Abstract
Helicobacter pylori-associated gastric cancer is male predominant and animal studies suggest that sex hormones influence gastric carcinogenesis. We investigated the effects of 17β-estradiol (E2) or castration on H.pylori-induced gastritis in male INS-GAS/FVB/N (Tg(Ins1-GAS)1Sbr) mice. Comparisons were made to previously evaluated sham (n = 8) and H.pylori-infected (n = 8), intact male INS-GAS mice which had developed severe corpus gastritis accompanied by atrophy, hyperplasia, intestinal metaplasia and dysplasia of the epithelium within 16 weeks postinfection (all P < 0.01). Castration at 8 weeks of age had no sparing effect on lesions in uninfected (n = 5) or H.pylori-infected mice (n = 7) but all lesion subfeatures were attenuated by E2 in H.pylori-infected mice (n = 7) (P < 0.001). Notably, inflammation was not reduced but glandular atrophy, hyperplasia, intestinal metaplasia and dysplasia were also less severe in uninfected, E2-treated mice (n = 7) (P < 0.01). Attenuation of gastric lesions by E2 was associated with lower messenger RNA (mRNA) expression of interferon (IFN)-γ (P < 0.05) and interleukin (IL)-1β (P < 0.004), and higher IL-10 (P < 0.02) as well as decreased numbers of Foxp3+ regulatory T cells when compared with infected intact males. Infected E2-treated mice also developed higher Th2-associated anti-H.pylori IgG1 responses (P < 0.05) and significantly lower Ki-67 indices of epithelial proliferation (P < 0.05). E2 elevated expression of mRNA for Foxp3 (P < 0.0001) and IL-10 (P < 0.01), and decreased IL-1β (P < 0.01) in uninfected, intact male mice compared with controls. Therefore, estrogen supplementation, but not castration, attenuated gastric lesions in H.pylori-infected male INS-GAS mice and to a lesser extent in uninfected mice, potentially by enhancing IL-10 function, which in turn decreased IFN-γ and IL-1β responses induced by H.pylori.
Introduction
Gastric cancer is the fourth most common cancer and the second most frequent cause of death from cancer worldwide with an ∼2-fold higher incidence in males than in females (1). This gender difference is observed globally regardless of geographical distribution (1,2); male predominance of gastric cancer correlates with a 10–15 years delay in onset of intestinal type gastric cancer in women compared with men (2). Epidemiological studies have suggested that a delayed menopause is associated with a reduced risk for gastric cancer (3–6). Moreover, hormone replacement therapy decreased gastric cancer risk in menopausal women (7) and estrogen therapy for patients with prostate cancer has also been associated with reduced risk for gastric cancer (8). In the rat model of N-methyl-N′-nitro-nitrosoguanidine (MNNG)-induced gastric cancer, there was a higher incidence in male compared with female rats (9,10). In male rats, both castration (9–11) and administration of 17β-estradiol (E2) (9,12) decreased the incidence of chemically induced premalignant lesions and gastric tumors. Interestingly, the incidence of gastric cancer in MNNG-treated castrated rats was lower than in E2 treated intact male rats (9), suggesting that E2 not only may provide protection against gastric carcinogenesis but also that male testicular hormones may promote gastric cancer.
The etiology of gastric cancer is considered multifactorial and includes infection with Helicobacter pylori, with additional risk of malignancy associated with dietary habits and smoking (1). Helicobacter pylori is a gram-negative bacterium that persistently colonizes the gastric epithelium in humans and is classified as a group I carcinogen by the World Health Organization (13). Host immune responses to H.pylori infection are largely determined by cytokine secretion from different functional subsets of T-helper (Th) 1 and Th2 cells as well as recently recognized Th17 cells. Helicobacter pylori infection in humans and H.pylori or Helicobacter felis infection in mice induce proinflammatory Th1-like immune responses (14,15) which promote gastric cancer (16). Although a number of clinical and experimental studies have shown that sex hormones are able to modulate immune responses in multiple species, the effect of sex hormones on immune responses and associated lesions in response to H.pylori infection are poorly understood.
Hypergastrinemic INS-GAS mice develop gastric adenocarcinoma by 7 months following H.pylori or H.felis infection (17–19) and lesions develop predominantly in male INS-GAS mice (19). We have previously demonstrated that ovariectomy and E2 supplementation in overiectomized female INS-GAS mice protected them from H.pylori-induced gastric cancer (20). However, the effect of both male and female sex hormones on gastric carcinogenesis has not been fully elucidated. In the present study, we investigated the effects of estrogen and castration on H.pylori-induced gastric pathology in male INS-GAS mice.
Materials and methods
Mice
Twenty-six-specific pathogen-free (for exogenous murine viruses, parasites and pathogenic bacteria, including Helicobacter spp.) male INS-GAS mice on the FVB/N background (Tg (Ins1-GAS) 1Sbr) were housed in microisolators at 70 +/−2°F and a 12 h light/dark cycle. Mice were fed chow diet (PROLAB RHM3000; Lab Diet, Richmond, IN) and water ad libitum. Data from age-matched sham control (n = 8) and H.pylori-infected (n = 8) intact male INS-GAS mice from a previous study (20) were used for comparative analyses of histopathology, cytokine messenger RNA (mRNA) expression levels, H.pylori colonization, serology and Ki-67 immunohistochemistry. Mice were euthanized by CO2 at 16 weeks postinfection. The study was approved by the MIT Committee on Animal Care and MIT facilities were accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
Castration and E2 implants
At 8 weeks of age, mice were randomly assigned to groups of surgically castrated and sham dosed (n = 5), surgically castrated and H.pylori-infected (n = 7) or E2-treated mice followed by sham (n = 7) or H.pylori infection (n = 7). Castration was performed using aseptic techniques under isoflurane anesthesia followed by buprenorphine (0.1 mg/kg) and 0.5 ml of warm lactated Ringers solution. A time-release E2 pellet (0.25 mg, 90 day release; Innovative Research of America, Sarasota, FL) was implanted subcutaneously in the area of the dorsal neck and was replaced 90 days after the first implantation. Testes from intact and E2-treated mice were weighed at necropsy.
