A set of simple clinical prediction criteria for patients with nosocomial Staphylococcus aureus bacteremia was developed to identify patients at low risk of infective endocarditis in whom transesophageal echocardiography might be dispensable and was validated with two independent cohorts.
Abstract
(see the editorial commentary and Soriano and Mensa, on pages 10–12.)
Background. Infective endocarditis (IE) is a severe complication in patients with nosocomial Staphylococcus aureus bacteremia (SAB). We sought to develop and validate criteria to identify patients at low risk for the development of IE in whom transesophageal echocardiography (TEE) might be dispensable.
Methods. Consecutive patients with nosocomial SAB from independent cohorts in Europe (Invasive S. aureus Infection Cohort [INSTINCT]) and North America (S. aureus Bacteremia Group [SABG]) were evaluated for the presence of clinical criteria predicting an increased risk for the development of IE (ie, prolonged bacteremia of >4 days' duration, presence of a permanent intracardiac device, hemodialysis dependency, spinal infection, and nonvertebral osteomyelitis). Patients were observed closely for clinical signs and symptoms of IE during hospitalization and a 3-month follow-up period.
Results. IE was present in 13 (4.3%) of 304 patients in the INSTINCT cohort and in 40 (9.3%) of 432 patients in the SABG cohort. Within 14 days after the first positive blood culture result, echocardiography was performed in 39.8% and 57.4% of patients in the INSTINCT and SABG cohorts, respectively. In patients with IE, the most common clinical prediction criteria present were prolonged bacteremia (69.2% vs 90% for INSTINCT vs SABG, respectively) and presence of a permanent intracardiac device (53.8% vs 32.5%). In total, 13 of 13 patients in the INSTINCT cohort and 39 of 40 patients in the SABG cohort with documented IE fulfilled at least 1 criterion (sensitivity, 100% vs. 97.5%; negative predictive value, 100% vs 99.2%).
Conclusions. A simple criteria set for patients with nosocomial SAB can identify patients at low risk of IE. Patients who meet these criteria may not routinely require TEE.
Staphylococcus aureus is the second most common cause of nosocomial bloodstream infection (BSI) worldwide [1, 2]. It differs from other bacterial pathogens by its propensity to cause deep-seated infections as well as metastatic infections at distant sites. Identifying the focus of infection in S. aureus bacteremia (SAB) can be a considerable challenge. Even after an extensive diagnostic evaluation, a focus of infection is ascertained in only 60%–80% of cases [3–6]. However, recommendations on which diagnostic procedures (eg, echocardiography and other imaging techniques) are needed to identify the source of infection and when they should be performed is based on evidence from a few studies [7–9].
Infective endocarditis (IE) can be the source of bacteremia at admission or a secondary complication of SAB occurring either during hospitalization or within weeks or months after discharge. In recent studies, IE occurred in 5%–17% of patients with SAB [5, 10–12]. Despite advances in imaging techniques, transthoracic echocardiography (TTE) is considered to be less sensitive than transesophageal echocardiography (TEE) for detection of vegetations on heart valves [13]. It has been suggested TEE should be performed routinely for all patients with SAB to exclude IE early in the course of the infection [8, 14–17].
In some clinical scenarios such as SAB with negative follow-up blood culture results, a removable focus of infection, and rapid clinical resolution ≤72 h after initiation of therapy in the absence of indwelling prosthetic devices (similar to simple SAB as defined in [18]), IE is unlikely to occur. To identify patients with nosocomial SAB who have a low risk for developing IE and for whom TEE may not be required, we propose simple clinical prediction criteria. Diagnostic accuracy of the criteria set was evaluated using data from a cohort of patients with SAB in Europe (Invasive S. aureus Infection Cohort [INSTINCT]) and North America (S. aureus Bacteremia Group [SABG]).
METHODS
Patients and Setting
Data was analyzed post hoc from 2 prospective cohort studies that were performed to investigate the epidemiology of SAB. Partial data from both studies have been published elsewhere [6, 12]. The INSTINCT study was conducted at 2 German tertiary care university hospitals in Cologne and Freiburg that have ∼45,000 and ∼59,000 annual admissions, respectively. The SABG study was conducted at Duke University (Durham, NC), a tertiary care hospital with 63,000 annual admissions. An infectious diseases (ID) consultation service was available throughout the study period in all centers.
Blood culture results were reviewed daily. Patients aged ≥18 years with nosocomial SAB who had ≥1 blood culture positive for S. aureus were included in the study.
Ethical Considerations
The study was approved by the respective institutional review boards. We followed the ethical standards set by the Helsinki Declaration of 1975, as revised in 2004, and the research guidelines of Duke University, the University of Cologne, and the University of Freiburg.
Data Acquisition
Patients with SAB were evaluated prospectively during their hospital stays for the source of infection, complicating factors (eg, the presence of prosthetic heart valves, vascular implants, or pacemakers), hemodialysis dependency, clinical signs of IE, early complications of SAB, duration of hospital stay, discharge date and status, and cause of death. Furthermore, the time and number of blood cultures obtained and the results of diagnostic imaging studies were recorded. SAB was considered nosocomial if clinical symptoms developed ≥48 h after hospital admission [19]. The source of bacteremia was defined as the most likely source responsible for the first positive blood culture result on the basis of clinical signs, microbiological findings, and imaging results. Definite IE was assessed in accordance with modified Duke criteria [20]. TEE was encouraged by the ID consultation service and performed at the discretion of the attending physician by an experienced echocardiographer.
