD8 T cell responses to an inserted protein whose expression is limited to nonimmune cells. J Immunol. 2001;166:4809–4812. doi: 10.4049/jimmunol.166.8.4809. [DOI] [PubMed] [Google Scholar]
- Sarukhan A, Camugli S, Gjata B, von Boehmer H, Danos O., and, Jooss K. Successful interference with cellular immune responses to immunogenic proteins encoded by recombinant viral vectors. J Virol. 2001;75:269–277. doi: 10.1128/JVI.75.1.269-277.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jooss K, Yang Y, Fisher KJ., and, Wilson JM. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J Virol. 1998;72:4212–4223. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandão JG, Scheper RJ, Lougheed SM, Curiel DT, Tillman BW, Gerritsen WR.et al. (2003CD40-targeted adenoviral gene transfer to dendritic cells through the use of a novel bispecific single-chain Fv antibody enhances cytotoxic T cell activation Vaccine 212268–2272. [DOI] [PubMed] [Google Scholar]
- Rea D, Havenga MJ, van Den Assem M, Sutmuller RP, Lemckert A, Hoeben RC.et al. (2001Highly efficient transduction of human monocyte-derived dendritic cells with subgroup B fiber-modified adenovirus vectors enhances transgene-encoded antigen presentation to cytotoxic T cells J Immunol 1665236–5244. [DOI] [PubMed] [Google Scholar]
- Worgall S, Busch A, Rivara M, Bonnyay D, Leopold PL, Merritt R.et al. (2004Modification to the capsid of the adenovirus vector that enhances dendritic cell infection and transgene-specific cellular immune responses J Virol 782572–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Bruder J, Kovesdi I., and, Huard J. Muscle stem cells can act as antigen-presenting cells: implication for gene therapy. Gene Ther. 2004;11:1321–1330. doi: 10.1038/sj.gt.3302293. [DOI] [PubMed] [Google Scholar]
- Wolff LJ, Wolff JA., and, Sebestyén MG. Effect of tissue-specific promoters and microRNA recognition elements on stability of transgene expression after hydrodynamic naked plasmid DNA delivery. Hum Gene Ther. 2009;20:374–388. doi: 10.1089/hum.2008.088. [DOI] [PubMed] [Google Scholar]
- Knolle PA., and, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21–34. doi: 10.1034/j.1600-0528.2002.017408.x. [DOI] [PubMed] [Google Scholar]
- Hoffman BE, Dobrzynski E, Wang L, Hirao L, Mingozzi F, Cao O.et al. (2007Muscle as a target for supplementary factor IX gene transfer Hum Gene Ther 18603–613. [DOI] [PubMed] [Google Scholar]
- Cao O, Dobrzynski E, Wang L, Nayak S, Mingle B, Terhorst C.et al. (2007Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer Blood 1101132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follenzi A, Battaglia M, Lombardo A, Annoni A, Roncarolo MG., and, Naldini L. Targeting lentiviral vector expression to hepatocytes limits transgene-specific immune response and establishes long-term expression of human antihemophilic factor IX in mice. Blood. 2004;103:3700–3709. doi: 10.1182/blood-2003-09-3217. [DOI] [PubMed] [Google Scholar]
- Fields PA, Armstrong E, Hagstrom JN, Arruda VR, Murphy ML, Farrell JP.et al. (2001Intravenous administration of an E1/E3-deleted adenoviral vector induces tolerance to factor IX in C57BL/6 mice Gene Ther 8354–361. [DOI] [PubMed] [Google Scholar]
- Pastore L, Morral N, Zhou H, Garcia R, Parks RJ, Kochanek S.et al. (1999Use of a liver-specific promoter reduces immune response to the transgene in adenoviral vectors Hum Gene Ther 101773–1781. [DOI] [PubMed] [Google Scholar]
- Krebs P, Scandella E, Odermatt B., and, Ludewig B. Rapid functional exhaustion and deletion of CTL following immunization with recombinant adenovirus. J Immunol. 2005;174:4559–4566. doi: 10.4049/jimmunol.174.8.4559. [DOI] [PubMed] [Google Scholar]
- Holst PJ, Ørskov C, Thomsen AR., and, Christensen JP. Quality of the transgene-specific CD8+ T cell response induced by adenoviral vector immunization is critically influenced by virus dose and route of vaccination. J Immunol. 2010;184:4431–4439. doi: 10.4049/jimmunol.0900537. [DOI] [PubMed] [Google Scholar]
- Wortmann A, Vöhringer S, Engler T, Corjon S, Schirmbeck R, Reimann J.et al. (2008Fully detargeted polyethylene glycol-coated adenovirus vectors are potent genetic vaccines and escape from pre-existing anti-adenovirus antibodies Mol Ther 16154–162. [DOI] [PubMed] [Google Scholar]
- Schirmbeck R, Wild J, Stober D, Blum HE, Chisari FV, Geissler M.et al. (2000Ongoing murine T1 or T2 immune responses to the hepatitis B surface antigen are excluded from the liver that expresses transgene-encoded hepatitis B surface antigen J Immunol 1644235–4243. [DOI] [PubMed] [Google Scholar]
- Schiedner G, Hertel S., and, Kochanek S. Efficient transformation of primary human amniocytes by E1 functions of Ad5: generation of new cell lines for adenoviral vector production. Hum Gene Ther. 2000;11:2105–2116. doi: 10.1089/104303400750001417. [DOI] [PubMed] [Google Scholar]
- Kreppel F, Biermann V, Kochanek S., and, Schiedner G. A DNA-based method to assay total and infectious particle contents and helper virus contamination in high-capacity adenoviral vector preparations. Hum Gene Ther. 2002;13:1151–1156. doi: 10.1089/104303402320138934. [DOI] [PubMed] [Google Scholar]
- Prill JM, Espenlaub S, Samen U, Engler T, Schmidt E, Vetrini F.et al. (2011Modifications of adenovirus hexon allow for either hepatocyte detargeting or targeting with potential evasion from Kupffer cells Mol Ther 1983–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transgene silencing of miRNA-regulated Ad vectors in skeletal muscle (mir-206) and primary human macrophages (mir-142-3p).
Ld/S28-39-specific CD8 T cells in liver, multifunctionality of HBsAg-specific CD8 T cells and total regulatory CD4 T cells.