Abstract
Phorbol esters such as phorbol 12-myristate 13-acetate (PMA) induce apoptosis in many tumor cells including the androgen-sensitive LNCaP prostate cancer cells. Although phorbol ester-induced apoptotic pathways have been well characterized, little is known of the pro-survival pathways modulated by these agents. We now provide experimental evidence to indicate that protein kinase D (PKD) promotes survival signals in LNCaP cells in response to PMA treatment. Knockdown of endogenous PKD1 or PKD2 decreased extracellular signal-regulated kinase (ERK) 1/2 and nuclear factor-kappaB (NF-κB)-dependent transcriptional activities and potentiated PMA-induced apoptosis, whereas overexpression of wild-type PKD1 enhanced ERK1/2 activity and suppressed PMA-induced apoptosis. PMA caused rapid activation, followed by progressive downregulation of endogenous PKD1 in a time- and concentration-dependent manner. The downregulation of PKD1 was dependent on the activity of protein kinase C (PKC), but not that of PKD. Selective depletion of endogenous PKC isoforms revealed that both PKCδ and PKCϵ were required for PKD1 activation and subsequent downregulation. Further analysis showed that the downregulation of PKD1 was mediated by a ubiquitin–proteasome degradation pathway, inhibition of which correlated to increased cell survival. In summary, our data indicate that PKD1 is activated and downregulated by PMA through a PKC-dependent ubiquitin–proteasome degradation pathway, and the activation of PKD1 or PKD2 counteracts PMA-induced apoptosis by promoting downstream ERK1/2 and NF-κB activities in LNCaP prostate cancer cells.
Introduction
Phorbol esters, natural products and pharmacological analogs of diacylglycerol (DAG), can trigger distinct cellular responses depending on the cell type and the specific protein kinase C (PKC) isozymes expressed (1). Despite their well-characterized tumor-promoting activity, phorbol esters can also induce cell growth arrest or trigger apoptosis in many tumor cell types (2,3). Phorbol esters such as PMA alone or in combination with other anticancer drugs have been exploited as potential cancer therapies. For example, PMA in combination with paclitaxel or all-trans-retinoic acid has been tested in cultured prostate cancer cells and in tumor xenografts in nude mice (4). Phase I clinical trials of PMA have been conducted in patients with relapsed/refractory malignancies including prostate cancer (5). It is thus conceivable that phorbol ester sensitivity in tumors will significantly impact the therapeutic efficacy of these agents in cancer patients.
Prostate cancer is the second leading cause of cancer-related deaths among men in the USA. Androgen-sensitive prostate cancer cells, such as LNCaP, C4-2 and CWR22-Rv1, undergo apoptosis upon treatment with phorbol esters such as PMA (2). Prostate cancer cells express multiple members of the PKC family, which play an important role in the control of cell survival and apoptosis in prostate cancer cells (2,3,6). The PKC family comprises 10 isozymes that are classified into three subfamilies based on their responsiveness to DAG and Ca2+: classical PKCs (α, βI/βII and γ; Ca2+ and DAG dependent), novel PKCs (δ, ϵ, η and θ; DAG dependent but Ca2+ independent) and atypical PKCs (λ/ι and ζ; both Ca2+ and DAG independent) (7). PKC, and in particular PKCδ, has been identified as a critical mediator of phorbol ester-induced apoptosis in LNCaP cells (8–13). Downstream of PKCδ, multiple signaling pathways are activated and contribute to the apoptotic process. These include the p38, c-jun N-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK) 1/2 mitogen-activated protein kinase (MAPK) cascades (9,12), the secretion of death factors including tumor necrosis factor α and TRAIL as well as death receptor signaling (11,14), and the PKCδ/RhoA/ROCK and cytoskeleton cascades (13). Thus, PMA-induced apoptosis is mediated through a complex signaling network that involves the activation of multiple signaling pathways.
It is noteworthy that PMA not only induces apoptotic signals but also modulates survival signals (12). Despite the extensive study of apoptotic pathways induced by PMA, much less is known of the survival signals activated by PMA. PMA has been shown to cause transient downregulation of Akt activity in LNCaP cells, a process that is important for the PMA-induced apoptotic response. The PI3K/Akt pathway plays a key role in protecting prostate cancer cells from apoptosis induced by cytokines and other apoptotic inducers (12,15,16). PKCα has been implicated in the inhibition of the Akt survival pathway by PMA through rapid dephosphorylation of Akt (10,12). In addition, the mitogen-activated protein kinase/ERK kinase (MEK) pathway is rapidly activated upon addition of PMA and the inhibition of this pathway by a MEK1 inhibitor significantly potentiates PMA-induced apoptosis (12), indicating that the MEK/ERK pathway promotes survival and counteracts the apoptotic response induced by PMA. Thus, it is conceivable that there is a fine balance between the apoptotic and survival pathways, which is crucial in cell fate determination in LNCaP cells.
Protein kinase D (PKD) plays a pro-proliferative and anti-apoptotic role in many cellular systems. This serine/threonine kinase family belongs to the Ca2+/calmodulin-dependent kinase superfamily (17) and is composed of three members, PKD1, PKD2, and PKD3, which share high sequence homology (18–21). It is a novel family of DAG receptors that bind DAG and phorbol esters with high affinity. In most cellular systems examined, the activity of PKD is controlled by PKC through phosphorylation of two conserved serine residues in the activation loop of PKD (serine 738 and 742 in human PKD1) (22,23). PKD regulates many fundamental cellular functions including cell proliferation, survival, apoptosis, cell migration, protein transport and gene regulation (24,25). It has been shown to transduce mitogenic signals and promote cell proliferation through modulating the ERK1/2 MAPK pathway (26–28). It also promotes cell survival through the induction of anti-apoptotic proteins in pancreatic cancer cells (29). PKD has also been shown to protect the cell from oxidative stress-induced cell death by activating the transcription factor nuclear factor-kappaB (NF-κB) (30).
In this study, the role of PKD in PMA-induced apoptosis in androgen-sensitive LNCaP prostate cancer cells was investigated. Our findings demonstrated a pro-survival and anti-apoptotic role of PKD1 and PKD2 in PMA-induced apoptosis in LNCaP cells and identified ERK1/2 and NF-κB as the downstream effectors in the process. We also found that PMA treatment caused concentration- and time-dependent downregulation of PKD1 through a PKC-mediated ubiquitin–proteasome degradation pathway.
Materials and methods
Materials
PMA and Ro 31-8220 were purchased from LC Laboratories (Woburn, MA), cycloheximide was obtained from Acros Organics (New Jersey, NJ), actinomycin D was from MP Biomedicals (Solon, OH) and N-acetyl-leucyl-leucyl-norleucinal (ALLN), MG132 and lactacystin were from Fisher Scientific (Pittsburgh, PA). The PKD inhibitors, CID755673 and kb-NB142-70, were synthesized by Dr Peter Wipf at the Department of Chemistry, University of Pittsburgh, as described previously (31–33).
