Tissue-specific expression of B7x protects against autoimmunity.
Abstract
B7x, an inhibitory member of the B7/CD28 superfamily, is highly expressed in a broad range of nonhematopoietic organs, suggesting a role in maintaining peripheral tolerance. As endogenous B7x protein is expressed in pancreatic islets, we investigated whether the molecule inhibits diabetogenic responses. Transfer of disease-inducing BDC2.5 T cells into B7x-deficient mice resulted in a more aggressive form of diabetes than in wild-type animals. This exacerbation of disease correlated with higher frequencies of islet-infiltrating Th1 and Th17 cells. Conversely, local B7x overexpression inhibited the development of autoimmunity, as crossing diabetes-susceptible BDC2.5/B6g7 mice to animals overexpressing B7x in pancreatic islets abrogated disease induction. This protection was caused by the inhibition of IFN-γ production by CD4 T cells and not to a skewing or expansion of Th2 or regulatory T cells. The suppressive function of B7x was also supported by observations from another autoimmune model, experimental autoimmune encephalomyelitis, in which B7x-deficient mice developed exacerbated disease in comparison with wild-type animals. Analysis of central nervous system–infiltrating immune cells revealed that the loss of endogenous B7x resulted in expanded Th1 and Th17 responses. Data from these two autoimmune models provide evidence that B7x expression in the periphery acts as an immune checkpoint to prevent tissue-specific autoimmunity.
As autoimmunity can be the result of a breakdown in peripheral tolerance, determining the mechanisms that keep self-reactive T cells in check is important for understanding pathogenesis. Members of the B7/CD28 superfamily of T cell receptors and their cognate ligands have been shown to be necessary for the regulation of peripheral T cell function (Keir and Sharpe, 2005). This superfamily has members that not only provide positive co-stimulatory signals that augment and sustain T cell function but several that contribute critical negative signals that down-regulate and inhibit T cell responses (Greenwald et al., 2005; Pentcheva-Hoang et al., 2009). These negative signals are especially important in regulating the induction of tolerance and autoimmunity. The B7/CD28 superfamily has been shown to play an important role in maintaining tolerance at the fetomaternal interface (Guleria et al., 2005; Petroff and Perchellet, 2010) and also in regulating autoreactive T cells in disease settings such as diabetes (Lühder et al., 1998; Ansari et al., 2003; Keir et al., 2006) and experimental autoimmune encephalomyelitis (EAE; Perrin et al., 1996; Hurwitz et al., 2002; Zhu et al., 2006; Carter et al., 2007).
The identification of B7x (B7-H4, B7S1), a member of the B7 family, as a negative regulator of T cell activation and function suggested a previously unrecognized mechanism by which peripheral tolerance can be induced or maintained. The initial characterization of B7x demonstrated that its messenger RNA (mRNA) is broadly expressed across a wide range of mouse organs with its highest expression observed in nonhematopoietic tissues (Prasad et al., 2003; Sica et al., 2003; Zang et al., 2003). It has since been shown that many human cancers exhibit aberrant B7x protein expression (Krambeck et al., 2006; Tringler et al., 2006; Simon et al., 2007; Zang et al., 2007; Awadallah et al., 2008; Jiang et al., 2010; Quandt et al., 2011). Our laboratory has reported that at the time of prostatectomy, B7x expression is elevated on prostate cancer cells; patients exhibiting the highest levels of the molecule on their tumors had increased risk of recurrence, spread of disease, and mortality (Zang et al., 2007). This correlation of increased B7x expression with poor prognosis has also been observed in other human cancers, suggesting that expression of this inhibitory molecule might facilitate tumor progression by inhibiting host immunity (Jiang et al., 2010; Quandt et al., 2011). In this study, we sought to determine whether tissue-specific B7x expression can suppress self-reactive host immune responses and protect from autoimmunity in two disease models: diabetes and EAE.
As B7x protein can be detected on the islets of Langerhans, we examined whether the molecule has a role in maintaining tolerance against diabetogenic T cells. In an adoptive transfer model of diabetes, the injection of activated diabetogenic T cells into B7x-deficient animals resulted in a more severe disease than in wild-type control mice. As the loss of B7x exacerbated disease, we also studied whether the overexpression of B7x could delay or prevent the aggressive form of diabetes that develops in BDC2.5/B6g7 animals. Pancreatic overexpression of B7x in BDC2.5/B6g7 mice abrogated disease induction and inhibited cytokine production by the pathogenic T cells. Examining the role of B7x in another model of autoimmunity supports the suppressive in vivo effect of B7x, as EAE induced in B7x-deficient mice is more severe relative to disease in wild-type mice. Therefore, our experiments demonstrate that B7x expression protects from self-reactive T cell–mediated tissue damage and that this molecule has a role in mediating peripheral tolerance.
RESULTS
B7x protein is expressed in the islets of Langerhans
B7x exhibits a unique mRNA expression profile in comparison with other members of the B7 family. Canonical members such as B7-1 and B7-2 are highly expressed in the hematopoietic compartment, predominantly by APCs, and rarely, if ever, on nonhematopoietic cells (Hathcock et al., 1994). In contrast, B7x is most highly expressed in nonlymphoid tissues and exhibits an inverse pattern of expression when compared with the tissue distribution of B7-2 mRNA (Fig. 1 A; Zang et al., 2003). There is very low expression of B7x mRNA in the hematopoietic compartment, and we did not observe B7x protein expression on murine DCs or macrophages even after culturing these cell types in various stimulating, suppressive, and maturation conditions (e.g., LPS, inflammatory cytokines, Th1/Th2 cytokines, and TGF-β), which differentially regulate the expression of the other members of the B7 family (Figs. S1 and S2). However, the high levels of B7x mRNA in nonhematopoietic tissues even in the absence of maturational or inflammatory stimuli suggest a role for the molecule in inhibiting immune responses in the periphery.
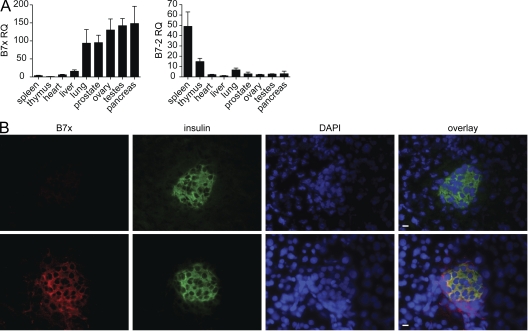
Figure 1.
B7x is highly expressed in the pancreas, specifically in the islets of Langerhans. (A) Real-time PCR on cDNA isolated from the organs of C57BL/6 mice with B7x or B7-2 primers. Expression data were normalized relative to HPRT. Data are representative of three independent experiments. Error bars represent the SD among the different mouse samples. (B) Pancreatic sections from B7x-deficient and B7x wild-type mice were stained for B7x (red), insulin (green), and DAPI (blue). Bars, 10 µm.
As real-time PCR data show relatively high levels of B7x mRNA in the pancreas (Fig. 1 A), we examined the tissue for B7x protein expression. We harvested pancreata from both wild-type and B7x-deficient mice for immunofluorescent staining. Histological staining of the pancreatic sections demonstrated that B7x colocalizes with insulin, indicating that B7x is specifically expressed in the islets of Langerhans (Fig. 1 B). It has already been shown that pancreatic expression of PD-L1, another inhibitory member of the B7 family, plays an important role in preventing diabetes by suppressing islet-reactive T cells (Ansari et al., 2003). Therefore, to determine whether B7x can also inhibit diabetogenic responses, we examined whether the loss of B7x could exacerbate disease in an adoptive transfer model of diabetes.
Loss of B7x results in exacerbated disease after adoptive transfer of diabetogenic T cells
To establish a highly aggressive and synchronized form of diabetes, we used BDC2.5 transgenic (tg) mice; these mice carry rearranged TCR α and β chain genes from a nonobese diabetic (NOD)–derived CD4 T cell clone (Katz et al., 1993). Transfer of activated BDC2.5 splenocytes into neonatal NOD, NOD.scid, or sublethally irradiated NOD and B6g7 recipients results in a rapid induction of diabetes with reproducible kinetics and incidence (Katz et al., 1995; Pakala et al., 1999; Calderon et al., 2006). We find that the adoptive transfer of 10 × 106 Con A–activated BDC2.5/B6g7 splenocytes into sublethally irradiated B6g7 mice results in diabetes in 100% of mice within 40 d after transfer (unpublished data). Therefore, in our experiments, we transferred 10 × 106 Con A–activated BDC2.5/B6g7 splenocytes into sublethally irradiated B7x-deficient and wild-type mice and compared disease progression. Diabetes induced in B7x-deficient mice is more aggressive than in wild-type control mice as shown by earlier onset of disease (Fig. 2 A), higher mean glucose measurements (Fig. 2 B), and earlier lethality of disease in the B7x knockout recipients (Fig. 2 C).
