Abstract
Cancer incidence and mortality rates show great variations across nations and between population groups. These variations are largely explained by differences in age distribution, diet and lifestyle, access to health care, cultural barriers and exposure to carcinogens and pathogens. Cancers caused by infections are significantly more common in developing than developed countries, and they overproportionally affect immigrant populations in the USA and other countries. The global pattern of cancer is not stagnant. Instead, it is dynamic because of fluctuations in the age distribution of populations, improvements in cancer prevention and early detection in affluent countries and rapid changes in diet and lifestyle in parts of the world. For example, increased smoking rates have caused tobacco-induced cancers to rise in various Asian countries, whereas reduced smoking rates have caused these cancers to plateau or even begin to decline in Western Europe and North America. Some population groups experience a disproportionally high cancer burden. In the USA and the Caribbean, cancer incidence and mortality rates are excessively high in populations of African ancestry when compared with other population groups. The causes of this disparity are multifaceted and may include tumor biological and genetic factors and their interaction with the environment. In this review, we will discuss the magnitude and causes of global cancer health disparities and will, with a focus on African-Americans and selected cancer sites, evaluate the evidence that genetic and tumor biological factors contribute to existing cancer incidence and outcome differences among population groups in the USA.
Introduction
Large global disparities in cancer incidence, prevalence and mortality are evident for almost all cancer sites (1–4). It is predicted that most of the future cancer incidence, morbidity and mortality will occur in the developing world. Currently, developing nations are burdened with cancers due to infectious diseases like cervical and liver cancer, but in the future, these nations will be increasingly burdened with non-communicable cancers because of a rising cancer risk associated with a Western style diet, use of tobacco and alcohol and a decrease in physical activity. Lung cancer has been and continues to be a common disease in the affluent countries of Europe and North America because of tobacco use. This deadly disease, with a global 5 years survival rate of only 1 in 10 cases, became the most common cancer worldwide ∼25 years ago. In 1980, ∼70% of lung cancers occurred in the developed world. With the decline of tobacco use in Western countries, the burden of lung cancer began shifting from the developed into the developing world. In fact, at least 50% of the disease now occurs in developing countries, with China having the highest number of active smokers among all nations (4). Lung cancer is the single most preventable cancer and increased public awareness of the harmful effects of tobacco in population-rich Asian countries could have a dramatic effect in decreasing global lung cancer rates and overall cancer mortality (4).
Stomach cancer and liver cancer are two other cancers with a high disease mortality whose pattern of occurrence shows large geographical differences (1,5). About two-thirds of stomach cancers occur in the developing world and also in specific high-risk areas including Japan, China, Eastern Europe and South America. Diversity in both Heliobacter pylori infections and dietary factors are thought to account for the international variations in stomach cancer. Liver cancer is the sixth most common and the third most deadly cancer in the in the world with ∼700 000 deaths/year. Like stomach cancer, an infection is causal in disease development. High hepatitis B and C virus infection rates in China and sub-Saharan Africa, together with exposure to dietary aflatoxin, explain the high liver cancer incidence and mortality rates in these countries. Comparatively high hepatitis infection rates amongst immigrants to the USA are the likely cause of the elevated liver cancer rates among the Asian and Hispanic/Latino minority populations (5).
Cervical cancer, caused by infections with oncogenic subtypes of the human papilloma virus, is most prevalent in sub-Saharan Africa, southern Asia, Latin America and the Caribbean. Cervical cancer was as common in developed countries as it is now in many parts of the developing world before the introduction of screening programs, thus highlighting how effective early detection can be in cancer prevention and control.
Breast and prostate cancer are more common in Europe, North America and Australia/New Zealand, than in less affluent countries. Low incidence rates for the disease are reported from parts of Africa and Asia with the lowest rates occurring in China (1,5). It remains unresolved whether the low reported occurrence rates for breast and prostate cancer in Africa are factually correct or an artifact of underreporting due to lack of screening and inadequate cancer surveillance. Notably, cancer mortality from breast and prostate cancer is quite high in some parts of Africa, with rates similar to those observed in Europe and North America (1,2). Nevertheless, incidence rates for these two cancers are increasing in Africa and Asia and other parts of the world, which is most probably caused by changes in diet and lifestyle and reproductive factors.
What causes the large global variations in cancer incidence and mortality between different population groups? What is the relative contribution of environmental and behavioral factors in causing cancer health disparities versus the effects of population differences in inherited cancer susceptibility? Comparatively rapid changes in cancer rates in some countries and the observation from migration studies that immigrants tend to acquire the cancer rates of their new home country within a few generations indicate the importance of modifiable exposures as the major risk factors for common cancers (4,6–9). From these studies, it does not appear that ancestral genetic differences between human populations are a major cancer cause, consistent with other reports (10). If those differences would be largely responsible for global variations in cancer rates, one would expect that these variations in cancer rates persist in immigrant populations because genetics-based differences in cancer susceptibility do not change quickly. On the other hand, new research findings argue that heritable factors could be responsible for some of the population differences in cancer rates. A Scandinavian study reported that a considerable proportion of the risk to develop common cancers like prostate and breast cancer can be attributed to heritable factors (11). Subsequently, genome-wide association studies (GWAS) of cancer identified numerous susceptibility loci for prostate, breast and other cancers that significantly influence cancer risk in the general population and are thought to substantially contribute to the global cancer burden (12–17). Several of these studies reported that the identified cancer risk loci may affect some population groups differently than others (12,17–19). For example, deleterious 8q24 variants seem to confer a higher risk for prostate cancer in men of African ancestry than men of European ancestry (12,18), as also suggested by admixture mapping (20). These genetic variations do not alone cause cancer, but instead may interact with harmful environmental exposures and thereby increase both the effects of these exposures and the odds of developing cancer. For example, a recent study observed that pesticide exposure modifies the association of 8q24 cancer susceptibility variants with prostate cancer (21). These examples of new research findings, linking genetic factors to the risk of developing common cancers, may help us to understand some cancer health disparities. Still, in our efforts to find explanations for the existing differences in cancer incidence and mortality among US population groups, some of the disparities cannot be readily explained by differences in dietary or behavioral factors, access to health care or genetics to the extent we know it. Instead, yet unknown factors seem to be involved. These observations from epidemiological and clinical studies led to the hypothesis that tumor biological and immunological factors may contribute to some of the existing cancer health disparities in the USA (22,23). They could also help to explain some observations of cancer health disparities seen on an international scale.
Defining cancer health disparities and population groups
A cancer health disparity is defined by the US National Cancer Institute (NCI) as ‘a difference in the incidence, prevalence, mortality and burden of cancer and related adverse health conditions that exist among specific population groups in the USA. Thus, the definition is not limited to cancer as the disease but also encompasses both comorbidities that develop in cancer patients and the influence of these comorbidities on quality of life and survival of the cancer patient. Comorbidities contribute to cancer health disparities and disproportionally affect underserved populations. Research into the mechanisms of how comorbidities influence a patient’s survival has shown that underserved patients in the USA are more likely to die from comorbidities after a cancer diagnosis (24,25). The application of comorbidity research in cancer health disparity studies clearly has the potential of significantly impacting clinical practice because the results can guide the clinician in improving health outcomes of cancer patients.
Population groups suffering from cancer health disparities can be described in many ways, including differences by age, socioeconomic status (SES), geographic location, gender and race/ethnicity. Variations in global cancer rates are usually derived from incidence and mortality estimates for major cancer types at the national level, e.g. GLOBOCAN (http://globocan.iarc.fr), mostly ignoring race/ethnicity-related differences in cancer incidence and mortality. However, research into cancer health disparities between race/ethnicity-defined population groups has received much attention lately, due to an increased understanding of the complexity of the issues involved. For example, it has been recognized that African-Americans as a population group have the highest overall cancer burden in the USA and experience an excessive mortality from certain cancer types. Interestingly, the epidemiology and molecular characteristics for some of these cancers resembles those of other populations of African descent living in the Caribbean and the United Kingdom (26–29). Furthermore, the cancer incidence and mortality trends observed among the Hispanic/Latino populations in the USA tend to resemble those observed in Latin American nations.
Classification into race/ethnic groups within the USA is typically defined by categorization into self-reported African-American/Black, Asian/Pacific Islander, Hispanic/Latino, American Indian/Alaska Natives and European-American/White. These classification terms are used by the Surveillance, Epidemiology and End Results (SEER) program (http://seer.cancer.gov/publications/disparities), the NCI authoritative source for information about cancer incidence and survival. Misclassification of self-identified African-American/Black and European-American/White race in the SEER database was found to be low (30). It is, however, well recognized that classification into the five race/ethnic categories is more of a compromise than an ideal solution. Researchers have repeatedly criticized this classification concept because self-identified race and ethnicity are primarily sociocultural/sociopolitical rather than biological determinants (31–33). To address these concerns, health disparity researchers should incorporate social descriptors in characterizing minority populations (34). Self-identified race/ethnicity can be an acceptable ancestry estimate in some regions but is a poor estimate of ancestry in other regions (35–39). Moreover, hidden population admixture can also lead to false-positive discoveries in genetic association studies (40–42). Therefore, it has been recommended that specifically genetic research into the causes of cancer health disparity should use, whenever possible, a characterization of study participants by their genetic ancestry instead of using self-identified or reported categorization into race/ethnic groups (43,44). Cancer health disparity research can be particularly prone to this artifact. Ancestry informative markers have been developed to correct for this potential confounder and can readily be used to characterize the ancestry of, for example, African-American, European-American and Native American study subjects (12,38,43,45–47).
Contribution of population genetics to cancer health disparities
In 2006, a whole-genome admixture scan of 1597 African-American prostate cancer cases and 873 disease-free controls identified 8q24 as a prostate cancer risk locus in African-American men (20). This method can detect common risk variants for a disease that are markedly different in frequency across populations (48). The design and results of the study by Freedman et al. provided evidence that the 8q24 locus confers an increased disease risk among men of West African ancestry when compared with men of European ancestry. From this landmark study, one may conclude that genetic differences by ancestry can explain some of the observed differences in disease frequency (here prostate cancer) between population groups defined by race/ethnicity. Other admixture studies have supported this hypothesis. For example, in two studies investigating US Hispanic/Latino women, it was found that a higher European ancestry is associated with an increased risk for breast cancer in this population group (49,50). Thus, findings from these and other investigations suggest that population differences in genetic ancestry can lead to population differences in cancer susceptibility.
There are also several reports that observed differences in gene expression among population groups due to common genetic variations (51–53). Two studies investigated gene expression variations between individuals with European ancestry and individuals with African ancestry (Nigeria) using lymphoblastoid cell lines (52,53). These authors assessed the enrichment of biological processes and pathways by genes that are systematically differentially expressed by race/ethnicity because of differences in genetic background. Notably, processes related to antimicrobial humoral response, inflammation mediated by chemokines and cytokines, histamine H1 receptor-mediated signaling pathway, toll-receptor signaling pathway and the vascular endothelial growth factor-signaling pathway were enriched. The results provide preliminary evidence that differences in population genetics between healthy volunteers of European and West African ancestry may cause gene expression differences affecting host immune response, inflammation and chemotaxis and angiogenesis. These findings are consistent with results from another gene expression profiling study (54), and while preliminary, raise the possibility that differences in common genetic variations among population groups could lead to population group-selective alterations in cancer-related pathways that control host response, inflammation and tumor angiogenesis.
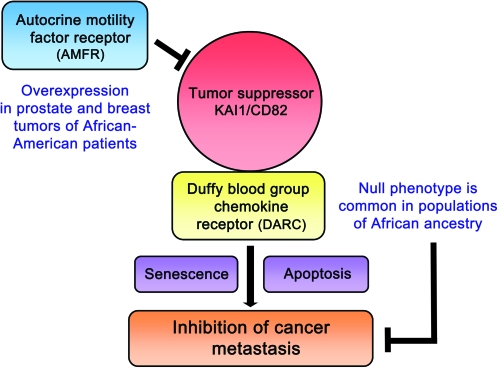
Functional genetic variants in key inflammatory and immune-related genes can show large frequency differences between population groups (55). Many of these genes have important functions in tumor biology (56,57). One may speculate that population-specific functional genetic polymorphisms are perhaps most commonly found in genes that regulate the immune system and host defense and developed because of the necessary adaptation of the host to become resistant to infections unique to a given environment. Other population-specific genetic variations may arise in the course of human adaptation to diet and ecoregions (58). There are several examples of functional genetic polymorphisms that are frequent in populations of African descent because they confer resistance to parasitic infections but may increase the susceptibility to other diseases including cancer. Trypanolytic apolipoprotein L1 variations are significantly more common in African populations and in African-Americans because they are thought to confer resistance to trypanosome infections (59). However, while seemingly protective, these variations have also been linked to the development of severe kidney disease in African-Americans (59). Another example of functional genetic polymorphisms is a single-nucleotide polymorphism (SNP) in caspase-12 that appears to be confined to populations of African descent. This genetic variation influences the inflammatory and innate immune response to endotoxin (60). Other functional polymorphisms in the NOS2 and Duffy antigen (DARC) genes that evolved in Africa to resist malaria infections (61–63). Interestingly, both genes may have key functions in tumor biology (64–66). The DARC polymorphism abolishes Duffy antigen expression, the erythrocyte receptor for the Plasmodium vivax malaria parasite (63). However, this antigen also serves as a chemokine receptor and reservoir (e.g. for interleukin-8 and monocyte chemotactic protein-1) and specifically interacts with the tumor suppressor KAI1 (66,67) (Figure 1). Lastly, a genetic variation in IL28B was recently identified as a predictor for hepatitis C treatment-induced clearance (71,72). Hepatitis C is a major risk factor for liver cancer (73,74). It was known for many years that African-Americans do not respond as well to interferon-based therapy for hepatitis C as other population groups (75). In new studies, it was found that the deleterious allele of an IL28B polymorphism had a substantially greater frequency among African-American than European-American patients, explaining at least some of the differences in the interferon-response rates between the two patient groups (71).
Fig. 1.
Inhibition of the KAI1 cancer metastasis suppressor pathway in African-American patients. KAI1 on tumor cells was shown to interact with DARC on the vascular endothelium, leading to metastasis suppression (66). Loss of DARC expression on erythrocytes, which is common in populations of African ancestry, and reduced DARC expression in tumors may compromise this metastasis suppressor pathway. Other mechanisms of KAI1 suppression include the loss of KAI1 expression, which is commonly found in many human cancers, and overexpression of the autocrine motility factor receptor (AMFR or gp78) which targets KAI1 for degradation (68). AMFR was found to be overexpressed in prostate and breast tumors of African-American patients when compared with tumors from European-American patients (54,69,70).
In summary, there is evidence that population genetics can lead to population differences in disease susceptibility. Common genetic variations may influence cancer-related pathways and cancer incidence and mortality in a population-specific manner where the population differences are explained by differences in ancestry. However, the relative contribution of these genetic factors to common cancers is still poorly understood.