Follow-up data were obtained 3 months after the first positive blood culture result and used as reference standard for the possible development of IE after discharge. In particular, the presence of fever at any time during the observation period, antimicrobial therapy, signs and symptoms of late complications of SAB (eg, recurrent bacteremia, endocarditis, and osteomyelitis), and any new hospital admission were either determined by telephone interview with the patient and/or his primary care physician or when the patient was readmitted. If patients were admitted to another hospital, detailed information about their clinical course was obtained. All data were collected prospectively by a trained study nurse and validated by an ID physician. IE was excluded if there were no clinical (including stroke and heart failure), microbiological, and imaging data that suggested a diagnosis of IE.
Criteria Set
The clinical prediction criteria included prolonged bacteremia, the presence of a permanent intracardiac device (eg, a prosthetic heart valve, pacemaker, or cardioverter-defibrillator), hemodialysis dependency, spinal infection (eg, vertebral osteomyelitis, epidural, subdural or intraspinal empyema, or abscess), and nonvertebral osteomyelitis. Irrespective of the timing and appropriateness of antimicrobial therapy, SAB was considered prolonged if >4 days elapsed between the first blood culture to yield S. aureus and the first negative follow-up blood culture result or if follow-up blood cultures were not performed. In this group, prolonged bacteremia was considered “documented” if follow-up blood cultures yielded S. aureus 2–4 days after the first positive blood culture result [12]. Cases in which no follow-up blood cultures were performed within 4 days were considered to represent “possible” prolonged bacteremia.
Statistical Analysis
Descriptive statistics include counts and percentages for qualitative variables and quartiles (25th, 50th, and 75th percentile) for quantitative data. The 2-sided Fisher exact test was used to assess the association of qualitative variables. Estimates of conditional probability (eg, sensitivity and specificity) were accompanied by 95% confidence intervals (CIs; determined by the Wilson method). Because age data for the SABG cohort were provided in decades, quartiles were calculated using mid-class values. The study is reported according to STARD (Statement for Reporting Diagnostic Accuracy Studies) [21].
RESULTS
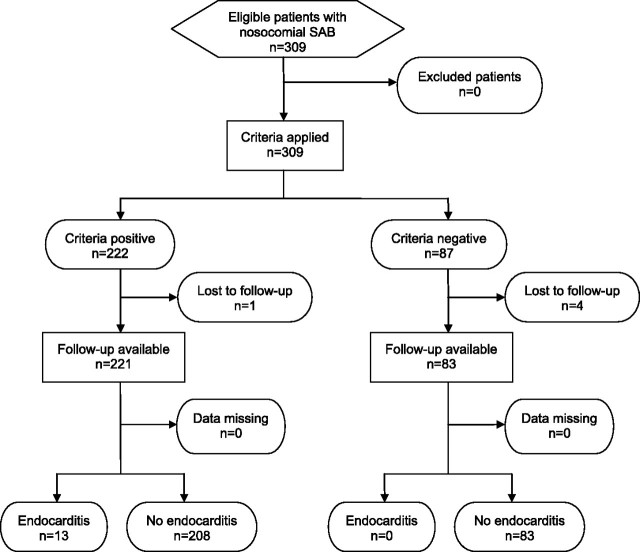
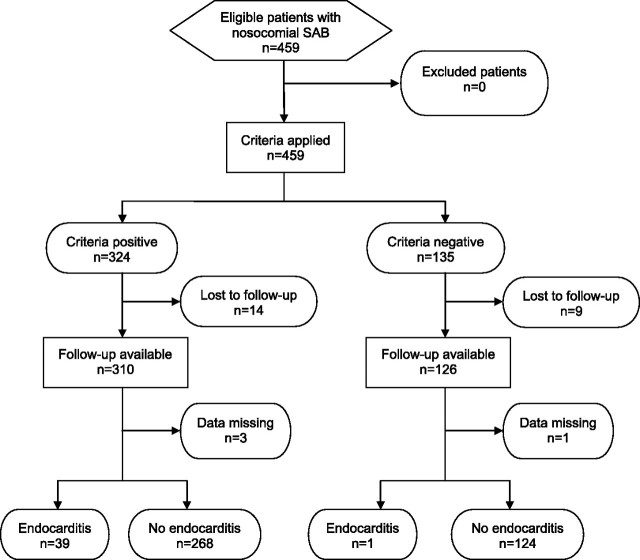
From 1 January 2006 to 30 June 2009, 572 consecutive patients with SAB were included in the INSTINCT study. Of these, SAB was considered nosocomial in 309 patients (54%). The SABG study recruited 1403 patients with SAB from 26 October 1994 to 30 December 2009; of these, 459 patients (32.7%) had nosocomial SAB. A total of 304 and 432 patients in the INSTINCT and SABG cohorts with complete data sets were included in additional analysis (Figures 1 and 2).
Figure 1.
Flow diagram of patients recruited in the Invasive S. aureus Infection Cohort (INSTINCT) cohort.
Figure 2.
Flow diagram of patients recruited in the S. aureus Bacteremia Group (SABG) cohort.
Age, source of infection, and 30-day and 90-day case-fatality rates were remarkably similar between the 2 cohorts (Table 1). Female sex was less prevalent in the INSTINCT cohort than in the SABG cohorts (33.9% vs 44.7%), and patients in the INSTINCT study were ethnically more homogenous (in the INSTINCT cohort, 97% of patients were white and 3% were “other”; in the SABG cohort, 67% of patients were white, 28% were black, and 5% were “other”). The rate of methicillin-resistant S. aureus (MRSA) was much higher in the US cohort, among both patients with IE (80% vs 7.7%) and patients without IE (64.3% vs 15.8 %).