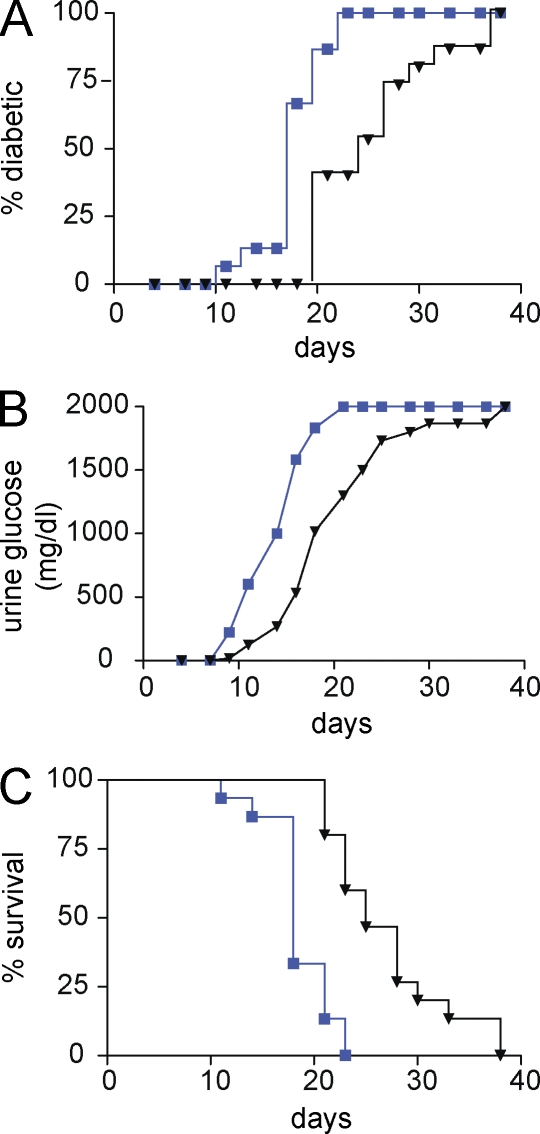
Figure 2.
Loss of B7x results in exacerbated disease after adoptive transfer of diabetogenic T cells. (A–C) After transfer of activated BDC2.5 cells into sublethally irradiated recipient mice, urine glucose levels were measured every 2 d. Mice were considered diabetic after two consecutive measurements exceeded 250 mg/dl. B7x-deficient mice (blue) exhibited a more severe disease in comparison with wild-type mice (black) when we examined disease incidence (A), mean glucose measurements (B), and survival (C; n = 15 mice per group). Data were pooled from three independent experiments.
IFN-γ and IL-17 expression by islet-infiltrating CD4 T cells is significantly increased in the pancreata of B7x-deficient mice
To determine the mechanisms by which the loss of B7x is exacerbating disease, we harvested the pancreata of the recipients 5 d after transfer and analyzed the infiltrating cells. B7x-deficient recipients had statistically greater numbers of congenically marked CD4 T cells in the pancreas relative to their wild-type controls (Fig. 3 A). An important factor in determining diabetes progression is the ratio of suppressive regulatory T cells (Treg cells) to pathogenic effector T cells (Teff cells; Herman et al., 2004; Tang et al., 2004; Tarbell et al., 2004, 2007). To assess the role of Treg cells in this disease setting, we examined pancreatic infiltrating CD4 T cells for FoxP3 expression. Pancreatic B7x expression could be mediating a protective effect by expanding a Treg cell population. However, in this model, we observed a similar low frequency of FoxP3+ cells in the pancreata of both recipient groups (Fig. 3 E). Thus, the exacerbation of disease in B7x-deficient animals cannot be attributed to a lower frequency of suppressive Treg cells in the pancreatic environment.
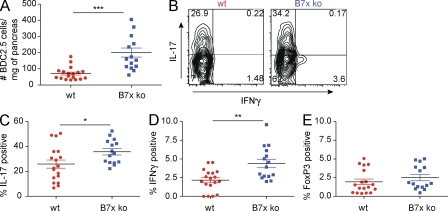
Figure 3.
Loss of B7x results in increased numbers of transferred diabetogenic T cells and enhanced production of IFN-γ and IL-17 in the pancreata. 5 d after transfer of activated BDC2.5 splenocytes, the pancreata were harvested. (A) Absolute number of transferred CD4+ T cells normalized to pancreatic weight. (B) Representative FACS plots showing cytokine production by transferred CD4+CD45.1+ cells in pancreatic islets of B7x-deficient (right) and wild-type (left) recipients. (C) Percentage of transferred CD4+CD45.1+ T cells that express IL-17 in the pancreata. (D) Percentage of transferred CD4+CD45.1+ T cells that express IFN-γ in the pancreata. (E) Percentage of transferred CD4+CD45.1+ T cells that are FoxP3 positive in the pancreata. Three independent experiments were pooled. Error bars represent the SEM, and black horizontal bars indicate the mean. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
As several studies have concluded that Th1 cells, or CD4 T cells characterized by IFN-γ production, promote disease (Healey et al., 1995; Katz et al., 1995; Liblau et al., 1995), in a diabetogenic setting, we examined the infiltrating cells for cytokine production. A higher percentage of pancreas-infiltrating BDC2.5 T cells in B7x-deficient animals produced IFN-γ upon in vitro restimulation in comparison with the wild-type controls (Fig. 3, B and D). Although it is not yet known whether this decrease in cytokine production in the wild-type mice is caused by a direct interaction between B7x-expressing islets and IFN-γ–producing CD4 T cells, this result correlated with previous in vitro data demonstrating B7x-mediated inhibition of IFN-γ production by CD4 T cells (Zang et al., 2003).
Several groups have demonstrated that IL-17 seems to promote disease progression in certain models of diabetes (Miljkovic et al., 2005; Mensah-Brown et al., 2006; Jain et al., 2008); however, others have questioned the requirement of Th17 cells in the development of disease (Bending et al., 2009; Martin-Orozco et al., 2009). As the role of Th17 cells has not been clearly defined in this adoptive transfer model of diabetes, we examined IL-17 production in the pancreata of the B7x-deficient and wild-type mice. We found that a large percentage of the BDC2.5 T cells in the pancreata of both groups of animals produced IL-17. However, a statistically higher percentage of the pancreatic T cells from the B7x knockout group produced the cytokine in comparison with the wild-type animals (Fig. 3, B and C). These data suggest that pancreatic expression of B7x can decrease IL-17 levels in vivo.
Although we have demonstrated that endogenous levels of B7x in pancreatic islets can inhibit cytokine production by diabetogenic T cells, the requirement for IFN-γ and IL-17 in mediating disease in this adoptive transfer model is unknown. To determine the roles of these inflammatory cytokines, we administered neutralizing antibodies to either IFN-γ or IL-17 after transfer of activated BDC2.5 splenocytes into B6g7 recipients. Blockade of IFN-γ resulted in a complete abrogation of disease, which is consistent with published reports on the importance of this cytokine in promoting diabetes (Fig. S3 A; Healey et al., 1995; Katz et al., 1995; Liblau et al., 1995). However, recipients that were treated with neutralizing IL-17A antibody developed disease at the same rate as the recipients that received an isotype control (Fig. S3 B). These results indicate that although IL-17 may contribute to diabetogenic responses, it is not required for disease induction, whereas IFN-γ is necessary for the pathogenic responses of BDC2.5 T cells.
B7x mRNA and protein are overexpressed in pancreatic islets of RipB7x tg mice
Recent papers have reported that the ectopic overexpression of B7x results in prolonged graft survival in an allogeneic islet transplantation model; however, the mechanism by which B7x mediates this effect remains unclear (Wang et al., 2009; Yuan et al., 2009). Therefore, we sought to determine whether the overexpression of B7x can suppress the development of an aggressive form of diabetes.
To overexpress B7x in pancreatic islets, we used a construct that drives B7x expression under the rat insulin II promoter to generate the RipB7x tg mouse. As this promoter has been shown to be slightly promiscuous, B7x is expressed at low levels in pancreatic LNs (PLNs) and spleens; however, the largest increase in B7x mRNA levels was in the pancreas, where we observed 200-fold higher B7x expression (Fig. 4 A). The elevated B7x mRNA levels correspond to increased B7x protein when comparing immunofluorescent staining of B7x in pancreata from RipB7x tg mice and from wild-type C57BL/6 mice (Fig. 4 B). As B7x staining in RipB7x pancreatic sections colocalizes with islet insulin staining, it seems that the molecule is overexpressed in the correct cell type to inhibit diabetogenic T cells.