The magnitude of cancer health disparities in the USA
Perhaps, the most complete understanding of the magnitude and causes of cancer health disparities across population groups exists in the USA This is largely because the NCI SEER program collects race/ethnicity information for all cancer patients, thereby allowing researchers to use the SEER database to analyze US incidence and mortality data across five population groups defined by race/ethnicity namely African-American/Black, Asian/Pacific Islander, Hispanic/Latino, American Indian/Alaska Natives and European-American/White. There have been numerous publications by the American Cancer Society and others that reported trends in age-adjusted US cancer rates by race/ethnicity and SES using SEER (5,26,28,76). These studies show that men develop cancer more frequently than women among all population groups and that African-Americans carry the highest cancer burden among all United States racial and ethnic groups. African-Americans have the highest death rates from all cancer sites combined (∼30% higher than European-Americans and about twice the rate of other minority groups) and from malignancies of the lung, colon and rectum, breast, prostate and cervix (5). In general, US minorities tend to present with a more advanced disease at diagnosis than European-American patients (28,77). Lack of access to high-quality regular screening and delayed diagnosis/treatment are thought to be the most important factors that account for the excess cancer mortality in economically disadvantaged minority populations. Nevertheless, cancer incidence rates are significantly lower among Asian/Pacific Islander, Hispanic/Latino and American Indian/Alaska Natives, when compared with African-Americans and European-Americans, which also explains the overall lower cancer mortality rates among them. Cancers from infectious disease, e.g. cervical, liver, stomach cancer, are the exception and are generally more frequent in these three minority populations as well as African-Americans when compared with European-Americans. It is currently unclear whether the low cancer incidence rates among American Indian/Alaska Natives are the result of a lower susceptibility for the disease in this population, underreporting or a reflection of a reduced cancer incidence because of competing causes of death.
Differences in cancer rates between US population groups are not stagnant. For example, the survival difference in breast cancer between African-American and European-American women did not exist before 1980 and the low rate of cervical cancer among European-American women is a more recent phenomenon (the result of high-quality screening in this population group). Numerous opportunities to reduce these disparities in the USA exist, including advancement in promoting primary prevention, improving screening/early detection methods, implementing more timely and better treatments and improving palliative care of cancer patients (5). Primary prevention should target infectious disease (such as H.pylori, hepatitis B and C and human papilloma virus infections), tobacco use and the obesity epidemic in minority populations. Secondary prevention should increase mammography and Pap test usage among women, prostate-specific antigen (PSA) testing for men and the fecal occult blood test and endoscopy for both sexes in minority populations where these cancer detection tests have not been used at appreciable rates. Using these early detection methods combined with standard care of the disease in the USA and other countries is poised to not only reduce the US cancer health disparities but also increase cancer survival rates elsewhere. Although early detection is without doubt one of our best approaches to reduce cancer mortality (78), screening for breast and prostate cancer has also substantially increased the number of detected cancers, raising questions about the biology and clinical relevance of many of the screening detected tumors (79). Recent epidemiological studies have shown that increased PSA testing may only modestly decrease the US mortality from prostate cancer in years to come (79). Therefore, future research should be aimed to identify additional biomarkers for screening that may help to discriminate minimal-risk from high-risk disease at time of detection (79).
Population differences in response to cancer therapy: do they exist?
Minority populations remain underrepresented in clinical trials, despite the critical importance of race/ethnic diversity in clinical trial participation. The lack of diversity in clinical trials makes it difficult to assess whether the efficacy of cancer therapies is the same or similar for African-American, Asian-American, Hispanic/Latino, Native American or European-American patients (80). US survival rates of cancer patients have significantly improved in recent years for all race/ethnic groups, but survival differences between the individual groups persist (81). American Indian/Alaska Natives and African-Americans have the least favorable prognosis of survival after cancer diagnosis, when compared with other US population groups. This finding is consistent with the evidence that delayed diagnosis and disparities in cancer therapy disproportionally affect these two patient groups. Hispanic/Latinos have a more favorable prognosis of survival than American Indian/Alaska Natives and African-Americans but are still more likely to be diagnosed with late-stage disease than European-Americans. Therefore, Hispanic/Latinos experience an excess mortality despite having the benefit of a relative low cancer incidence (82). Some of the recent trends in cancer mortality are influenced by changes in lifestyle choices, as opposed to changes in diagnosis and therapy. For example, the survival health disparity between African-Americans and European-Americans narrowed in recent years because of a more rapid decline in tobacco-related cancer mortality in African-Americans than European-Americans (83).
Analyses of the scientific literature and a focused assessment of clinical trial data strongly suggest that only modest survival differences are evident for African-American and European-American patients when treated comparably for similar-stage cancers (84–86). The findings from these studies indicate that therapy outcomes are similar for these two patient groups and conclude that differences in treatment and disease stage at presentation should be the primary target of interventions designed to reduce survival heath disparities (84). Other studies are in support of these conclusions and also emphasize the findings that some persistent differences in survival between African-Americans and European-Americans could be caused by the deleterious effects of comorbidities that overproportionally affect African-Americans (87–93). However, these studies do not go unchallenged. Multiple other studies in more recent years, including findings from randomized clinical trials, reported yet unexplained differences in survival between African-American and European-American cancer patients (94–106). Not surprising, some authors hypothesized that differences in tumor biology contributed to their observations. Independent of the discussion about the role of tumor biology in cancer health disparities, it is an appropriate conclusion from all these studies that those race/ethnic differences in survival that cannot be explained entirely by differences in access to health care/comorbidities should have a smaller effect size than the suggested effect size for race/ethnic differences in survival based on SEER data.
Undoubtedly, unexplained residual differences in cancer survival among US population groups may relate to race/ethnic disparities in cancer care, including inadequate treatment of comorbidities (107–112). Even in a clinical trial setting, it is plausible that differences in ancillary care, comorbidities and environmental factors could cause poorer outcomes in one population group compared with another (113). However, if one was to support the argument that genetic and tumor biological factors are contributing to these survival differences, what are the mechanisms? Currently, there is little evidence that the response to cancer therapy is grossly divergent among population groups. For breast cancer, it has been observed that disease molecular subtypes respond differently to chemotherapy (114). The prevalence of these subtypes varies among US population groups with the greatest difference observed between women of African and European ancestry (115–118). Thus, breast cancer subtype differences by race/ethnicity may contribute to population differences in therapy response and disease survival. Furthermore, there is the observation that African-Americans encounter more toxic side effects from anthracycline-based therapies than European-Americans or Hispanic/Latinos (119,120), perhaps explaining why these patients are more likely to terminate their chemotherapy prematurely (110). The decreased efficacy and increase in toxic side effects may relate to population differences in genetic variants that affect the response to cancer therapy. Genotypes that affect a patient's response to anthracycline-based therapies have been described (121,122). Because of these observations, researchers have started to examine genetic effects in the response to therapy and how genetic factors may lead to race/ethnic differences to either the effects or unwanted side effects of chemotherapy (123). Therefore, while preliminary, these results suggest that race/ethnic differences in response to therapy may exist (124).
Population differences in therapy response and survival may also relate to stress, chronic depression and lack of social support (125). Stress has been linked to an increased risk of developing cancer because it influences the immune response and can lead to immune dysfunction (125–128). Chronic stress can enhance the release of interleukin-6, an inflammatory cytokine with a key function in cancer progression (129,130). Thus, it is reasonable to assume that stress and other biobehavioral factors may affect disease outcomes and could contribute to cancer health disparities. Still, there is a deficiency of data examining the relationship between biological and biobehavioral factors and health disparities in survival.
In summary, there is not much indication that population differences in the response to cancer therapy significantly contribute to the existing US cancer health disparities in disease survival. Then again, minority populations remain underrepresented in clinical trials, which makes it difficult to assess whether the efficacy of tested therapies provide equal benefit to all population groups. It will be a major challenge of the future to increase minority recruitment into clinical trials, which is needed to assure that novel prevention strategies and cancer therapies provide the same benefit to all.
The excessive burden of prostate cancer among men of African descent
The largest US cancer health disparity exists in prostate cancer with African-American men having the highest incidence and mortality rates (5). This disparity has been widely studied and has been attributed to differences in insurance status and medical care, tumor growth rates and disease aggressiveness, location and histopathological variables of the tumor and genetic predisposition (22,86,131–138). There is evidence from clinical studies that prostate cancer outcomes are similar for African-American and European-American men when the disease is organ-confined (90,139), but African-Americans appear to have worse outcomes when the disease is advanced (22,96–98,105).
Prostate tumors tend to develop earlier as a clinical disease in African-American men than in other men (22,137). It is recommended that African-American men are screened for prostate cancer starting at age 45, instead of age 50 as recommended for other men, in an attempt to increase early detection of the disease in this population (22). As a result of these efforts, 40- to 49-year-old African-American men are now more likely to have a PSA test than European-American men in this age group (140,141). Although substantial progress has been made in increasing the use of PSA testing among African-American men across all age groups (140,142), we still continue to see an almost unchanged disparity in the prostate cancer mortality between these men and European-American men.
The US is not the only geographical location with a higher disease incidence, an earlier disease onset, and an increased aggressiveness of prostate cancer in men of African ancestry. Rather, research in the Caribbean, South America and United Kingdom found an excessive disease burden in men of African ancestry when compared with men from other population groups (27,143–146). Additional studies in West and Central Africa revealed a high prevalence of prostate tumors in men visiting hospitals in Nigeria, Senegal, Cameroon and Kenya (147–150). Importantly, the authors of these studies noted that the prostate cancer incidence in these areas may have been grossly underestimated. Since many of the African men with prostate tumors have an advanced disease at diagnosis, screening for early detection of prostate tumors in parts of Africa would probably save many lives as well as shed light on the factual incidence rate for this disease in African populations.
It is generally accepted that modifiable risk factors account for the majority of prostate cancers globally (151), although recent findings from genetic research suggest a significant influence of common genetic variations in disease development (12,14,18). A literature review of exposure differences in modifiable risk factors between African-Americans and European-Americans found only modest differences between these population groups with respect to prostate cancer development (152). One such risk factor that has been widely studied is dietary patterns among African-Americans and European-Americans (153–155). African-Americans were found to have a lower overall fruit and vegetable consumption, with the caveat that they may consume a higher frequency of specific types of fruits and vegetables (e.g. cruciferous vegetables) that are considered to be protective against cancer (154,155). In addition to the assessment of fruit and vegetable consumption, meat and animal fat intake have been identified as a candidate prostate cancer risk factor for African-Americans in two studies (156,157). It is well established that African-Americans have higher rates of vitamin D deficiency than European-Americans. Both cell culture and animal studies indicate that vitamin D has anticancer properties. Thus, it has been hypothesized that this deficiency puts African-Americans at an increased risk of cancer (158). Although the relationship between vitamin D and cancer has been studied extensively, the results do not support a causative relationship between low vitamin D and an increased prostate cancer risk to date (158,159).
Nutrition is associated with cancer not just through the type of diet but additionally through the effects of obesity. A high calorie intake together with a sedentary lifestyle leads to overweight and obesity. African-Americans have the highest proportion of individuals with a body mass index of >30, which is considered a threshold for being obese (160). About 37% percent of African-American men fall into this category versus 32% of the entire US male population. Obesity is a risk factor for cancer and has been associated with an increased prostate cancer-specific mortality and treatment failure (161–163). Thus, it is possible that the high obesity rate among African-American men has some contribution to the higher disease incidence and mortality among them. Recent studies have investigated the mechanisms of the effects of obesity on cancer and found that dietary factors and obesity may influence prostate tumor biology by modifying gene expression patterns (164,165). Gene signatures in prostate tumors may develop in response to nutrition and lifestyle intervention (164).
Prostate gland biology is androgen dependent. A racial disparity in tumor androgen receptor expression in men with a localized disease has been observed (166). Furthermore, several epidemiological studies reported African-American men to have modestly higher blood testosterone levels than European-American men (167–169). The biological significance of this observation has since become uncertain because blood male sex hormone levels were not found to be associated with the risk of prostate cancer in prospective studies and in a recent meta-analysis (170). It was also hypothesized that prostate glands from African-American men may have an increased availability of male sex hormones, increased androgen receptor signaling and an increased potential of cell growth and tumor development. However, when two studies examined prostatic tissue, testosterone and dihydrotestosterone levels were not different between African-Americans and European-Americans (171,172). Prostate size is also similar in both groups of men, suggesting that an enhanced androgenic effect is not present in the gland of African-American men (173). Very recently, two large studies re-examined the question whether male sex hormones are increased in blood samples from African-American males (174,175). Both studies did not find elevated testosterone levels in African-American men compared with other population groups (e.g. European-American, Hispanic/Latino, Asian-American men). Instead, it was discovered that serum estrogen differed significantly by race/ethnicity with the highest levels being detected in African-American men and in a group of men from Tobago (174,175). This is of interest because estrogen may induce inflammation-induced oxidative/nitrosative stress in the prostate gland (176). In addition, a higher proportion of African-American than European-American men may have chronic inflammation in their non-cancerous prostate, as observed in one study (177). Given that prostatic tissue inflammation and prostatitis are risk factors for prostate cancer (178,179), prevalent inflammation in the prostate gland of African-American males may put these men at an increased prostate cancer risk.
Numerous studies have examined the possibility of low penetrance genes contributing to the excessive burden of prostate cancer in African-American men. So far, attempts have failed to identify common genetic variations that are associated with prostate cancer mortality (180). To date, the best described risk locus for prostate cancer is located at 8q24. This locus confers an increased disease risk in many populations (12,18,181,182). There is also indication that this locus confers a higher risk for prostate cancer in men of African ancestry than in men of European ancestry (12,18,20). Several additional GWAS-identified prostate cancer susceptibility loci (i.e. 11q13, 17q12, 19q33 and Xp11) have been validated in studies of men with African ancestry (19,183). Because of the importance of inflammation in disease development, researchers also evaluated the relationship between prostate cancer and common genetic variations in genes comprising inflammation and host defense pathways. These studies yielded preliminary evidence, suggesting that the low penetrance genetic variability in these pathways may influence cancer risk in African-Americans and other population groups (184–191).
Prostate tumors in African-Americans tend to be more aggressive at diagnosis than they are in European-Americans (22). This observation generated an interest in understanding the biology of these tumors. One recent study by Powell et al. addressed this question by analyzing autopsy material from prostates of 1056 African-American and European-American men who did not have a prior diagnosis of prostate cancer. Interestingly, Powell et al. observed that the cancer prevalence was similar between the two race/ethnicity groups at this subclinical stage. However, amongst the men with detectable prostate tumors, African-American men seemed to have faster growing and more aggressive tumors, when compared with European-American men (137). Additional research will have to investigate further whether mechanisms related to an increased proliferation, as described in this study, or inhibition of apoptosis, as described by others (192), may promote the enhanced growth of tumors in African-American men. Epidermal growth factor receptor signaling is antiapoptotic and promotes cell proliferation by mechanisms including MAPK, PI3K/Akt and nuclear factor kappa-β activation. Furthermore, this oncogene and candidate drug target is critical in the progression to an androgen-independent disease and therefore has been widely studied in respect to advanced prostate cancer (193). Notably, the receptor was found to be more commonly expressed in tumors from African-American patients when compared with European-American patients (194). These studies suggest that epidermal growth factor receptor perhaps contributes to apoptosis inhibition and enhanced proliferation in prostate tumors from African-American patients as well as possibly increasing the odds of disease recurrence.