Table 1.
Characteristics of Patients With Nosocomial Staphylococcus aureus Bacteremia (SAB) in the Invasive S. aureus Infection Cohort (INSTINCT) and S. aureus Bacteremia Group (SABG) Cohorts
| INSTINCT |
SABG |
|||||
| Characteristic | All patients (n = 304) | Patients without IE (n = 291) | Patients with IE (n = 13) | All patients (n = 432) | Patients without IE (n = 392) | Patients with IE (n = 40) |
| Age, years | ||||||
| Median (range) Interquartile range | 67 (21–91) 54–74 | 67 (21–91) 54–73 | 72 (47–86) 66–78 | 65 (15–95) 55–75 | 65 (25–95) 45–75 | 65 (15–75) 55–75 |
| Ratio of male to female patients | 103:201 | 99:192 | 4:9 | 193:239 | 171:221 | 22:18 |
| Percentage of female patients | 33.9 | 34 | 30.8 | 44.7 | 43.6 | 55 |
| Methicillin-resistant S. aureus | 47 (15.5) | 46 (15.8) | 1 (7.7) | 284 (65.7) | 252 (64.3) | 32 (80) |
| Long-term catheter present at onset | 29 (9.5) | 28 (9.6) | 1 (7.7) | 37 (8.6) | 32 (8.2) | 5 (12.5) |
| Source of bacteremia identified at onset | ||||||
| Short-term intravascular catheter | 117 (38.5) | 114 (39.2) | 3 (23.1) | 155 (35.9) | 149 (38.0) | 6 (15) |
| Long-term intravascular catheter | 23 (7.6) | 23 (7.9) | 0 | 37 (8.6) | 32 (8.2) | 5 (12.5) |
| Skin/soft tissue or wound | 33 (10.9) | 32 (11) | 1 (7.7) | 57 (13.2) | 51 (13) | 6 (15) |
| Respiratory tract | 11 (3.6) | 11 (3.8) | 0 | 57 (13.2) | 56 (14.3) | 1 (2.5) |
| Unknown | 92 (30.3) | 84 (28.9) | 8 (61.5) | 66 (15.3) | 57 (14.5) | 9 (22.5) |
| Other | 28 (9.2) | 27 (9.3) | 1 (7.7) | 60 (13.9) | 47 (12) | 13 (32.5) |
| Echocardiography within 14 days | 121 (39.8) | 111 (38.1) | 10 (76.9) | 248 (57.4) | 214 (54.6) | 34 (85) |
| TTE only | 65 (21.4) | 64 (22) | 1 (7.7) | 129 (29.9) | 117 (29.8) | 12 (30) |
| TEE only | 30 (9.9) | 29 (10) | 1 (7.7) | 41 (9.5) | 35 (8.9) | 6 (15) |
| TTE and TEE | 26 (8.6) | 18 (6.2) | 8 (61.5) | 78 (18.1) | 62 (15.8) | 16 (40) |
| Follow-up blood culture within 4 days | 171 (56.2) | 160 (55) | 11 (84.6) | 291 (67.4) | 260 (66.3) | 31 (77.5) |
| Duration of antimicrobial therapy, days | ||||||
| Median (range) Interquartile range | 14 (0–253) 9–19 | 14 (0–253) 9–18.5 | 22a (4–50) 13–33 | 17 (0–176) 12–33 | 17 (0–98) 11.8–30 | 42 (0–176) 15.5–54 |
| >14 days | 134 (44.1) | 125 (43) | 9 (69.2) | 267 (61.8) | 237 (60.5) | 30 (75) |
| >21 days | 59 (19.4) | 52 (17.9) | 7 (53.8) | 170 (39.4) | 141 (36) | 29 (72.5) |
| Late metastatic S. aureus infection or recurrent SAB | 9 (3) | 9 (3.1) | 0 | 27 (6.2) | 23 (5.9) | 4 (10) |
| 30-day case-fatality | 52 (17.1) | 48 (16.5) | 4 (30.8) | 93 (21.5) | 82 (20.9) | 11 (27.5) |
| 90-day case-fatality | 87 (28.6) | 80 (27.5) | 7 (53.8) | 125 (28.9) | 109 (27.8) | 16 (40) |
NOTE. Data are no. (%) of patients, unless otherwise indicated. TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
Median duration of antimicrobial therapy in patients that survived >14 days, 31 (range, 13–50 days; interquartile range, 19–37.5).
Within 14 days after the first positive blood culture result, echocardiography (TTE or TEE) was performed in 121 patients (39.8%) in the INSTINCT cohort and in 248 patients (57.4%) in the SABG cohort. Although echocardiography was strongly encouraged for all patients with SAB, the remaining patients either were discharged, were transferred, or died before an echocardiogram could be performed or the attending physician refused to initiate echocardiography. Follow-up blood cultures were obtained ≤4 days after the first positive blood culture result in 171 patients (56.2%) in the INSTINCT cohort and 291 patients (67.4%) in the SABG cohort.