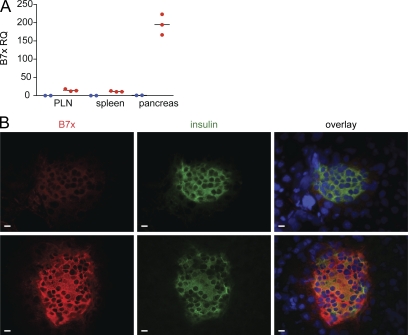
Figure 4.
RipB7x transgenic mice show increased expression of B7x in pancreatic islets. (A) Real-time PCR data of B7x expression in the pancreata of tg (red) and wild-type (blue) mice. Data are representative of three independent experiments. Black horizontal lines indicate the mean. (B) Immunofluorescent staining of pancreatic sections from wild-type (top) and RipB7x tg (bottom) mice for B7x, insulin, and DAPI (blue). Bars, 10 µm.
Overexpression of B7x in the pancreatic islets of BDC2.5 mice does not prevent insulitis but abrogates progression to disease
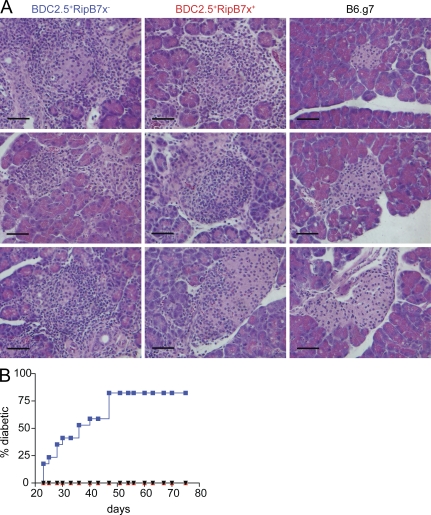
We crossed RipB7x tg mice that had been bred onto the B6g7 background to BDC2.5 tg mice. Backcrossing the BDC2.5 tg onto a B6g7 (C57BL/6 strain with the NOD haplotype) background results in an aggressive onset of diabetes, with >60% of mice exhibiting disease at 10 wk of age (Gonzalez et al., 1997). It was hypothesized that overexpression of B7x could modulate the aggressive and highly penetrant form of the disease in BDC2.5/B6g7 animals. We first compared the development of insulitis in BDC2.5/B6g7 mice that were positive for the RipB7x transgene (BDC2.5+RipB7x+) to BDC2.5/B6g7 mice that were negative for the RipB7x transgene (BDC2.5+RipB7x−). Starting around day 18, BDC2.5/B6g7 mice developed a highly aggressive form of insulitis characterized by severe inflammation marked by edema around the islets and large numbers of infiltrating lymphocytes and inflammatory cells (Gonzalez et al., 1997). Hematoxylin- and eosin (H&E)-stained pancreatic sections from 32-d-old mice showed hyperaggressive insulitis in both BDC2.5+RipB7x− and BDC2.5+RipB7x+ mice (Fig. 5 A), demonstrating that overexpression of B7x on pancreatic islets does not prevent insulitis.
Figure 5.
Overexpression of B7x in the pancreata of BDC2.5 mice does not prevent insulitis but does abrogate induction of disease. (A) Representative H&E staining of pancreatic sections from 32-d-old mice. The pancreata were isolated from BDC2.5+RipB7x− mice (left), BDC2.5+RipB7x+ mice (middle), and B6g7 control mice (right). Bars, 50 µm. (B) Disease incidence in BDC2.5+RipB7x− (blue; n = 17), BDC2.5+RipB7x+ (red; n = 11), and B6g7 (black; n = 9) mice for over 10 wk. Data were pooled from four independent experiments.
Despite the aggressive insulitis observed in both groups of mice, by 10 wk of age, ∼80% of BDC2.5+RipB7x− mice had become diabetic, whereas the BDC2.5+RipB7x+ group (0/11) remained free of disease (Fig. 5 B). As previously published, BDC2.5 mice that are not diabetic by 10 wk of age do not develop the disease later (Gonzalez et al., 1997). Therefore, the data suggest that although the overexpression of B7x in pancreatic islets does not prevent insulitis, it results in complete abrogation of clinical disease.
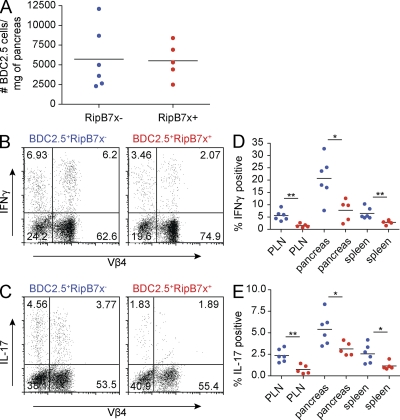
IFN-γ and IL-17 expression by islet-infiltrating BDC2.5 T cells is markedly reduced in RipB7x+ mice
To determine the mechanism by which B7x overexpression exerts its protective effect, we characterized the infiltrating cells from the pancreata of BDC2.5+RipB7x+ and BDC2.5+RipB7x− mice at 1 mo of age. Although there was no difference in the number of BDC2.5 T cells (CD4+Vβ4+ cells) in the pancreata (Fig. 6 A), there was decreased cytokine production by the T cells in RipB7x+ mice. Intracellular cytokine staining for IFN-γ showed that a significantly lower percentage of CD4+Vβ4+ T cells from the pancreata of BDC2.5+RipB7x+ animals produced the cytokine upon in vitro restimulation in comparison with CD4+Vβ4+ T cells from BDC2.5+RipB7x− mice (Fig. 6, B and D). This decrease in IFN-γ–producing BDC2.5 T cells can also be observed in the PLNs and spleens of BDC2.5+RipB7x+ mice (Fig. 6 D). We also examined IL-17 production in the pancreata of the BDC2.5+RipB7x+ and BDC2.5+RipB7x− mice. BDC2.5+RipB7x− mice have a significantly greater percentage of IL-17–producing CD4+Vβ4+ cells in the pancreata, PLNs, and spleens than BDC2.5+RipB7x+ mice (Fig. 6, C and E). These results suggest that the overexpression of B7x is inhibiting IFN-γ and IL-17 cytokine production by diabetogenic T cells, resulting in an amelioration of disease.
Figure 6.
Overexpression of B7x in the pancreata results in inhibition of IFN-γ and IL-17 production by BDC2.5 T cells. (A) Absolute number of CD4+Vβ4+ T cells normalized to pancreatic weight. (B and C) Representative dot plots of intracellular IFN-γ (B) and IL-17 (C) staining of the pancreata from BDC2.5+RipB7x− (left) and BDC2.5+RipB7x+ (right) animals. Populations were gated on pancreatic CD4+ T cells. (D and E) Percentage of CD4+Vβ4+ T cells that produced IFN-γ (D) or IL-17 (E) in each organ. BDC2.5+RipB7x− are shown in blue; BDC2.5+RipB7x+ are shown in red. Data are representative of three independent experiments. Black horizontal lines indicate the mean. *, P < 0.05; **, P < 0.01.
A potential mechanism for B7x-mediated inhibition of diabetes could be the preferential inhibition of Th1 cells, allowing for the expansion of Th2 cells. The balance between the two subsets in a diabetes setting is important as several studies have concluded that Th1 cells, characterized by IFN-γ production, promote disease (Healey et al., 1995; Katz et al., 1995; Liblau et al., 1995), whereas Th2 cells, characterized by IL-4 production, either protect from diabetes or promote a clinically silent peri-insulitis (Healey et al., 1995; Katz et al., 1995; Mueller et al., 1996, 1997). Thus, we assessed the role of Th2 cells in our system by examining IL-4 production in the pancreata of our mice. We found that IL-4 levels in the pancreata of both BDC2.5+RipB7x+ and BDC2.5+RipB7x− mice were very low (Fig. S4, A and B) and that there was no difference in the percentage of IL-4–producing cells between the two groups of mice. Therefore, it seems that the overexpression of B7x does not mediate its protective effect through the expansion of a Th2 population.
