Abstract
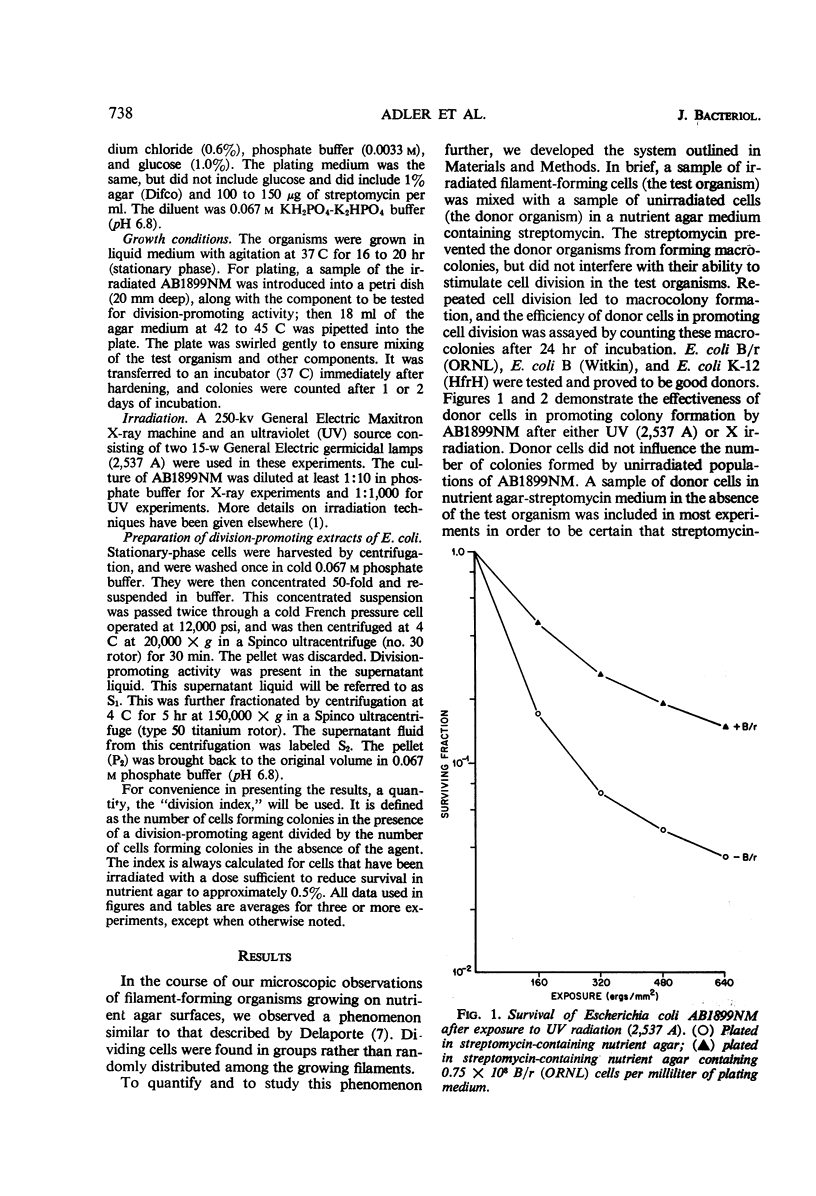
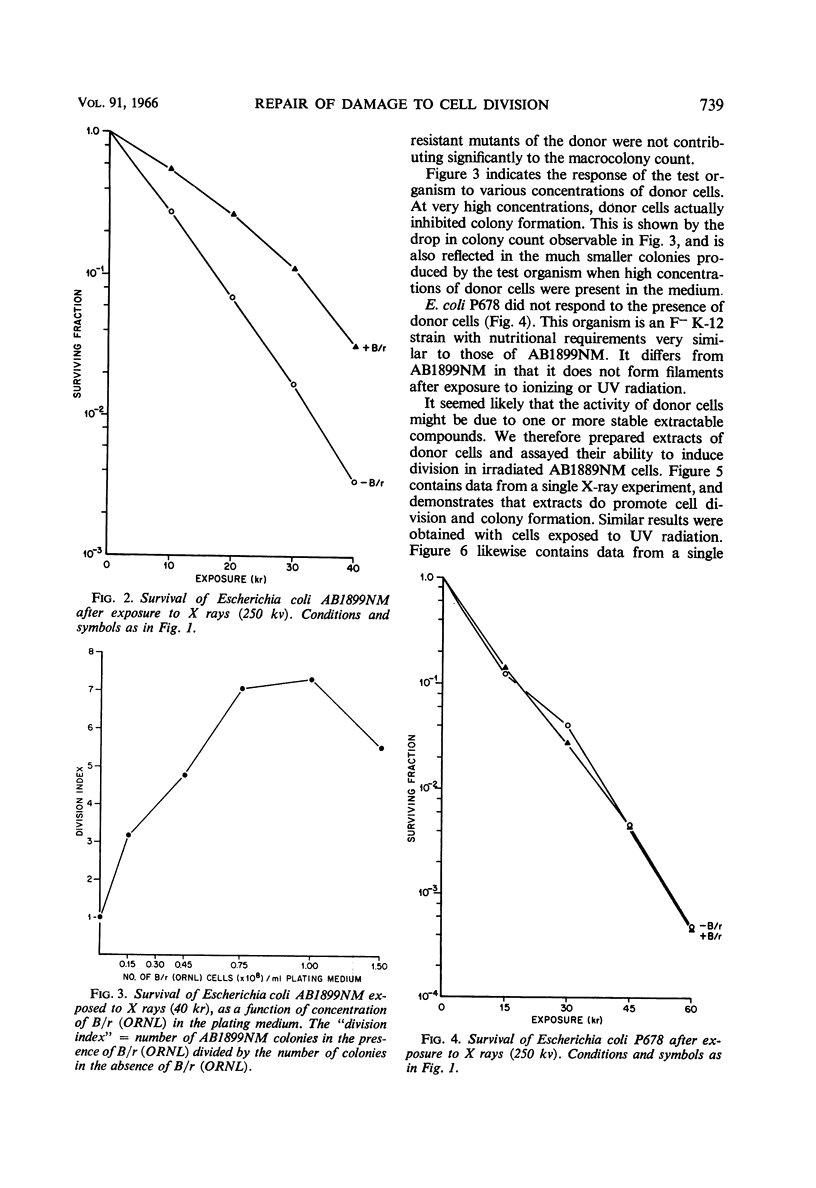
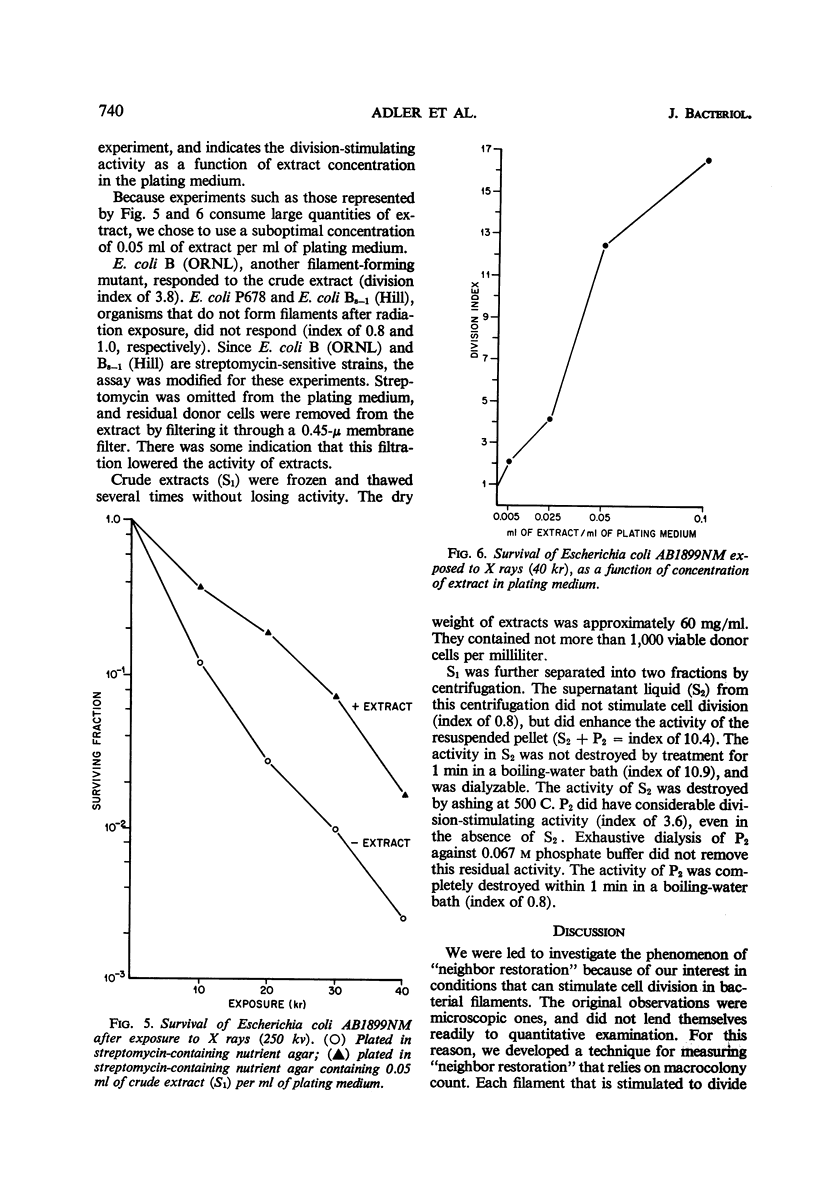
Adler, Howard I. (Oak Ridge National Laboratory, Oak Ridge, Tenn.), William D. Fisher, Alice A. Hardigree, and George E. Stapleton. Repair of radiation-induced damage to the cell division mechanism of Escherichia coli. J. Bacteriol. 91:737–742. 1966.—Microscopic observations of irradiated populations of filamentous Escherichia coli cells indicated that filaments can be induced to divide by a substance donated by neighboring cells. We have made this observation the basis for a quantitative technique in which filaments are incubated in the presence of nongrowing donor cells. The presence of “donor” organisms promotes division and subsequent colony formation in filaments. “Donor” bacteria do not affect nonfilamentous cells. An extract of “donor” cells retains the division-promoting activity. The extract has been partially fractionated, and consists of a heat-stable and a heat-labile component. The heat-stable component is inactive in promoting cell division, but enhances the activity of the heat-labile component. The division-promoting system is discussed as a radiation repair mechanism and as a normal component of the cell division system in E. coli.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I., COPELAND J. C. Genetic analysis of radiation response in Escherichia coli. Genetics. 1962 Jun;47:701–712. doi: 10.1093/genetics/47.