Cell culture
LNCaP cells (American Type Culture Collection) were maintained in RPMI 1640 medium (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA), 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 1 mM sodium pyruvate, 0.2% glucose, penicillin (100 U/ml)–streptomycin (100 μg/ml) at 37°C in a humidified atmosphere of 5% CO2. LNCaP cells were discarded after eight passages.
siRNA transfection
The non-targeting (si-nt) and validated stealth PKD1 (si-PKD1, si-PKD1/2), PKD2 (si-PKD2, si-PKD2/1) and PKCϵ siRNAs were obtained from Invitrogen. The PKD1(2) siRNA sequence was ′GUCGAGAGAAGAGGUCAAA′. The PKCα and PKCδ siRNA sequences were ′CCGAGUGAAACUCACGGACUUCAAU’ and ′CCAUGAGUUUAUCGCCACCTT′. The siRNAs were transfected into LNCaP cells using DharmaFECT Reagent 3 (Dharmacon, Lafayette, CO) according to the manufacturer’s instructions. Experiments were performed 48 h after transfection.
Adenoviral infection
Subconfluent LNCaP cultures were infected with PKD1 adenoviruses at different multiplicities of infection in a minimum volume of serum-free medium. After 90 min of incubation at 37°C, growth medium was added, and the cells were cultured continuously for 24 h before harvesting for assays.
Luciferase reporter gene assay
LNCaP cells (3 × 105 cells per well) were seeded in 12-well plate. After overnight attachment, the cells were transfected with the appropriate PKC and PKD siRNAs. After two days, the cells were transfected with NF-κB reporter pGL2-NF-κB (1.5 μg/well) (a kind gift from Dr Gutian Xiao at the Department of Microbiology and Molecular Genetics, University of Pittsburgh) using LipofectAMINE 2000 following the manufacturer’s instructions. The pTK-RL plasmid (0.3 μg/well) was cotransfected to normalize variation in transfection efficiency. After 24 h, the cells were treated without (dimethyl sulfoxide) or with PMA (10 nM) for 6 h. The luciferase activity was assayed using dual luciferase assay system according to supplier’s instructions (Promega, Madison, WI).
Western blot analysis
Attached and floating cells treated with PMA were collected in lysis buffer containing 50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1.5 mM MgCl2, 10% glycerol, 1% Triton X-100, 5 mM ethyleneglycol-bis(aminoethylether)-tetraacetic acid, 1 mM Na3VO4, 10 mM NaF and one tablet of Protease Inhibitor Cocktail (Roche, Indianapolis, IN). Protein concentration was determined using BCA Protein Assay kit (Thermo Scientific, Rockford, IL) according to manufacturer’s instructions. Equal amounts of protein were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. Membranes were preblotted with 5% dry milk in Tris-buffered saline containing 0.2% Tween 20 at room temperature for 1 h, then blotted with a primary antibody and followed by a secondary goat anti-rabbit or anti-mouse antibody conjugated to horseradish peroxidase (1:1000; Bio-Rad, Hercules, CA). Bands were visualized by the enhanced chemiluminescence western blotting detection system (GE Healthcare, Piscataway, NJ). The primary antibodies included p-Ser742-PKD1 (Invitrogen), PKD2 (Abcam, Cambridge, MA), PKCδ (BD Biosciences, San Jose, CA), anti-ubiquitin antibody (P4D1; Santa Cruz, Santa Cruz, CA), PKCϵ (Santa Cruz), green fluorescent protein (GFP) (Roche), PKD1, p-Ser916-PKD1, cleaved PARP (Asp214), Akt, p-Ser473-Akt, p44/42 (137F5, ERK1/2) and p-Thr202/Tyr204-p44/42 (p-ERK1/2) antibodies (Cell Signaling Technology, Danvers, MA).
Immunoprecipitation
PKD1-GFP protein was immunoprecipitated by a GFP monoclonal antibody preconjugated to protein A sepharose beads for 3 h at 4°C. After washing with radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl and 1% Triton X-100) three times and 50 mM Tris–HCl buffer once, the precipitated protein was subjected to western blotting analysis as described above and probed with anti-GFP and anti-ubiquitin antibodies.
Cell cycle analysis
Cell cycle analysis was conducted as described previously (34). Briefly, LNCaP cells were fixed in 70% ice-cold ethanol overnight at 4°C, followed by propidium iodide staining. The labeled cells were analyzed using a BD FACSCalibur Flow Cytometer (BD Biosciences). Results were analyzed using ModFit LT software (Verity Software House).
Results
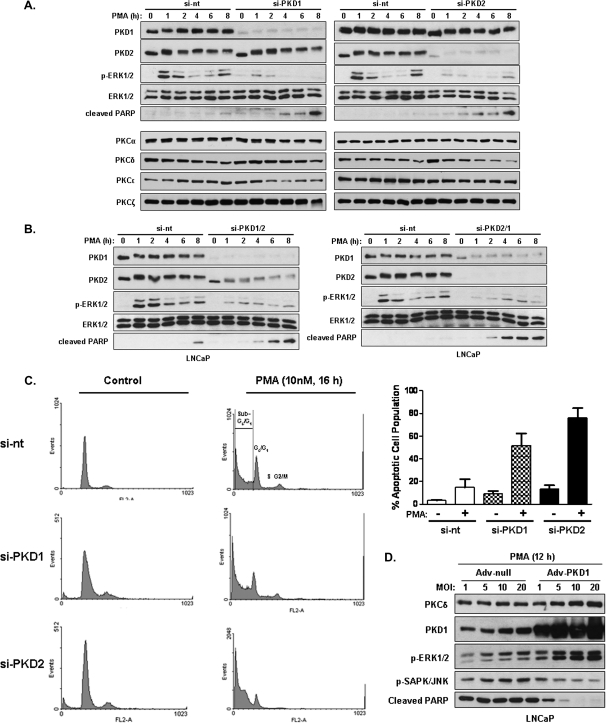
Depletion of endogenous PKD1 and PKD2 potentiated PMA-induced apoptosis in LNCaP cells
LNCaP prostate cancer cells express predominantly PKD1 and PKD2 but little PKD3 (34). To better understand the specific role of PKD in PMA-induced apoptosis in LNCaP cells, endogenous PKD1 and PKD2 were depleted by isoform-specific PKD siRNAs. LNCaP cells were transfected with control non-targeting siRNA (si-nt) or two isoform-specific PKD siRNAs (si-PKD1 and si-PKD2). As shown in Figure 1A, >90% of endogenous PKD1 or PKD2 was depleted by si-PKD1 or si-PKD2. Si-PKD1 selectively depleted PKD1 and did not affect the level of endogenous PKD2 and vice versa for si-PKD2. PMA-induced apoptosis in LNCaP cells was detected by immunoblotting for cleaved PARP, a marker of apoptosis (35). Depletion of either PKD1 or PKD2 caused early onset of PARP cleavage compared with the control, implying that both enzymes promote cell survival in response to PMA. Meanwhile, an additive effect on PARP cleavage was observed when knocking down both PKD1 and PKD2 by dual PKD siRNAs including si-PKD1/2 which depleted nearly 100% of PKD1 and ∼50% of PKD2 and si-PKD2/1 that depleted almost all PKD2 and ∼60% of PKD1 based on densitometry analysis, implying that PKD1 and PKD2 may act through nonredundant-signaling pathways to promote cell survival (Figure 1B).