Epigenetic DNA alterations, which include DNA methylation changes are present in a greater fraction of prostate tumors than any other of the known genetic defects (195). Changes in DNA methylation are amongst the earliest somatic changes that can be detected in cancerous lesions of the prostate and occur at the stage of proliferative inflammatory atrophy, a precursor lesion in human prostate carcinogenesis. Increased DNA methylation can lead to numerous downstream effects including inactivation of tumor suppressor genes like PTEN or a loss of protection against reactive chemical species. The latter typically occurs in response to hypermethylation of GSTP1, which is observed in 90% of prostate cancers (195). Given the importance of epigenetic DNA alterations in contributing to prostate cancer, the occurrence of DNA hypermethylation in tumors from African-American men has been evaluated. In three studies, a pattern emerged consistent with increased DNA hypermethylation in African-American tumors when compared with similar-stage tumors from European-American men (196–198). Currently, it is unknown what mechanisms may lead to race/ethnic differences in promoter DNA hypermethylation and whether this pattern is induced by environmental exposures, intrinsic differences in tumor biology or a combination of the two.
Several research groups have investigated the occurrence of harmful chromosomal alterations in prostate tumors, comparing tumors from African-Americans with tumors from European-Americans. Two studies analyzed tumors from the two patient groups using the same platform (199,200), whereas one study compared their analysis of African-American tumors with previously published data from European-Americans (201). The first study analyzing 16 tumors from each patient group did not observe significant differences in chromosomal aberrations between the patients (199). The second study reported several differences consistent with distinct genomic aberrations in African-American tumors at chromosomal sites that encode oncogenes (e.g. ETV1, MYC) and tumor suppressor genes (e.g. PTEN, RB1). The third and most recent study evaluated genomic alterations in both a discovery and validation cohort to describe chromosomal aberrations whose occurrence is most distinct between African-American and European-American tumors. The data from this study revealed common genomic alterations specific to African-American tumors that may specifically target immune response genes (200). Recurrent genomic rearrangements like TMPRSS2:ERG are signature mutations of many human prostate tumors and have important biological and clinical implications (202). Two studies examined the frequency of these recurrent gene fusions in European, European-American, Chinese, Japanese and African-American prostate cancer patients. Although common in European and European-American patients (in ∼50% of patients), these mutations were less frequent in African-American patients, as assessed in one study, and rather uncommon in patients from Asia (2–15%), as found in both studies (203,204). This pattern is not restricted to TMPRSS2:ERG rearrangements, but rather evidence supports that inactivation of the PTEN tumor suppressor may follow the same trend (203). The observations raise the possibility that prostate tumors in Asian populations arise from pathogenic mechanisms that are different from Western populations, which could affect disease aggressiveness. Thus, prostate tumor biology may display an unexpected global heterogeneity between men of European, African and Asian descent.
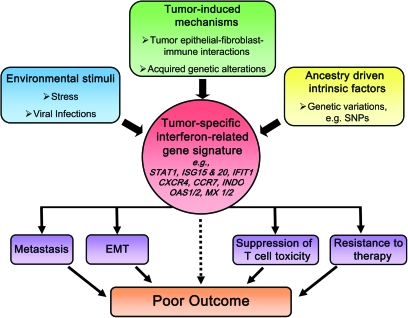
Gene expression profiling is a hypothesis-generating instrument that allows researchers to examine the phenotypic diversity of tumors. This technique can be very advantageous in exploring tumor biological differences in health disparity research. Using differential display analysis, a novel prostate-specific gene, PCGEM1, was discovered that had a cell growth-promoting function (205,206). PCGEM1 was mapped to chromosome 2q32 and was shown to be overexpressed in prostate tumors. Further analyses revealed that this gene acts as a non-coding RNA and is most highly expressed in prostate tumors of African-American patients (206). Although further research is needed, this candidate gene may have important oncogenic functions in prostate cancer biology, particular in respect to the African-American population. Our group and others have used genome-wide gene expression profiling to investigate candidate differences in tumor biology between African-American and European-American prostate cancer patients (69,70,207). Analyzing macrodissected tumors and cultured prostate cancer epithelial cells from African-American and European-American patients, Wallace et al. and Timofeeva et al. found consistent gene expression differences between the two patient groups. Notably, several known metastasis-promoting genes, including AMFR, CXCR4 and MMP9, were more highly expressed in tumors from African-Americans than European-Americans. The two tumor studies by Wallace et al. and Reams et al. made another important observation. In agreement with the findings by Rose et al. (200), their data pointed to significant differences in tumor immunobiology and inflammation pathways between African-American and European-American patients. Moreover, an interferon γ signature was found to be prominent in tumors from African-American patients (69). This particular signature appears to be identical to previously identified interferon-related gene signatures that are induced by both tumor–stroma interactions and resistance to DNA damage (208–211). Interestingly, the interferon-related signatures in these studies were all found to be associated with metastasis and poor disease outcome. Furthermore, while additional research is required, it is possible that the presence of the interferon-related signature in prostate tumors may also suppress T-cell cytotoxicity directed against cancer cells by mechanisms that may include upregulation of the interferon-response gene, indolamine-2,3-dioxygenase (Figure 2). We think that tumor-induced mechanisms, environmental stimuli and ancestry-driven intrinsic factors are candidate exposures that induce the interferon-related gene signature in prostate tumors.
Fig. 2.
Potential origin and effects of the interferon-related gene signature in tumor biology. Tumor-induced mechanisms, environmental stimuli and/or ancestry-driven intrinsic factors are candidate exposures that induce the interferon-related gene signature in prostate and breast tumors. This signature may lead to poor outcome by mechanisms including EMT and increased metastatic potential, suppression of T-cell toxicity and resistance to therapy. EMT, epithelial to mesenchymal transition.
In summary, various studies examined the relative contribution of genetic and tumor biological factors to the excess burden of prostate cancer among African-American men. There is good evidence that variations in cancer susceptibility loci by genetic ancestry may contribute to population differences in disease occurrence. Other studies reported consistent differences in tumor markers and tumor biology between African-American and European-American patients. Whether any of these differences are causatively linked to the increased disease aggressiveness of African-American tumors is uncertain and will need further research. Other observations suggest that a low-grade chronic inflammation in the non-cancerous and cancerous prostate gland is more prevalent in African-American men (177). This condition could be related to environmental factors, tumor microenvironmental factors, immunobiological factors related to ancestry or a combination of these factors.
Survival health disparity in breast cancer: is tumor biology a contributing factor?
Breast cancer incidence rates are ∼3-fold higher in more developed than less developed countries (2). Results from cancer epidemiology have taught us that age, reproductive history and lifestyle are the major risk factors for breast cancer (212,213). Thus, disparities in these modifiable factors and differences in age distribution are obvious causes of the large global differences in breast cancer (1,2). A predominance of early onset and aggressive estrogen receptor (ER)-negative breast cancers that exists in many low-risk countries may account for the relatively high mortality to incidence ratio in parts of Asia and Africa (2). In the USA, European-American and African-American women have a significantly higher risk to develop breast cancer than Asian/Pacific Islander, Hispanic/Latino or American Indian/Alaska Natives (214).
Causes for the premenopausal and postmenopausal diseases are different (212,213). There are also differences in the risk factor profile for ER-positive tumors when compared with ER-negative tumors and for African-American women compared with European-American women (215–218). In general, factors that raise a woman's lifetime exposure to estrogen, like the use of hormone replacement therapy, increase the risk for the disease (212). The decline in hormone replacement therapy use, triggered by studies showing that hormone replacement therapy increases breast cancer risk, has been credited for contributing to the marked disease decline in the USA in recent years (219).
The relative contribution of predisposing genetic markers to breast cancer health disparities across populations is currently unknown. High-penetrance mutations in the BRCA1 and BRCA2 genes occur in many populations but they are rare (220–222). Several of the GWAS discovered breast cancer risk loci for European women, including FGFR2 and other loci, have been validated in studies of African-American women (223,224). However, a more recent analysis within the Multiethnic Cohort Study observed significant population heterogeneity for the association of 12 SNPs from previous GWAS studies with breast cancer (225). An overall modest disease association for these SNPs was observed in all examined population groups, with the exception of a null result for the African-Americans. It will need large-scale future studies of multiple race/ethnic populations to determine whether the frequency and pattern of low-penetrance risk loci for breast cancer varies among population groups.
Breast cancer is a heterogeneous disease and its biology is closely related to the hormone receptor status of the tumor. Landmark gene expression profiling studies discovered that breast tumors can be classified into subtypes with distinct gene expression profiles (226,227). Molecular signatures characterize three luminal subtypes among the ER-positive tumors and three subtypes (basal-like, HER2-positive and normal-like) among the ER-negative tumors. Of all subtypes, both basal-like and HER2-positive, ER-negative tumors tend to produce the most aggressive disease (115,228). Basal-like tumors overlap largely with a group of tumors referred to as triple-negative, meaning they are negative for ER, HER2 and progesterone receptor expression (229). Triple-negative tumors are of particular interest because they are not treatable by endocrine-targeted therapy, such as tamoxifen and aromatase inhibitors, or by HER2-targeted therapy such as trastuzumab. Patients with triple-negative breast cancer have a worse prognosis than patients with ER-positive breast cancer independent of therapy, particularly in the first 5 years following diagnosis (230,231).
It was recognized in 2006 and confirmed by other reports that the prevalence of these subtypes varies among US population groups with the greatest difference observed between women of African and European ancestry (115,117,118,232–234). Multiple investigations in West and Central Africa have provided further corroboration that women of African ancestry tend to develop early-onset, high-grade and ER-negative tumors more frequently than women of European ancestry (116,235–239). In the most representative study of 507 patients from West Africa, it was found that a large proportion of these breast cancer patients (55%) had a triple-negative disease which is a significantly higher proportion than one would expect to find in similar age cohorts of African-American, European-American or European patients (116). The findings raise the possibility of a causative relationship between West African ancestry and an increased susceptibility for developing early onset and ER-negative breast tumors, as seen in West African populations and in women who were brought through the slave trade into the USA and the Caribbean and through migration into the United Kingdom (29,240). Early onset ER-negative tumors also develop more frequently in Asian Indian and Pakistani women and in women from other parts of Asia, although not as prevalent as it is in West Africa (2,241). Cancer epidemiology showed that rates of ER-negative breast tumors in a population can change over time, pointing to an influence of environmental and reproductive factors on disease phenotypes (242). For example, relationships between age at menarche or breastfeeding and basal-like breast cancer have been observed (233,243). It was found in one study that the development of basal-like breast cancer in African-American women is associated with breastfeeding and adiposity (233). According to an estimate by the authors, about two-thirds of basal-like breast cancer in premenopausal African-American women could be prevented by promoting breastfeeding and reducing abdominal adiposity (233). Notably, however, other studies did not observe a relationship between body mass index and basal-like/triple-negative breast cancers (117,218,243).
SES is associated with breast cancer mortality. Thus, several studies assessed if SES influences tumor characteristics such as tumor ER status. It was found that income and education are associated with tumor ER status and populations with a low SES are more likely to develop an ER-negative disease than populations with a high SES (217,244–246). This association may explain some of the excess risk among US Hispanic and African-American women to develop an ER-negative disease yet the magnitude of the association between SES and tumor ER status seems to fall short of explaining most of the observed differences in tumor ER status between women of African and European ancestry. Furthermore, it remains uncertain whether the association of SES with tumor ER status is related to an increased risk of developing an ER-negative disease in populations with a low SES or an increased risk of developing an ER-positive disease in populations with a high SES or both. Generally, more affluent populations have a higher risk of breast cancer and an increased prevalence of ER-positive tumors (2,217), with some variation across race/ethnic strata (247).
African-American women face a lower risk of being diagnosed with breast cancer, yet they are at increased risk of dying from the disease when compared with women from other US population groups (102,248–251). An analysis of survival disparities by disease stage using SEER data showed that the survival difference is larger for stage II/III disease than stage 0/I disease, with African-American having the worst outcomes, followed by Hispanic/Latino, European-American, and lastly, Asian-American/Pacific Islander patients (251). This survival disparity continued to exist independent of age, screening status, tumor characteristics, grade, ER status, therapy, comorbidities and demographics. The African-American to European-American disparity in age-standardized breast cancer mortality emerged in the beginning of the 1980s and since then has widened (252). The greatest absolute difference in the mortality hazard between the two patient groups occurs in the first years after diagnosis (252,253), which may reflect differences in treatment, differences in response or both. Differences in treatment exist and persist, with many African-American women receiving inadequate or delayed therapy (110,111,254,255). However, when compared with European-American women, a poorer survival of African-American women has also been observed in equal access health care systems and clinical trials (104,105,256,257). It has been hypothesized that the increased prevalence of early onset, ER-negative tumors and basal-like/triple-negative tumors in women of African ancestry is largely responsible for the excessive mortality among African-American women. Although this argument is very plausible, one has to be cautious because the breast cancer survival disparity in US is irrespective of the tumor ER status (252). Basal-like/triple-negative tumors confer an increased risk for early disease spread and deadly brain metastases (258), but the disease is not more aggressive in African-American patients than in European-American patients, as found by one study (259).
Few studies have examined the biology of breast tumors in African-American women beyond the concept of subtypes that are described by their gene expression pattern. Early studies concentrated almost exclusively on hormone receptor expression and disease grading in African-American patients, describing an increased proportion of tumors that are ER-negative and poorly differentiated among this patient group (260,261). More recent studies describe that the ER isoform profile is different between tumors from African-American and European-American patients (262) and additionally observed differences in prostaglandin E1 receptor expression, expression of cell cycle-regulatory proteins and aberrant expression of the p53 tumor suppressor gene (263–265). The latter two observations were confirmed by a recent study in finding that African-American tumors are more likely to have a p53 mutation and gene expression aberrations in cell cycle-related pathway (54). Interestingly, the tumor p53 status has been found by others to be associated with socioeconomic deprivation, suggesting an environmental influence related to SES in the development of p53 mutations (266,267). It was further noticed by one group that ER-negative breast tumors in young African-American women tend to exhibit promoter DNA hypermethylation (268), consistent with the more recent findings of DNA hypermethylation in prostate tumors of African-American men (198).