Another possible mechanism by which B7x overexpression in the pancreata of BDC2.5+RipB7x+ mice could be mediating its protective effect is by expanding a Treg cell population and thereby preventing disease induction. However, we found that mice from the diabetes-susceptible BDC2.5+RipB7x− group had higher percentages of Treg cells than their RipB7x+ counterparts (Fig. S4, C and D), most likely reflecting the more destructive state of inflammation in the pancreata of BDC2.5+RipB7x− animals. The balance between Teff and Treg cells is also important is determining disease state in BDC2.5/NOD mice. It has been shown that blockade of ICOS, a B7 co-stimulatory molecule which is highly expressed on pancreatic Treg cells, changes the gene expression profiles of infiltrating Treg cells toward a less suppressive phenotype (Herman et al., 2004). This results in a skewing of the Teff/Treg cell ratio toward Teff cells, which manifests in the conversion of benign insulitis to diabetes. The Teff/Treg cell ratio was not markedly different between the pancreata of BDC2.5+RipB7x− and BDC2.5+RipB7x+ animals (Fig. S4 E). Therefore, overexpression of B7x does not protect from disease induction by expanding the Treg cell population.
Genetic ablation of B7x exacerbates myelin oligodendrocyte glycoprotein (MOG)–induced EAE disease
As the results from our two models of diabetes showed that modulation of pancreatic B7x expression can determine disease susceptibility, we investigated whether the molecule has a more general role in protecting peripheral tissues from immune-mediated destruction by examining its function in EAE. EAE is a CD4 T cell–mediated autoimmune attack of the myelin sheath in the central nervous system (CNS) and serves as an animal model for multiple sclerosis (Ben-Nun et al., 1981; Ando et al., 1989). The first stage of disease is characterized by a perivascular infiltration of the CNS by myelin-reactive CD4 T cells. This is followed by an influx of mononuclear cells such as activated macrophages that strip the myelin from axons (Renno et al., 1995). The resulting demyelination of the CNS clinically manifests as progressive paralysis. To address whether endogenous B7x expression has a role in inhibiting myelin-reactive T cells in the CNS, we induced EAE in B7x-deficient and wild-type mice and examined disease incidence.
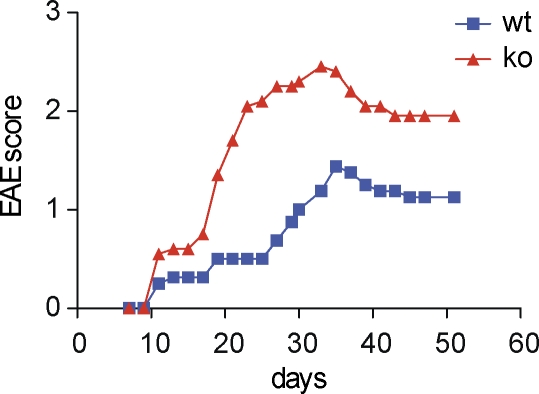
We induced suboptimal disease to reveal any inhibitory effects mediated by endogenous B7x. After titrating varying amounts of MOG peptide, we established a protocol that induces low-grade EAE. EAE induced on the 129/SvE background showed a reproducible exacerbation of disease in the B7x-deficient mice in comparison with their wild-type counterparts, as animals from the B7x-deficient group exhibited higher mean clinical scores than wild-type cohorts (Fig. 7).
Figure 7.
Induction of EAE in B7x knockout mice results in exacerbated disease on the 129/SvE background. After induction of disease, mice were observed for 2 mo for clinical signs of EAE. Data are the mean clinical score and are representative of three independent experiments (n = 8–10 mice per group). P < 0.0001.
Characterization of CNS cellular infiltrate in B7x−/− 129/SvE mice reveals significant increases in the absolute numbers of activated CD4 T cells and Th1 and Th17 cells
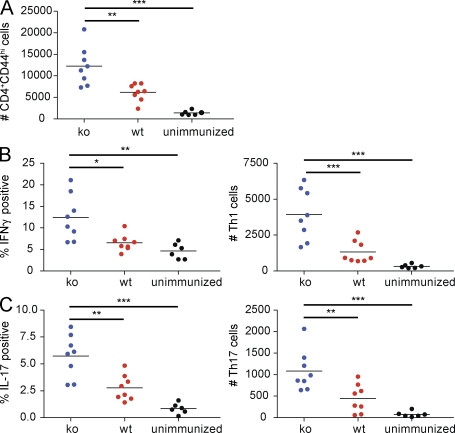
As Th1 and Th17 subsets of CD4 T cells are determining factors in EAE induction, we characterized the cellular infiltrates in the CNS of B7x−/− 129/SvE and wild-type animals at day 14 after disease induction. Absolute numbers of activated CD4 T cells (CD4+CD44hi) infiltrating the brain and spinal cord were significantly higher in B7x−/− 129/SvE animals than in wild-type controls (Fig. 8 A). As expected, the absolute numbers of activated CD4 T cells in the CNS of the immunized mice were also significantly higher than the unimmunized wild-type controls, reflecting the diseased state of the MOG-immunized mice (Fig. 8 A).
Figure 8.
Flow cytometric analysis of cellular infiltrate in the CNS of B7x−/− 129/SvE mice 14 d after induction of EAE. On day 14 after antigen immunization, mononuclear cells from the CNS were isolated and restimulated with PMA/ionomycin for intracellular cytokine staining. (A) Absolute numbers of activated CD4 T cells (CD4+CD44hi). (B) Percentage of CD4+ T cells producing IFN-γ (left) and absolute numbers of Th1 cells (right). (C) Percentage of CD4+ T cells producing IL-17 (left) and absolute numbers of Th17 cells. B7x-deficient mice are shown in blue; wild-type mice are shown in red; unimmunized control mice are shown in black. Data were pooled from three independent experiments. Black horizontal lines indicate the mean. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Analysis of Th1 and Th17 populations from the CNS cellular infiltrates revealed a significantly higher frequency of both IFN-γ– and IL-17–producing CD4 T cells in the B7x−/− 129/SvE animals in comparison with wild-type controls (Fig. 8, B and C, left). The absolute number of pathogenic Th1 and Th17 cells in the CNS of these mice also showed highly significant differences between the B7x-deficient and wild-type animals (Fig. 8, B and C, right). These results indicate that B7x deficiency promotes the expansion of Th1 and Th17 cells during EAE induction and that this increase of cytokine expression could be promoting the greater severity of disease in the knockout mice.
There is not a significant difference between the frequency of Treg cells in the CNS of B7x−/− 129/SvE mice and wild-type control mice
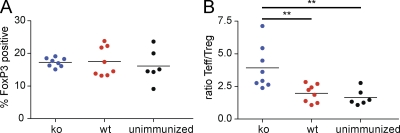
To determine whether B7x deficiency was affecting the frequency of Treg cells in the CNS of B7x−/− 129/SvE animals, we examined FoxP3 expression in the infiltrating CD4 T cells. The percentage of CD4 T cells that were FoxP3 positive did not differ between the two groups (Fig. 9 A). Although there was an increased number of Treg cells in the CNS of B7x-deficient animals because of the increased numbers of total CD4 T cells in these mice, analysis of the Teff/Treg cell ratios showed that that the increase in Teff cells is greater than the increase in Treg cells in the B7x knockout mice (Fig. 9 B). Thus, these data indicate that B7x does not play a role in modulating the frequency of Treg cells in the autoimmune model of EAE.
Figure 9.
No significant difference in the percentages of Treg cells in the CNS of B7x−/− 129/SvE mice and wild-type controls at day 14 after induction of disease. (A) Percentage of CD4+ T cells that are FoxP3 positive in the CNS. (B) Ratio of Teff/Treg cells. B7x-deficient mice are shown in blue; wild-type mice are shown in red; unimmunized control mice are shown in black. Data were pooled from three independent experiments. Black horizontal lines indicate the mean. **, P < 0.01.
DISCUSSION
Although high expression of most B7 family members is largely restricted to immune cells, especially APCs, we were not able to detect protein expression of B7x in any cell type of hematopoietic origin. This strongly suggested that B7x does not play a role in regulating T cell priming in the lymphoid organs. However, histological staining of pancreatic sections demonstrated constitutive expression of B7x in the islets of Langerhans, indicating that this inhibitory molecule may have a role in maintaining peripheral tolerance to islet-reactive T cells. It has already been shown that PD-L1, another member of the B7 family, plays an important role in preventing diabetes by inhibiting islet-reactive T cells (Ansari et al., 2003). Although PD-L1 has been reported to be expressed at very low levels (Liang et al., 2003) or absent (Ansari et al., 2003) in naive pancreata, its expression is up-regulated in an age-dependent manner in NOD mice. Using bone marrow chimeras, it was shown that PD-L1 expression on parenchymal rather than hematopoietic cells protects against disease in NOD mice (Keir et al., 2006). Furthermore, PD-L1 expression on islets results in protection from autoreactive T cells in diabetic mice in an islet transplantation model. Therefore, to determine whether the B7x pathway also plays a role in inhibiting diabetogenic responses, we examined whether the modulation of B7x expression in the pancreas could affect disease incidence.