Experimental infection with H.pylori
Helicobacter pylori Sydney strain (SS1) was incubated in Brucella broth containing 5% fetal calf serum for 24 h at 37°C under microaerobic conditions on a shaker (100 r.p.m.). Normal motility and morphology were confirmed by phase contrast microscopy followed by centrifugation and resuspension of the bacterial pellet in sterile Brucella broth containing 30% glycerol to ∼109 organisms per milliliter as estimated by spectrophotometry (OD660nm = 0.9–1.1). At 10 weeks of age, 2 weeks after castration or E2 pellet implantation, mice were orally gavaged with 0.2 ml of H.pylori suspension or sterile broth every other day for three doses.
Quantitative culture of H.pylori
Quantitative culture of H.pylori was performed as described previously (19). Briefly, biopsy specimens from the corpus and antrum were individually weighed and homogenized in 250 μl of Brucella broth, diluted 10- and 100-fold in Brucella broth and 25 μl of each dilution was plated on selective medium. After incubation under microaerobic conditions (80:10:10 for N2:CO2:H2) at 37°C for 7 days, the number of H.pylori colonies was counted and reported as colony-forming units per gram of antrum or corpus tissue.
Real-time quantitative polymerase chain reaction for mRNA expression levels of gastric inflammatory mediators
Total RNA from gastric tissue was isolated using Trizol reagent following the supplier's instructions (Invitrogen); RNA was further purified using a RNAeasy kit (Qiagen). Complementary DNA from gastric mRNA (2 μg) was reverse transcribed using the High Capacity complementary DNA Archive kit following the supplier's instructions (Applied Biosystems, Foster City, CA). Quantitative polymerase chain reaction assays were performed using the 7500 Fast Real-Time PCR System (Applied Biosystems) for proinflammatory interferon (IFN)-γ, interleukin (IL)-1β and IL-17A; Foxp3 for natural regulatory T cells and anti-inflammatory IL-10. Assay data were normalized to the endogenous control glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and expressed as fold change in reference to values obtained from sham dosed, uninfected, intact male control mice using the Comparative CT method (User Bulletin No. 2; Applied Biosystems).
ELISA for anti-H.pylori IgG1 and IgG2a in serum
An outer membrane protein preparation of H.pylori was used as an antigen substrate as described previously (21). Serum was collected at necropsy, stored at −20°C and then evaluated by ELISA as described (22). Briefly, Immulon II 96-well plates (Dynax Technology, Chantilly, VA) were coated with 100 μl of a 10 μg/ml concentration of H.pylori outer membrane proteins in carbonate buffer overnight at 4°C. Serum was diluted 1:100 with 1% bovine serum albumin in phosphate-buffered saline and assayed in duplicate. Secondary antibodies were biotinylated anti-mouse IgG1 and anti-IgG2a clones A85 and R19-15, respectively, from (BD Biosciences PharMingen, San Diego, CA), diluted 1:2000 as was Extravidin peroxidase (Sigma, St Louis, MO). ABTS peroxidase substrate (Kirkegaard and Perry Laboratories, Gaithersburg, MD) was applied for 30 min at room temperature with absorbance read at 405/562λ using an ELISA plate reader (Dynatech MR 7000; Dynatech Laboratories, Chantilly, VA). Results were reported as a mean delta absorbance value.
Histological evaluation
At necropsy, linear strips of stomach extending from the squamocolumnar junction to the proximal duodenum were placed in 10% neutral-buffered formalin overnight, paraffin embedded, cut at 4 μm, and stained with hematoxylin and eosin. Inflammation, oxyntic gland atrophy, foveolar hyperplasia, intestinal metaplasia and dysplasia in the gastric cardia/corpus were scored using a described previously ordinal ascending scale (23) from 0 to 4 in increments of 0.5 by a veterinary pathologist (A.B.R.) blinded to treatment groups.
Immunohistochemistry for epithelial proliferation and Foxp3+ cells
Four gastric corpus tissue samples were selected randomly from each experimental group for Ki-67 immunohistochemistry as an index of epithelial proliferation. Deparaffinization and hydration of sections was done in xylene and graded ethanol to water. Antigen retrieval was performed by heating the slides for 20 min at 95°C in target retrieval solution, pH 6 (S1699; DAKO, Carpinteria, CA). Using an automated immunostainer (i6000; Biogenex, San Ramon, CA), sections were stained with anti-human Ki-67 antibody diluted 1:50 (BD Bioscience PharMingen) using an ARK kit (DAKO) according to the supplier's protocol. A Ki-67 labeling index (LI) was defined as the mean percentage of positively stained nuclei counted in 10 well-oriented proximal corpus gastric glands as described previously (23).
Foxp3+ immunochemistry was performed using Foxp3 antibody (FJK-16S; eBiosciences, San Diego, CA) as described previously (24). Cells expressing nuclear Foxp3 are presented as the number of Foxp3+ cells per field averaged (magnification = ×200) from 10 fields per stomach.