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADLER H. I., HARDIGREE A. A. ANALYSIS OF A GENE CONTROLLING CELL DIVISION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Mar;87:720–726. doi: 10.1128/jb.87.3.720-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADLER H. I., HARDIGREE A. A. POSTIRRADIATION GROWTH, DIVISION, AND RECOVERY IN BACTERIA. Radiat Res. 1965 May;25:92–102. [PubMed] [Google Scholar]
- ADLER H. I., HASKINS S. D. Heterogeneity of cultures of Escherichia coli B/r. Nature. 1960 Oct 15;188:249–251. doi: 10.1038/188249a0. [DOI] [PubMed] [Google Scholar]
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELAPORTE B. La restauration par voisinage chez des bactéries irradiées par des rayons X. Ann Inst Pasteur (Paris) 1956 Nov;91(5):727–735. [PubMed] [Google Scholar]
- Elder R. L., Beers R. F. Nonphotoreactivating Repair of Ultraviolet Light-Damaged Transforming Deoxyribonucleic Acid by Micrococcus lysodeikticus Extracts. J Bacteriol. 1965 Sep;90(3):681–686. doi: 10.1128/jb.90.3.681-686.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBERG J. A LOCUS FOR RADIATION RESISTANCE IN ESCHERICHIA COLI. Genetics. 1964 May;49:771–778. doi: 10.1093/genetics/49.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRULA E. A., GRULA M. M. Cell division in a species of Erwinia III. Reversal of inhibition of cell division caused by D-amino acids, penicillin, and ultraviolet light. J Bacteriol. 1962 May;83:981–988. doi: 10.1128/jb.83.5.981-988.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKINS B., ALPER T. Anaerobic growth as a factor influencing radiosensitivity. J Gen Microbiol. 