In an adoptive transfer model of diabetes, we observed that B7x-deficient mice exhibited more aggressive disease than wild-type mice. Analysis of the infiltrating BDC2.5 T cells revealed that the loss of endogenous B7x resulted in increased numbers of Th1 and Th17 cells in the pancreata. This exacerbation of disease cannot be attributed to changes in Treg cell frequencies and correlates with increased production of IFN-γ and IL-17 by the activated BDC2.5 T cells. As the loss of B7x expression resulted in more severe diabetes, we also examined whether the overexpression of the molecule could prevent disease. We found that the overexpression of B7x in the pancreata of BDC2.5 mice resulted in complete abrogation of diabetes. To determine how B7x exerts its ameliorating effect, we examined Th1, Th2, Th17, and Treg cell populations in the pancreata, PLNs, and spleens of BDC2.5+RipB7x+ and BDC2.5+RipB7x− mice. We demonstrated that the protective effect of B7x overexpression was not mediated by either an expansion of or skewing toward a Th2 or Treg cell population. In contrast, we observed that there was a significant inhibition of IFN-γ and IL-17 production by CD4 T cells in the pancreata of the B7x-overexpressing mice. Therefore, in both models of diabetes, we find that the presence of B7x results in an inhibition of inflammatory cytokine production.
The importance of IFN-γ in autoimmune diabetes has been shown by the marked inhibition of both insulitis and disease in NOD mice lacking the IFN-γ receptor α chain. BDC2.5 transgenics with the same null mutation in the IFN-γ receptor α chain also display a complete abrogation of disease (Wang et al., 1997). This lack of disease induction has been attributed to the loss of IFN-γR on β cells and APCs such as macrophages. It has been postulated that the loss of IFN-γ responsiveness inhibits the up-regulation of MHC class I and II molecules and also prevents efficient killing of β cells as this cytokine has been shown to regulate apoptosis and iNOS (inducible nitric oxide synthetase) production. Recently, it was reported that activated macrophages are key mediators of β cell death in an adoptive transfer model of diabetes using activated BDC2.5 T cells (Calderon et al., 2006). As IFN-γ is known to be a potent activator of macrophage function, inhibition of this cytokine by pancreatic B7x could be preventing proper antigen presentation and the efficient killing of β cells by macrophages.
Several studies have recently begun to address the role of IL-17 and Th17 cells in diabetes. It has been shown that IL-17 augments IFN-γ–, TNF-, and IL-1β–induced iNOS mRNA and protein expression in an insulinoma cell line (MIN6) and also in pancreatic islet cells (Miljkovic et al., 2005). Another study has shown that IL-23, which promotes the development of Th17 cells, can enhance disease induction in a model of subdiabetogenic treatment with low doses of streptozotocin (Mensah-Brown et al., 2006). More direct evidence for the role of IL-17 in the induction of diabetes was demonstrated when polarized Th17 cells were shown to induce diabetes in NOD.scid recipients; this disease was abrogated upon administration of neutralizing antibody to IL-17A (Jain et al., 2008). Thus, the presence of IL-17 seems to promote disease progression. The inhibition of IL-17 production that we observed in the pancreata of BDC2.5+RipB7x+ protected mice could be contributing to the abrogation of diabetes.
However, several recent studies questioned the requirement of Th17 cells in the induction of diabetes. Although disease can be induced in NOD.scid mice by the transfer of polarized BDC2.5 Th17 cells, diabetes onset was concomitant with the conversion of pancreatic infiltrates from a Th17 to a Th1 phenotype (Bending et al., 2009; Martin-Orozco et al., 2009). Thus, although diabetogenic Th17 cells appear to be pathogenic, these studies demonstrate that it is the conversion to a Th1 phenotype that allows for disease development. Another study has shown that transfer of polarized Th17 cells from CFA-immunized NOD mice can delay the development of disease in a transfer model of diabetes, suggesting a protective role for IL-17–producing cells (Nikoopour et al., 2010). Our own experiments neutralizing IL-17 in an adoptive transfer model of diabetes support reports that the cytokine is not necessary for mediating disease (Bending et al., 2009; Martin-Orozco et al., 2009). However, this conclusion does not preclude a role for IL-17 in shaping a diabetogenic response; further study is needed to determine how the cytokine could be modulating tissue inflammation during disease.
We also examined another model of autoimmunity to determine whether B7x has a more general role in protecting tissues from damage by self-reactive T cells. As data from our two models of diabetes demonstrated an inhibitory role for B7x on CD4 T cells, we investigated another model of autoimmunity initiated by Th1 and Th17 cells. The induction of EAE in B7x-deficient mice reveals that the loss of this suppressive molecule results in an exacerbation of disease, demonstrating that the B7x pathway can also regulate MOG-reactive T cell responses.
Another study has shown that administration of B7x blocking antibody exacerbates disease in an EAE model; however, it did not investigate the cellular mechanisms of this result (Prasad et al., 2003). We examined the populations of CNS-infiltrating CD4 T cells to dissect out the differences between the B7x-deficient and wild-type mice. Aggravation of disease correlates with increased numbers of activated CD4 T cells and Th1 and Th17 cells in the CNS of B7x−/− 129/SvE mice. It seems that the loss of the inhibitory B7x molecule during EAE induction promotes both Th1 and Th17 expansion in the CNS without affecting the frequency of the Treg cell population. Initially, Th1 cells were thought to be the mediators of EAE by promoting tissue inflammation. The predominant production of IFN-γ in the spleen and CNS during EAE supported the theory that Th1 cells were the pathogenic T cell subset responsible for disease (Merrill et al., 1992). Moreover, the recovery phase of disease correlated with a decrease in IFN-γ production in the CNS (Kennedy et al., 1992; Khoury et al., 1992). These data strongly suggested that the myelin-specific T cells mediating disease were Th1 T cells secreting the disease-promoting cytokine IFN-γ.
However, more recent publications have shown that in mice, autoreactive IL-17–producing CD4 T cells are the dominant pathogenic T cells in EAE (Langrish et al., 2005; Komiyama et al., 2006). Although IL-17 may be the effector cytokine that mediates tissue damage and induces immunopathology in EAE, there is still a role for IFN-γ–producing Th1 cells in the induction of disease. Using an adoptive transfer model of EAE, it was recently shown that although polarized Th1 cells were capable of inducing disease, a Th17 culture lacking Th1 cells was unable to initiate EAE (O’Connor et al., 2008). This inability of the Th17 cells to induce disease was attributed to the observation that only Th1 cells could access noninflamed CNS. It was speculated that the establishment of inflammatory lesions in the CNS by Th1 cells is what allows for the eventual recruitment of pathological Th17 cells. Therefore, the expansion of both Th1 and Th17 cells in the CNS of B7x-deficient mice is most likely the cause of exacerbated disease after EAE induction.
In summary, our data indicate that B7x plays an important role in determining autoimmune susceptibility and in controlling disease severity. In models of diabetes and EAE, we found that the molecule plays a role in ameliorating disease by inhibiting cytokine production by CD4 T cells. Our data support the hypothesis that B7x expression in the periphery acts as an immune checkpoint to prevent tissue-specific autoimmunity and thus provides a potential target for therapeutic intervention in the clinic.
MATERIALS AND METHODS
Mice.
7–9-wk-old female C57BL/6 mice were purchased from Taconic. BDC2.5/B6g7 tg mice were a gift from D. Mathis (Joslin Diabetes Center, Boston, MA). To maintain this diabetes-susceptible line, the BDC2.5/B6g7 transgenics were bred with B6g7 mice, another gift from D. Mathis.
To generate the RipB7x tg mice, B7x was cloned into the Rip7 cassette, which drives B7x expression under the rat insulin II promoter. The mice were bred onto the C57BL/6 background in 12 generations. To use these mice for diabetes experiments, the RipB7x line was also bred to homozygosity for the I-Ag7 haplotype by breeding with B6g7 mice. All mice were treated in accordance with the National Institutes of Health and American Association of Laboratory Animal Care regulations, and experiments were approved by University of California, Berkeley and Memorial Sloan-Kettering Cancer Center (MSKCC) Institutional Animal Care and Use Committee.
Generation of B7x antibodies.
