FoxP3-deficient mice are rescued from tissue and organ destruction and subsequent lethal autoimmune disease by the combined absence of OX40 and CD30 signals.
Abstract
Our previous studies have implicated signaling through the tumor necrosis family receptors OX40 and CD30 as critical for maintaining CD4 memory responses. We show that signals through both molecules are also required for CD4 effector-mediated autoimmune tissue damage. Under normal circumstances, male mice deficient in the forkhead transcription factor FoxP3, which lack regulatory CD4 T cells, develop lethal autoimmune disease in the first few weeks of life. However, in the combined absence of OX40 and CD30, FoxP3-deficient mice develop normally and breed successfully. The extensive tissue infiltration and organ destruction characteristic of FoxP3 disease does not appear in these mice, and their mortality is not associated with autoimmunity. Although the absence of OX40 plays the dominant role, FoxP3-deficient mice sufficient in CD30 but deficient in OX40 signals still eventually develop lethal disease. This result was supported by the observation that blocking antibodies to OX40 and CD30 ligands also abrogated disease mediated by FoxP3-deficient T cells. These observations identify OX40 and CD30 signals as essential for the development of clinically relevant CD4-dependent autoimmunity and suggest that combination therapies that abrogate these signals might be used to treat established human autoimmune diseases.
Lack or defective expression of the forkhead transcription factor (FoxP3) causes lethal X-linked CD4 T cell–dependent Th1- and Th2-driven autoimmune disease in both mice (Brunkow et al., 2001; Fontenot et al., 2003; Khattri et al., 2003) and men (Bennett et al., 2001; Wildin et al., 2001) as a result of lack of FoxP3-dependent regulatory T cells (Treg cells). This is mainly dependent on CD4 T cells, as depletion of CD4, but not CD8, T cells abrogates disease either genetically or with mAbs (Blair et al., 1994). The murine model is therefore particularly valuable for identifying pathways that might ameliorate clinical autoimmune disease in humans. We have previously shown that the TNF receptors OX40 and CD30 play synergistic roles in the generation of CD4 memory/effector responses (Kim et al., 2003; Gaspal et al., 2005, 2008) and are also required for the maintenance of memory CD4 cells within the lamina propria of the gut (Withers et al., 2009). We show in this paper that FoxP3 Treg cells are dispensable in mice deficient in OX40 and CD30 signals. FoxP3-deficient mice lacking OX40 and CD30 develop and grow normally, fail to develop clinically relevant autoimmune disease, and have a normal lifespan. We also show that blocking mAbs to OX40 and CD30 ligands abrogates development of FoxP3KO disease. This study highlights the critical importance of both these signals in effector CD4 function and suggests that blocking both these signaling pathways in human autoimmune disease might be particularly effective at ameliorating disease.
RESULTS AND DISCUSSION
Generation and phenotype of FoxP3KO mice deficient in OX40 and CD30
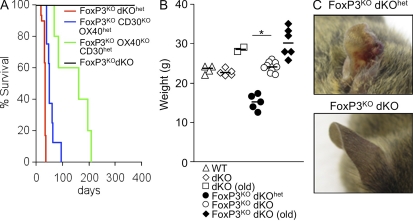
To investigate the impact of abrogation of OX40 and CD30 signals on the development of autoimmunity in FoxP3KO mice, male C57BL/6 mice, deficient in both OX40 and CD30 (double [d] KO), were crossed with females with a heterozygous null allele for the X-linked gene FoxP3 (FoxP3het). As expected, ∼50% of male offspring heterozygous for expression of OX40 and CD30 (dKOhet) were FoxP3 deficient and developed lethal autoimmune disease at ∼4 wk (Fig. 1 A), characterized by weight loss (Fig. 1 B) and scurfy phenotype (Fig. 1 C). Because OX40 and CD30 are linked genetically, offspring have a high probability of inheriting deficiency in both genes as a haplotype. Consequently, after an F1 intercross between male dKO mice and FoxP3hetdKOhet females, ∼50% of the F2 male progeny were FoxP3 deficient. Within this population ∼50% were dKOhet and ∼50% dKO. In contrast to FoxP3KOdKOhet mice, which developed autoimmune disease and lost body weight, FoxP3KOdKO mice developed no signs of disease and were indistinguishable in their body weight, health, and behavior from normal male mice of the same age (not depicted) or FoxP3+dKO mice (Fig. 1 B). As an example of their good health, breeding pairs between male FoxP3KOdKO mice and FoxP3hetdKO females were established, with consequent generation of female mice that were FoxP3KOdKO. Like their male counterparts, these female mice developed and bred normally. We have now maintained a viable colony of FoxP3KOdKO mice for 24 mo, in which breeding pairs have averaged three litters (with a mean of five pups per litter). This contrasts with CD28KO FoxP3KO mice, which develop delayed disease from 90 d, with 50% lethality by 160 d (Singh et al., 2007). From ∼8 mo of age, approximately two thirds of the FoxP3KOdKO mice started to develop eczema affecting their ears only but were otherwise healthy with no evidence of abnormal behavior or weight loss. The remaining mice have developed no overt evidence of any abnormality and have survived for 12 mo or more.
Figure 1.