Infective endocarditis was found in 13 (4.3%) and 40 patients (9.3%) in the INSTINCT and SABG cohorts, respectively. In all patients with documented IE in the INSTINCT cohort, the diagnosis was established during hospitalization, and no additional patient developed IE during the follow-up period. All cases of IE were confirmed by echocardiography, which was performed a median of 6 days (range, 1–25 days) after the first positive blood culture result. The mitral (5 patients [38.5%]) and aortic valves (4 patients [30.8%]) were the most frequently affected sites; there was 1 case (7.7%) of tricuspid valve endocarditis, and 1 patient had 2 valves affected; there were 3 cases (23.1%) of pacemaker lead infections and 1 case (7.7%) of mural endocarditis. Four cases (30.8%) were prosthetic valve infections. Seven patients (53.8%) with IE died within 90 days. No patient with IE developed late metastatic S. aureus infection or recurrent SAB.
In the SABG cohort, echocardiography was performed in 38 (95%) of 40 patients with IE a median of 5 days (range, 0–49 days) after the first positive blood culture result. In 17 patients (42.5%), the mitral valve was affected; in 11 patients (27.5%), the aortic valve was affected; and in 3 patients (7.5%), the tricuspid valve was affected. More than 1 valve was involved in 3 patients (7.5%). There were 4 cases (10%) of pacemaker lead infections and 3 cases (7.5%) of mural IE. Ten cases (25%) were prosthetic valve infections. In 5 patients (12.5%), no lesion was observed by echocardiography, and the diagnosis of IE was based on clinical Duke criteria alone. Within 90 days, 16 patients with IE (40%) died and 4 patients (10%) developed late metastatic infections or recurrent SAB.
Concerning the presence of the clinical prediction criteria defined above, both cohorts were remarkably similar. Most patients in both cohorts (INSTINCT vs. SABG, 57.2% vs 56%) fulfilled 1 criterion; 2 criteria were satisfied by 14.1% versus 14.1%, 3 criteria were fulfilled by 1.3% versus 0.9%, and no criteria were fulfilled by 27.3% versus 28.9% of patients.
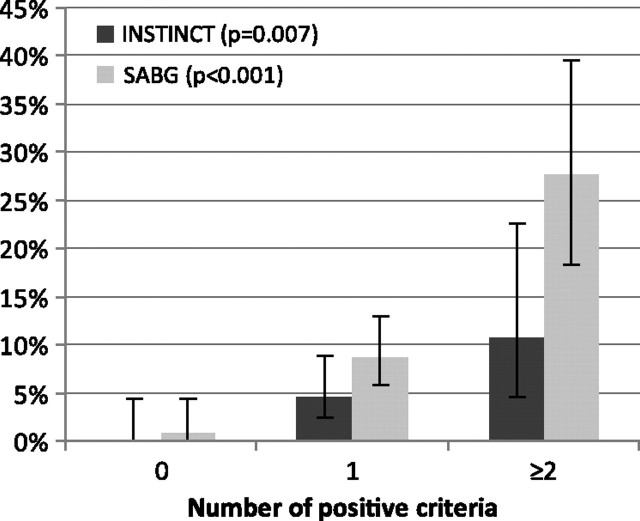
In both cohorts, patients with IE were more likely to have documented prolonged bacteremia, a permanent intracardiac device, and spinal infections or nonvertebral osteomyelitis than patients without IE (Table 2). In the SABG cohort, each prediction criterion was present significantly more frequently in patients with IE, whereas in the INSTINCT cohort, this was the case only for the presence of a permanent intracardiac device. The frequency of hemodialysis dependency was similar in patients with and without IE in the INSTINCT cohort (7.7% vs 8.6%; P > .99), whereas in the SABG cohort, hemodialysis dependency was more common among patients with IE (15% vs 6.1%; P = .05). In both cohorts, with an increasing number of criteria fulfilled, the diagnosis of IE became more likely (Figure 3).
Table 2.
Clinical Prediction Criteria Associated With Increased Risk of Infective Endocarditis (IE) in Patients With Nosocomial Staphylococcus aureus Bacteremia.
| No. (%) of Patients, by Study |
||||||||
| INSTINCT |
SABG |
|||||||
| Clinical prediction criterion | All patients (n = 304) | Patients without IE (n = 291) | Patients with IE (n = 13) | Pa | All patients (n = 432) | Patients without IE (n = 392) | Patients with IE (n = 40) | P |
| Prolonged bacteremia (>4 days) | 195 (64.1) | 186 (63.9) | 9 (69.2) | .78 | 272 (63) | 236 (60.2) | 36 (90) | <.01 |
| Documented prolonged bacteremia | 50 (16.4) | 47 (16.2) | 3 (23.1) | .46 | 111 (25.7) | 85 (21.7) | 26 (65) | <.01 |
| Possible prolonged bacteremia | 145 (47.7) | 139 (47.8) | 6 (46.2) | >.99 | 161 (37.3) | 151 (38.5) | 10 (25) | .12 |
| Permanent intracardiac device | 41 (13.5) | 34 (11.7) | 7 (53.8) | <.01 | 51 (11.8) | 38 (9.7) | 13 (32.5) | <.01 |
| Prosthetic heart valve | 18 (5.9) | 14 (4.8) | 4 (30.8) | <.01 | 25 (5.8) | 15 (3.8) | 10 (25.0) | <.01 |
| Pacemaker/cardioverter-defibrillator | 25 (8.2) | 22 (7.6) | 3 (23.1) | .08 | 29 (6.7) | 25 (6.4) | 4 (10.0) | .33 |
| Hemodialysis dependency | 26 (8.6) | 25 (8.6) | 1 (7.7) | >.99 | 30 (6.9) | 24 (6.1) | 6 (15) | .05 |
| Spinal infection or nonvertebral osteomyelitis | 8 (2.6) | 7 (2.4) | 1 (7.7) | .3 | 14 (3.2) | 9 (2.3) | 5 (12.5) | <.01 |
| No criteria fulfilled | 83 (27.3) | 83 (28.5) | 0 | 125 (28.9) | 124 (31.6) | 1 (2.5) | ||
| ≥1 criterion fulfilled | 221 (72.7) | 208 (71.5) | 13 (100) | 307 (71.1) | 268 (68.4) | 39 (97.5) | ||
NOTE. Data are shown for 304 patients in the Invasive S. aureus Infection Cohort (INSTINCT) and 432 patients in the S. aureus Bacteremia Group (SABG) with complete follow-up. More than 1 criterion may be present.