B7x knockout mice were immunized with irradiated TrampC2 cells that overexpress B7x mixed with irradiated TrampC2 cells that overexpress GM-CSF. 1 d after immunization, the mice were given 100 µg α–CTLA-4 (clone 9D9; MSKCC Monoclonal Facility). 4 mo later, the mice received another cellular vaccination followed by two boosts of 50 µg B7x Ig (Zang et al., 2003) emulsified in RIBI (Sigma-Aldrich) at 1 and 2 mo after second immunization. The spleens were harvested and fused to a hybridoma partner by the MSKCC Monoclonal Facility. The resultant antibodies were screened on RMA cells that overexpress B7x. The biotinylated antibodies that we used were 19D6, 15D12, 12D11, and 1H3.
Antibodies and flow cytometry.
Cells were preincubated with 50 µg/ml 24.G2 (MSKCC Monoclonal Antibody Core) to block Fc-γ receptor binding and stained with the following antibodies: B7x (clone 9; eBioscience), Vβ4 (clone KT4; BD), CD4 (clone RM4-5; eBioscience), CD4 (clone L3T4; eBioscience), IAg7 (clone 10.2.16; MSKCC Monoclonal Antibody Core), IAb (clone Y3P; MSKCC Monoclonal Antibody Core), IgG1 (clone eBRG1; eBioscience), CD11.c (clone N418; eBioscience), F4/80 (clone BM8; eBioscience), and FoxP3 (clone FJK-16s; eBioscience), Intracellular cytokine staining was performed after restimulation with 50 ng/ml PMA and 1 µg/ml ionomycin for 5 h in the presence of monensin (eBioscience). The cells were fixed and permeabilized using Cytofix/Cytoperm (BD) according to the manufacturer’s instructions. The cells were stained with anti–IFN-γ (XMG1.2; eBioscience), anti–IL-4 (11B11; BD), or anti–IL-17 (TC11-18H10; BD). The samples were analyzed with a CyAn (Dako) or LSRII (BD) flow cytometer. Flow cytometry data were analyzed with FlowJo (Tree Star).
The hybridoma producing neutralizing antibody to IL-17A was a gift from J. van Snick (Ludwig Institute for Cancer Research, Brussels, Belgium). Anti–IFN-γ (XMG1.2), rat IgG1 isotype control (HRPN), and mouse IgG1 isotype control (MOPC-21) were obtained from BioXcell.
RNA preparation and real-time PCR.
RNA was isolated from homogenized tissue or harvested cell lines using TRI Reagent (Sigma-Aldrich) according to the manufacturer’s instructions. The RNA samples were reverse transcribed using Ready-To-Go You-Prime First-Strand Beads (GE Healthcare). Quantitative PCR was performed on a real-time PCR system (model 7500; Applied Biosystems). Premade TaqMan primer/probe gene expression assays for each gene analyzed were purchased from Applied Biosystems (B7x, Mm00628552_m1; B7-2, Mm00444543_m1; and HRPT, Mm00446968_A1). HPRT was used as the endogenous control, and relative changes in gene expression were calculated using the ΔΔCt method.
Isolation and activation of DCs.
Bone marrow from the femur and tibia was isolated and cultured with 10 ng/ml GM-CSF for 6 d and then matured for 48 h with 2.5 ng/ml TNF, 2.5 ng/ml TNF + 10 ng/ml IL-10, 1 µg/ml LPS, LPS + TNF, or LPS + TNF + IL-10. All cytokines were purchased from R&D systems.
Isolation and activation of macrophages.
To induce inflammatory macrophages, mice were injected with 3–4 ml of 4% thioglycolate (BD). 72 h later, peritoneal macrophages were washed out with RPMI 1640 medium. The exudate was then adhered onto 12-well plates for 12 h. Unless otherwise stated, the macrophages were then cultured with medium alone or activated with 20 ng/ml IL-4, 100 ng/ml IFN-γ + LPS, 500 ng/ml IL-6 + 50 ng/ml IL-10, or 5 ng/ml TGF-β. All cytokines were purchased from R&D systems. Treg cells were isolated using a Miltenyi Biotec isolation kit. Macrophages were cultured in media alone with Treg cells (CD4+CD25+ cells) or with CD4+CD25− cells in the presence of 10 µg/ml soluble anti-CD3 and 5 µg/ml anti-CD28. The mixed cultures were set up at a 1:2 ratio with 0.5 × 106 macrophages and 1.0 × 106 T cells. After treatment, the macrophages were harvested with a cell scraper for FACS analysis.
Monitoring for diabetes.
Starting from 3 wk of age, urine glucose levels of mice were measured with Diastix (Bayer) testing strips. Mice were considered diabetic after two consecutive measurements exceeding 250 mg/dl. The first positive measurement was defined as the onset of diabetes.
Isolation of pancreatic islet cells.
Mice were sacrificed, and the pancreata were resected. After mincing into small pieces, the pancreata were digested with Collagenase P (Roche) by vigorous shaking in a water bath at 37°C. The digested islets were hand-picked using a dissection microscope (SZ30; Olympus) and dispersed by pulling through an 18-gauge needle. The single cell suspensions were then analyzed by flow cytometry.
Adoptive transfer of activated BDC2.5 splenocytes.
Spleens were harvested from BDC2.5/B6g7 transgenic mice and activated with 5 µg/ml Con A (Sigma-Aldrich). After 3 d of culture, cells were washed with 0.2 M methyl-α-d-mannopyranoside (Sigma-Aldrich). The activated BDC2.5/B6g7 splenocytes were then transferred intravenously into sublethally irradiated recipients (750 rads).
Cytokine blockade experiments were performed with either 2 mg/mouse of anti–IFN-γ (XMG1.2) or control IgG1 (HRPN) or 2 mg/mouse of anti–IL-17A (MM17-F3) or control mouse IgG1 (MOPC-21). The antibodies were administered i.p. on days 0, 2, 4, 6, and 8 after transfer.
Histology.
Pancreata were fixed with 4% paraformaldehyde and embedded in paraffin. The samples were processed and sectioned by the MSKCC Laboratory of Comparative Pathology. Pancreatic sections were stained with H&E by the MSKCC Molecular Cytology facility. The slides were analyzed with a microscope (AxioPlan 2; Carl Zeiss). Images were collected with a camera (Spot; Diagnostic Instruments, Inc.) operated by Spot Advanced software (Diagnostic Instruments, Inc.).
For fluorescent microscopy, pancreata were dissected and snap frozen in OCT (Tissue-Tek). Sections were fixed with acetone and stained with primary antibodies overnight at 4°C. After washing, the cells were stained with fluorescently conjugated secondary antibodies. The slides were counterstained with DAPI and mounted in Slowfade Light Antifade (Invitrogen). The following primary antibodies were used: goat polyclonal anti-B7x (R&D Systems) and polyclonal guinea pig anti–swine insulin (Dako). The following secondary antibodies were used: FITC-conjugated donkey anti–guinea pig (Jackson ImmunoResearch Laboratories, Inc.) and Cy5-conjugated donkey anti–goat (Jackson ImmunoResearch Laboratories, Inc.). The slides were analyzed with an inverted microscope (DMIFB/E; Leica). Images were collected with a charge-coupled device camera (Quantix; Roper Scientific) operated by Slidebook software (Intelligent Imaging Innovations).
EAE induction.
Mice were subcutaneously immunized with 10 µg MOG emulsified 1:1 in IFA supplemented with 500 µg Mycobacterium tuberculosis H37RA (Difco) in the right flank. On days 1 and 3 after immunization, the mice received 300 ng pertussis toxin (List Biologicals) intravenously. Clinical scores were assessed in a blinded fashion on a scale from 0 to 5 as follows: 0, no disease; 0.5, partial limp tail paralysis; 1.0 complete limp tail paralysis (flaccid tail); 2, limp tail and hind limb weakness; 3, both hind limb weakness; 3.5, complete hind limb paralysis; 4, complete hind limb paralysis and forelimb weakness; 5, moribund. MOG peptide used was: MOG 35–55, MEVGWYRSPFSRVVHLYRNGK.
Isolation of CNS-associated leukocytes for intracellular cytokine staining.
After anesthetizing with ketamine/xylazine, the mice were perfused with PBS, and brains and spinal cords were harvested. Spinal cord tissue was flushed out with cRPMI and an 18-gauge needle. The CNS tissue (brain and spinal cord) was minced and ground against a 70-µM filter using the back of a syringe. After washing with cRPMI, the pellet was resuspended in 4 ml of 70% Percoll (Sigma-Aldrich) and overlaid with 37% Percoll and 30% Percoll. The cell suspensions were then centrifuged for 20 min at 460 g at 20°C. The leukocytes were harvested at the 37%/70% interface and washed again with cRMPI. The cells were then analyzed by flow cytometry.