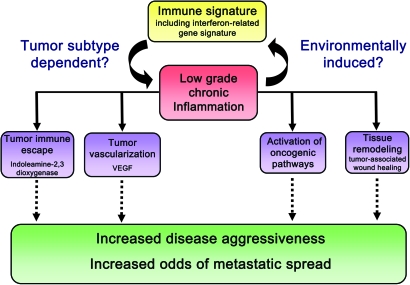
Our group embarked on a pilot project analyzing 32 laser capture microdissected tumors from African-American and European-American breast cancer patients for differences in gene expression (in both the microdissected tumor epithelium and stroma) (54). Key observations were validated in a larger set of paraffin-embedded tumors from 143 African-American patients and 105 European-American patients. The gene expression analysis of these breast tumors revealed that differences in tumor biology may exist between African-American and European-American beyond current knowledge. Among our findings were that African-American tumors contained a prominent interferon signature in both ER-negative and ER-positive tumors, which mirrored our findings from an earlier prostate cancer study of African-American men (69) (Figure 2). It appears that the interferon signature is functionally related to a STAT1 signature in basal-like breast cancer and a tumor–stroma interaction signature described by two other groups (209,211,269). This signature may also influence therapeutic outcome because of its great homology with a recently discovered interferon-related DNA damage resistance signature, which predicts resistance to chemotherapy and radiation in breast cancer and perhaps other epithelial cancers (210,270). Future research should examine the precise origin of the interferon signature, whether this signature is prevalent in tumors of African-American and West African patients and other global populations of West African descent, and how it influences the response to therapy beyond the boundaries of health disparity research. Other findings from our study pointed to tumor microenvironmental differences that relate to tumor vascularization and macrophage infiltration (Figure 3). These differences between African-American and European-American breast patients may arise from a low-grade inflammation, or from population differences in tumor–stroma interactions, but could also be related to tumor subtype differences between the two patient groups. Some of our observations have been further corroborated by others by showing that endothelial cells from African-Americans and European-Americans have different gene expression profiles, consistent with differences in function (271). Those differences may influence tumor biology and the process of wound healing in general. In fact, it has been known for many years that some population groups, including African-Americans, are more prone to develop keloids, which are benign collagenous dermal tumors that form during the wound healing process (272–275). It is aberrant wound healing involving altered fibroblast and endothelial functions that are thought to cause the condition. Future research may explore the causes of altered wound healing in populations that are prone to this disease and examine how these functions may possibly relate to the development of malignant tumors, especially the development of early-onset epithelial cancers of the breast and prostate.
Fig. 3.
Possible disease-modifying implications of an immune signature in breast and prostate tumors of African-American patients. Differences in tumor immunobiology and in the development of a low-grade inflammation between African-American and European-American may arise from differences in either environmental or tumor environmental exposures or could be related to tumor subtype differences between the two patient groups. A low-grade chronic inflammation in tumors has been linked to activation of oncogenic pathways, increased tissue remodeling and tumor vascularization and the development of tumor immune escape mechanisms. VEGF, vascular endothelial growth factor.
Population differences in lung cancer incidence and mortality
Lung cancer causes more cancer deaths globally and in the USA than any other cancer type, accounting for ∼28% or 159 000 of the estimated 562 000 annual cancer deaths in the USA in recent years (5). Age-adjusted incidence and mortality is highest among African-American men, followed by European-American men who have 30–40% lower incidence and mortality rates. Asian/Pacific Islanders, American Indians/Alaska Natives and Hispanic/Latinos, and women in general, all experience a substantially lower lung cancer burden. As expected, the lung cancer burden generally mirrors the tobacco consumption among these population groups. Even though roughly 85% of lung cancers are caused by tobacco, it should be noted that the death rate from lung cancer among never-smokers is higher in men than women (276).
The high lung cancer incidence and mortality experienced by African-American men has been examined in numerous studies (277). This cancer health disparity is mainly driven by an excess disease incidence among these men, although differences in lung cancer treatment have been reported. Specially, African-American patients were found to obtain a potentially lifesaving surgery less frequently after disease diagnosis than European-American patients (277,278). A lung cancer risk prediction model for African-Americans has been proposed which identifies tobacco use, chronic obstructive pulmonary disease, no hay fever history and exposure to asbestos and wood dust as the most significant risk factors (279). Several studies examined lung cancer occurrence in various population groups and observed consistent race/ethnic differences in smokers and non-smokers. In a large study of 183 813 participants in the Multiethnic Cohort Group, significant race/ethnic differences in the risk to develop cancer persisted after stratifying participants into moderate (10 cigarettes/day) and heavy smokers (30 cigarettes/day), thus after at least partially accounting for differences in smoking (280). The results from this study suggested that African-American and Native Hawaiian smokers are more susceptible to develop lung cancer than European-Americans, Japanese-Americans or Hispanic/Latinos if they smoke equal or similar amounts of tobacco. The conclusions of the study have become subject to criticism. Mainly, differences in smoking could have persisted among the various population groups after grouping them into moderate and heavy smokers because the poor and minority populations tend to smoke cigarettes more efficiently (i.e. inhaling more cigarette smoke than more affluent smokers) (277). However, other studies found that an increased susceptibility for lung cancer may also exist among African-American never-smokers, raising the possibility that this population group may indeed experience an increased susceptibility to lung cancer (276,281). Noteworthy, there have also been reports pointing to differences in pulmonary functions between various race/ethnic groups and to an increased familial risk of lung cancer among African-Americans when compared with European-Americans, as found in one study that examined relatives of individuals with early-onset lung cancer (282,283).
Another factor that may explain the increased burden of lung cancer among African-Americans is their preferential use of mentholated cigarettes (277). The majority of African-American smokers prefers this type of cigarettes. It has been shown that smoking mentholated cigarettes inhibits nicotine metabolism, leading to a higher systemic nicotine exposure (284). This may impact disease etiology because nicotine has oncogenic properties and may promote lung cancer progression (285). Furthermore, smokers of mentholated cigarettes also experience increased blood cotinine and carbon monoxide levels (286,287). Nonetheless, several large epidemiological studies could not provide any evidence that cigarette mentholation increases lung cancer risk of smokers (288–290).
Other studies have explored differences in the DNA repair capacity, inflammation, tumor marker expression and low-penetrance genetic susceptibility between population groups. It is believed that examining these factors may help explain the observed race/ethnic differences in the lung cancer burden in the USA. One study found differences in DNA radiation damage-induced growth arrest in lymphocytes from African-Americans and European-Americans (291). Furthermore, they found that a less efficient G2-M checkpoint was associated with an increased risk of lung cancer in African-Americans. Other studies examined blood cytokine concentrations and functional polymorphisms in the mannose-binding lectin and found that variations in these key regulators of inflammation and innate immunity may influence lung cancer survival (292,293). Interestingly, the markers identified in these studies appeared to have different effects in African-Americans than European-Americans. Lastly, work by Chang et al. proposed a link between nucleotide excision repair variants and lung cancer risk in African-Americans. Specifically, two variants in genes involved in the nucleotide excision repair pathway were found to be associated with lung cancer risk in African-Americans from the San Francisco Bay Area (294).
Population differences in colorectal cancer incidence and mortality
Globally, colorectal cancer is the third most common cancer, affecting men more commonly than woman overall (3). There are very large regional differences in disease occurrence with cancer rates in some areas (e.g. parts of Europe, Japan, USA) being 10-fold the rates of other areas (e.g. India, Egypt, Pakistan, parts of Africa). These disparities are probably explained by differences in lifestyle factors, including an increased prevalence of high fiber, low fat, low-meat diets in the areas with the lowest rates. Throughout the years, colorectal cancer rates have stabilized or declined in many high-risk areas (3). In the USA, both cancer incidence and mortality rates among European-Americans steadily declined starting between 1980 and 1990, and the decline continues today (103,295). Unfortunately, we have not seen the same declines in other US population groups, leading to the existing health disparities for the disease. Today, African-Americans have the highest colorectal cancer rates (103,295,296). Their age-adjusted disease cancer mortality is ∼40% higher than it is for European-Americans and about twice the rate of Asian/Pacific Islanders and Hispanic/Latinos. Because colorectal cancer tends to affect young African-Americans more frequently than young individuals in other population groups, their recommended age to begin with screening has been set at age 45, in contrast to age 50 for men from other population groups. The early onset of the disease in African-Americans is a factor in causing the larger health disparity in the 20–64 age group than in the age group above this age (296).
As is observed for several other cancers, the poorer clinical outcome for African-Americans can be partially attributed to a more advanced disease stage at diagnosis, when compared with European-Americans, accounting for much of the excess cancer mortality among them (297,298). An advanced stage at diagnosis is perhaps the single most important contributing factor to the racial disparity in colon cancer survival (103). This disparity is not restricted to African-Americans, and other US minority groups are also more frequently diagnosed with an advanced disease (77). Colonoscopy screening rates are not obviously lower among African-Americans than other population groups (28,295,296), but appear to lag behind in high-risk individuals with known family history and affected first-degree relatives (299). African-Americans are less likely to receive standard adjuvant therapy than non-Hispanic white and Hispanic patients, as found by several studies (109,296). Possible differences in survival among African-American patients treated with standard adjuvant therapy, when compared with European-American patients, were assessed in clinical trials and equal access medical institutions. These studies produced mixed results. Some studies did not find any differences (300), whereas others observed them (87,301,302). When survival differences persisted between the two patient groups, those were better explained by the influence of comorbidities than by differences in response to therapy, as measured by recurrence-free survival (87,296).
Although most of the differences in colorectal cancer incidence rates occur because of differences in diet and physical activity, researchers have begun studying the tumor biology of colorectal cancer to examine if there are genetic and biological factors that may explain the relative aggressiveness of tumors in African-American patients. It has been known that African-Americans tend to develop colon tumors more frequently in the descending colon, which is more difficult to reach by a sigmoidscope (303). They also have a greater occurrence of tumor formation on the right side of the colon (304,305). It is well established that proximal (right side) colon cancers are genetically distinct from distal lesions and are more likely to show microsatellite instability. Because of observations like these, recent efforts have been investigating tumor biology and genetics in a hope to better elucidate underlying differences in colon cancer biology amongst race/ethnic groups. Several studies have focused on genetic instability as a cause of colon cancer. Genetic instability frequently results from microsatellite instability, which commonly occurs due to silencing or mutations of DNA mismatch repair genes. Recent studies have found African-Americans to have higher occurrence of microsatellite instability-positive tumors and decreased expression of the mismatch repair gene, MLH1, when compared with European-Americans (306,307). In addition, African-American patients may show differences in p53 pathway alterations in their tumors when compared with European-Americans. It was reported that nuclear accumulation of the p53 tumor suppressor protein in cancer cells, a surrogate for a pathologically aberrant p53 pathway, is a predictor of disease outcome in European-American colon cancer patients but not in African-American patients (308). Other research is focusing on the involvement of low-penetrance genes in the colon cancer health disparity. Preliminary results suggest that SNPs in genes of the arachidonic acid-signaling pathway may influence colon cancer risk of African-Americans differently than the risk of European-Americans (309,310).
Conclusions
Populations in the developing world and many minorities in more developed countries have not equally benefited from the advances in our understanding and treatment of cancer. There are substantial disparities in disease incidence and mortality between population groups nationally and globally that are largely explained by differences in lifestyle and diet, age distribution, carcinogen exposure and inadequate or delayed access to health care. To date, there is sparse evidence that patients from any two population groups respond differently to cancer therapy when treated comparably for similar-stage cancers, arguing that inadequate or delayed access to health care is the most important factor in causing survival health disparities. However, minority populations remain underrepresented in clinical trials, and we therefore may not be able to appropriately assess whether the existing cancer therapies provide equal benefit to all population groups. Numerous studies have identified differences in tumor biology and epigenetic factors among diverse patient populations. These factors, singularly or in combination, may contribute to some of the existing cancer health disparities.
Although preliminary, there is evidence that population differences in genetic ancestry can lead to population differences in cancer susceptibility. GWAS have dramatically impacted research on genetic determinants of cancer risk, but certainly, more of this research needs to be done for populations in the developing world. As cancer health disparities research creates more understanding of the interrelationships between environmental exposures and genetic susceptibility, an extensive epidemiological assessment of various population groups worldwide is required to find out how our current understanding of environmental and genetic risk factors and their interactions in cancer development applies to all of these groups or whether significant differences exist. In order to address these complex issues surrounding domestic and global cancer health disparities, international collaborations must be established.
Funding
Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, USA.
Acknowledgments
We would like to thank Mohammed Khan for his help in preparing the figures.