Determined using the 2-sided Fisher exact test.
Figure 3.
Relative frequency of infective endocarditis by number of positive criteria (error bars denote exact 95% confidence intervals) in the Invasive S. aureus Infection Cohort (INSTINCT; n = 304) and S. aureus Bacteremia Group (SABG; n = 432) cohorts. P values refer to intragroup (INSTINCT and SABG) comparisons of the relative frequency of infective endocarditis by the number of positive criteria (determined by the Fisher exact test).
All patients in the INSTINCT study with a final diagnosis of S. aureus IE fulfilled at least 1 criterion for the identification of patients with increased risk of endocarditis (sensitivity, 100%; 95% CI, 77.2%–100%]) (Table 3). Among 83 patients who did not fulfill any of the prediction criteria, no cases with IE were found (negative predictive value, 100%; 95% CI, 95.6%–100%). Similar results were obtained in the SABG cohort. All but 1 patient with IE satisfied at least 1 criterion (sensitivity, 97.5% [95% CI, 87.1%–99.9%]; negative predictive value, 99.2% [95% CI, 95.6%–100%]).
Table 3.
Sensitivity, Specificity, and Positive and Negative Predictive Values of the Clinical Prediction Criteria Set
| Percentage (95% CI) |
||
| Variable | INSTINCT | SABG |
| Sensitivity | 100 (77.2–100) | 97.5 (87.1–99.9) |
| Specificity | 28.5 (23.6–34) | 31.6 (27.2–36.4) |
| Positive predictive value | 5.9 (3.5–9.8) | 12.7 (9.4–16.9) |
| Negative predictive value | 100 (95.6–100) | 99.2 (95.6–100) |
NOTE. Ninety-five percent confidence intervals (CIs) were determined on the basis of the Wilson method. INSTINCT, Invasive Staphylococcus aureus Infection Cohort; SABG, S. aureus Bacteremia Group
In patients who did not satisfy any prediction criteria, IE may have gone undetected (Table 4). Seventeen patients died during the follow-up period without echocardiographic evaluation, and in 11 patients IE, may have been masked by >21 days of antimicrobial therapy.
Table 4.
Characteristics of Patients Who Satisfied No Clinical Prediction Criteria
| No. (%) of patients, by study |
||
| Characteristic | INSTINCT (n = 83) | SABG (n = 125) |
| Death within 90 days | 15 (18.1) | 32 (25.6) |
| and no echocardiography performeda | 8 (9.6) | 9 (7.2) |
| Survived 90 days and no echocardiography performed | 26 (31.3) | 24 (19.2) |
| and >14 days of antimicrobial therapy | 13 (15.7) | 13 (10.4) |
| and >21 days of antimicrobial therapy | 5 (6) | 6 (4.8) |
| and >28 days antimicrobial therapy | 2 (2.4) | 4 (3.2) |
NOTE. INSTINCT, Invasive Staphylococcus aureus Infection Cohort; SABG, S. aureus Bacteremia Group
Transthoracic echocardiography or transesophageal echocardiography within 90 days after onset of S. aureus bacteremia.
DISCUSSION
IE is a severe manifestation of SAB, and it has therefore been recommended to perform echocardiography, preferably TEE, in all patients with SAB [17, 22]. Although it is generally accepted standard to perform echocardiography in all patients with community-onset SAB and in those with nosocomial SAB associated with deep-seated infection, some clinicians are reluctant to perform TEE in patients who have uncomplicated SAB, show prompt clinical response to antimicrobial treatment, and have the focus of infection (eg, the intravascular catheter) promptly removed, as proposed previously [23, 24]. Even in a setting in which infectious diseases consultation is available, the compliance with the recommendation to perform echocardiography ranges between 34% and 73% [18, 25–27].
By use of independent cohorts from 2 different continents, we evaluated simple clinical prediction criteria that can be used to identify patients with nosocomial SAB who are at very low risk for the development of IE and in whom TEE might therefore be dispensable. The criteria set is geared to evaluate patients 6–8 days after the first positive blood culture result and is based on data that is usually available at this time point.
The rationale for selecting the prediction criteria was (1) prolonged bacteremia has been shown to be the strongest predictor for complicated SAB and S. aureus IE [12, 28–30]; (2) permanent intracardiac devices, such as a prosthetic heart valve, a pacemaker, or an implantable cardioverter-defibrillator in patients with SAB have been found associated with a higher risk for endocarditis [7, 29, 31–34]; (3) patients with SAB who have end-stage renal disease and a history of hemodialysis are considered to be at risk to develop endocarditis [31, 35, 36]; (4) spinal infections and nonvertebral osteomyelitis due to S. aureus have been found to be associated with endocarditis [37–39].