Fig. 1.
Depletion of endogenous PKD1 and PKD2 potentiated PMA-induced apoptosis in LNCaP cells. (A) LNCaP cells were transfected with non-targeting (si-nt), PKD1 (si-PKD1), PKD2 (si-PKD2) siRNAs. Two days after transfection, cells were treated with vehicle (dimethyl sulfoxide) or 10 nM PMA for 1, 2, 4, 6 and 8 h, respectively. Cell lysates were subjected to western blotting analysis for indicated protein targets. Data from one of three independent experiments are shown. (B) LNCaP cells were transfected with dual PKD siRNAs, si-PKD1/2 and si-PKD2/1. Control cells were transfected with non-targeting siRNA (si-nt). Two days later, cells were treated with PMA as above and subjected to immunoblotting for indicated proteins. Data from one of three independent experiments are shown. (C) Depletion of endogenous PKD1 or PKD2 increased apoptotic cell population at sub-G0/G1 phase. LNCaP cells were transfected with si-nt, si-PKD1 or si-PKD2. Two days after transfection, cells were treated with vehicle (dimethyl sulfoxide) or 10 nM PMA for 16 h, respectively. LNCaP cells were fixed and cell cycle distribution was evaluated by flow cytometry after propidium iodide labeling. The experiments were repeated three time and results from a representative experiment are shown (left). Cell cycle distribution was analyzed by ModFit LT software. The % apoptotic cell population was calculated as the mean ± SEM of % cells in the sub-G0/G1 phase of cell cycle from four independent experiments (right). (D) Overexpression of PKD1 suppressed PMA-induced apoptosis in LNCaP cells. LNCaP cells were infected with increasing MOIs of adenoviruses encoding empty vector or wild-type PKD1. After two days infection, cells were treated with 10 nM PMA for 12 h. Cell lysates were subjected to western blotting analysis. Results from a representative experiment of at least three such independent experiments are shown.
PMA activates the ERK MAPK pathway and the inhibition of ERK activation by a MEK1 inhibitor has been shown to potentiate PMA-induced apoptosis, implying a role of MEK1/ERK1/2 pathway in survival of LNCaP cells (12). Here, we showed that depletion of either PKD1 or PKD2 or both significantly blocked PMA-induced ERK1/2 activation (measured by p-ERK1/2 levels) in both early (<1 h) and late (>6 h) phases of ERK1/2 activation (Figure 1A and B). As controls, the levels of endogenous ERK1/2 were not altered. Knockdown of PKD1 or PKD2 did not affect the levels of endogenous PKC isoforms (α, δ, ϵ, ξ) at basal state and upon short-term PMA treatment (<8 h), though it did cause a progressive time-dependent downregulation of PKCδ in LNCaP cells (Figure 1A and supplementary Figure 1, available at Carcinogenesis Online). Taken together, these results indicate that PKD1 and PKD2 are required for PMA-induced ERK1/2 activation and knockdown of both genes potentiates PMA-induced apoptosis in LNCaP cells.
To provide further evidence that the knockdown of endogenous PKD1 or PKD2 potentiates PMA-induced apoptosis in LNCaP cells, we evaluated the effect of PKD depletion on PMA-induced apoptosis by cell cycle analysis. This approach has previously been used to measure PMA-induced apoptosis in LNCaP cells (9–11). As shown in Figure 1C, PMA induced accumulation of cells in sub-G0/G1 phase of the cell cycle, the cell population generally associated with apoptosis. Among PMA-treated samples, cells with knockdown of PKD1 (si-PKD1) or PKD2 (si-PKD2) showed increased accumulation of apoptotic cell population in sub-G0/G1 phase compared with the control cells transfected with non-targeting siRNA (si-nt). Based on the quantitative measurement, knockdown of PKD1 resulted in an >3-fold increase in apoptotic cells, whereas knockdown of PKD2 caused a 5-fold increase as compared with the control cells treated with PMA (Figure 1C, right). These data further confirm that depletion of endogenous PKD1 or PKD2 exacerbates PMA-induced apoptosis in LNCaP cells.
Overexpression of PKD1 reduced PMA-induced apoptosis in LNCaP cells
The role of PKD1 in PMA-induced apoptosis in LNCaP cells was investigated further by over-expressing PKD1 in LNCaP cells. As illustrated in Figure 1D, overexpression of PKD1 by infecting cells with adenovirus carrying wild-type PKD1 (Adv-PKD1) significantly reduced PMA-induced PARP cleavage compared with the control cells-expressing empty adenoviruses (Adv-null). Accordingly, overexpression of wild-type PKD1 caused upregulation of p-ERK1/2 levels correlating to reduced PARP cleavage and reduced SAKP/JNK activity (measured by p-SAPK/JNK levels), a pro-apoptotic signal in LNCaP cells (11,14). Taken together, these results suggest that PKD1 protects LNCaP cells from PMA-induced apoptosis through upregulating ERK1/2 activity and downregulating JNK activity.
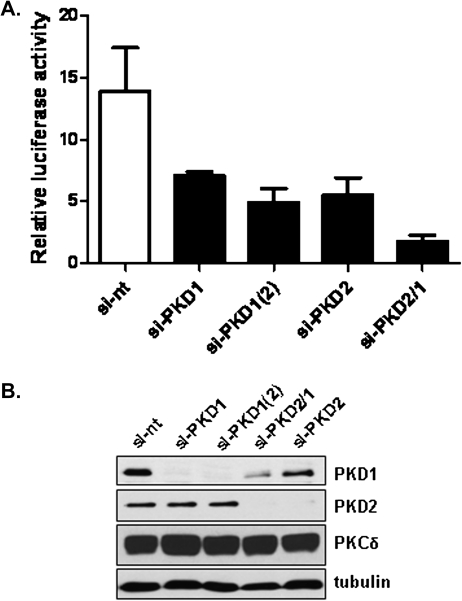
Knockdown of PKD1 and PKD2 blocked PMA-induced NF-κB transcriptional activity
Phorbol esters have been shown to activate NF-κB transcriptional activity through PKC-dependent mechanisms, a process that promotes cell survival (36–39). Here, we investigated a potential role of PKD1 and PKD2 in PMA-induced NF-κB-dependent transcriptional response by knocking down endogenous PKD in LNCaP cells. As shown in Figure 2A, depletion of PKD1 by two siRNAs [si-PKD1 and si-PKD1(2)] or PKD2 by one siRNA (si-PKD2) inhibited PMA-induced NF-κB transcriptional activity by ∼2- to 3-fold. Depletion of both PKD2 and PKD1 by the dual PKD siRNA (si-PKD2/1) had an additive effect, resulted in nearly 8-fold reduction in NF-κB transcriptional activity. As illustrated in Figure 2B, the siRNAs caused effective knockdown of PKD1, PKD2 or both isoforms. These results indicate that PKD1 and PKD2 are required for PMA-induced NF-κB-dependent transcriptional activity.