Testosterone and E2 assays
Five randomly selected serum samples from each H.pylori-infected group were assayed for testosterone and E2 levels using commercial immunoassay kits (Cayman Chemical Company, Ann Arbor, MA) following the supplier's protocol. Assay detection limits were 6.0 pg/ml for testosterone and 7.8 pg/ml for E2.
Statistical analysis
Body and testis weights, serum testosterone and E2 levels, H.pylori colonization, anti-H.pylori antibodies and the Ki-67 and Foxp3 LI were evaluated for normal distribution using the Kolomogorov–Smirnov test. Data comparisons were then evaluated by either one-way analysis of variance or the nonparametric Kruskal–Wallis test followed by selected comparisons using a Dunn’s posttest. Histology scores were analyzed using the nonparametric Mann–Whitney U-test and mRNA expression levels were compared using the Student’s t-test. Statistical significance was defined as P < 0.05.
Results
E2 caused testicular atrophy and lowered serum testosterone in male INS-GAS mice
Castration or administration of E2 did not impact mean body weight at necropsy (intact mice: 32.9 ± 1.9 g, castrated mice 35.7 ± 6.5 g, E2-treated intact mice 30.9 ± 3.4 g). The testis weight of E2-treated mice was significantly lower than that of intact mice (intact: 190.0 ± 7.5 mg, E2-treated mice: 79.3 ± 17.7 mg, P < 0.001). Serum testosterone levels in castrated and E2-treated mice were significantly lower compared with intact mice (P < 0.01) (Table I). When compared with intact mice, castration did not alter serum E2 levels, whereas the E2 level in E2-treated male mice was significantly increased (P < 0.05) (Table I). The time-release E2 pellet resulted in serum E2 levels that ranged from values observed in female proestrus to supraphysiological levels as described previously (20).
Table I.
Levels of testosterone and E2 were measured in serum from Helicobacter pylori-infected INS-GAS mice that were intact, castrated or treated with E2 supplementation
| Group | Serum testosterone level (pg/ml) | Serum E2 level (pg/ml) |
| Intact mice (n = 5) | 177.2 ± 36.0 | 15.5 ± 2.3 |
| Castrated mice (n = 5) | 8.7 ± 0.9* | 11.6 ± 1.6 |
| E2-treated mice (n = 5) | 15.7 ± 4.2* | 72.5 ± 19.7** |
Castration and E2 significantly reduced testosterone (*P < 0.01) compared with intact mice and E2 supplementation resulted in supraphsyiological levels (**P < 0.05). Values represent the mean ± standard error.
E2 administration was associated with attenuation of gastric lesions in both H.pylori-infected and uninfected male INS-GAS mice
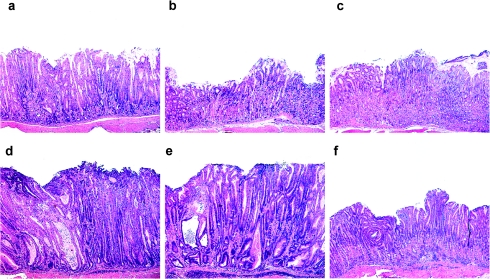
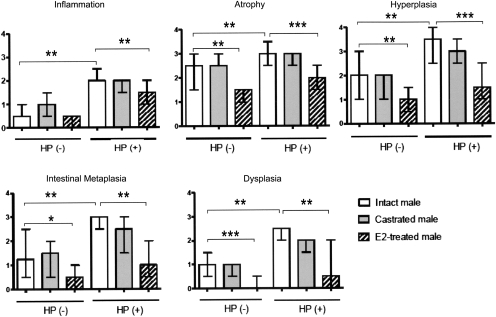
As previously reported (20) and reproduced in Figures 1a and 2, uninfected control intact male INS-GAS mice developed minimal to mild corpus gastritis by 24 weeks of age. Helicobacter pylori-infected intact male INS-GAS mice developed severe corpus gastritis consistent with male predisposition to premalignant gastric lesions in INS-GAS mice (19) (Figure 1d). Scores for H.pylori-associated inflammation, atrophy, hyperplasia, intestinal metaplasia and dysplasia in infected intact mice were significantly higher than those in uninfected intact mice (all P < 0.01). Castration had no effect on lesion scores in either uninfected or infected mice (Figures 1b, e and 2). E2-treated mice developed less severe H.pylori-associated inflammation (P < 0.01), atrophy (P < 0.001), hyperplasia (P < 0.001), intestinal metaplasia (P < 0.01) and dysplasia (P < 0.001) (Figures 1c and 2) compared with untreated H.pylori-infected mice. E2 also had significant effects in reducing glandular atrophy (P < 0.01), hyperplasia (P < 0.01), intestinal metaplasia (P < 0.05) and dysplasia (P < 0.001) in uninfected, E2-treated INS-GAS mice. Only severity of inflammation, attributable to hypergastrinemia in uninfected male INS-GAS mice, was unaffected by E2 supplementation.
Fig. 1.
Histopathology of the proximal gastric corpus of male INS-GAS mice. (a) Uninfected intact male with moderate corpus gastritis. (b) Uninfected castrated male with mild corpus gastritis. (c) Uninfected E2-treated male with attenuated gastritis. (d) Helicobacter pylori-infected male with marked inflammation and mucosal hyperplasia and dysplasia. (e) H.pylori-infected castrated male with severe gastritis comparable with infected intact males. (f) H.pylori-infected E2-treated male with less severe gastritis than infected intact male INS-GAS.