Although long-term intravascular access devices (eg, tunneled or totally implantable catheters) have been shown to be associated with an increased risk of IE [40, 41], the presence of these catheters was not included in the criteria set. Prolonged bacteremia or hemodialysis dependency may represent independent risk factors for IE rather than the presence of these devices per se, particularly if long-term catheters are promptly removed. In fact, only 1 of 29 patients in the INSTINCT cohort and 5 of 37 patients in the SABG cohort with a long-term intravascular access device present at onset developed IE.
The comparison between the European and North American cohorts showed strikingly similar results regarding patient characteristics and the source of SAB. Of note, MRSA was much more prevalent in the US cohort (65.7% vs 15.5%). Many studies, including a large meta-analysis, have shown that methicillin resistance is associated with a worse outcome in SAB [42]. However, the 30-day and 90-day case-fatality rates did not differ significantly between the 2 study sites, despite the prominent difference in MRSA prevalence.
Prolonged bacteremia was more common among patients with IE in the US cohort (90% vs 69.2%). It might be argued that this was due to the increased prevalence of MRSA in SABG cohort members and because empirical antimicrobial therapy is often not appropriate and definitive therapy less effective in patients with BSI caused by MRSA. However, our data do not support this hypothesis because 20 (62.5%) of 32 SABG patients with IE caused by MRSA had documented prolonged bacteremia, compared with 6 (75%) of 8 patients with IE caused by MSSA (data not shown). It is tempting to speculate that the higher incidence of IE in the SABG cohort might be explained by the higher incidence of MRSA; however, our data do not prove this.
In both cohorts, the absence of any of the criteria had a high negative predictive value (100% and 99.2% in the INSTINCT and SABG cohorts, respectively) for the development of IE. Therefore, we propose that TEE to exclude IE may be dispensable in patients with nosocomial SAB who do not fulfill any of the clinical prediction criteria.
There are several limitations to the study. The reliable detection of IE is of critical importance. Neither of the 2 cohort studies was designed to measure the rate of IE at 3 months after the onset of SAB, and routine follow-up echocardiography data were not available. Also, a small number of patients were lost to follow-up or data were missing (INSTINCT, 1.6%; SABG, 5.9%). More importantly, only 39.5% (in INSTINCT) and 57.4% (in SABG) of patients underwent echocardiography ≤14 days after onset of SAB, despite considerable efforts of the investigators to include this diagnostic method into standard care. Thus, some cases of IE may have gone undetected. However, we reasoned that IE would become clinically apparent after discontinuation of antibiotic therapy; therefore, patients were observed closely for the development of clinical signs and symptoms suggestive of IE over a period of 3 months. One might argue that a considerable number of patients who did not fulfill any predictive criteria and in whom echocardiography was not performed died during follow-up, and in these patients undetected IE could have been present. However, a sudden death related to IE that goes undetected by the primary care physician and/or the hospital should occur only rarely. Furthermore, there was no case of IE observed among the surviving patients during the follow-up periods. One could also argue that prolonged antimicrobial therapy could have adequately treated undetected early stage IE, and indeed, 11 patients for whom echocardiography was not performed were given >3 weeks of antimicrobial treatment.
The comparatively low specificity of the criteria set is largely due to the conservative definition of prolonged bacteremia, ie the absence of negative blood cultures obtained at day 1–4 after the first positive blood culture. Using this definition, 145 patients in INSTINCT and 161 patients in the SABG cohort with “possible” prolonged bacteremia were included in the prolonged bacteremia group, although most likely a considerable number of these patients could have been excluded if follow-up blood cultures had been obtained. If “documented” prolonged bacteremia (ie, the presence of a follow-up blood culture positive for S. aureus 2–4 days after the first positive blood culture result) was used as a criterion, the specificity of the test would be markedly increased (data not shown). However, we decided not to use this approach, because some patients with ongoing bacteremia could be missed if no follow-up blood samples are obtained for culture, leading to a lower negative predictive value.
In summary, we propose that in patients with at least 1 clinical prediction criterion, echocardiography to exclude IE is highly recommended. In the subset of patients with a low probability of IE (ie, those without any of the criteria), TEE evaluation may not be necessary. However, physicians should not be discouraged from performing echocardiography in criteria-negative patients when IE is clinically suspected during the course of the infection.
Furthermore, clinicians should be encouraged by ID physicians and clinical microbiologists to perform follow-up cultures for all patients with SAB 2–4 days after the first positive blood culture result. This approach can be used to exclude prolonged bacteremia and thus helps to identify patients in whom TEE might be dispensable, and it could lead to a more economical use of echocardiographic evaluations. Nevertheless, it is of critical importance to confirm the validity of the proposed prediction criteria prospectively in a controlled study, in which all patients with SAB would undergo echocardiography and follow-up blood cultures.
Acknowledgments
We thank Katharina Achilles, Andreas Langhorst, Stephan Neumann, Georg Peppinghaus, Felicia Ruffin, and Julia Wendel for help with the data acquisition; Oleg Krut and Hilmar Wisplinghoff for database management; and Fotini Dodos and Catherine Seck for excellent echocardiographic service.