Fig. 2.
Effect of PKD knockdown on NF-κB-dependent transcription. (A) LNCaP cells transfected with non-targeting (si-nt), PKD1 [si-PKD1, si-PKD1(2)], PKD2 (si-PKD2) siRNAs and dual PKD siRNAs including si-PKD2/1. Two days later, the cells were transfected with NF-κB reporter gene pGL2-NF-kB and an internal control plasmid pTK-RL. After 24 h, the cells were subjected to PMA treatment at 10 nM for 6 h, followed by measurement of luciferase activity. Relative luciferase activity was calculated as ratio of PMA-stimulated versus unstimulated luciferase activity. Results are the mean ± SEM from three independent experiments. (B) Levels of PKD1, PKD2 in siRNA-transfected LNCaP cells. The knockdown was confirmed by immunoblotting for PKD1 and PKD2. Tubulin and PKCδ were blotted as controls.
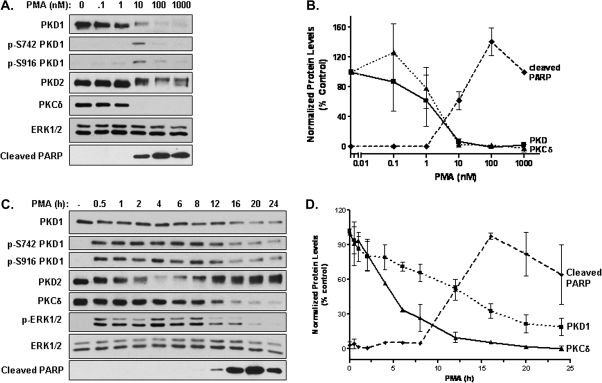
PMA induced concentration- and time-dependent downregulation of PKD1 in LNCaP cells
Our data so far indicated that the activation of PKD induced pro-survival signals that suppressed PMA-induced apoptotic response. Next, we sought to examine whether and how PMA regulates PKD-mediated survival signals in LNCaP cells. As shown in Figure 3, LNCaP cells were treated with increasing times and concentrations of PMA for 24 h. Levels of PKD protein and activity were analyzed by western blotting. Our results showed that PMA treatment caused time- and concentration-dependent downregulation of endogenous PKD1 and PKCδ proteins, which was inversely correlated to the appearance of cleaved PARP. The PMA concentrations required for achieving half maximal downregulation of PKD1 or PKCδ were similar (1.5 or 2.0 nM, respectively) (Figure 3B), albeit the PKCδ downregulation occurred earlier and was more complete (nearly 100% after 24 h) compared with those of PKD1 (Figure 3C and D). The downregulation of PKD1 was also correlated to the drop of PKD1 activity measured by phosphorylation at Ser742 (p-S742-PKD1) and Ser916 (p-S916-PKD1) as well as the decreased p-ERK1/2 levels. In contrast, the effect of PMA treatment on PKD2 expression was less apparent. Although prolonged PMA treatment (24 h) at high concentrations (100 nM or 1 μM) caused moderate PKD2 downregulation, low concentrations of PMA (10 nM) in general did not significantly affect PKD2 levels (Figure 3A and B). Note that a seemingly transient drop of PKD2 protein level in Figure 3C was due to the fact that PKD2 antibody was stage specific and less reactive to phosphorylated (active) forms of PKD2. Overall, our data indicate that the downregulation of PKD1 inversely correlated to the apoptotic response induced by PMA.
Fig. 3.
PMA induced concentration- and time-dependent downregulation of PKD1 in LNCaP cells. (A) LNCaP cells were treated with increasing concentrations of PMA (0.1, 1, 10, 100, 1000 nM) for 24 h. Cells were harvested and subjected to immunoblotting for indicated proteins. (B) The expression levels of PKD1, PKCδ and cleaved PARP were quantified by densitometry analysis. (C) LNCaP cells were treated without (dimethyl sulfoxide) or with 10 nM PMA for 0.5, 1, 2, 4, 6, 8, 12, 16, 20, 24 h. Cells were lysed for immunoblotting for indicated proteins. (D) Densitometry analysis on levels of PKD1, PKCδ and cleaved PARP, For all blots, total ERK1/2 served as loading control. For densitomery analysis, the values of each protein were normalized against ERK1/2 levels. The experiments were repeated four times and results from representative experiments are shown.
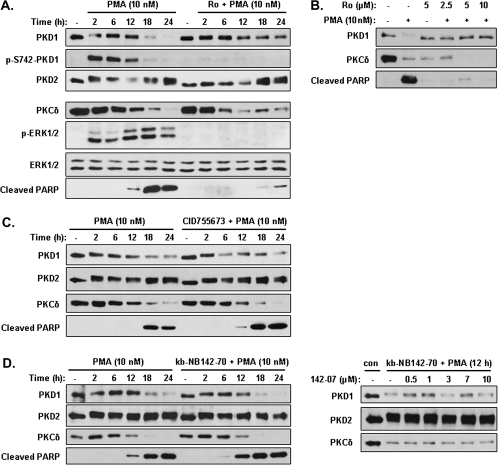
PKD1 downregulation was dependent on PKC, but not PKD, activity
PKC is known to activate PKD through phosphorylating the conserved serine residues in the activation loop of PKD. To determine the mechanisms of PMA-induced downregulation of PKD1 in LNCaP cells, we first sought to assess the role of PKC by blocking PKC activity using a pan-PKC inhibitor Ro 31-8220. Pretreatment of LNCaP cells with Ro 31-8220 at 2 μM completely abolished PMA-induced PKD1 activation (as measured by p-S742-PKD1 antibody), significantly reduced the downregulation of PKD1 and inhibited PMA-induced PARP cleavage, which is consistent with previous reports demonstrating a critical role of PKC activity in PMA-induced apoptosis in LNCaP cells (8–13) (Figure 4A). As controls to show that the inhibitor is working, Ro 31-8220 inhibited PMA-induced ERK1/2 phosphorylation but did not alter levels of ERK1/2 protein (Figure 4A). Interestingly, the downregulation of PKCδ was not blocked by Ro 31-8220, implying that PKCδ activity was not required for its downregulation. Similar results were obtained using increasing concentrations of Ro 31-8220, which blocked both PMA-induced PKD1 downregulation and PARP cleavage but did not reverse the downregulation of PKCδ (Figure 4B). These results also imply that PKCδ activity, but not its downregulation, is critical for the apoptotic response. Taken together, our data indicate that PKC activity is required for PMA-induced PKD1 activation and its subsequent downregulation in LNCaP cells.
Fig. 4.