Fig. 2.
Gastric lesion scores for H.pylori-infected HP (+) and uninfected HP (−) intact, castrated and E2-treated male INS-GAS mice. The data are presented as the median with a bar representing a range. *P < 0.05, **P < 0.01, ***P < 0.001.
E2 decreased gastric tissue mRNA expression of IFN-γ and IL-1β and augmented mRNA expression of IL-10 and Foxp3 in H.pylori-infected male INS-GAS mice
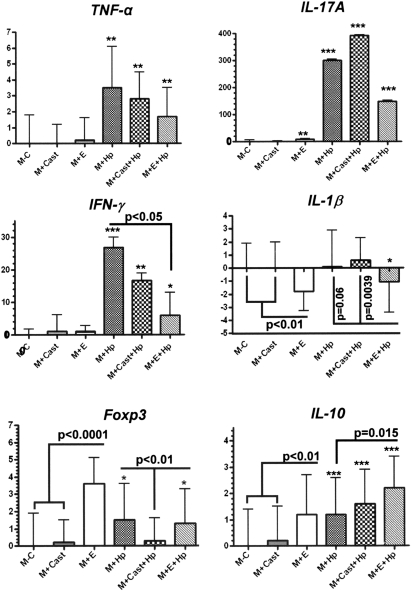
We previously demonstrated that ovariectomized INS-GAS mice developed more severe gastric pathology and produced higher mRNA levels of gastric IFN-γ, IL-1β and tumor necrosis factor (TNF)-α when compared with E2-treated cohorts at 28 weeks post H.pylori infection (20). Foxp3+ regulatory T cells and proinflammatory Th17 cells were reported to have an important role in the development of H.pylori-induced gastritis in mice (25,26). Thus, we measured mRNA levels for gastric TNF-α, IL-17A, IFN-γ, IL-1β, Foxp3 and IL-10 in uninfected and H.pylori-infected male INS-GAS mice, including mice that were castrated or treated with E2 (Figure 3).
Fig. 3.
Gastric tissues (n = 5–8 per group) were evaluated by quantitative polymerase chain reaction for mRNA levels of selected inflammatory mediators normalized to expression of GAPDH. The y-axis represents mean fold change of mRNA levels in reference to control mice (uninfected, E2 untreated intact male INS-GAS mice denoted as (M − C). M + Cast indicates castrated males; M + E indicates E2-treated males; M + Hp indicates H.pylori-infected males. Error bars represent standard deviations. *P < 0.05, **P < 0.01, ***P < 0.001 indicate significant differences compared with control values. Additional significant differences between experimental groups are indicated by P values.
mRNA levels for gastric TNF-α were similarly elevated by H.pylori infection in intact males, castrated males and E2-treated male INS-GAS mice (P < 0.01) although castration or E2 supplementation had no additional effects on TNF-α. Promotion of IL-17A and IFN-γ by H.pylori was even greater (P < 0.001) with IFN-γ expression reduced by E2 (P < 0.05) but not by castration. IL-1β expression was not enhanced by H.pylori infection alone but E2 reduced IL-1β expression below control values for intact or castrated, uninfected males (P < 0.01) and H.pylori-infected, intact (P = 0.06) or castrated males (P < 0.005). Foxp3 T regulatory cell marker and anti-inflammatory IL-10 mRNA expression in intact uninfected males were significantly increased by E2 supplementation (P < 0.0001, P < 0.01, respectively) and by H.pylori infection (P < 0.05, P <0.001, respectively). Castration of H.pylori-infected mice lowered Foxp3 to baseline values (P < 0.01) and had no effect on IL-10 expression.
The number of gastric Foxp3+ cells was positively associated with the severity of H.pylori-induced gastric lesions
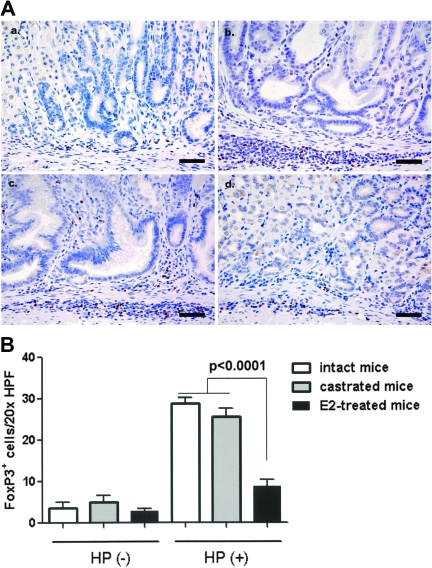
Large numbers of Foxp3+ cells were noted in the mucosa and submucosa of the murine stomachs (Figure 4A) and were significantly higher in H.pylori infected intact (n = 8) and castrated male INS-GAS mice (n = 5) when compared with the H.pylori-infected E2-treated mice (n = 7) (Figure 4b, P < 0.0001). There was no significant difference in the number of gastric Foxp3+ cells between H.pylori-infected intact and castrated mice (P = 0.207) or among all the uninfected groups (all P > 0.3) regardless of additional treatment status (Figure 4B). The higher numbers of Foxp3+ cells were directly correlated to the severity of gastric inflammation and associated pathological alterations such as epithelial hyperplasia, atrophy and dysplasia in H.pylori-infected E2-treated mice (Figure 2).
Fig. 4.