Financial support. This work was supported in part by the Paul-Ehrlich Gesellschaft für Chemotherapie (to H. S.) and National Institutes of Health (R01-AI068804 to V. F.). A. J. K., H. B., and S. R. are supported by the Federal Ministry of Education and Research (BMBF 01KI1017, BMBF 01KN0706, and BMBF 01EO0803). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest. V. F. received grants or research support from Astellas, Cubist, Merck, Theravance, Cerexa, Pfizer, Novartis, NIH, Advanced Liquid Logic; was a paid consultant for Astellas, Cubist, Inhibitex, Merck, Johnson & Johnson, Leo Pharmaceuticals, NovaDigm, The Medicines Company, Baxter Pharmaceuticals, and Biosynexus; is on the speaker's bureau for Cubist; has received royalties from UpToDate; has received honoraria from Arpida, Astellas, Cubist, Inhibitex, Merck, Pfizer, Targanta, Theravance, Wyeth, Ortho-McNeil, Novartis, Vertex Pharmaceuticals, and Medimmune; and serves on an advisory committee for Cubist. All other authors: no conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed in the Acknowledgments section.
References
- 1.Kern WV. Management of Staphylococcus aureus bacteremia and endocarditis: progresses and challenges. Curr Opin Infect Dis. 2010;23:346–58. doi: 10.1097/QCO.0b013e32833bcc8a. [DOI] [PubMed] [Google Scholar]
- 2.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 3.Hill PC, Birch M, Chambers S, et al. Prospective study of 424 cases of Staphylococcus aureus bacteraemia: determination of factors affecting incidence and mortality. Intern Med J. 2001;31:97–103. [PubMed] [Google Scholar]
- 4.Jensen AG, Wachmann CH, Espersen F, Scheibel J, Skinhoj P, Frimodt-Moller N. Treatment and outcome of Staphylococcus aureus bacteremia: a prospective study of 278 cases. Arch Intern Med. 2002;162:25–32. doi: 10.1001/archinte.162.1.25. [DOI] [PubMed] [Google Scholar]
- 5.Nickerson EK, Hongsuwan M, Limmathurotsakul D, et al. Staphylococcus aureus bacteraemia in a tropical setting: patient outcome and impact of antibiotic resistance. PLoS One. 2009;4:e4308. doi: 10.1371/journal.pone.0004308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seifert H, Wisplinghoff H, Kaasch A, et al. [Epidemiology, course and prognosis of Staphylococcus aureus bacteremia—preliminary results from the INSTINCT (INvasive STaphylococcus aureus INfection CohorT) cohort] Dtsch Med Wochenschr. 2008;133:340–5. doi: 10.1055/s-2008-1046715. [DOI] [PubMed] [Google Scholar]
- 7.Abraham J, Mansour C, Veledar E, Khan B, Lerakis S. Staphylococcus aureus bacteremia and endocarditis: the Grady Memorial Hospital experience with methicillin-sensitive S. aureus and methicillin-resistant S. aureus bacteremia. Am Heart J. 2004;147:536–9. doi: 10.1016/j.ahj.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Fowler VG, Jr, Li J, Corey GR, et al. Role of echocardiography in evaluation of patients with Staphylococcus aureus bacteremia: experience in 103 patients. J Am Coll Cardiol. 1997;30:1072–8. doi: 10.1016/s0735-1097(97)00250-7. [DOI] [PubMed] [Google Scholar]
- 9.Sullenberger AL, Avedissian LS, Kent SM. Importance of transesophageal echocardiography in the evaluation of Staphylococcus aureus bacteremia. J Heart Valve Dis. 2005;14:23–8. [PubMed] [Google Scholar]
- 10.Chang FY, MacDonald BB, Peacock JE, Jr, et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore) 2003;82:322–32. doi: 10.1097/01.md.0000091185.93122.40. [DOI] [PubMed] [Google Scholar]
- 11.Das I, O'Connell N, Lambert P. Epidemiology, clinical and laboratory characteristics of Staphylococcus aureus bacteraemia in a university hospital in UK. J Hosp Infect. 2007;65:117–23. doi: 10.1016/j.jhin.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Fowler VG, Jr, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med. 2003;163:2066–72. doi: 10.1001/archinte.163.17.2066. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds HR, Jagen MA, Tunick PA, Kronzon I. Sensitivity of transthoracic versus transesophageal echocardiography for the detection of native valve vegetations in the modern era. J Am Soc Echocardiogr. 2003;16:67–70. doi: 10.1067/mje.2003.43. [DOI] [PubMed] [Google Scholar]
- 14.Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation. 2005;111:e394–434. doi: 10.1161/CIRCULATIONAHA.105.165564. [DOI] [PubMed] [Google Scholar]
- 15.Chu VH, Bayer AS. Use of echocardiography in the diagnosis and management of infective endocarditis. Curr Infect Dis Rep. 2007;9:283–90. doi: 10.1007/s11908-007-0044-x. [DOI] [PubMed] [Google Scholar]
- 16.Rosen AB, Fowler VG, Jr, Corey GR, et al. Cost-effectiveness of transesophageal echocardiography to determine the duration of therapy for intravascular catheter-associated Staphylococcus aureus bacteremia. Ann Intern Med. 1999;130:810–20. doi: 10.7326/0003-4819-130-10-199905180-00004. [DOI] [PubMed] [Google Scholar]
- 17.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fowler VG, Jr, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis. 1998;27:478–86. doi: 10.1086/514686. [DOI] [PubMed] [Google Scholar]
- 19.Friedman ND, Kaye KS, Stout JE, et al. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–7. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 20.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–8. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 21.Bossuyt PM, Reitsma JB, Bruns DE, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD Initiative. Ann Intern Med. 2003;138:40–4. doi: 10.7326/0003-4819-138-1-200301070-00010. [DOI] [PubMed] [Google Scholar]