The downregulation of PKD1 was dependent on PKC, but not PKD, activity and the inhibition of PKD potentiated PMA-induced apoptosis. (A and B) Inhibition of PKC blocked PKD1 downregualtion. LNCaP cells were pretreated with or without the PKC inhibitor Ro 31-8220 at 2 μM for 1 h, followed by the addition of 10 nM PMA for indicated times (A), with increasing concentrations of Ro 31-8220 for 1 h, followed by 10 nM PMA treatment for 24 h (B). Cell lysates were subjected to western blotting analysis for indicated proteins. (C and D) Inhibition of PKD potentiated PMA-induced apoptosis but did not affect PKD1 downregulation. LNCaP cells were pretreated for 1 h with the PKD inhibitor CID755673 at 30 μM (C) or kb-NB142-70 at 10 μM (D, left panels) or increasing concentrations of kb-NB142-70 (D, right panels). PMA (10 nM) was then added for 0, 2, 6, 12, 18 and 24 h, respectively. Cells were lysed and subjected to immunoblotting for PKD1, PKD2, PKCδ and cleaved PARP. The above experiments were repeated three times and results from representative experiments are shown.
Next, we sought to investigate whether PKD activity per se is necessary for PMA-induced apoptotic response and if the activation of PKD1 is necessary for its own downregulation. Here, we used two direct PKD inhibitors that we have previously identified and characterized, CID755673 (IC50: 182 nM for PKD1) and its analog kb-NB142-70 (IC50: 28.3 nM for PKD1), which have little inhibitory activity for PKC (32,33). As shown in Figure 4C, pretreatment with CID755673 (30 μM) did not block the time-dependent downregulation of PKD1 nor did it affect the downregulation of PKCδ. Similar results were obtained using kb-NB142-70 (10 μM). Furthermore, increasing concentrations of kb-NB142-70 did not block the downregulation of PKD1 or PKCδ (Figure 4D). Additionally, the inhibition of PKD activity by both inhibitors potentiated PMA-induced PARP cleavage, indicating that PKD activity protects the cells from apoptosis. As controls, PKD2 levels remained relatively constant regardless of the treatment conditions. Thus, inhibition of PKD1 activity potentiated PMA-induced apoptosis, but did not affect its downregulation, indicating that PKD1 activity is required for its pro-survival effects.
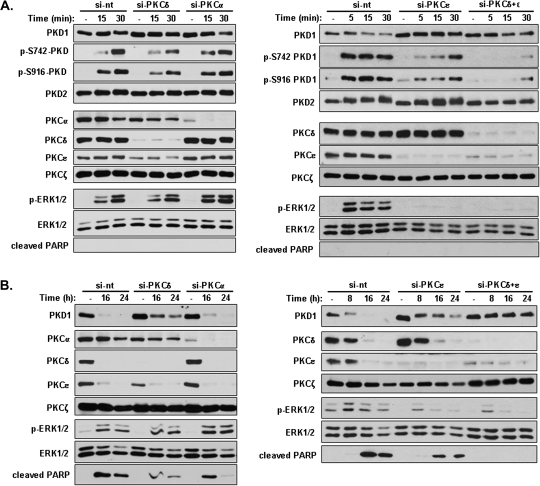
PKCδ and ϵ were responsible for PMA-induced PKD1 activation and downregulation
LNCaP cells express PKCα, PKCϵ, PKCδ and PKCζ (9). To identify the specific PKC isozymes involved in mediating PMA-induced PKD1 activation and downregulation, LNCaP cells were depleted of PKC isoforms using siRNAs that target PKCα (si-PKCα), PKCδ (si-PKCδ), PKCϵ (si-PKCϵ) and both PKCδ and ϵ (si-PKCδ+ϵ). Transfected cells were subjected to either short- (5, 15, 30 min) or long-time (8, 16, 24 h) PMA treatment to evaluate the contribution of PKC isoforms in PKD1 activation or downregulation, respectively. As shown in Figure 5A, selective depletion of PKCδ or ϵ partially blocked PMA-induced PKD1 activation as measured by p-S916-PKD1 and p-S742-PKD1 levels, whereas silencing of both PKCδ and ϵ resulted in almost complete blockade of PKD1 activation. Interestingly, depletion of PKCϵ alone completely blocked PMA-induced rapid activation of ERK1/2, whereas depletion of PKCδ only partially reduced p-ERK1/2 levels. In contrast, depletion of PKCα had no effects on PKD1 activation and on PMA-induced ERK1/2 activation. As expected, short-time PMA treatment did not alter the endogenous levels of PKD and PKC isoforms and did not induce PARP cleavage. Taken together, these results indicate that both PKCδ and ϵ are required for PMA-induced PKD1 activation.
Fig. 5.
PKCδ and ϵ, but not PKCα, were required for PMA-induced PKD1 activation and downregulation. (A) PKCδ and ϵ were key mediators of PMA-induced PKD1 activation. LNCaP cells were transfected with non-targeting siRNA (si-nt) and siRNAs-targeting endogenous PKCα (si-PKCα), PKCδ (si-PKCδ), PKCϵ (si-PKCϵ) and both PKCδ and ϵ (si-PKCδ+ϵ). Two days after transfection, cells were treated with vehicle (dimethyl sulfoxide) or 10 nM PMA for 5, 15, 30 min to activate PKD. Cells were harvested and subjected to western blotting analysis for indicated protein targets. The above experiments were repeated three times and results from representative experiments are shown. (B) Both PKCδ and ϵ were required for PMA-induced PKD1 downregulation and apoptosis. LNCaP cells were transfected with the indicated PKC siRNAs. Two days after transfection, cells were subjected to prolonged PMA treatment at 10 nM for 0, 8, 16, 24 h. Cells were collected and analyzed by immunoblotting. The above experiments were repeated four times and results from representative experiments are shown.
To identify the PKC isoforms required for PKD1 downregulation, LNCaP cells transfected with PKC siRNAs were subjected to prolonged PMA treatment. As shown in Figure 5B, PMA treatment for 8, 16 and 24 h caused progressive downregulation of endogenous PKD1 in control cells transfected with the non-targeting siRNA (si-nt), correlating to enhanced PARP cleavage. The depletion of PKCϵ or δ partially blocked PKD1 downregulation, whereas complete inhibition was achieved by depleting both isoforms. Accordingly, depletion of either PKCϵ or δ partially blocked the apoptotic response, whereas depletion of both PKCs completely blocked PARP cleavage and inhibited PMA-induced apoptosis. We also noted that knockdown of PKCα alone resulted a slight inhibition of cleaved PARP signal but had no apparent effect on PKD1 downregulation. The effects of PKC knockdown on apoptosis agreed with previous reports (40). Addtionally, depletion of either PKCδ or ϵ or both also reduced late time ERK1/2 activity, whereas knockdown of PKCα had no effect. Overall, our data indicate that both PKCδ and ϵ are necessary for PMA-induced PKD1 downregulation in LNCaP cells.