Glossary
Abbreviations
- ER
estrogen receptor
- GWAS
genome-wide association study
- SEER
Surveillance, Epidemiology and End Results
- SES
socioeconomic status
- SNP
single-nucleotide polymorphism
References
- 1.Jemal A, et al. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Kamangar F, et al. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol. Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 4.Thun MJ, et al. The global burden of cancer: priorities for prevention. Carcinogenesis. 2010;31:100–110. doi: 10.1093/carcin/bgp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jemal A, et al. Cancer statistics, 2010. CA Cancer J. Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 6.Haenszel W, et al. Studies of Japanese migrants. I. Mortality from cancer and other diseases among Japanese in the United States. J. Natl Cancer Inst. 1968;40:43–68. [PubMed] [Google Scholar]
- 7.Buell P. Changing incidence of breast cancer in Japanese-American women. J. Natl Cancer Inst. 1973;51:1479–1483. doi: 10.1093/jnci/51.5.1479. [DOI] [PubMed] [Google Scholar]
- 8.Thomas DB, et al. Cancer in first and second generation Americans. Cancer Res. 1987;47:5771–5776. [PubMed] [Google Scholar]
- 9.Ziegler RG, et al. Migration patterns and breast cancer risk in Asian-American women. J. Natl Cancer Inst. 1993;85:1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 10.Ioannidis JP, et al. 'Racial' differences in genetic effects for complex diseases. Nat. Genet. 2004;36:1312–1318. doi: 10.1038/ng1474. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenstein P, et al. Environmental and heritable factors in the causation of cancer-analyses of cohorts of twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 12.Amundadottir LT, et al. A common variant associated with prostate cancer in European and African populations. Nat. Genet. 2006;38:652–658. doi: 10.1038/ng1808. [DOI] [PubMed] [Google Scholar]
- 13.Hunter DJ, et al. A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat. Genet. 2007;39:870–874. doi: 10.1038/ng2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng SL, et al. Cumulative association of five genetic variants with prostate cancer. N. Engl. J. Med. 2008;358:910–919. doi: 10.1056/NEJMoa075819. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, et al. Common 5p15.33 and 6p21.33 variants influence lung cancer risk. Nat. Genet. 2008;40:1407–1409. doi: 10.1038/ng.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wokolorczyk D, et al. A range of cancers is associated with the rs6983267 marker on chromosome 8. Cancer Res. 2008;68:9982–9986. doi: 10.1158/0008-5472.CAN-08-1838. [DOI] [PubMed] [Google Scholar]
- 17.Takata R, et al. Genome-wide association study identifies five new susceptibility loci for prostate cancer in the Japanese population. Nat. Genet. 2010;42:751–754. doi: 10.1038/ng.635. [DOI] [PubMed] [Google Scholar]
- 18.Haiman CA, et al. Multiple regions within 8q24 independently affect risk for prostate cancer. Nat. Genet. 2007;39:638–644. doi: 10.1038/ng2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang BL, et al. Validation of genome-wide prostate cancer associations in men of african descent. Cancer Epidemiol. Biomarkers Prev. 2011;20:23–32. doi: 10.1158/1055-9965.EPI-10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freedman ML, et al. Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc. Natl Acad. Sci. USA. 2006;103:14068–14073. doi: 10.1073/pnas.0605832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koutros S, et al. Pesticide use modifies the association between genetic variants on chromosome 8q24 and prostate cancer. Cancer Res. 2010;70:9224–9233. doi: 10.1158/0008-5472.CAN-10-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell IJ. Epidemiology and pathophysiology of prostate cancer in African-American men. J. Urol. 2007;177:444–449. doi: 10.1016/j.juro.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 23.Amend K, et al. Breast cancer in african-american women: differences in tumor biology from European-american women. Cancer Res. 2006;66:8327–8330. doi: 10.1158/0008-5472.CAN-06-1927. [DOI] [PubMed] [Google Scholar]
- 24.Tammemagi CM, et al. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005;294:1765–1772. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 25.Berndt SI, et al. Disparities in treatment and outcome for renal cell cancer among older black and white patients. J. Clin. Oncol. 2007;25:3589–3595. doi: 10.1200/JCO.2006.10.0156. [DOI] [PubMed] [Google Scholar]
- 26.Ghafoor A, et al. Cancer statistics for African Americans. CA Cancer J. Clin. 2002;52:326–341. doi: 10.3322/canjclin.52.6.326. [DOI] [PubMed] [Google Scholar]
- 27.Bunker CH, et al. Prostate cancer risk is three-fold higher among men, aged 50-64, of African descent compared with men of Asian-Indian descent in Trinidad and Tobago. Ethn. Dis. 2002;12:S3. [PubMed] [Google Scholar]
- 28.Ward E, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J. Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 29.Bowen RL, et al. Early onset of breast cancer in a group of British black women. Br. J. Cancer. 2008;98:277–281. doi: 10.1038/sj.bjc.6604174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel DA, et al. Evaluation of African-American and white racial classification in a surveillance, epidemiology, and end results cancer registry. Ethn. Dis. 2005;15:713–719. [PubMed] [Google Scholar]
- 31.Rebbeck TR, et al. Ethnicity, ancestry, and race in molecular epidemiologic research. Cancer Epidemiol. Biomarkers Prev. 2005;14:2467–2471. doi: 10.1158/1055-9965.EPI-05-0649. [DOI] [PubMed] [Google Scholar]
- 32.Sankar P, et al. Genetic research and health disparities. JAMA. 2004;291:2985–2989. doi: 10.1001/jama.291.24.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brawley OW. Toward a better understanding of race and cancer. Clin. Cancer Res. 2010;16:5920–5922. doi: 10.1158/1078-0432.CCR-10-2541. [DOI] [PubMed] [Google Scholar]
- 34.Race, Ethnicity, and Genetics Working Group (2005) The use of racial, ethnic, and ancestral categories in human genetics research. Am. J. Hum. Genet. 77:519–532. doi: 10.1086/491747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parra EJ, et al. Estimating African American admixture proportions by use of population-specific alleles. Am. J. Hum. Genet. 1998;63:1839–1851. doi: 10.1086/302148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collins-Schramm HE, et al. Ethnic-difference markers for use in mapping by admixture linkage disequilibrium. Am. J. Hum. Genet. 2002;70:737–750. doi: 10.1086/339368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parra FC, et al. Color and genomic ancestry in Brazilians. Proc. Natl Acad. Sci. USA. 2003;100:177–182. doi: 10.1073/pnas.0126614100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benn-Torres J, et al. Admixture and population stratification in African Caribbean populations. Ann. Hum. Genet. 2008;72:90–98. doi: 10.1111/j.1469-1809.2007.00398.x. [DOI] [PubMed] [Google Scholar]
- 39.Giri VN, et al. Race, genetic West African ancestry, and prostate cancer prediction by prostate-specific antigen in prospectively screened high-risk men. Cancer Prev. Res. (Phila.) 2009;2:244–250. doi: 10.1158/1940-6207.CAPR-08-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kittles RA, et al. CYP3A4-V and prostate cancer in African Americans: causal or confounding association because of population stratification? Hum. Genet. 2002;110:553–560. doi: 10.1007/s00439-002-0731-5. [DOI] [PubMed] [Google Scholar]
- 41.Freedman ML, et al. Assessing the impact of population stratification on genetic association studies. Nat. Genet. 2004;36:388–393. doi: 10.1038/ng1333. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, et al. Evaluating bias due to population stratification in epidemiologic studies of gene-gene or gene-environment interactions. Cancer Epidemiol. Biomarkers Prev. 2006;15:124–132. doi: 10.1158/1055-9965.EPI-05-0304. [DOI] [PubMed] [Google Scholar]
- 43.Collins-Schramm HE, et al. Markers that discriminate between European and African ancestry show limited variation within Africa. Hum. Genet. 2002;111:566–569. doi: 10.1007/s00439-002-0818-z. [DOI] [PubMed] [Google Scholar]
- 44.Barnholtz-Sloan JS, et al. Ancestry estimation and correction for population stratification in molecular epidemiologic association studies. Cancer Epidemiol. Biomarkers Prev. 2008;17:471–477. doi: 10.1158/1055-9965.EPI-07-0491. [DOI] [PubMed] [Google Scholar]
- 45.Yang N, et al. Examination of ancestry and ethnic affiliation using highly informative diallelic DNA markers: application to diverse and admixed populations and implications for clinical epidemiology and forensic medicine. Hum. Genet. 2005;118:382–392. doi: 10.1007/s00439-005-0012-1. [DOI] [PubMed] [Google Scholar]
- 46.Barnholtz-Sloan JS, et al. Examining population stratification via individual ancestry estimates versus self-reported race. Cancer Epidemiol. Biomarkers Prev. 2005;14:1545–1551. doi: 10.1158/1055-9965.EPI-04-0832. [DOI] [PubMed] [Google Scholar]
- 47.Tian C, et al. A genomewide single-nucleotide-polymorphism panel with high ancestry information for African American admixture mapping. Am. J. Hum. Genet. 2006;79:640–649. doi: 10.1086/507954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith MW, et al. A high-density admixture map for disease gene discovery in African Americans. Am. J. Hum. Genet. 2004;74:1001–1013. doi: 10.1086/420856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fejerman L, et al. Genetic ancestry and risk of breast cancer among U.S. Latinas. Cancer Res. 2008;68:9723–9728. doi: 10.1158/0008-5472.CAN-08-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fejerman L, et al. European ancestry is positively associated with breast cancer risk in Mexican women. Cancer Epidemiol. Biomarkers Prev. 2010;19:1074–1082. doi: 10.1158/1055-9965.EPI-09-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spielman RS, et al. Common genetic variants account for differences in gene expression among ethnic groups. Nat. Genet. 2007;39:226–231. doi: 10.1038/ng1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Storey JD, et al. Gene-expression variation within and among human populations. Am. J. Hum. Genet. 2007;80:502–509. doi: 10.1086/512017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang W, et al. Evaluation of genetic variation contributing to differences in gene expression between populations. Am. J. Hum. Genet. 2008;82:631–640. doi: 10.1016/j.ajhg.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin DN, et al. Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PLoS One. 2009;4:e4531. doi: 10.1371/journal.pone.0004531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Dyke AL, et al. Cytokine SNPs: comparison of allele frequencies by race and implications for future studies. Cytokine. 2009;46:236–244. doi: 10.1016/j.cyto.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coussens LM, et al. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hussain SP, et al. Radical causes of cancer. Nat. Rev. Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 58.Hancock AM, et al. Colloquium paper: human adaptations to diet, subsistence, and ecoregion are due to subtle shifts in allele frequency. Proc. Natl Acad. Sci. USA. 2010;107(suppl. 2):8924–8930. doi: 10.1073/pnas.0914625107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Genovese G, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saleh M, et al. Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature. 2004;429:75–79. doi: 10.1038/nature02451. [DOI] [PubMed] [Google Scholar]
- 61.Hobbs MR, et al. A new NOS2 promoter polymorphism associated with increased nitric oxide production and protection from severe malaria in Tanzanian and Kenyan children. Lancet. 2002;360:1468–1475. doi: 10.1016/S0140-6736(02)11474-7. [DOI] [PubMed] [Google Scholar]
- 62.Cramer JP, et al. iNOS promoter variants and severe malaria in Ghanaian children. Trop. Med. Int. Health. 2004;9:1074–1080. doi: 10.1111/j.1365-3156.2004.01312.x. [DOI] [PubMed] [Google Scholar]
- 63.Tournamille C, et al. Disruption of a GATA motif in the Duffy gene promoter abolishes erythroid gene expression in Duffy–negative individuals. Nat. Genet. 1995;10:224–228. doi: 10.1038/ng0695-224. [DOI] [PubMed] [Google Scholar]
- 64.Wink DA, et al. The multifaceted roles of nitric oxide in cancer. Carcinogenesis. 1998;19:711–721. doi: 10.1093/carcin/19.5.711. [DOI] [PubMed] [Google Scholar]
- 65.Glynn SA, et al. Increased NOS2 predicts poor survival in estrogen receptor-negative breast cancer patients. J. Clin. Invest. 2010;120:3843–3854. doi: 10.1172/JCI42059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bandyopadhyay S, et al. Interaction of KAI1 on tumor cells with DARC on vascular endothelium leads to metastasis suppression. Nat. Med. 2006;12:933–938. doi: 10.1038/nm1444. [DOI] [PubMed] [Google Scholar]
- 67.Hadley TJ, et al. From malaria to chemokine receptor: the emerging physiologic role of the Duffy blood group antigen. Blood. 1997;89:3077–3091. [PubMed] [Google Scholar]
- 68.Tsai YC, et al. The ubiquitin ligase gp78 promotes sarcoma metastasis by targeting KAI1 for degradation. Nat. Med. 2007;13:1504–1509. doi: 10.1038/nm1686. [DOI] [PubMed] [Google Scholar]
- 69.Wallace TA, et al. Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res. 2008;68:927–936. doi: 10.1158/0008-5472.CAN-07-2608. [DOI] [PubMed] [Google Scholar]
- 70.Timofeeva OA, et al. Enhanced expression of SOS1 is detected in prostate cancer epithelial cells from African-American men. Int. J. Oncol. 2009;35:751–760. [PMC free article] [PubMed] [Google Scholar]
- 71.Ge D, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 72.Suppiah V, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat. Genet. 2009;41:1100–1104. doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- 73.Schafer DF, et al. Hepatocellular carcinoma. Lancet. 1999;353:1253–1257. doi: 10.1016/S0140-6736(98)09148-X. [DOI] [PubMed] [Google Scholar]
- 74.Di Bisceglie AM, et al. Hepatitis C-related hepatocellular carcinoma in the United States: influence of ethnic status. Am. J. Gastroenterol. 2003;98:2060–2063. doi: 10.1111/j.1572-0241.2003.t01-1-07641.x. [DOI] [PubMed] [Google Scholar]
- 75.Conjeevaram HS, et al. Peginterferon and ribavirin treatment in African American and Caucasian American patients with hepatitis C genotype 1. Gastroenterology. 2006;131:470–477. doi: 10.1053/j.gastro.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 76.Smigal C, et al. Trends in breast cancer by race and ethnicity: update 2006. CA Cancer J. Clin. 2006;56:168–183. doi: 10.3322/canjclin.56.3.168. [DOI] [PubMed] [Google Scholar]
- 77.Chien C, et al. Differences in colorectal carcinoma stage and survival by race and ethnicity. Cancer. 2005;104:629–639. doi: 10.1002/cncr.21204. [DOI] [PubMed] [Google Scholar]
- 78.Kerlikowske K, et al. Efficacy of screening mammography. A meta-analysis. JAMA. 1995;273:149–154. [PubMed] [Google Scholar]
- 79.Esserman L, et al. Rethinking screening for breast cancer and prostate cancer. JAMA. 2009;302:1685–1692. doi: 10.1001/jama.2009.1498. [DOI] [PubMed] [Google Scholar]
- 80.Murthy VH, et al. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 81.Clegg LX, et al. Cancer survival among US whites and minorities: a SEER (Surveillance, Epidemiology, and End Results) Program population-based study. Arch. Intern. Med. 2002;162:1985–1993. doi: 10.1001/archinte.162.17.1985. [DOI] [PubMed] [Google Scholar]
- 82.Reyes-Ortiz CA, et al. Neighborhood Composition and cancer among Hispanics: tumor stage and size at time of diagnosis. Cancer Epidemiol. Biomarkers Prev. 2008;17:2931–2936. doi: 10.1158/1055-9965.EPI-07-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Delancey JO, et al. Recent trends in black-white disparities in cancer mortality. Cancer Epidemiol. Biomarkers Prev. 2008;17:2908–2912. doi: 10.1158/1055-9965.EPI-08-0131. [DOI] [PubMed] [Google Scholar]
- 84.Bach PB, et al. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002;287:2106–2113. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]