- 22.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 23.Pigrau C, Rodriguez D, Planes AM, et al. Management of catheter-related Staphylococcus aureus bacteremia: when may sonographic study be unnecessary? Eur J Clin Microbiol Infect Dis. 2003;22:713–9. doi: 10.1007/s10096-003-1041-0. [DOI] [PubMed] [Google Scholar]
- 24.van Hal SJ, Mathur G, Kelly J, Aronis C, Cranney GB, Jones PD. The role of echocardiography in excluding left sided infective endocarditis in Staphylococcus aureus bacteraemia. J Infect. 2005;51:218–21. doi: 10.1016/j.jinf.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Honda H, Krauss MJ, Jones JC, Olsen MA, Warren DK. The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med. 2010;123:631–7. doi: 10.1016/j.amjmed.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagao M, Iinuma Y, Saito T, et al. Close cooperation between infectious disease physicians and attending physicians results in better outcomes for patients with Staphylococcus aureus bacteraemia. Clin Microbiol Infect. 2009;16:1783–8. doi: 10.1111/j.1469-0691.2010.03156.x. [DOI] [PubMed] [Google Scholar]
- 27.Rieg S, Peyerl-Hoffmann G, de With K, et al. Mortality of S. aureus bacteremia and infectious diseases specialist consultation—a study of 521 patients in Germany. J Infect. 2009;59:232–9. doi: 10.1016/j.jinf.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 28.Neuner EA, Casabar E, Reichley R, McKinnon PS. Clinical, microbiologic, and genetic determinants of persistent methicillin-resistant Staphylococcus aureus bacteremia. Diagn Microbiol Infect Dis. 2010;67:228–33. doi: 10.1016/j.diagmicrobio.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 29.Hill EE, Vanderschueren S, Verhaegen J, et al. Risk factors for infective endocarditis and outcome of patients with Staphylococcus aureus bacteremia. Mayo Clin Proc. 2007;82:1165–9. doi: 10.4065/82.10.1165. [DOI] [PubMed] [Google Scholar]
- 30.Fowler VG, Jr., Justice A, Moore C, et al. Risk factors for hematogenous complications of intravascular catheter-associated Staphylococcus aureus bacteremia. Clin Infect Dis. 2005;40:695–703. doi: 10.1086/427806. [DOI] [PubMed] [Google Scholar]
- 31.Chang CF, Kuo BI, Chen TL, Yang WC, Lee SD, Lin CC. Infective endocarditis in maintenance hemodialysis patients: fifteen years' experience in one medical center. J Nephrol. 2004;17:228–35. [PubMed] [Google Scholar]
- 32.El-Ahdab F, Benjamin DK, Jr, Wang A, et al. Risk of endocarditis among patients with prosthetic valves and Staphylococcus aureus bacteremia. Am J Med. 2005;118:225–9. doi: 10.1016/j.amjmed.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 33.Uslan DZ, Dowsley TF, Sohail MR, et al. Cardiovascular implantable electronic device infection in patients with Staphylococcus aureus bacteremia. Pacing Clin Electrophysiol. 2010;33:407–13. doi: 10.1111/j.1540-8159.2009.02565.x. [DOI] [PubMed] [Google Scholar]
- 34.Uslan DZ, Sohail MR, St Sauver JL, et al. Permanent pacemaker and implantable cardioverter defibrillator infection: a population-based study. Arch Intern Med. 2007;167:669–75. doi: 10.1001/archinte.167.7.669. [DOI] [PubMed] [Google Scholar]
- 35.Robinson DL, Fowler VG, Sexton DJ, Corey RG, Conlon PJ. Bacterial endocarditis in hemodialysis patients. Am J Kidney Dis. 1997;30:521–4. doi: 10.1016/s0272-6386(97)90311-5. [DOI] [PubMed] [Google Scholar]
- 36.Benito N, Miro JM, de Lazzari E, et al. Health care-associated native valve endocarditis: importance of non-nosocomial acquisition. Ann Intern Med. 2009;150:586–94. doi: 10.7326/0003-4819-150-9-200905050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen AG, Espersen F, Skinhoj P, Rosdahl VT, Frimodt-Moller N. Increasing frequency of vertebral osteomyelitis following Staphylococcus aureus bacteraemia in Denmark 1980–1990. J Infect. 1997;34:113–8. doi: 10.1016/s0163-4453(97)92395-1. [DOI] [PubMed] [Google Scholar]
- 38.Pigrau C, Almirante B, Flores X, et al. Spontaneous pyogenic vertebral osteomyelitis and endocarditis: incidence, risk factors, and outcome. Am J Med. 2005;118:1287. doi: 10.1016/j.amjmed.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 39.Fery-Blanco C, Revest M, Alla F, et al. Program and abstracts of 50th Interscience Conference on Antimicrobial Agents and Chemotherapy. Boston: American Society for Microbiology; 2010. Vertebral osteomyelitis associated with infective endocarditis: characteristics analyzed within IE2008, a one-year population-based survey in France [abstract K2168] [Google Scholar]
- 40.Crowley AL, Peterson GE, Benjamin DK, Jr, et al. Venous thrombosis in patients with short- and long-term central venous catheter-associated Staphylococcus aureus bacteremia. Crit Care Med. 2008;36:385–90. doi: 10.1097/01.CCM.0B013E3181611F914. [DOI] [PubMed] [Google Scholar]
- 41.Miro JM, Anguera I, Cabell CH, et al. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin Infect Dis. 2005;41:507–14. doi: 10.1086/431979. [DOI] [PubMed] [Google Scholar]
- 42.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003;36:53–9. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]