Quantification of FoxP3+ TREG cells in the gastric corpus of INS-GAS male mice at 16 weeks postinoculation (a–d). Representative high magnification images demonstrating immunohistochemical staining for FoxP3, a regulatory T-cell marker, in the gastric corpus in uninfected mice (a), H.pylori-infected intact mice (b) H.pylori-infected castrated mice (c) and H.pylori-infected and E2-treated mice (d). (e) Mean number of FoxP3+ cells in the gastric mucosa and submucosa assessed from 10 well-oriented fields (magnification, ×200; 1.00 mm2) per mouse gastric corpus. Bar, 40 μm.
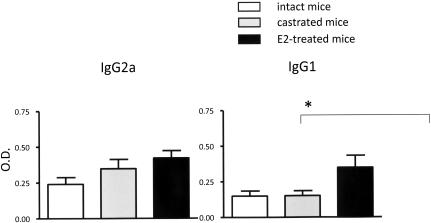
E2 promoted the Th2-associated IgG1 response to H.pylori
Helicobacter pylori infection caused seroconversion in all infected INS-GAS mice (Figure 5). There were serum levels of H.pylori-specific IgG2a (Th1-associated isotype) in all infected male mice irrespective of castration or E2 treatment. In contrast, E2 treatment, but not castration, promoted a higher Th2-associated IgG1 response (P < 0.05).
Fig. 5.
Serum IgG2a (Th1 isotype) and IgG1 (Th2 isotype) response to H.pylori measured by ELISA. The IgG1 response in male E2 treated, H.pylori-infected INS-GAS mice was significantly higher than that observed in sera from infected intact or castrated mice. Values represent the mean ± standard error. *P < 0.05.
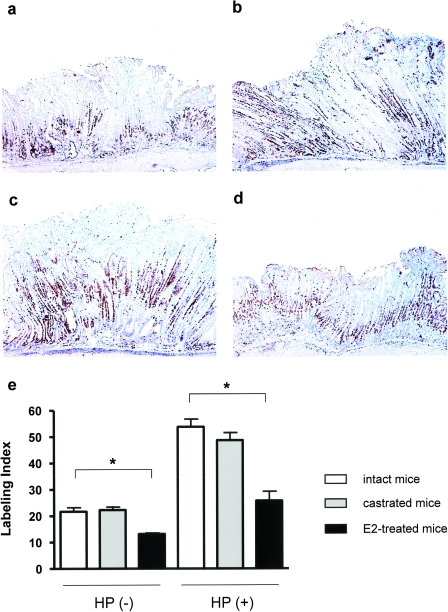
E2 suppressed epithelial cell proliferation
Ki-67 positively stained nuclei were mainly observed at the isthmus of gastric glands in uninfected intact mice (Figure 6). The Ki-67 LI was increased in H.pylori-infected intact, castrated or E2-treated mice (all P < 0.05), with positively stained nuclei distributed from the upper region of gastric glands to the gastric pit. Castration had no effect on the Ki-67 LI in uninfected or infected mice (Figure 6c and e). In contrast, administration of E2 significantly decreased the Ki-67 LI in both uninfected (P < 0.002) and infected mice (P < 0.0001) (Figure 6d and e). Thus, lower P values generated by analysis of H.pylori-infected mice treated with E2 (or not) compared with E2 treated (or not) uninfected controls indicates the suppressive effect of E2 on epithelial cell proliferation was more pronounced in infected mice compared with uninfected mice.
Fig. 6.
Immunohistochemical detection of Ki-67 in proximal corpus of stomach. (a) Uninfected intact male (b) H.pylori-infected intact male (c) infected castrated male; (d) infected E2-treated male (e) epithelial cell proliferation LI of proximal corpus. Castration had no effect but E2 treatment reduced the LI in both uninfected HP (−) and H.pylori-infected HP (+) intact male mice with greater reduction in H.pylori-infected mice. *P < 0.0002; **P < 0.001. Values represent the mean ± standard error.
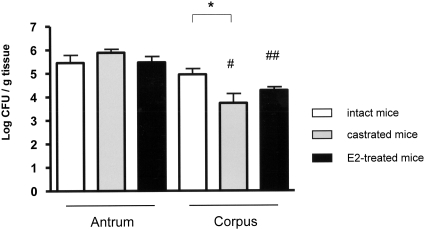
Colonization of H.pylori was unaffected by castration or E2
Helicobacter pylori colonization in gastric mucosa was confirmed and quantified by culture of antrum and corpus tissue from all inoculated mice. In the antrum, H.pylori colonization was not impacted by castration or E2 treatment (Figure 7). Similarly, E2 treatment had no effect on colonization levels in the corpus but castration was associated with lower colonization levels by 1 log in the corpus (P < 0.05). Colonization levels of H.pylori were similar between antrum and corpus in intact mice but was lower in the corpus compared with antrum in mice that had been castrated or treated with E2 (both P < 0.001).
Fig. 7.
H.pylori colonization levels of the gastric antrum and corpus in intact, castrated and E2-treated male INS-GAS mice. Castration significantly decreased H.pylori colonization levels in the corpus (*P < 0.05). H.pylori colonization levels in the corpus were lower than in the antrum in castrated and E2-treated males. #, ## are both P < 0.001. Values represent the mean ± standard error.