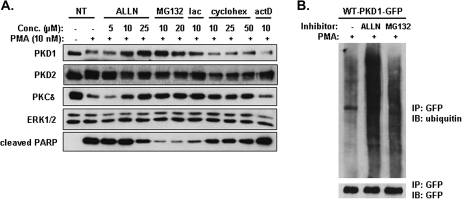
PMA-induced downregulation of PKD1 was mediated by proteasome degradation pathway
The ubiquitin/proteasome system plays an important role in the degradation of cellular-signaling proteins (41,42). To determine if the effect of PMA on PKD1 protein was due to proteasome-mediated degradation, we tested several proteasome inhibitors, including ALLN, MG132 and lactacystin. LNCaP cells were preincubated with the inhibitors for 4 h prior to PMA treatment. As shown in Figure 6A, ALLN inhibited PMA-induced PKD1 downregulation in a concentration-dependent manner. MG132 and lactacystin similarly blocked PKD1 degradation. Additionally, the downregulation of PKCδ was also blocked by increasing concentrations of the proteasome inhibitors. In contrast, PKD2 protein remained relatively constant under these treatments. A protein translation inhibitor cycloheximide and a transcription inhibitor actinomycin D had no effects on PKD1 downregulation. These results suggested that the downregulation of PKD1 and PKCδ was mediated through an ubiquitin-triggered proteasome degradation pathway. Interestingly, the inhibition of PKCδ and PKD1 downregulation closely coupled to reduced PARP cleavage and inhibition of PMA-induced apoptosis, implying that the degradation of these proteins could play a role in the apoptotic process. To determine if PKD1 was subjected to ubiquitination, GFP-PKD1 overexpressed in LNCaP cells was immunoprecipitated by a GFP antibody and probed using an ubiquitin antibody. As shown in Figure 6B, GFP-PKD1 was strongly ubiquitinated in ALLN- and MG132-treated samples compared with the untreated control sample. Thus, these results suggested that, upon activation by PMA, PKD1 was ubiquitinated and downregulated through a proteasome-mediated degradation pathway in LNCaP cells.
Fig. 6.
PMA-induced downregulation of PKD1 was mediated through an ubiquitin–proteasome degradation pathway. (A) PMA-induced downregulation of PKD1 was blocked by proteasome inhibitors. LNCaP cells were preincubated for 4 h with vehicle (dimethyl sulfoxide), proteasome inhibitors including ALLN (5, 10, 25 μM), MG132 (10, 20 μM), lactacystin (10 μM), a protein translation inhibitor cycloheximide (10, 25, 50 μM), or a transcription inhibitor actinomycin D (10 μM), followed by the addition of 10 nM PMA for 16 h. Cell were collected and analyzed by western blotting for indicated proteins. (B) PMA induced ubiquintination of PKD1. LNCaP cells were transfected with WT-PKD1-GFP plasmid. Two days after transfection, cells were preincubated without or with 25 μM ALLN, 10 μM MG132 for 4 h, followed by 10 nM PMA treatment 16 h. Cells were lysed and subjected to immunoprecipitation using GFP monoclonal antibody. Immunoprecipitated proteins were analyzed by immunoblotting for ubiquitin and GFP. The above experiments were repeated at least three times and data from a representative experiment are shown.
Discussion
PKD isozymes are differentially expressed in human prostate cancer cells (34). LNCaP cells predominantly express PKD1 and PKD2, and their roles in PMA-induced apoptosis have not been elucidated. By depleting endogenous PKD1 or PKD2, we showed that the loss of PKD greatly potentiated PMA-induced apoptosis in LNCaP cells, whereas overexpression of wild-type PKD1 decreased the apoptotic response induced by PMA. Thus, PKD promoted the survival of LNCaP cells. Depletion of PKD1 and PKD2 blocked, whereas overexpression of wild-type PKD1 potentiated, PMA-induced ERK1/2 activation and NF-κB transcriptional activity, indicating that PKD is required for these pro-suvival signals induced by PMA. Although PMA has been shown to activate all three MAPK cascades (p38, JNK, ERK1/2) in LNCaP cells, each pathway plays distinct roles in PMA-induced apoptosis (12,13). Although p38 and JNK mediate the apoptotic effect of PMA, ERK1/2 activation counteracts the apoptotic response and promotes cell survival (11–13). Here, our study has demonstrated a significant role of PKD, particularly PKD1, in promoting PMA-induced EKR1/2 survival pathway and suppression of PMA-induced JNK pro-apoptotic pathway. Our findings further imply that activities and levels of PKD in prostate cancer cells contribute to the fine balance between apoptotic and survival signals induced by PMA and ultimately affect PMA sensitivity of the tumor cells. Interestingly, although our data implied that PKD1 and PKD2 played redundant roles in PMA-induced apoptosis and in modulating downstream ERK1/2 and NF-κB activity, depletion of PKD1 and PKD2 had an additive effect on the apoptotic response, suggesting that PKD1 and PKD2 may signal through nonredundant pathways to modulate PMA-induced apoptosis.
In LNCaP cells, PMA treatment led to rapid PKD activation, followed by slow and progressive downregulation of PKD1. The role of PKC in PKD1 activation and downregulation was examined using a pharmacological inhibitor of PKC (Ro 31-8220) and PKC siRNAs. Ro 31-8220 treatment blocked both PKD1 activation and downregulation, indicating a critical role of PKC in the process. Depletion of endogenous PKCϵ and/or PKCδ showed that PKD1 activation and downregulation were controlled by both PKCδ and ϵ. Knockdown of either PKC isoform partially blocked PKD1 activation/downregulation and PMA-induced apoptosis, whereas knockdown of both isoforms almost completely inhibited these responses. The effect of PKCα, δ and ϵ knockdown on apoptosis agreed with a previous report using stable PKC knockdown approach in prostate cancer cell lines (40).