Statistical analysis.
Statistical analysis was performed using Prism software 4 (GraphPad Software). Data were analyzed by the two-tailed Student’s t test, and P < 0.05 was considered statistically significant.
Online supplemental material.
Fig. S1 shows that murine DCs do not up-regulate B7x expression even in various stimulating, suppressive, and maturation conditions. Fig. S2 demonstrates that murine macrophages do not express B7x even when cultured in different conditions or with Treg cells. Fig. S3 shows that blockade of IFN-γ in a transfer model of diabetes abrogates disease incidence, whereas blockade of IL-17A has no effect. Fig. S4 shows that overexpression of B7x in the pancreata of BDC2.5 mice does not expand either a Th2 or Treg cell population. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20100639/DC1.
Acknowledgments
We would like to thank Emily Corse, Tsvetelina Pentcheva-Hoang, Sergio Quezada, and Rebecca Waitz for helpful discussion and/or critical reading of the manuscript.
This work was supported by the Howard Hughes Medical Institute (J.P. Allison) and National Institutes of Health grant CA40041 (to J.P. Allison). X. Zang was a fellow of the Cancer Research Institute and is supported in part by National Institutes of Health grant DP2DK083076 and Department of Defense grant PC094137. P. Loke was a recipient of the Wellcome Trust International Research Fellowship.
The authors have no financial conflicts of interest.
Footnotes
Abbreviations used:
- CNS
- central nervous system
- EAE
- experimental autoimmune encephalomyelitis
- MOG
- myelin oligodendrocyte glycoprotein
- mRNA
- messenger RNA
- NOD
- nonobese diabetic
- PLN
- pancreatic LN
- tg
- transgenic
References
- Ando D.G., Clayton J., Kono D., Urban J.L., Sercarz E.E. 1989. Encephalitogenic T cells in the B10.PL model of experimental allergic encephalomyelitis (EAE) are of the Th-1 lymphokine subtype. Cell. Immunol. 124:132–143 10.1016/0008-8749(89)90117-2 [DOI] [PubMed] [Google Scholar]
- Ansari M.J., Salama A.D., Chitnis T., Smith R.N., Yagita H., Akiba H., Yamazaki T., Azuma M., Iwai H., Khoury S.J., et al. 2003. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J. Exp. Med. 198:63–69 10.1084/jem.20022125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awadallah N.S., Shroyer K.R., Langer D.A., Torkko K.C., Chen Y.K., Bentz J.S., Papkoff J., Liu W., Nash S.R., Shah R.J. 2008. Detection of B7-H4 and p53 in pancreatic cancer: potential role as a cytological diagnostic adjunct. Pancreas. 36:200–206 10.1097/MPA.0b013e318150e4e0 [DOI] [PubMed] [Google Scholar]
- Ben-Nun A., Otmy H., Cohen I.R. 1981. Genetic control of autoimmune encephalomyelitis and recognition of the critical nonapeptide moiety of myelin basic protein in guinea pigs are exerted through interaction of lymphocytes and macrophages. Eur. J. Immunol. 11:311–316 10.1002/eji.1830110409 [DOI] [PubMed] [Google Scholar]
- Bending D., De la Peña H., Veldhoen M., Phillips J.M., Uyttenhove C., Stockinger B., Cooke A. 2009. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J. Clin. Invest. 119:565–572 10.1172/JCI37865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon B., Suri A., Unanue E.R. 2006. In CD4+ T-cell-induced diabetes, macrophages are the final effector cells that mediate islet beta-cell killing: studies from an acute model. Am. J. Pathol. 169:2137–2147 10.2353/ajpath.2006.060539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter L.L., Leach M.W., Azoitei M.L., Cui J., Pelker J.W., Jussif J., Benoit S., Ireland G., Luxenberg D., Askew G.R., et al. 2007. PD-1/PD-L1, but not PD-1/PD-L2, interactions regulate the severity of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 182:124–134 10.1016/j.jneuroim.2006.10.006 [DOI] [PubMed] [Google Scholar]
- Gonzalez A., Katz J.D., Mattei M.G., Kikutani H., Benoist C., Mathis D. 1997. Genetic control of diabetes progression. Immunity. 7:873–883 10.1016/S1074-7613(00)80405-7 [DOI] [PubMed] [Google Scholar]
- Greenwald R.J., Freeman G.J., Sharpe A.H. 2005. The B7 family revisited. Annu. Rev. Immunol. 23:515–548 10.1146/annurev.immunol.23.021704.115611 [DOI] [PubMed] [Google Scholar]
- Guleria I., Khosroshahi A., Ansari M.J., Habicht A., Azuma M., Yagita H., Noelle R.J., Coyle A., Mellor A.L., Khoury S.J., Sayegh M.H. 2005. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J. Exp. Med. 202:231–237 10.1084/jem.20050019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathcock K.S., Laszlo G., Pucillo C., Linsley P., Hodes R.J. 1994. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J. Exp. Med. 180:631–640 10.1084/jem.180.2.631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey D., Ozegbe P., Arden S., Chandler P., Hutton J., Cooke A. 1995. In vivo activity and in vitro specificity of CD4+ Th1 and Th2 cells derived from the spleens of diabetic NOD mice. J. Clin. Invest. 95:2979–2985 10.1172/JCI118006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman A.E., Freeman G.J., Mathis D., Benoist C. 2004. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J. Exp. Med. 199:1479–1489 10.1084/jem.20040179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz A.A., Sullivan T.J., Sobel R.A., Allison J.P. 2002. Cytotoxic T lymphocyte antigen-4 (CTLA-4) limits the expansion of encephalitogenic T cells in experimental autoimmune encephalomyelitis (EAE)-resistant BALB/c mice. Proc. Natl. Acad. Sci. USA. 99:3013–3017 10.1073/pnas.042684699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R., Tartar D.M., Gregg R.K., Divekar R.D., Bell J.J., Lee H.H., Yu P., Ellis J.S., Hoeman C.M., Franklin C.L., Zaghouani H. 2008. Innocuous IFNγ induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J. Exp. Med. 205:207–218 10.1084/jem.20071878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Zhu Y., Wu C., Shen Y., Wei W., Chen L., Zheng X., Sun J., Lu B., Zhang X. 2010. Tumor expression of B7-H4 predicts poor survival of patients suffering from gastric cancer. Cancer Immunol. Immunother. 59:1707–1714 10.1007/s00262-010-0900-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J.D., Wang B., Haskins K., Benoist C., Mathis D. 1993. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 74:1089–1100 10.1016/0092-8674(93)90730-E [DOI] [PubMed] [Google Scholar]
- Katz J.D., Benoist C., Mathis D. 1995. T helper cell subsets in insulin-dependent diabetes. Science. 268:1185–1188 10.1126/science.7761837 [DOI] [PubMed] [Google Scholar]
- Keir M.E., Sharpe A.H. 2005. The B7/CD28 costimulatory family in autoimmunity. Immunol. Rev. 204:128–143 10.1111/j.0105-2896.2005.00242.x [DOI] [PubMed] [Google Scholar]
- Keir M.E., Liang S.C., Guleria I., Latchman Y.E., Qipo A., Albacker L.A., Koulmanda M., Freeman G.J., Sayegh M.H., Sharpe A.H. 2006. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 203:883–895 10.1084/jem.20051776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M.K., Torrance D.S., Picha K.S., Mohler K.M. 1992. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J. Immunol. 149:2496–2505 [PubMed] [Google Scholar]
- Khoury S.J., Hancock W.W., Weiner H.L. 1992. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. J. Exp. Med. 176:1355–1364 10.1084/jem.176.5.1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama Y., Nakae S., Matsuki T., Nambu A., Ishigame H., Kakuta S., Sudo K., Iwakura Y. 2006. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 177:566–573 [DOI] [PubMed] [Google Scholar]
- Krambeck A.E., Thompson R.H., Dong H., Lohse C.M., Park E.S., Kuntz S.M., Leibovich B.C., Blute M.L., Cheville J.C., Kwon E.D. 2006. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc. Natl. Acad. Sci. USA. 103:10391–10396 10.1073/pnas.0600937103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish C.L., Chen Y., Blumenschein W.M., Mattson J., Basham B., Sedgwick J.D., McClanahan T., Kastelein R.A., Cua D.J. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201:233–240 10.1084/jem.20041257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S.C., Latchman Y.E., Buhlmann J.E., Tomczak M.F., Horwitz B.H., Freeman G.J., Sharpe A.H. 2003. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur. J. Immunol. 33:2706–2716 10.1002/eji.200324228 [DOI] [PubMed] [Google Scholar]