Discussion
Epidemiological data indicate that the incidence of gastric cancer in humans is male predominant with a male-to-female ratio of ∼2:1 (1,27). In addition, this male-to-female ratio of gastric cancer incidence peaks at ∼60 years of age and then starts decreasing during aging, suggesting that the risk of gastric cancer increases in postmenopausal women (2). INS-GAS mice expressing human gastrin develop male-dominant gastric pathology, which is further promoted by infection with H.felis (17) or H.pylori (18,19). We demonstrated that treatment with exogenous estrogen, but not with castration, significantly reduced the severity of H.pylori-induced gastric lesions. Consistent with our findings, a population-based cohort study reported that estrogen therapy for prostate cancer reduced the risk for gastric cancer in humans (8). We show that higher levels of gastric IL-10 mRNA in E2-treated male uninfected mice compared with sham controls suggest that E2 administration upregulated gastric IL-10 function. This data is also supported by observing higher mRNA levels for IL-10 in E2 treated, H.pylori-infected male mice compared with cohort mice. In addition, we previously reported that E2 treated, H.pylori-infected ovariectomized mice expressed more IL-10 transcripts compared with untreated cohorts (20). These lines of evidence suggest that E2-mediated attenuation of H.pylori-induced gastric lesions in male INS-GAS mice results, at least in part, from upregulation of gastric IL-10 which downregulated the proinflammatory cytokines IFN-γ and IL-1β responses noted during chronic H.pylori infection.
Proinflammatory Th1 cytokines including IFN-γ, IL-1β and TNF-α are upregulated in H.pylori-induced gastric lesions in animal models and humans (28). Recently, Th17 cells expressing IL-17A were implicated in the pathogenesis of H.pylori infection (26). Our results indicate that E2 treatment in H.pylori-infected male INS-GAS mice significantly decreased mRNA levels of gastric IFN-γ and IL-1β compared with non-E2 treated mice. Importantly, mRNA levels of gastric IL-1β, whose levels are upregulated in individuals with IL-1β polymorphisms, are associated with increased risk of gastric cancer (29). Notably, IL-1β cytokine levels were lower in stomachs of E2-treated males with or without infection when compared with sham controls, suggesting that E2 may modulate the IL-1-signaling pathway. The mRNA levels of gastric TNF-α (P = 0.11) and IL-17A (P = 0.28) in E2 treated infected male mice trended to be lower without statistical significance when compared with the untreated H.pylori-infected male mice at 16 weeks postinfection. This is consistent with our previous findings that the mRNA levels of gastric IFN-γ, TNF-α and IL-1β in H.pylori-infected intact female INS-GAS mice were significantly lower at 28 weeks but not at 16 weeks postinfection when compared with ovariectomized H.pylori-infected mice (20). Studies designed with a longer duration of H.pylori infection may result in significant difference in mRNA levels of these proinflammatory cytokines between the H.pylori-infected male INS-GAS mice with and without E2 treatment.
Recently, natural TREG cells have been recognized to play an important role in suppressing H.pylori-induced gastric disease. There were larger numbers of Foxp3+ TREG cells noted in inflamed gastric tissue of H.pylori-positive patients and experimentally infected mice (30,31). Adoptive transfer of TREG cells into TEFFECTOR cell-reconstituted H.pylori-infected Rag2−/− mice (lacking T and B lymphocytes) suppressed gastric pathology in an IL-10-dependent manner (25). Consistent with these observations, H.pylori-infected wild-type or castrated mice developed more severe gastric lesions and had larger numbers of gastric Foxp3+ cells than E2 treated, H.pylori-infected mice. However, E2-treated uninfected mice contained significantly higher mRNA levels of gastric Foxp3 (a reliable biomarker for TREG cells) but did not display more Foxp3+ cells when compared with untreated uninfected controls. This discrepancy may result from the possibility that E2 administration could activate transcription of gastric Foxp3 in other cell types in addition to natural TREG cells since it was reported that in humans Foxp3 expression occurs in mammary, prostatic and other epithelial cells (32). The transcription of gastric Foxp3 in these non-TREG cells may not be translated or the translated Foxp3 may have been insensitive to immunostaining with the Foxp3 antibody used in this study.
Exogenous E2 also enhanced a Th2-dependent IgG1 serum antibody response to H.pylori compared with untreated cohort mice. In contrast, there was no difference in the Th1-dependent IgG2a response between infected intact, castrated or E2-treated mice. These results suggest that infected mice treated with E2 mounted a more robust Th2-biased immune response. Estrogen has a biphasic dose effect on immune responses (33); high estrogen levels promoted a Th2-mediated immune response, whereas low estrogen levels stimulated a Th1-mediated immune response (33). In the present study, serum E2 levels in E2-treated mice were higher than the physiological range for intact male mice, indicating that increased levels of E2 promoted the Th2-biased immune response in infected mice. The present study demonstrates that E2 treated H.pylori-infected mice had an attenuated gastritis compared with infected intact mice, which is consistent with a previous study by our laboratory that demonstrated that a Th2-biased immune response elicited by concurrent parasitic infection ameliorated H.felis-induced gastritis in C57BL/6 mice (16). Similarly, in our previous study using ovariectomized female INS-GAS mice, administration of E2 after H.pylori infection induced a Th2-biased cytokine profile in gastric mucosa and attenuated gastric pathology (20). These Th2-biased immune responses induced by E2 in INS-GAS mice were observed to be independent of whether E2 supplementation was administered before or after H.pylori infection. In addition, testosterone has also been considered an immunomodulator, exerting anti-inflammatory and Th2-biased immune responses in several animal models of autoimmunity (33,34). However, castration of mice in this study did not impact the Th1 or Th2 humoral immune response to H.pylori.