- 85.Shavers VL, et al. Racial and ethnic disparities in the receipt of cancer treatment. J. Natl Cancer Inst. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 86.Evans S, et al. Investigating Black-White differences in prostate cancer prognosis: a systematic review and meta-analysis. Int. J. Cancer. 2008;123:430–435. doi: 10.1002/ijc.23500. [DOI] [PubMed] [Google Scholar]
- 87.Dignam JJ, et al. Outcomes among African-Americans and Caucasians in colon cancer adjuvant therapy trials: findings from the National Surgical Adjuvant Breast and Bowel Project. J. Natl Cancer Inst. 1999;91:1933–1940. doi: 10.1093/jnci/91.22.1933. [DOI] [PubMed] [Google Scholar]
- 88.Freedland SJ, et al. Race is not an independent predictor of biochemical recurrence after radical prostatectomy in an equal access medical center. Urology. 2000;56:87–91. doi: 10.1016/s0090-4295(00)00587-2. [DOI] [PubMed] [Google Scholar]
- 89.Dignam JJ. Efficacy of systemic adjuvant therapy for breast cancer in African-American and Caucasian women. J. Natl Cancer Inst. Monogr. 2001;30:36–43. doi: 10.1093/oxfordjournals.jncimonographs.a003458. [DOI] [PubMed] [Google Scholar]
- 90.Roach M, III, et al. Race and survival of men treated for prostate cancer on radiation therapy oncology group phase III randomized trials. J. Urol. 2003;169:245–250. doi: 10.1016/S0022-5347(05)64078-5. [DOI] [PubMed] [Google Scholar]
- 91.Roach M, III, et al. Racial differences in CYP3A4 genotype and survival among men treated on Radiation Therapy Oncology Group (RTOG) 9202: a phase III randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2007;69:79–87. doi: 10.1016/j.ijrobp.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 92.Dawood S, et al. Triple receptor-negative breast cancer: the effect of race on response to primary systemic treatment and survival outcomes. J. Clin. Oncol. 2009;27:220–226. doi: 10.1200/JCO.2008.17.9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chu QD, et al. Race/ethnicity has no effect on outcome for breast cancer patients treated at an academic center with a public hospital. Cancer Epidemiol. Biomarkers Prev. 2009;18:2157–2161. doi: 10.1158/1055-9965.EPI-09-0232. [DOI] [PubMed] [Google Scholar]
- 94.Elledge RM, et al. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J. Natl Cancer Inst. 1994;86:705–712. doi: 10.1093/jnci/86.9.705. [DOI] [PubMed] [Google Scholar]
- 95.Moul JW, et al. Black race is an adverse prognostic factor for prostate cancer recurrence following radical prostatectomy in an equal access health care setting. J. Urol. 1996;155:1667–1673. [PubMed] [Google Scholar]
- 96.Powell IJ, et al. Prostate cancer biochemical recurrence stage for stage is more frequent among African-American than white men with locally advanced but not organ-confined disease. Urology. 2000;55:246–251. doi: 10.1016/s0090-4295(99)00436-7. [DOI] [PubMed] [Google Scholar]
- 97.Thompson I, et al. Association of African-American ethnic background with survival in men with metastatic prostate cancer. J. Natl Cancer Inst. 2001;93:219–225. doi: 10.1093/jnci/93.3.219. [DOI] [PubMed] [Google Scholar]
- 98.Thatai LC, et al. Racial disparity in clinical course and outcome of metastatic androgen-independent prostate cancer. Urology. 2004;64:738–743. doi: 10.1016/j.urology.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 99.Hoffman RM, et al. Racial and ethnic differences in advanced-stage prostate cancer: the Prostate Cancer Outcomes Study. J. Natl Cancer Inst. 2001;93:388–395. doi: 10.1093/jnci/93.5.388. [DOI] [PubMed] [Google Scholar]
- 100.Field TS, et al. Disparities and survival among breast cancer patients. J. Natl Cancer Inst. Monogr. 2005;35:88–95. doi: 10.1093/jncimonographs/lgi044. [DOI] [PubMed] [Google Scholar]
- 101.Woodward WA, et al. African-American race is associated with a poorer overall survival rate for breast cancer patients treated with mastectomy and doxorubicin-based chemotherapy. Cancer. 2006;107:2662–2668. doi: 10.1002/cncr.22281. [DOI] [PubMed] [Google Scholar]
- 102.Newman LA, et al. Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. J. Clin. Oncol. 2006;24:1342–1349. doi: 10.1200/JCO.2005.03.3472. [DOI] [PubMed] [Google Scholar]
- 103.Alexander DD, et al. African-American and Caucasian disparities in colorectal cancer mortality and survival by data source: an epidemiologic review. Cancer Biomark. 2007;3:301–313. doi: 10.3233/cbm-2007-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Polite BN, et al. Racial differences in clinical outcomes from metastatic breast cancer: a pooled analysis of CALGB 9342 and 9840-Cancer and Leukemia Group B. J. Clin. Oncol. 2008;26:2659–2665. doi: 10.1200/JCO.2007.13.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Albain KS, et al. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J. Natl Cancer Inst. 2009;101:984–992. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Balmanoukian A, et al. African American women who receive primary anthracycline- and taxane-based chemotherapy for triple-negative breast cancer suffer worse outcomes compared with white women. J. Clin. Oncol. 2009;27:e35–e37. doi: 10.1200/JCO.2008.21.5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Joslyn SA. Racial differences in treatment and survival from early-stage breast carcinoma. Cancer. 2002;95:1759–1766. doi: 10.1002/cncr.10827. [DOI] [PubMed] [Google Scholar]
- 108.Zeliadt SB, et al. Racial disparity in primary and adjuvant treatment for nonmetastatic prostate cancer: SEER-Medicare trends 1991 to 1999. Urology. 2004;64:1171–1176. doi: 10.1016/j.urology.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 109.Potosky AL, et al. Age, sex, and racial differences in the use of standard adjuvant therapy for colorectal cancer. J. Clin. Oncol. 2002;20:1192–1202. doi: 10.1200/JCO.2002.20.5.1192. [DOI] [PubMed] [Google Scholar]
- 110.Hershman D, et al. Racial disparities in treatment and survival among women with early-stage breast cancer. J. Clin. Oncol. 2005;23:6639–6646. doi: 10.1200/JCO.2005.12.633. [DOI] [PubMed] [Google Scholar]
- 111.Bickell NA, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J. Clin. Oncol. 2006;24:1357–1362. doi: 10.1200/JCO.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- 112.Gross CP, et al. Racial disparities in cancer therapy: did the gap narrow between 1992 and 2002? Cancer. 2008;112:900–908. doi: 10.1002/cncr.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dignam JJ, et al. Prognosis after rectal cancer in blacks and whites participating in adjuvant therapy randomized trials. J. Clin. Oncol. 2003;21:413–420. doi: 10.1200/JCO.2003.02.004. [DOI] [PubMed] [Google Scholar]
- 114.Rouzier R, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin. Cancer Res. 2005;11:5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 115.Carey LA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 116.Huo D, et al. Population differences in breast cancer: survey in indigenous African women reveals over-representation of triple-negative breast cancer. J. Clin. Oncol. 2009;27:4515–4521. doi: 10.1200/JCO.2008.19.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stead LA, et al. Triple-negative breast cancers are increased in black women regardless of age or body mass index. Breast Cancer Res. 2009;11:R18. doi: 10.1186/bcr2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stark A, et al. African ancestry and higher prevalence of triple-negative breast cancer: findings from an international study. Cancer. 2010;116:4926–4932. doi: 10.1002/cncr.25276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Krischer JP, et al. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J. Clin. Oncol. 1997;15:1544–1552. doi: 10.1200/JCO.1997.15.4.1544. [DOI] [PubMed] [Google Scholar]
- 120.Hasan S, et al. Doxorubicin cardiotoxicity in African Americans. J. Natl Med. Assoc. 2004;96:196–199. [PMC free article] [PubMed] [Google Scholar]
- 121.Fagerholm R, et al. NAD(P)H:quinone oxidoreductase 1 NQO1*2 genotype (P187S) is a strong prognostic and predictive factor in breast cancer. Nat. Genet. 2008;40:844–853. doi: 10.1038/ng.155. [DOI] [PubMed] [Google Scholar]
- 122.Glynn SA, et al. A mitochondrial target sequence polymorphism in manganese superoxide dismutase predicts inferior survival in breast cancer patients treated with cyclophosphamide. Clin. Cancer Res. 2009;15:4165–4173. doi: 10.1158/1078-0432.CCR-09-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.O'Donnell PH, et al. Cancer pharmacoethnicity: ethnic differences in susceptibility to the effects of chemotherapy. Clin. Cancer Res. 2009;15:4806–4814. doi: 10.1158/1078-0432.CCR-09-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huang RS, et al. Effect of population and gender on chemotherapeutic agent-induced cytotoxicity. Mol. Cancer Ther. 2007;6:31–36. doi: 10.1158/1535-7163.MCT-06-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Antoni MH, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat. Rev. Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Duijts SF, et al. The association between stressful life events and breast cancer risk: a meta-analysis. Int. J. Cancer. 2003;107:1023–1029. doi: 10.1002/ijc.11504. [DOI] [PubMed] [Google Scholar]
- 127.Glaser R, et al. Stress-induced immune dysfunction: implications for health. Nat. Rev. Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 128.Steptoe A, et al. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav. Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 129.Kiecolt-Glaser JK, et al. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc. Natl Acad. Sci. USA. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ara T, et al. Interleukin-6 in bone metastasis and cancer progression. Eur. J. Cancer. 2010;46:1223–1231. doi: 10.1016/j.ejca.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brawley OW. Prostate cancer and black men. Semin. Urol. Oncol. 1998;16:184–186. [PubMed] [Google Scholar]
- 132.Jones BA, et al. Explaining the race difference in prostate cancer stage at diagnosis. Cancer Epidemiol. Biomarkers Prev. 2008;17:2825–2834. doi: 10.1158/1055-9965.EPI-08-0203. [DOI] [PubMed] [Google Scholar]
- 133.Rapiti E, et al. Impact of socioeconomic status on prostate cancer diagnosis, treatment, and prognosis. Cancer. 2009;115:5556–5565. doi: 10.1002/cncr.24607. [DOI] [PubMed] [Google Scholar]
- 134.Fedewa SA, et al. Association of insurance and race/ethnicity with disease severity among men diagnosed with prostate cancer, National Cancer Database 2004–2006. Cancer Epidemiol. Biomarkers Prev. 2010;19:2437–2444. doi: 10.1158/1055-9965.EPI-10-0299. [DOI] [PubMed] [Google Scholar]
- 135.Tiguert R, et al. Racial differences and prognostic significance of tumor location in radical prostatectomy specimens. Prostate. 1998;37:230–235. doi: 10.1002/(sici)1097-0045(19981201)37:4<230::aid-pros4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 136.Tewari A, et al. Racial differences in serum prostate-specific antigen (PSA) doubling time, histopathological variables and long-term PSA recurrence between African-American and white American men undergoing radical prostatectomy for clinically localized prostate cancer. BJU Int. 2005;96:29–33. doi: 10.1111/j.1464-410X.2005.05561.x. [DOI] [PubMed] [Google Scholar]
- 137.Powell IJ, et al. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J. Urol. 2010;183:1792–1796. doi: 10.1016/j.juro.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shibata A, et al. Genetic predisposition to prostate cancer: possible explanations for ethnic differences in risk. Prostate. 1997;32:65–72. doi: 10.1002/(sici)1097-0045(19970615)32:1<65::aid-pros9>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 139.Fowler JE, Jr., et al. Survival in blacks and whites after treatment for localized prostate cancer. J. Urol. 1996;156:133–136. [PubMed] [Google Scholar]
- 140.Ross LE, et al. Use of the prostate-specific antigen test among U.S. men: findings from the 2005 National Health Interview Survey. Cancer Epidemiol. Biomarkers Prev. 2008;17:636–644. doi: 10.1158/1055-9965.EPI-07-2709. [DOI] [PubMed] [Google Scholar]
- 141.Scales CD, Jr., et al. Prostate-specific antigen screening among young men in the United States. Cancer. 2008;113:1315–1323. doi: 10.1002/cncr.23667. [DOI] [PubMed] [Google Scholar]
- 142.Potts JM, et al. Trends in PSA, age and prostate cancer detection among black and white men from 1990-2006 at a tertiary care center. Cancer. 2010;116:3910–3915. doi: 10.1002/cncr.25124. [DOI] [PubMed] [Google Scholar]
- 143.Bouchardy C, et al. Ethnicity and cancer risk in Sao Paulo, Brazil. Cancer Epidemiol. Biomarkers Prev. 1991;1:21–27. [PubMed] [Google Scholar]
- 144.Odedina FT, et al. Prostate cancer disparities in Black men of African descent: a comparative literature review of prostate cancer burden among Black men in the United States, Caribbean, United Kingdom, and West Africa. Infect. Agent. Cancer. 2009;4(suppl. 1):S2. doi: 10.1186/1750-9378-4-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ben Shlomo Y, et al. The risk of prostate cancer amongst Black Men in the United Kingdom: the PROCESS cohort study. Eur. Urol. 2007;53:99–105. doi: 10.1016/j.eururo.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 146.Bunker CH, et al. High prevalence of screening-detected prostate cancer among Afro-Caribbeans: the Tobago Prostate Cancer Survey. Cancer Epidemiol. Biomarkers Prev. 2002;11:726–729. [PubMed] [Google Scholar]
- 147.Osegbe DN. Prostate cancer in Nigerians: facts and nonfacts. J. Urol. 1997;157:1340–1343. [PubMed] [Google Scholar]
- 148.Gueye SM, et al. Clinical characteristics of prostate cancer in African Americans, American whites, and Senegalese men. Urology. 2003;61:987–992. doi: 10.1016/s0090-4295(02)02588-8. [DOI] [PubMed] [Google Scholar]
- 149.Angwafo FF, III, et al. High-grade intra-epithelial neoplasia and prostate cancer in Dibombari, Cameroon. Prostate Cancer Prostatic Dis. 2003;6:34–38. doi: 10.1038/sj.pcan.4500587. [DOI] [PubMed] [Google Scholar]
- 150.Wasike RW, et al. Descriptive case series of patients presenting with cancer of the prostate and their management at Kenyatta National Hospital, Nairobi. East Afr. Med. J. 2007;84:S31–S35. doi: 10.4314/eamj.v84i9.9559. [DOI] [PubMed] [Google Scholar]
- 151.Hsing AW, et al. International trends and patterns of prostate cancer incidence and mortality. Int. J. Cancer. 2000;85:60–67. doi: 10.1002/(sici)1097-0215(20000101)85:1<60::aid-ijc11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 152.Mordukhovich I, et al. A review of African American-white differences in risk factors for cancer: prostate cancer. Cancer Causes Control. 2011;22:341–357. doi: 10.1007/s10552-010-9712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Block G, et al. Estimates of nutrient intake from a food frequency questionnaire: the 1987 National Health Interview Survey. J. Am. Diet. Assoc. 1992;92:969–977. [PubMed] [Google Scholar]
- 154.Swanson CA, et al. A comparison of diets of blacks and whites in three areas of the United States. Nutr. Cancer. 1993;20:153–165. doi: 10.1080/01635589309514282. [DOI] [PubMed] [Google Scholar]
- 155.Kant AK, et al. Trends in black-white differentials in dietary intakes of U.S. adults, 1971–2002. Am. J. Prev. Med. 2007;32:264–272. doi: 10.1016/j.amepre.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hayes RB, et al. Dietary factors and risks for prostate cancer among blacks and whites in the United States. Cancer Epidemiol. Biomarkers Prev. 1999;8:25–34. [PubMed] [Google Scholar]
- 157.Rodriguez C, et al. Meat consumption among Black and White men and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol. Biomarkers Prev. 2006;15:211–216. doi: 10.1158/1055-9965.EPI-05-0614. [DOI] [PubMed] [Google Scholar]
- 158.Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States) Cancer Causes Control. 2005;16:83–95. doi: 10.1007/s10552-004-1661-4. [DOI] [PubMed] [Google Scholar]