1963 Feb;30:307–315. doi: 10.1099/00221287-30-2-307. [DOI] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROERSCH A., VAN DER KAM P. C., ADEMA J. DARK REACTIVATION OF ULTRAVIOLET IRRADIATED BACTERIOPHAGE DEOXYRIBONUCLEIC ACID IN VITRO. Biochim Biophys Acta. 1964 Feb 17;80:346–348. [PubMed] [Google Scholar]
- RORSCH A., EDELMAN A., van der KAMP, COHEN J. A. Phenotypic and genotypic characterization of radiation sensitivity in Escherichia coli B. Biochim Biophys Acta. 1962 Aug 20;61:278–289. doi: 10.1016/0926-6550(62)90090-7. [DOI] [PubMed] [Google Scholar]
- RUPERT C. S. Photoreactivation of transforming DNA by an enzyme from bakers' yeast. J Gen Physiol. 1960 Jan;43:573–595. doi: 10.1085/jgp.43.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riklis E. Studies on mechanism of repair of ultraviolet-irradiated viral and bacterial DNAin vivo and in vitro. Can J Biochem. 1965 Jul;43(7):1207–1219. doi: 10.1139/o65-132. [DOI] [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAPLETON G. E. The influence of pretreatments and posttreatments on bacterial inactivation by ionizing radiations. Ann N Y Acad Sci. 1955 Feb 3;59(4):604–618. doi: 10.1111/j.1749-6632.1955.tb45973.x. [DOI] [PubMed] [Google Scholar]
- Watkins J. H., Winslow C. E. Factors Determining the Rate of Mortality of Bacteria Exposed to Alkalinity and Heat. J Bacteriol. 1932 Sep;24(3):243–265. doi: 10.1128/jb.24.3.243-265.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]