- Liblau R.S., Singer S.M., McDevitt H.O. 1995. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol. Today. 16:34–38 10.1016/0167-5699(95)80068-9 [DOI] [PubMed] [Google Scholar]
- Lühder F., Höglund P., Allison J.P., Benoist C., Mathis D. 1998. Cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J. Exp. Med. 187:427–432 10.1084/jem.187.3.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Orozco N., Chung Y., Chang S.H., Wang Y.H., Dong C. 2009. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur. J. Immunol. 39:216–224 10.1002/eji.200838475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensah-Brown E.P., Shahin A., Al-Shamisi M., Wei X., Lukic M.L. 2006. IL-23 leads to diabetes induction after subdiabetogenic treatment with multiple low doses of streptozotocin. Eur. J. Immunol. 36:216–223 10.1002/eji.200535325 [DOI] [PubMed] [Google Scholar]
- Merrill J.E., Kono D.H., Clayton J., Ando D.G., Hinton D.R., Hofman F.M. 1992. Inflammatory leukocytes and cytokines in the peptide-induced disease of experimental allergic encephalomyelitis in SJL and B10.PL mice. Proc. Natl. Acad. Sci. USA. 89:574–578 10.1073/pnas.89.2.574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miljkovic D., Cvetkovic I., Momcilovic M., Maksimovic-Ivanic D., Stosic-Grujicic S., Trajkovic V. 2005. Interleukin-17 stimulates inducible nitric oxide synthase-dependent toxicity in mouse beta cells. Cell. Mol. Life Sci. 62:2658–2668 10.1007/s00018-005-5259-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller R., Krahl T., Sarvetnick N. 1996. Pancreatic expression of interleukin-4 abrogates insulitis and autoimmune diabetes in nonobese diabetic (NOD) mice. J. Exp. Med. 184:1093–1099 10.1084/jem.184.3.1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller R., Bradley L.M., Krahl T., Sarvetnick N. 1997. Mechanism underlying counterregulation of autoimmune diabetes by IL-4. Immunity. 7:411–418 10.1016/S1074-7613(00)80362-3 [DOI] [PubMed] [Google Scholar]
- Nikoopour E., Schwartz J.A., Huszarik K., Sandrock C., Krougly O., Lee-Chan E., Singh B. 2010. Th17 polarized cells from nonobese diabetic mice following mycobacterial adjuvant immunotherapy delay type 1 diabetes. J. Immunol. 184:4779–4788 10.4049/jimmunol.0902822 [DOI] [PubMed] [Google Scholar]
- O’Connor R.A., Prendergast C.T., Sabatos C.A., Lau C.W., Leech M.D., Wraith D.C., Anderton S.M. 2008. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J. Immunol. 181:3750–3754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakala S.V., Chivetta M., Kelly C.B., Katz J.D. 1999. In autoimmune diabetes the transition from benign to pernicious insulitis requires an islet cell response to tumor necrosis factor α. J. Exp. Med. 189:1053–1062 10.1084/jem.189.7.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentcheva-Hoang T., Corse E., Allison J.P. 2009. Negative regulators of T-cell activation: potential targets for therapeutic intervention in cancer, autoimmune disease, and persistent infections. Immunol. Rev. 229:67–87 10.1111/j.1600-065X.2009.00763.x [DOI] [PubMed] [Google Scholar]
- Perrin P.J., Maldonado J.H., Davis T.A., June C.H., Racke M.K. 1996. CTLA-4 blockade enhances clinical disease and cytokine production during experimental allergic encephalomyelitis. J. Immunol. 157:1333–1336 [PubMed] [Google Scholar]
- Petroff M.G., Perchellet A. 2010. B7 family molecules as regulators of the maternal immune system in pregnancy. Am. J. Reprod. Immunol. 63:506–519 10.1111/j.1600-0897.2010.00841.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad D.V., Richards S., Mai X.M., Dong C. 2003. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 18:863–873 10.1016/S1074-7613(03)00147-X [DOI] [PubMed] [Google Scholar]
- Quandt D., Fiedler E., Boettcher D., Marsch W.Ch., Seliger B. 2011. B7-h4 expression in human melanoma: its association with patients’ survival and antitumor immune response. Clin. Cancer Res. 17:3100–3111 10.1158/1078-0432.CCR-10-2268 [DOI] [PubMed] [Google Scholar]
- Renno T., Krakowski M., Piccirillo C., Lin J.Y., Owens T. 1995. TNF-alpha expression by resident microglia and infiltrating leukocytes in the central nervous system of mice with experimental allergic encephalomyelitis. Regulation by Th1 cytokines. J. Immunol. 154:944–953 [PubMed] [Google Scholar]
- Sica G.L., Choi I.H., Zhu G., Tamada K., Wang S.D., Tamura H., Chapoval A.I., Flies D.B., Bajorath J., Chen L. 2003. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 18:849–861 10.1016/S1074-7613(03)00152-3 [DOI] [PubMed] [Google Scholar]
- Simon I., Katsaros D., Rigault de la Longrais I., Massobrio M., Scorilas A., Kim N.W., Sarno M.J., Wolfert R.L., Diamandis E.P. 2007. B7-H4 is over-expressed in early-stage ovarian cancer and is independent of CA125 expression. Gynecol. Oncol. 106:334–341 10.1016/j.ygyno.2007.03.035 [DOI] [PubMed] [Google Scholar]
- Tang Q., Henriksen K.J., Bi M., Finger E.B., Szot G., Ye J., Masteller E.L., McDevitt H., Bonyhadi M., Bluestone J.A. 2004. In vitro–expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J. Exp. Med. 199:1455–1465 10.1084/jem.20040139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbell K.V., Yamazaki S., Olson K., Toy P., Steinman R.M. 2004. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J. Exp. Med. 199:1467–1477 10.1084/jem.20040180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbell K.V., Petit L., Zuo X., Toy P., Luo X., Mqadmi A., Yang H., Suthanthiran M., Mojsov S., Steinman R.M. 2007. Dendritic cell–expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J. Exp. Med. 204:191–201 10.1084/jem.20061631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tringler B., Liu W., Corral L., Torkko K.C., Enomoto T., Davidson S., Lucia M.S., Heinz D.E., Papkoff J., Shroyer K.R. 2006. B7-H4 overexpression in ovarian tumors. Gynecol. Oncol. 100:44–52 10.1016/j.ygyno.2005.08.060 [DOI] [PubMed] [Google Scholar]
- Wang B., André I., Gonzalez A., Katz J.D., Aguet M., Benoist C., Mathis D. 1997. Interferon-gamma impacts at multiple points during the progression of autoimmune diabetes. Proc. Natl. Acad. Sci. USA. 94:13844–13849 10.1073/pnas.94.25.13844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Hao J., Metzger D.L., Mui A., Ao Z., Verchere C.B., Chen L., Ou D., Warnock G.L. 2009. Local expression of B7-H4 by recombinant adenovirus transduction in mouse islets prolongs allograft survival. Transplantation. 87:482–490 10.1097/TP.0b013e318195e5fa [DOI] [PubMed] [Google Scholar]
- Yuan C.L., Xu J.F., Tong J., Yang H., He F.R., Gong Q., Xiong P., Duan L., Fang M., Tan Z., et al. 2009. B7-H4 transfection prolongs beta-cell graft survival. Transpl. Immunol. 21:143–149 10.1016/j.trim.2009.03.007 [DOI] [PubMed] [Google Scholar]
- Zang X., Loke P., Kim J., Murphy K., Waitz R., Allison J.P. 2003. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc. Natl. Acad. Sci. USA. 100:10388–10392 10.1073/pnas.1434299100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang X., Thompson R.H., Al-Ahmadie H.A., Serio A.M., Reuter V.E., Eastham J.A., Scardino P.T., Sharma P., Allison J.P. 2007. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc. Natl. Acad. Sci. USA. 104:19458–19463 10.1073/pnas.0709802104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B., Guleria I., Khosroshahi A., Chitnis T., Imitola J., Azuma M., Yagita H., Sayegh M.H., Khoury S.J. 2006. Differential role of programmed death-ligand 1 [corrected] and programmed death-ligand 2 [corrected] in regulating the susceptibility and chronic progression of experimental autoimmune encephalomyelitis. J. Immunol. 176:3480–3489 [DOI] [PubMed] [Google Scholar]