- 159.Yin L, et al. Meta-analysis of longitudinal studies: serum vitamin D and prostate cancer risk. Cancer Epidemiol. 2009;33:435–445. doi: 10.1016/j.canep.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 160.Flegal KM, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 161.Calle EE, et al. Obesity and cancer. Oncogene. 2004;23:6365–6378. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 162.Spangler E, et al. Association of obesity with tumor characteristics and treatment failure of prostate cancer in African-American and European American men. J. Urol. 2007;178:1939–1944. doi: 10.1016/j.juro.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 163.Cao Y, et al. Body-mass index, prostate cancer-specific mortality and biochemical recurrence:a systematic review and meta-analysis. Cancer Prev. Res. (Phila.) 2011;4:486–501. doi: 10.1158/1940-6207.CAPR-10-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ornish D, et al. Changes in prostate gene expression in men undergoing an intensive nutrition and lifestyle intervention. Proc. Natl Acad. Sci. USA. 2008;105:8369–8374. doi: 10.1073/pnas.0803080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sharad S, et al. Prostate cancer gene expression signature of patients with high body mass index. Prostate Cancer Prostatic Dis. 2011;14:22–29. doi: 10.1038/pcan.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gaston KE, et al. Racial differences in androgen receptor protein expression in men with clinically localized prostate cancer. J. Urol. 2003;170:990–993. doi: 10.1097/01.ju.0000079761.56154.e5. [DOI] [PubMed] [Google Scholar]
- 167.Ross R, et al. Serum testosterone levels in healthy young black and white men. J. Natl Cancer Inst. 1986;76:45–48. [PubMed] [Google Scholar]
- 168.Ellis L, et al. Racial/ethnic variations in male testosterone levels: a probable contributor to group differences in health. Steroids. 1992;57:72–75. doi: 10.1016/0039-128x(92)90032-5. [DOI] [PubMed] [Google Scholar]
- 169.Calistro AL. Population differences in the testosterone levels of young men are associated with prostate cancer disparities in older men. Am. J. Hum. Biol. 2010;22:449–455. doi: 10.1002/ajhb.21016. [DOI] [PubMed] [Google Scholar]
- 170.Roddam AW, et al. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J. Natl Cancer Inst. 2008;100:170–183. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mohler JL, et al. Racial differences in prostate androgen levels in men with clinically localized prostate cancer. J. Urol. 2004;171:2277–2280. doi: 10.1097/01.ju.0000127739.88383.79. [DOI] [PubMed] [Google Scholar]
- 172.Marks LS, et al. Prostatic tissue testosterone and dihydrotestosterone in African-American and white men. Urology. 2006;68:337–341. doi: 10.1016/j.urology.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 173.Mavropoulos JC, et al. Do racial differences in prostate size explain higher serum prostate-specific antigen concentrations among black men? Urology. 2007;69:1138–1142. doi: 10.1016/j.urology.2007.01.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rohrmann S, et al. Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of Americans. J. Clin. Endocrinol. Metab. 2007;92:2519–2525. doi: 10.1210/jc.2007-0028. [DOI] [PubMed] [Google Scholar]
- 175.Orwoll ES, et al. Evidence for geographical and racial variation in serum sex steroid levels in older men. J. Clin. Endocrinol. Metab. 2010;95:E151–E160. doi: 10.1210/jc.2009-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tam NN, et al. Sex hormones induce direct epithelial and inflammation-mediated oxidative/nitrosative stress that favors prostatic carcinogenesis in the noble rat. Am. J. Pathol. 2007;171:1334–1341. doi: 10.2353/ajpath.2007.070199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Eastham JA, et al. Clinical characteristics and biopsy specimen features in African-American and white men without prostate cancer. J. Natl Cancer Inst. 1998;90:756–760. doi: 10.1093/jnci/90.10.756. [DOI] [PubMed] [Google Scholar]
- 178.Dennis LK, et al. Epidemiologic association between prostatitis and prostate cancer. Urology. 2002;60:78–83. doi: 10.1016/s0090-4295(02)01637-0. [DOI] [PubMed] [Google Scholar]
- 179.De Marzo AM, et al. Inflammation in prostate carcinogenesis. Nat. Rev. Cancer. 2007;7:256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Penney KL, et al. Genome-wide association study of prostate cancer mortality. Cancer Epidemiol. Biomarkers Prev. 2010;19:2869–2876. doi: 10.1158/1055-9965.EPI-10-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Robbins C, et al. Confirmation study of prostate cancer risk variants at 8q24 in African Americans identifies a novel risk locus. Genome Res. 2007;17:1717–1722. doi: 10.1101/gr.6782707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Xu J, et al. Prostate cancer risk associated loci in African Americans. Cancer Epidemiol. Biomarkers Prev. 2009;18:2145–2149. doi: 10.1158/1055-9965.EPI-09-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hooker S, et al. Replication of prostate cancer risk loci on 8q24, 11q13, 17q12, 19q33, and Xp11 in African Americans. Prostate. 2010;70:270–275. doi: 10.1002/pros.21061. [DOI] [PubMed] [Google Scholar]
- 184.Zheng SL, et al. A comprehensive association study for genes in inflammation pathway provides support for their roles in prostate cancer risk in the CAPS study. Prostate. 2006;66:1556–1564. doi: 10.1002/pros.20496. [DOI] [PubMed] [Google Scholar]
- 185.Sun J, et al. Genetic variability in inflammation pathways and prostate cancer risk. Urol. Oncol. 2007;25:250–259. doi: 10.1016/j.urolonc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 186.Fernandez P, et al. COX-2 promoter polymorphisms and the association with prostate cancer risk in South African men. Carcinogenesis. 2008;29:2347–2350. doi: 10.1093/carcin/bgn245. [DOI] [PubMed] [Google Scholar]
- 187.Zabaleta J, et al. Interactions of cytokine gene polymorphisms in prostate cancer risk. Carcinogenesis. 2008;29:573–578. doi: 10.1093/carcin/bgm277. [DOI] [PubMed] [Google Scholar]
- 188.Panguluri RC, et al. COX-2 gene promoter haplotypes and prostate cancer risk. Carcinogenesis. 2004;25:961–966. doi: 10.1093/carcin/bgh100. [DOI] [PubMed] [Google Scholar]
- 189.Shook SJ, et al. Association of RNASEL variants with prostate cancer risk in Hispanic Caucasians and African Americans. Clin. Cancer Res. 2007;13:5959–5964. doi: 10.1158/1078-0432.CCR-07-0702. [DOI] [PubMed] [Google Scholar]
- 190.Rennert H, et al. Association of susceptibility alleles in ELAC2/HPC2, RNASEL/HPC1, and MSR1 with prostate cancer severity in European American and African American men. Cancer Epidemiol. Biomarkers Prev. 2005;14:949–957. doi: 10.1158/1055-9965.EPI-04-0637. [DOI] [PubMed] [Google Scholar]
- 191.Robbins CM, et al. Association of HPC2/ELAC2 and RNASEL non-synonymous variants with prostate cancer risk in African American familial and sporadic cases. Prostate. 2008;68:1790–1797. doi: 10.1002/pros.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Guo Y, et al. Racial differences in prostate cancer growth: apoptosis and cell proliferation in Caucasian and African-American patients. Prostate. 2000;42:130–136. doi: 10.1002/(sici)1097-0045(20000201)42:2<130::aid-pros7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 193.Mimeault M, et al. Recent advances on multiple tumorigenic cascades involved in prostatic cancer progression and targeting therapies. Carcinogenesis. 2006;27:1–22. doi: 10.1093/carcin/bgi229. [DOI] [PubMed] [Google Scholar]
- 194.Shuch B, et al. Racial disparity of epidermal growth factor receptor expression in prostate cancer. J. Clin.Oncol. 2004;22:4673–4677. doi: 10.1200/JCO.2004.06.134. [DOI] [PubMed] [Google Scholar]
- 195.Nelson WG, et al. Epigenetic alterations in human prostate cancers. Endocrinology. 2009;150:3991–4002. doi: 10.1210/en.2009-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Woodson K, et al. Hypermethylation of GSTP1, CD44, and E-cadherin genes in prostate cancer among US Blacks and Whites. Prostate. 2003;55:199–205. doi: 10.1002/pros.10236. [DOI] [PubMed] [Google Scholar]
- 197.Enokida H, et al. Ethnic group-related differences in CpG hypermethylation of the GSTP1 gene promoter among African-American, Caucasian and Asian patients with prostate cancer. Int. J. Cancer. 2005;116:174–181. doi: 10.1002/ijc.21017. [DOI] [PubMed] [Google Scholar]
- 198.Kwabi-Addo B, et al. Identification of differentially methylated genes in normal prostate tissues from African American and Caucasian men. Clin. Cancer Res. 2010;16:3539–3547. doi: 10.1158/1078-0432.CCR-09-3342. [DOI] [PubMed] [Google Scholar]
- 199.Cher ML, et al. A similar pattern of chromosomal alterations in prostate cancers from African-Americans and Caucasian Americans. Clin. Cancer Res. 1998;4:1273–1278. [PubMed] [Google Scholar]
- 200.Rose AE, et al. Copy number and gene expression differences between African American and Caucasian American prostate cancer. J. Transl. Med. 2010;8:70. doi: 10.1186/1479-5876-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Castro P, et al. Genomic profiling of prostate cancers from African American men. Neoplasia. 2009;11:305–312. doi: 10.1593/neo.81530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kumar-Sinha C, et al. Recurrent gene fusions in prostate cancer. Nat. Rev. Cancer. 2008;8:497–511. doi: 10.1038/nrc2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mao X, et al. Distinct genomic alterations in prostate cancers in Chinese and Western populations suggest alternative pathways of prostate carcinogenesis. Cancer Res. 2010;70:5207–5212. doi: 10.1158/0008-5472.CAN-09-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Magi-Galluzzi C, et al. TMPRSS2-ERG gene fusion prevalence and class are significantly different in prostate cancer of caucasian, african-american and japanese patients. Prostate. 2011;71:489–497. doi: 10.1002/pros.21265. [DOI] [PubMed] [Google Scholar]
- 205.Srikantan V, et al. PCGEM1, a prostate-specific gene, is overexpressed in prostate cancer. Proc. Natl Acad. Sci. USA. 2000;97:12216–12221. doi: 10.1073/pnas.97.22.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Petrovics G, et al. Elevated expression of PCGEM1, a prostate-specific gene with cell growth-promoting function, is associated with high-risk prostate cancer patients. Oncogene. 2004;23:605–611. doi: 10.1038/sj.onc.1207069. [DOI] [PubMed] [Google Scholar]
- 207.Reams RR, et al. Microarray comparison of prostate tumor gene expression in African-American and Caucasian American males: a pilot project study. Infect. Agent. Cancer. 2009;4(suppl. 1):S3. doi: 10.1186/1750-9378-4-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Einav U, et al. Gene expression analysis reveals a strong signature of an interferon-induced pathway in childhood lymphoblastic leukemia as well as in breast and ovarian cancer. Oncogene. 2005;24:6367–6375. doi: 10.1038/sj.onc.1208797. [DOI] [PubMed] [Google Scholar]
- 209.Buess M, et al. Characterization of heterotypic interaction effects in vitro to deconvolute global gene expression profiles in cancer. Genome Biol. 2007;8:R191. doi: 10.1186/gb-2007-8-9-r191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Weichselbaum RR, et al. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc. Natl Acad. Sci. USA. 2008;105:18490–18495. doi: 10.1073/pnas.0809242105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Khodarev NN, et al. STAT1 pathway mediates amplification of metastatic potential and resistance to therapy. PLoS One. 2009;4:e5821. doi: 10.1371/journal.pone.0005821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Key TJ, et al. Epidemiology of breast cancer. Lancet Oncol. 2001;2:133–140. doi: 10.1016/S1470-2045(00)00254-0. [DOI] [PubMed] [Google Scholar]
- 213.Michels KB, et al. Diet and breast cancer: a review of the prospective observational studies. Cancer. 2007;109:2712–2749. doi: 10.1002/cncr.22654. [DOI] [PubMed] [Google Scholar]
- 214.Ghafoor A, et al. Trends in breast cancer by race and ethnicity. CA Cancer J. Clin. 2003;53:342–355. doi: 10.3322/canjclin.53.6.342. [DOI] [PubMed] [Google Scholar]
- 215.Bernstein L, et al. Ethnicity-related variation in breast cancer risk factors. Cancer. 2003;97:222–229. doi: 10.1002/cncr.11014. [DOI] [PubMed] [Google Scholar]
- 216.Colditz GA, et al. Risk factors for breast cancer according to estrogen and progesterone receptor status. J. Natl Cancer Inst. 2004;96:218–228. doi: 10.1093/jnci/djh025. [DOI] [PubMed] [Google Scholar]
- 217.Vona-Davis L, et al. The influence of socioeconomic disparities on breast cancer tumor biology and prognosis: a review. J. Womens Health (Larchmt.) 2009;18:883–893. doi: 10.1089/jwh.2008.1127. [DOI] [PubMed] [Google Scholar]
- 218.Yang XR, et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the breast cancer association consortium studies. J. Natl Cancer Inst. 2011;103:250–263. doi: 10.1093/jnci/djq526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Jemal A, et al. Recent trends in breast cancer incidence rates by age and tumor characteristics among U.S. women. Breast Cancer Res. 2007;9:R28. doi: 10.1186/bcr1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Katagiri T, et al. Mutations in the BRCA1 gene in Japanese breast cancer patients. Hum. Mutat. 1996;7:334–339. doi: 10.1002/(SICI)1098-1004(1996)7:4<334::AID-HUMU7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 221.Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171–182. doi: 10.1016/s0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 222.Olopade OI, et al. Breast cancer genetics in African Americans. Cancer. 2003;97:236–245. doi: 10.1002/cncr.11019. [DOI] [PubMed] [Google Scholar]
- 223.Zheng W, et al. Evaluation of 11 breast cancer susceptibility loci in African-American women. Cancer Epidemiol. Biomarkers Prev. 2009;18:2761–2764. doi: 10.1158/1055-9965.EPI-09-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Barnholtz-Sloan JS, et al. FGFR2 and other loci identified in genome-wide association studies are associated with breast cancer in African-American and younger women. Carcinogenesis. 2010;31:1417–1423. doi: 10.1093/carcin/bgq128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Chen F, et al. Caution in generalizing known genetic risk markers for breast cancer across all ethnic/racial populations. Eur. J. Hum. Genet. 2011;19:243–245. doi: 10.1038/ejhg.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Perou CM, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 227.Sorlie T, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl Acad. Sci. USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Nielsen TO, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin. Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 229.Cleator S, et al. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007;8:235–244. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- 230.Bentzon N, et al. Prognostic effect of estrogen receptor status across age in primary breast cancer. Int. J. Cancer. 2008;122:1089–1094. doi: 10.1002/ijc.22892. [DOI] [PubMed] [Google Scholar]
- 231.Blows FM, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7:e1000279. doi: 10.1371/journal.pmed.1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ihemelandu CU, et al. Molecular breast cancer subtypes in premenopausal and postmenopausal african-american women: age-specific prevalence and survival. J. Surg. Res. 2007;143:109–118. doi: 10.1016/j.jss.2007.03.085. [DOI] [PubMed] [Google Scholar]
- 233.Millikan RC, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res. Treat. 2008;109:123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Cunningham JE, et al. Racial differences in the incidence of breast cancer subtypes defined by combined histologic grade and hormone receptor status. Cancer Causes Control. 2010;21:399–409. doi: 10.1007/s10552-009-9472-2. [DOI] [PubMed] [Google Scholar]
- 235.Ijaduola TG, et al. Pattern of breast cancer among white-American, African-American, and nonimmigrant west-African women. J. Natl Med. Assoc. 1998;90:547–551. [PMC free article] [PubMed] [Google Scholar]
- 236.Ikpatt OF, et al. Oestrogen and progesterone receptors in Nigerian breast cancer: relationship to tumour histopathology and survival of patients. Cent. Afr. J. Med. 2003;49:122–126. [PubMed] [Google Scholar]