Supplementation of E2 markedly decreased gastric epithelial cell proliferation in both H.pylori-infected and uninfected mice compared with E2-untreated mice. Although the molecular mechanism is not known, this suppressive effect of E2 on epithelial proliferation was also observed when applied to gastric cancer cell lines in vitro (35). This E2 effect on epithelial cell proliferation is supported by a reduced risk of colorectal cancer in menopausal women receiving hormone replacement therapy (36). Two estrogen receptors, ERα and ERβ, respond to expression of ERβ, which is mainly expressed in normal colonic mucosa, were decreased in patients with colorectal cancer (37,38). Moreover, it has been reported that ERβ inhibited epithelial cell proliferation in a colon cancer cell line (39). Similarly, estrogen receptors expressed on gastric epithelium may also play an important role in gastric epithelial cell proliferation and associated risk of gastric cancer. A series of epidemiological studies performed in Europe suggested that treatment with estrogen receptor blocking agents in women with breast cancer increased the risk of these individuals for developing gastric cancer (40,41).
Earlier studies reported that castration decreased the incidence of gastric cancer in MNNG-treated male rats (9,10). In the present study, castration reduced testosterone levels but not E2 levels in H.pylori-infected mice; a protective effect of castration on gastric dysplasia was not observed. There are two possible explanations for this discrepancy. First, the mechanism of carcinogenesis in the H.pylori mouse model is different from that of the MNNG rat model. Second, the roles of various sex hormones in the development of gastritis and premalignant lesions may be different between rats and mice and in particular, male INS-GAS mice.
In summary, we have shown that exogenous E2 treatment, but not castration, exerts a protective effect against the development of H.pylori-induced gastritis and premalignant lesions in INS-GAS male mice. This beneficial effect of E2 may result from several mechanisms, including stimulation of IL-10 activity, enhancement of Th2-mediated immune responses, and inhibitory effects on epithelial cell proliferation. Because estrogen replacement therapy slightly increases the risk of ischemic heart disease, stroke and breast cancer (42), its prophylactic use for H.pylori-associated disease appears unwarranted. However, further investigation into molecular mechanisms involving estrogen suppression on gastric carcinogenesis by E2 could lead to the development of novel E2-based therapeutic strategies applicable to gastric diseases.
Funding
National Institutes of Health (R01AI37750; P01CA26T31; P30ES02109 to J.G.F.; R01CA93405 to T.C.W. and J.G.F.).
Acknowledgments
We thank Elaine Robbins for technical support in preparation of figures.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- E2
17β-estradiol
- IFN
interferon
- IL
interleukin
- LI
labeling index
- mRNA
messenger RNA
- MNNG
N-methyl-N′-nitro-nitrosoguanidine
- TNF
tumor necrosis factor
References
- 1.Parkin DM, et al. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Sipponen P, et al. Delayed rise in incidence of gastric cancer in females results in unique sex ratio (M/F) pattern: etiologic hypothesis. Gastric Cancer. 2002;5:213–219. doi: 10.1007/s101200200037. [DOI] [PubMed] [Google Scholar]
- 3.Palli D, et al. Reproductive history and gastric cancer among post-menopausal women. Int. J. Cancer. 1994;56:812–815. doi: 10.1002/ijc.2910560609. [DOI] [PubMed] [Google Scholar]
- 4.La Vecchia C, et al. Menstrual and reproductive factors and gastric-cancer risk in women. Int. J. Cancer. 1994;59:761–764. doi: 10.1002/ijc.2910590609. [DOI] [PubMed] [Google Scholar]
- 5.Frise S, et al. Menstrual and reproductive risk factors and risk for gastric adenocarcinoma in women: findings from the canadian national enhanced cancer surveillance system. Ann. Epidemiol. 2006;16:908–916. doi: 10.1016/j.annepidem.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Freedman ND, et al. Menstrual and reproductive factors and gastric cancer risk in a large prospective study of women. Gut. 2007;56:1671–1677. doi: 10.1136/gut.2007.129411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindblad M, et al. Hormone replacement therapy and risks of oesophageal and gastric adenocarcinomas. Br. J. Cancer. 2006;94:136–141. doi: 10.1038/sj.bjc.6602906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindblad M, et al. Estrogen and risk of gastric cancer: a protective effect in a nationwide cohort study of patients with prostate cancer in Sweden. Cancer Epidemiol. Biomarkers Prev. 2004;13:2203–2207. [PubMed] [Google Scholar]
- 9.Furukawa H, et al. Effect of sex hormones on the experimental induction of cancer in rat stomach - a preliminary study. Digestion. 1982;23:151–155. doi: 10.1159/000198722. [DOI] [PubMed] [Google Scholar]
- 10.Furukawa H, et al. Effect of sex hormones on carcinogenesis in the stomachs of rats. Cancer Res. 1982;42:5181–5182. [PubMed] [Google Scholar]
- 11.Ando Y, et al. Progesterone enhancement of stomach tumor development in SD rats treated with N-methyl-N'-nitro-N-nitrosoguanidine. Jpn. J. Cancer Res. 1995;86:924–928. doi: 10.1111/j.1349-7006.1995.tb03002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell-Thompson M, et al. 17Beta-estradiol modulates gastroduodenal preneoplastic alterations in rats exposed to the carcinogen N-methyl-N'-nitro-nitrosoguanidine. Endocrinology. 1999;140:4886–4894. doi: 10.1210/endo.140.10.7030. [DOI] [PubMed] [Google Scholar]