PMA treatment can induce protein degradation via an ubiquitin/proteasome-mediated pathway. These effects have been demonstrated for a number of PKC isoforms (43,44). Here, we showed that prolonged PMA treatment caused progressive downregulation of PKD1 protein. This is a time- and concentration-dependent effect that occurred at later time points of PMA treatment. Reverse transcription–polymerase chain reaction analysis indicated that PMA did not alter the messenger RNA levels of PKD1 and PKD2, indicating that the downregulation did not occur at the transcriptional level (data not shown). Further analysis indicated that the downregulation could be reversed by proteasome inhibitors in a concentration-dependent manner, supporting an ubiquitin–proteasome degradation pathway in the control of PKD1 protein levels. Our findings coincided with a previous report showing that prolonged PMA treatment promoted ubiquitination- and proteasome-mediated degradation of DKF-1, a Caenorhabditis elegans homolog of PKD (45), while contrasted to earlier studies that found PKD to be resistant to PMA-induced downregulation (46). We also noted that PKCδ, a key apoptotic mediator, was downregulated by PMA similar to PKD1, and the effect was reversed by proteasome inhibitors. However, our data suggest that the downregulation of PKCδ was not critical for its apoptotic effects since inhibition of PKC alone without impact on its degradation was sufficient to block PMA-induced apoptosis. Moreover, the downregulation of PKD1 was dependent on PKC, but not its intrinsic kinase activity. It is possible that different protein degradation machineries were involved in PKCδ and PKD1 downregulation. This was supported by the observation that PKD1 downregulation was controlled by PKCδ and ϵ activities, while that of PKCδ was independent of any PKC activity (PKC inhibitors did not prevent the downregulation of PKCδ). Meanhwhile, the kinetics of their degradation were not well correlated (the degradation of PKCδ occurred at an earlier time point). Nonetheless, it is important to note that the downregulation of PKD1 was associated with increased apoptotic response and that the blockade of its degradation by proteasome inhibitors correlated with reduced PARP cleavage and increased cell survival, suggesting that the degradation of PKD1 may play a role in PMA-induced apoptotic response. It is plausible that the degradation of PKD1 serves to offset the PKD-mediated survival pathway to facilitate PMA-induced apoptosis. Finally, although our data did not show a drastic effect of PMA on PKD2 protein expression, based on the results of PKD depletion and inhibition, it was clear that PKD2, like PKD1, also played an important pro-survival and anti-apoptotic function in PMA-induced apoptosis in LNCaP cells.
In summary, we showed that PMA activated and then downregulated PKD1 through a PKCδ/ϵ-mediated pathway in androgen-sensitive LNCaP prostate cancer cells. The activation of PKD1 promoted tumor cell survival by increasing ERK1/2 and NF-κB-dedependent survival signals and suppressing JNK apoptotic signal. Additionally, prolonged PMA treatment induced PKD1 downregulation, a process that may facilitate the apoptotic process and enhance PMA sensitivity. Finally, the combination of phorbol esters and PKD inhibitors showed synergistic effects in the induction of apoptosis in prostate tumor cells, supporting the potential value of targeting PKD in combinatorial treatment for prostate cancer.
Supplementary material
Supplementary Figure 1 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health (R03 MH082038–01, R01CA129127–01 and R01CA142580-01).
Supplementary Material
Acknowledgments
We thank the technical assistance from Dr Dongzhu Ma at the University of Pittsburgh. The authors declare no competing financial interests in the work described.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- ALLN
N-acetyl-leucyl-leucyl-norleucinal
- ERK
extracellular signal-regulated kinase
- DAG
diacylglycerol
- GFP
green fluorescent protein
- JNK
c-jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MEK
mitogen-activated protein kinase/ERK kinase
- NF-κB
nuclear factor-kappaB
- PKC
protein kinase C
- PKD
protein kinase D
- PMA
phorbol 12-myristate 13-acetate
References
- 1.Barry OP, et al. Protein kinase C isozymes, novel phorbol ester receptors and cancer chemotherapy. Curr. Pharm. Des. 2001;7:1725–1744. doi: 10.2174/1381612013397041. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Guerrico AM, et al. Molecular mechanisms of protein kinase C-induced apoptosis in prostate cancer cells. J. Biochem. Mol. Biol. 2005;38:639–645. doi: 10.5483/bmbrep.2005.38.6.639. [DOI] [PubMed] [Google Scholar]
- 3.Xiao L, et al. Phorbol ester-induced apoptosis and senescence in cancer cell models. Methods Enzymol. 2008;446:123–139. doi: 10.1016/S0076-6879(08)01607-8. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, et al. Protein kinase D specifically mediates apoptosis signal-regulating kinase 1-JNK signaling induced by H2O2 but not tumor necrosis factor. J. Biol. Chem. 2005;280:19036–19044. doi: 10.1074/jbc.M414674200. [DOI] [PubMed] [Google Scholar]
- 5.Schaar D, et al. A phase I clinical trial of 12- O-tetradecanoylphorbol-13-acetate for patients with relapsed/refractory malignancies. Cancer Chemother. Pharmacol. 2006;57:789–795. doi: 10.1007/s00280-005-0125-1. [DOI] [PubMed] [Google Scholar]
- 6.Griner EM, et al. Protein kinase C and other diacylglycerol effectors in cancer. Nat. Rev. Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 7.Newton AC. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem. Rev. 2001;101:2353–2364. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- 8.Gavrielides MV, et al. Androgens regulate protein kinase Cdelta transcription and modulate its apoptotic function in prostate cancer cells. Cancer Res. 2006;66:11792–11801. doi: 10.1158/0008-5472.CAN-06-1139. [DOI] [PubMed] [Google Scholar]
- 9.Fujii T, et al. Involvement of protein kinase C delta (PKCdelta) in phorbol ester-induced apoptosis in LNCaP prostate cancer cells. Lack of proteolytic cleavage of PKCdelta. J. Biol. Chem. 2000;275:7574–7582. doi: 10.1074/jbc.275.11.7574. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Bermejo ML, et al. Diacylglycerol (DAG)-lactones, a new class of protein kinase C (PKC) agonists, induce apoptosis in LNCaP prostate cancer cells by selective activation of PKCalpha. J. Biol. Chem. 2002;277:645–655. doi: 10.1074/jbc.M107639200. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Guerrico AM, et al. Phorbol ester-induced apoptosis in prostate cancer cells via autocrine activation of the extrinsic apoptotic cascade: a key role for protein kinase C delta. J. Biol. Chem. 2005;280:38982–38991. doi: 10.1074/jbc.M506767200. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka Y, et al. Protein kinase C promotes apoptosis in LNCaP prostate cancer cells through activation of p38 MAPK and inhibition of the Akt survival pathway. J. Biol. Chem. 2003;278:33753–33762. doi: 10.1074/jbc.M303313200. [DOI] [PubMed] [Google Scholar]