- 237.Gukas ID, et al. Clinicopathological features and molecular markers of breast cancer in Jos, Nigeria. West Afr. J. Med. 2005;24:209–213. doi: 10.4314/wajm.v24i3.28220. [DOI] [PubMed] [Google Scholar]
- 238.Bird PA, et al. Poor hormone receptor expression in East African breast cancer: evidence of a biologically different disease? Ann. Surg. Oncol. 2008;15:1983–1988. doi: 10.1245/s10434-008-9900-7. [DOI] [PubMed] [Google Scholar]
- 239.Awadelkarim KD, et al. Pathological, clinical and prognostic characteristics of breast cancer in Central Sudan versus Northern Italy: implications for breast cancer in Africa. Histopathology. 2008;52:445–456. doi: 10.1111/j.1365-2559.2008.02966.x. [DOI] [PubMed] [Google Scholar]
- 240.Hennis AJ, et al. Breast cancer incidence and mortality in a Caribbean population: comparisons with African-Americans. Int. J. Cancer. 2009;124:429–433. doi: 10.1002/ijc.23889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kakarala M, et al. Breast cancer histology and receptor status characterization in Asian Indian and Pakistani women in the U.S.-a SEER analysis. BMC Cancer. 2010;10:191. doi: 10.1186/1471-2407-10-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Hausauer AK, et al. Recent breast cancer trends among Asian/Pacific Islander, Hispanic, and African-American women in the US: changes by tumor subtype. Breast Cancer Res. 2007;9:R90. doi: 10.1186/bcr1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Yang XR, et al. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol. Biomarkers Prev. 2007;16:439–443. doi: 10.1158/1055-9965.EPI-06-0806. [DOI] [PubMed] [Google Scholar]
- 244.Gordon NH. Association of education and income with estrogen receptor status in primary breast cancer. Am. J. Epidemiol. 1995;142:796–803. doi: 10.1093/oxfordjournals.aje.a117718. [DOI] [PubMed] [Google Scholar]
- 245.Thomson CS, et al. Prognostic factors in women with breast cancer: distribution by socioeconomic status and effect on differences in survival. J. Epidemiol. Community Health. 2001;55:308–315. doi: 10.1136/jech.55.5.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Bauer KR, et al. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 247.Vainshtein J. Disparities in breast cancer incidence across racial/ethnic strata and socioeconomic status: a systematic review. J. Natl Med. Assoc. 2008;100:833–839. doi: 10.1016/s0027-9684(15)31378-x. [DOI] [PubMed] [Google Scholar]
- 248.Eley JW, et al. Racial differences in survival from breast cancer. Results of the National Cancer Institute Black/White Cancer Survival Study. JAMA. 1994;272:947–954. doi: 10.1001/jama.272.12.947. [DOI] [PubMed] [Google Scholar]
- 249.Newman LA, et al. African-American ethnicity, socioeconomic status, and breast cancer survival: a meta-analysis of 14 studies involving over 10,000 African-American and 40,000 White American patients with carcinoma of the breast. Cancer. 2002;94:2844–2854. doi: 10.1002/cncr.10575. [DOI] [PubMed] [Google Scholar]
- 250.Chlebowski RT, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J. Natl Cancer Inst. 2005;97:439–448. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 251.Curtis E, et al. Racial and ethnic differences in breast cancer survival: how much is explained by screening, tumor severity, biology, treatment, comorbidities, and demographics? Cancer. 2008;112:171–180. doi: 10.1002/cncr.23131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Menashe I, et al. Underlying causes of the black-white racial disparity in breast cancer mortality: a population-based analysis. J. Natl Cancer Inst. 2009;101:993–1000. doi: 10.1093/jnci/djp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Demicheli R, et al. Racial disparities in breast cancer outcome: insights into host-tumor interactions. Cancer. 2007;110:1880–1888. doi: 10.1002/cncr.22998. [DOI] [PubMed] [Google Scholar]
- 254.Shavers VL, et al. Racial/ethnic variation in clinical presentation, treatment, and survival among breast cancer patients under age 35. Cancer. 2003;97:134–147. doi: 10.1002/cncr.11051. [DOI] [PubMed] [Google Scholar]
- 255.van Ravesteyn NT, et al. Race-specific impact of natural history, mammography screening, and adjuvant treatment on breast cancer mortality rates in the United States. Cancer Epidemiol. Biomarkers Prev. 2011;20:112–122. doi: 10.1158/1055-9965.EPI-10-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wojcik BE, et al. Breast carcinoma survival analysis for African American and white women in an equal-access health care system. Cancer. 1998;82:1310–1318. doi: 10.1002/(sici)1097-0142(19980401)82:7<1310::aid-cncr14>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 257.Jatoi I, et al. Widening disparity in survival between white and African-American patients with breast carcinoma treated in the U. S. Department of Defense Healthcare system. Cancer. 2003;98:894–899. doi: 10.1002/cncr.11604. [DOI] [PubMed] [Google Scholar]
- 258.Anders CK, et al. The prognostic contribution of clinical breast cancer subtype, age, and race among patients with breast cancer brain metastases. Cancer. 2010;117:1602–1611. doi: 10.1002/cncr.25746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.O'Brien KM, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin. Cancer Res. 2010;16:6100–6110. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Rose DP, et al. Tumor biology and prognosis in black breast cancer patients: a review. Cancer Detect. Prev. 2001;25:16–31. [PubMed] [Google Scholar]
- 261.Joslyn SA. Hormone receptors in breast cancer: racial differences in distribution and survival. Breast Cancer Res. Treat. 2002;73:45–59. doi: 10.1023/a:1015220420400. [DOI] [PubMed] [Google Scholar]
- 262.Poola I, et al. Functionally active estrogen receptor isoform profiles in the breast tumors of African American women are different from the profiles in breast tumors of Caucasian women. Cancer. 2002;94:615–623. doi: 10.1002/cncr.10274. [DOI] [PubMed] [Google Scholar]
- 263.Ma X, et al. Prostaglandin E receptor EP1 suppresses breast cancer metastasis and is linked to survival differences and cancer disparities. Mol. Cancer Res. 2010;8:1310–1318. doi: 10.1158/1541-7786.MCR-10-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Porter PL, et al. Racial differences in the expression of cell cycle-regulatory proteins in breast carcinoma. Cancer. 2004;100:2533–2542. doi: 10.1002/cncr.20279. [DOI] [PubMed] [Google Scholar]
- 265.Jones BA, et al. African-American/White differences in breast carcinoma: p53 alterations and other tumor characteristics. Cancer. 2004;101:1293–1301. doi: 10.1002/cncr.20500. [DOI] [PubMed] [Google Scholar]
- 266.Baker L, et al. p53 mutation, deprivation and poor prognosis in primary breast cancer. Br. J. Cancer. 2010;102:719–726. doi: 10.1038/sj.bjc.6605540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Greenblatt MS, et al. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994;54:4855–4878. [PubMed] [Google Scholar]
- 268.Mehrotra J, et al. Estrogen receptor/progesterone receptor-negative breast cancers of young African-American women have a higher frequency of methylation of multiple genes than those of Caucasian women. Clin. Cancer Res. 2004;10:2052–2057. doi: 10.1158/1078-0432.ccr-03-0514. [DOI] [PubMed] [Google Scholar]
- 269.Camp JT, et al. Interactions with fibroblasts are distinct in Basal-like and luminal breast cancers. Mol. Cancer Res. 2011;9:3–13. doi: 10.1158/1541-7786.MCR-10-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Khodarev NN, et al. STAT1 is overexpressed in tumors selected for radioresistance and confers protection from radiation in transduced sensitive cells. Proc. Natl Acad. Sci. USA. 2004;101:1714–1719. doi: 10.1073/pnas.0308102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wei P, et al. Differential endothelial cell gene expression by African Americans versus Caucasian Americans: a possible contribution to health disparity in vascular disease and cancer. BMC Med. 2011;9:2. doi: 10.1186/1741-7015-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Kelly AP. Keloids. Dermatol. Clin. 1988;6:413–424. [PubMed] [Google Scholar]
- 273.Butler PD, et al. Current progress in keloid research and treatment. J. Am. Coll. Surg. 2008;206:731–741. doi: 10.1016/j.jamcollsurg.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 274.Smith JC, et al. Gene profiling of keloid fibroblasts shows altered expression in multiple fibrosis-associated pathways. J. Invest. Dermatol. 2008;128:1298–1310. doi: 10.1038/sj.jid.5701149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Russell SB, et al. Epigenetically altered wound healing in keloid fibroblasts. J. Invest. Dermatol. 2010;130:2489–2496. doi: 10.1038/jid.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Thun MJ, et al. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med. 2008;5:e185. doi: 10.1371/journal.pmed.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Berger M, et al. Racial disparities in lung cancer. Curr. Probl. Cancer. 2007;31:202–210. doi: 10.1016/j.currproblcancer.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 278.Lathan CS, et al. The effect of race on invasive staging and surgery in non-small-cell lung cancer. J. Clin. Oncol. 2006;24:413–418. doi: 10.1200/JCO.2005.02.1758. [DOI] [PubMed] [Google Scholar]
- 279.Etzel CJ, et al. Development and validation of a lung cancer risk prediction model for African-Americans. Cancer Prev. Res. (Phila.) 2008;1:255–265. doi: 10.1158/1940-6207.CAPR-08-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Haiman CA, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N. Engl. J. Med. 2006;354:333–342. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 281.Schwartz AG, et al. Lung carcinoma in African Americans and whites. A population- based study in metropolitan Detroit, Michigan. Cancer. 1997;79:45–52. doi: 10.1002/(sici)1097-0142(19970101)79:1<45::aid-cncr7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 282.Oscherwitz M, et al. Differences in pulmonary functions in various racial groups. Am. J. Epidemiol. 1972;96:319–327. doi: 10.1093/oxfordjournals.aje.a121462. [DOI] [PubMed] [Google Scholar]
- 283.Cote ML, et al. Risk of lung cancer among white and black relatives of individuals with early-onset lung cancer. JAMA. 2005;293:3036–3042. doi: 10.1001/jama.293.24.3036. [DOI] [PubMed] [Google Scholar]
- 284.Benowitz NL, et al. Mentholated cigarette smoking inhibits nicotine metabolism. J. Pharmacol. Exp. Ther. 2004;310:1208–1215. doi: 10.1124/jpet.104.066902. [DOI] [PubMed] [Google Scholar]
- 285.West KA, et al. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J. Clin. Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Clark PI, et al. Effect of menthol cigarettes on biochemical markers of smoke exposure among black and white smokers. Chest. 1996;110:1194–1198. doi: 10.1378/chest.110.5.1194. [DOI] [PubMed] [Google Scholar]
- 287.Perez-Stable EJ, et al. Nicotine metabolism and intake in black and white smokers. JAMA. 1998;280:152–156. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- 288.Kabat GC, et al. Use of mentholated cigarettes and lung cancer risk. Cancer Res. 1991;51:6510–6513. [PubMed] [Google Scholar]
- 289.Stellman SD, et al. Lung cancer risk in white and black Americans. Ann. Epidemiol. 2003;13:294–302. doi: 10.1016/s1047-2797(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 290.Werley MS, et al. Possible effects on smokers of cigarette mentholation: a review of the evidence relating to key research questions. Regul. Toxicol. Pharmacol. 2007;47:189–203. doi: 10.1016/j.yrtph.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 291.Zheng YL, et al. Less efficient g2-m checkpoint is associated with an increased risk of lung cancer in African Americans. Cancer Res. 2005;65:9566–9573. doi: 10.1158/0008-5472.CAN-05-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Pine SR, et al. Association of lung cancer outcome with functional polymorphisms in Mammose-binding Lectin-2, an innate immunity gene. J. Natl Cancer Inst. 2007;99:1401–1409. doi: 10.1093/jnci/djm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Enewold L, et al. Serum concentrations of cytokines and lung cancer survival in African Americans and Caucasians. Cancer Epidemiol. Biomarkers Prev. 2009;18:215–222. doi: 10.1158/1055-9965.EPI-08-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Chang JS, et al. Nucleotide excision repair genes and risk of lung cancer among San Francisco Bay Area Latinos and African Americans. Int. J. Cancer. 2008;123:2095–2104. doi: 10.1002/ijc.23801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Kauh J, et al. Racial disparities in colorectal cancer. Curr. Probl. Cancer. 2007;31:123–133. doi: 10.1016/j.currproblcancer.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 296.Polite BN, et al. Colorectal cancer and race: understanding the differences in outcomes between African Americans and whites. Med. Clin. North Am. 2005;89:771–793. doi: 10.1016/j.mcna.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 297.Mayberry RM, et al. Determinants of black/white differences in colon cancer survival. J. Natl Cancer Inst. 1995;87:1686–1693. doi: 10.1093/jnci/87.22.1686. [DOI] [PubMed] [Google Scholar]
- 298.Le H, et al. Effects of socioeconomic status and treatment disparities in colorectal cancer survival. Cancer Epidemiol. Biomarkers Prev. 2008;17:1950–1962. doi: 10.1158/1055-9965.EPI-07-2774. [DOI] [PubMed] [Google Scholar]
- 299.Murff HJ, et al. Colonoscopy screening in African Americans and Whites with affected first-degree relatives. Arch. Intern. Med. 2008;168:625–631. doi: 10.1001/archinte.168.6.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.McCollum AD, et al. Outcomes and toxicity in african-american and caucasian patients in a randomized adjuvant chemotherapy trial for colon cancer. J. Natl Cancer Inst. 2002;94:1160–1167. doi: 10.1093/jnci/94.15.1160. [DOI] [PubMed] [Google Scholar]
- 301.Dominitz JA, et al. Race, treatment, and survival among colorectal carcinoma patients in an equal-access medical system. Cancer. 1998;82:2312–2320. doi: 10.1002/(sici)1097-0142(19980615)82:12<2312::aid-cncr3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 302.Rabeneck L, et al. Survival of colorectal cancer patients hospitalized in the Veterans Affairs Health Care System. Am. J. Gastroenterol. 2003;98:1186–1192. doi: 10.1111/j.1572-0241.2003.07448.x. [DOI] [PubMed] [Google Scholar]
- 303.Shavers VL. Racial/ethnic variation in the anatomic subsite location of in situ and invasive cancers of the colon. J. Natl Med. Assoc. 2007;99:733–748. [PMC free article] [PubMed] [Google Scholar]
- 304.Troisi RJ, et al. Incidence of colorectal carcinoma in the U.S.: an update of trends by gender, race, age, subsite, and stage, 1975–1994. Cancer. 1999;85:1670–1676. [PubMed] [Google Scholar]
- 305.Lieberman DA, et al. Prevalence of colon polyps detected by colonoscopy screening in asymptomatic black and white patients. JAMA. 2008;300:1417–1422. doi: 10.1001/jama.300.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Brim H, et al. Impact of BRAF, MLH1 on the incidence of microsatellite instability high colorectal cancer in populations based study. Mol. Cancer. 2008;7:68. doi: 10.1186/1476-4598-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Ashktorab H, et al. High incidence of microsatellite instability in colorectal cancer from African Americans. Clin. Cancer Res. 2003;9:1112–1117. [PubMed] [Google Scholar]
- 308.Manne U, et al. Nuclear accumulation of p53 in colorectal adenocarcinoma: prognostic importance differs with race and location of the tumor. Cancer. 1998;83:2456–2467. doi: 10.1002/(sici)1097-0142(19981215)83:12<2456::aid-cncr8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 309.Goodman JE, et al. Arachidonate lipoxygenase (ALOX) and cyclooxygenase (COX) polymorphisms and colon cancer risk. Carcinogenesis. 2004;25:2467–2472. doi: 10.1093/carcin/bgh260. [DOI] [PubMed] [Google Scholar]
- 310.Sansbury LB, et al. COX-2 polymorphism, use of nonsteroidal anti-inflammatory drugs, and risk of colon cancer in African Americans (United States) Cancer Causes Control. 2006;17:257–266. doi: 10.1007/s10552-005-0417-0. [DOI] [PubMed] [Google Scholar]