- 13.Anonymous Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monogr. Eval. Carcinog. Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 14.Mohammadi M, et al. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J. Immunol. 1996;156:4729–4738. [PubMed] [Google Scholar]
- 15.Bamford KB, et al. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–492. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 16.Fox JG, et al. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter- induced gastric atrophy. Nat. Med. 2000;6:536–542. doi: 10.1038/75015. [DOI] [PubMed] [Google Scholar]
- 17.Wang TC, et al. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118:36–47. doi: 10.1016/s0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- 18.Fox JG, et al. Host and microbial constituents influence Helicobacter pylori-induced cancer in a murine model of hypergastrinemia. Gastroenterology. 2003;124:1879–1890. doi: 10.1016/s0016-5085(03)00406-2. [DOI] [PubMed] [Google Scholar]
- 19.Fox JG, et al. Helicobacter pylori-associated gastric cancer in INS-GAS mice is gender specific. Cancer Res. 2003;63:942–950. [PubMed] [Google Scholar]
- 20.Ohtani M, et al. Protective role of 17 beta -estradiol against the development of Helicobacter pylori-induced gastric cancer in INS-GAS mice. Carcinogenesis. 2007;28:2597–2604. doi: 10.1093/carcin/bgm150. [DOI] [PubMed] [Google Scholar]
- 21.Pronovost AD, et al. Evaluation of a new immunodiagnostic assay for Helicobacter pylori antibody detection: correlation with histopathological and microbiological results. J. Clin. Microbiol. 1994;32:46–50. doi: 10.1128/jcm.32.1.46-50.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whary MT, et al. Chronic active hepatitis induced by Helicobacter hepaticus in the A/JCr mouse is associated with a Th1 cell-mediated immune response. Infect. Immun. 1998;66:3142–3148. doi: 10.1128/iai.66.7.3142-3148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers AB, et al. Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice. Cancer Res. 2005;65:10709–10715. doi: 10.1158/0008-5472.CAN-05-1846. [DOI] [PubMed] [Google Scholar]
- 24.Lemke LB, et al. Concurrent Helicobacter bilis infection in C57BL/6 mice attenuates proinflammatory H. pylori-induced gastric pathology. Infect. Immun. 2009;77:2147–2158. doi: 10.1128/IAI.01395-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee CW, et al. Wild-type and interleukin-10-deficient regulatory T cells reduce effector T-cell-mediated gastroduodenitis in Rag2−/− mice, but only wild-type regulatory T cells suppress Helicobacter pylori gastritis. Infect. Immun. 2007;75:2699–2707. doi: 10.1128/IAI.01788-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kao JY, et al. Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in mice. Gastroenterology. 138:1046–1054. doi: 10.1053/j.gastro.2009.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandanos E, et al. Oestrogen and the enigmatic male predominance of gastric cancer. Eur. J. Cancer. 2008;44:2397–2403. doi: 10.1016/j.ejca.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 28.Fox JG, et al. Inflammation, atrophy, and gastric cancer. J. Clin. Invest. 2007;117:60–69. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Omar EM, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 30.Rad R, et al. CD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo. Gastroenterology. 2006;131:525–537. doi: 10.1053/j.gastro.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Lundgren A, et al. Helicobacter pylori-specific CD4+ CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect. Immun. 2003;71:1755–1762. doi: 10.1128/IAI.71.4.1755-1762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin F, et al. Human FOXP3 and cancer. Oncogene. 29:4121–4129. doi: 10.1038/onc.2010.174. [DOI] [PubMed] [Google Scholar]
- 33.Whitacre CC, et al. A gender gap in autoimmunity. Science. 1999;283:1277–1278. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- 34.Dalal M, et al. Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen-specific T lymphocyte response. J. Immunol. 1997;159:3–6. [PubMed] [Google Scholar]
- 35.Messa C, et al. Epidermal growth factor and 17beta-estradiol effects on proliferation of a human gastric cancer cell line (AGS) Scand. J. Gastroenterol. 2000;35:753–758. doi: 10.1080/003655200750023444. [DOI] [PubMed] [Google Scholar]
- 36.Grodstein F, et al. Postmenopausal hormone therapy and the risk of colorectal cancer: a review and meta-analysis. Am. J. Med. 1999;106:574–582. doi: 10.1016/s0002-9343(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 37.Foley EF, et al. Selective loss of estrogen receptor beta in malignant human colon. Cancer Res. 2000;60:245–248. [PubMed] [Google Scholar]
- 38.Konstantinopoulos PA, et al. Oestrogen receptor beta (ERbeta) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour's dedifferentiation. Eur. J. Cancer. 2003;39:1251–1258. doi: 10.1016/s0959-8049(03)00239-9. [DOI] [PubMed] [Google Scholar]
- 39.Martineti V, et al. ERbeta is a potent inhibitor of cell proliferation in the HCT8 human colon cancer cell line through regulation of cell cycle components. Endocr. Relat. Cancer. 2005;12:455–469. doi: 10.1677/erc.1.00861. [DOI] [PubMed] [Google Scholar]
- 40.Chandanos E, et al. Tamoxifen exposure in relation to gastric adenocarcinoma development. Eur. J. Cancer. 2008;44:1007–1014. doi: 10.1016/j.ejca.2008.02.049. [DOI] [PubMed] [Google Scholar]
- 41.Chandanos E, et al. Endogenous estrogen exposure in relation to distribution of histological type and estrogen receptors in gastric adenocarcinoma. Gastric Cancer. 2008;11:168–174. doi: 10.1007/s10120-008-0475-6. [DOI] [PubMed] [Google Scholar]
- 42.Nelson HD, et al. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288:872–881. doi: 10.1001/jama.288.7.872. [DOI] [PubMed] [Google Scholar]