- 13.Xiao L, et al. ROCK mediates phorbol ester-induced apoptosis in prostate cancer cells via p21Cip1 up-regulation and JNK. J. Biol. Chem. 2009;284:29365–29375. doi: 10.1074/jbc.M109.007971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao L, et al. PKC-mediated secretion of death factors in LNCaP prostate cancer cells is regulated by androgens. Mol. Carcinog. 2009;48:187–195. doi: 10.1002/mc.20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thakkar H, et al. Pro-survival function of Akt/protein kinase B in prostate cancer cells. Relationship with TRAIL resistance. J. Biol. Chem. 2001;276:38361–38369. doi: 10.1074/jbc.M103321200. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, et al. Constitutively active Akt is an important regulator of TRAIL sensitivity in prostate cancer. Oncogene. 2001;20:6073–6083. doi: 10.1038/sj.onc.1204736. [DOI] [PubMed] [Google Scholar]
- 17.Manning G, et al. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 18.Johannes FJ, et al. PKCu is a novel, atypical member of the protein kinase C family. J. Biol. Chem. 1994;269:6140–6148. [PubMed] [Google Scholar]
- 19.Valverde AM, et al. Molecular cloning and characterization of protein kinase D: a target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proc. Natl Acad. Sci. USA. 1994;91:8572–8576. doi: 10.1073/pnas.91.18.8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sturany S, et al. Molecular cloning and characterization of the human protein kinase D2. A novel member of the protein kinase D family of serine threonine kinases. J. Biol. Chem. 2001;276:3310–3318. doi: 10.1074/jbc.M008719200. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi A, et al. PKCnu, a new member of the protein kinase C family, composes a fourth subfamily with PKCmu. Biochim. Biophys. Acta. 1999;1450:99–106. doi: 10.1016/s0167-4889(99)00040-3. [DOI] [PubMed] [Google Scholar]
- 22.Waldron RT, et al. Protein kinase C phosphorylates protein kinase D activation loop Ser744 and Ser748 and releases autoinhibition by the pleckstrin homology domain. J. Biol. Chem. 2003;278:154–163. doi: 10.1074/jbc.M208075200. [DOI] [PubMed] [Google Scholar]
- 23.Zugaza JL, et al. Protein kinase D (PKD) activation in intact cells through a protein kinase C-dependent signal transduction pathway. EMBO. J. 1996;15:6220–6230. [PMC free article] [PubMed] [Google Scholar]
- 24.Wang QJ. PKD at the crossroads of DAG and PKC signaling. Trends Pharmacol. Sci. 2006;27:317–323. doi: 10.1016/j.tips.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Rozengurt E, et al. Protein kinase D signaling. J. Biol. Chem. 2005;280:13205–13208. doi: 10.1074/jbc.R500002200. [DOI] [PubMed] [Google Scholar]
- 26.Sinnett-Smith J, et al. Protein kinase D2 potentiates MEK/ERK/RSK signaling, c-Fos accumulation and DNA synthesis induced by bombesin in Swiss 3T3 cells. J. Cell. Physiol. 2007;211:781–790. doi: 10.1002/jcp.20984. [DOI] [PubMed] [Google Scholar]
- 27.Sinnett-Smith J, et al. Protein kinase D potentiates DNA synthesis induced by Gq-coupled receptors by increasing the duration of ERK signaling in swiss 3T3 cells. J. Biol. Chem. 2004;279:16883–16893. doi: 10.1074/jbc.M313225200. [DOI] [PubMed] [Google Scholar]
- 28.Wong C, et al. Protein kinase C-dependent protein kinase D activation modulates ERK signal pathway and endothelial cell proliferation by vascular endothelial growth factor. J. Biol. Chem. 2005;280:33262–33269. doi: 10.1074/jbc.M503198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trauzold A, et al. PKCmu prevents CD95-mediated apoptosis and enhances proliferation in pancreatic tumour cells. Oncogene. 2003;22:8939–8947. doi: 10.1038/sj.onc.1207001. [DOI] [PubMed] [Google Scholar]
- 30.Storz P, et al. Protein kinase D mediates a stress-induced NF-kappaB activation and survival pathway. Embo. J. 2003;22:109–120. doi: 10.1093/emboj/cdg009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bravo -Altamirano K, et al. Synthesis and structure-activity relationships of benzothienothiazepinone inhibitors of protein kinase D. ACS Med. Chem. Lett. 2011;2:154–159. doi: 10.1021/ml100230n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lavalle CR, et al. Novel protein kinase D inhibitors cause potent arrest in prostate cancer cell growth and motility. BMC Chem. Biol. 2010;10:5. doi: 10.1186/1472-6769-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharlow ER, et al. Potent and selective disruption of protein kinase d functionality by a benzoxoloazepinolone. J. Biol. Chem. 2008;283:33516–33526. doi: 10.1074/jbc.M805358200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, et al. Protein kinase D3 (PKD3) contributes to prostate cancer cell growth and survival through a PKCepsilon/PKD3 pathway downstream of Akt and ERK 1/2. Cancer Res. 2008;68:3844–3853. doi: 10.1158/0008-5472.CAN-07-5156. [DOI] [PubMed] [Google Scholar]
- 35.Duriez PJ, et al. Cleavage of poly(ADP-ribose) polymerase: a sensitive parameter to study cell death. Biochem. Cell Biol. 1997;75:337–349. [PubMed] [Google Scholar]
- 36.Trauzold A, et al. CD95 and TRAIL receptor-mediated activation of protein kinase C and NF-kappaB contributes to apoptosis resistance in ductal pancreatic adenocarcinoma cells. Oncogene. 2001;20:4258–4269. doi: 10.1038/sj.onc.1204559. [DOI] [PubMed] [Google Scholar]
- 37.Busuttil V, et al. Blocking NF-kappaB activation in Jurkat leukemic T cells converts the survival agent and tumor promoter PMA into an apoptotic effector. Oncogene. 2002;21:3213–3224. doi: 10.1038/sj.onc.1205433. [DOI] [PubMed] [Google Scholar]
- 38.Wang Q, et al. PKCdelta-mediated regulation of FLIP expression in human colon cancer cells. Int. J. Cancer. 2006;118:326–334. doi: 10.1002/ijc.21373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Q, et al. Induction of cIAP-2 in human colon cancer cells through PKC delta/NF-kappa B. J. Biol. Chem. 2003;278:51091–51099. doi: 10.1074/jbc.M306541200. [DOI] [PubMed] [Google Scholar]
- 40.Yin L, et al. Phorbol ester-induced apoptosis of C4-2 cells requires both a unique and a redundant protein kinase C signaling pathway. J. Biol. Chem. 2005;280:5533–5541. doi: 10.1074/jbc.M405266200. [DOI] [PubMed] [Google Scholar]
- 41.Zhimin L, et al. Degradation of activated protein kinases by ubiquitination. Annu. Rev. Biochem. 2009;78:435–475. doi: 10.1146/annurev.biochem.013008.092711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hochstrasser M. Ubiquitin, proteasomes, and the regulation of intracellular protein degradation. Curr. Opin. Cell Biol. 1995;7:215–223. doi: 10.1016/0955-0674(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 43.Lu Z, et al. Activation of protein kinase C triggers its ubiquitination and degradation. Mol. Cell. Biol. 1998;18:839–845. doi: 10.1128/mcb.18.2.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee HW, et al. Ubiquitination of protein kinase C-alpha and degradation by the proteasome. J. Biol. Chem. 1996;271:20973–20976. [PubMed] [Google Scholar]
- 45.Feng H, et al. Conserved domains subserve novel mechanisms and functions in DKF-1, a Caenorhabditis elegans protein kinase D. J. Biol. Chem. 2006;281:17815–17826. doi: 10.1074/jbc.M511898200. [DOI] [PubMed] [Google Scholar]
- 46.Rennecke J, et al. Immunological demonstration of protein kinase C mu in murine tissues and various cell lines. Differential recognition of phosphorylated forms and lack of down-regulation upon 12-O-tetradecanoylphorphol-13-acetate treatment of cells. Eur. J. Biochem. 1996;242:428–432. doi: 10.1111/j.1432-1033.1996.0428r.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.