Deficiency in OX40 and CD30 confers protection from FoxP3-dependent autoimmunity. (A) Kaplan-Meier survival curve for FoxP3KOdKOhet (n = 30 males), FoxP3KOOX40KOCD30het (n = 8 males), FoxP3KOOX40hetCD30KO (n = 5 males), and FoxP3KOdKO mice (n > 20 males and n > 30 females). (B) Body weight of WT male littermate controls (FoxP3hetdKOhet) and male FoxP3KOdKOhet mice taken at the time of sacrifice, FoxP3KOdKO male aged 5 wk, and old FoxP3KOdKO male mice (aged 8 mo) or dKO male mice (aged 8 mo). The horizontal bars show the median. *, P < 0.002. (C) Ear photograph of a 5-wk-old FoxP3KOdKOhet male with eczema and scurfy skin (top) and a FoxP3KOdKO male littermate (bottom). Data are representative of at least five separate experiments.
FoxP3 is not required for prevention of inflammatory responses to gut microbiota in the absence of OX40 and CD30 signals
In addition to regulating autoimmune T cells, FoxP3 is required for Treg cell–mediated counteraction of inflammation against commensal gut microbiota (Izcue et al., 2009). However, FoxP3KOdKO failed to develop any signs of macroscopic or microscopic inflammatory bowel disease, and examination of the lamina propria showed it to be normal (unpublished data), with the exception of a depletion of CD4 T cells similar to that observed in dKO mice (Withers et al., 2009).
Prevention of disease is dependent on both OX40 and CD30
To assess the individual contributions of OX40 and CD30 to autoimmunity in FoxP3KO mice, FoxP3KOdKO female mice were bred with male OX40KO or CD30KO mice. As FoxP3KOdKO female mice have two copies of the FoxP3KO allele, all the male offspring of this breeding strategy are FoxP3KO with either a single OX40 (OX40het) or CD30 (CD30het) gene. Disease onset was delayed in both situations (P = 0.002 for both genes) compared with FoxP3KOdKOhet mice, clearly demonstrating the relevance of both genes (Fig. 1 A). FoxP3KO mice lacking CD30, but which had a single copy of the OX40 gene (OX40hetCD30KO), developed severe eczematous skin lesions between 7 and 9 wk and were sacrificed for humane reasons. Disease onset was delayed even further in FoxP3KO mice lacking OX40 but sufficient in a single copy of the CD30 gene (OX40KOCD30het; P < 0.001); some developed eczematous skin between 10 and 12 wk without obvious weight loss, indicating an attenuated phenotype compared with FoxP3KOdKOhet mice (all dead by ∼5 wk with 40% body weight loss). This shows that OX40 plays the dominant role but that the contribution of CD30 is significant and acts cooperatively with OX40 to permit the emergence of autoimmune pathology in the context of FoxP3 deficiency.
Abrogation of organ infiltration and high-titer autoantibodies and retention of lymphoid tissue architecture in FoxP3KOdKO mice
To exclude subclinical disease in FoxP3KOdKO mice, animals were sacrificed contemporaneously with FoxP3KOdKOhet littermates that developed overt disease. A striking feature of FoxP3 deficiency is an eczematous skin infiltration by T cells resulting in the scurfy phenotype with infiltration of the subcutaneous tissues of the skin most obvious in the ear macroscopically (Fig. 1 C) but also by microscopy (Fig. 2 A). In contrast, the ears of FoxP3KOdKO mice appeared grossly and microscopically normal and were indistinguishable from WT normal mice. This did not simply reflect a delay in disease onset, as FoxP3KOdKO or FoxP3+dKO mice sacrificed at 180 d also appeared macroscopically and microscopically normal (Fig. S1, A–D). At later time points from 8 mo of age, some FoxP3KOdKO mice developed eczema affecting their ears, but the infiltrates were small and contained few CD4+ T cells (unpublished data). No mice developed fatal autoimmune pathology.
Figure 2.
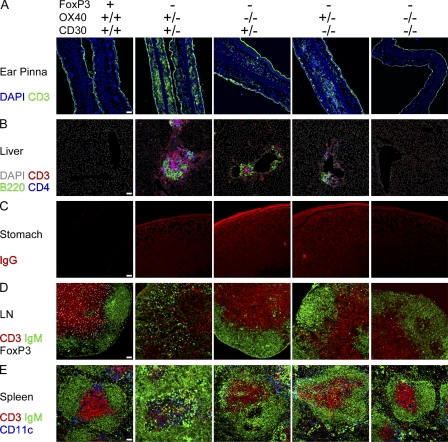
Confocal micrographs of cryostat tissue sections and autoantibodies from ∼4–5-wk-old FoxP3KO mice intact and deficient in OX40 and CD30 signals. (A) Ear pinna (CD3, green; DAPI, blue). (B) Liver (DAPI, gray; CD3, red; B220, green; CD4, blue). (C) RAGKO stomach sections incubated with sera to identify autoantibodies. (D) Lymph node (IgM, green; CD3, red; FoxP3, white). (E) Spleen (IgM, green; CD3, red; CD11c, blue). Data are representative of at least 5 mice deficient in OX40 or CD30 and at least 10 different FoxP3KOdKO and FoxP3KOdKOhet mice. Bars, 50 µm.
In addition to skin T cell infiltration, at the time of clinical disease onset, FoxP3KO mice develop lymphocyte infiltrations in many other tissues including the liver. These infiltrates form substantial tertiary lymphoid structures in the liver of FoxP3KOCD30hetOX40het mice with focal aggregates of B220+ B and CD3+ T cells (Fig. 2 B). In contrast, in FoxP3KOdKO mice, there was virtually no infiltration by lymphocytes and certainly no organized aggregates were detected. FoxP3KOdKO mice sacrificed at 6 mo of age also showed a lack of tissue infiltration (Fig. S1 B).
After 8 mo, some mice also showed small CD4− T cell infiltrates in the liver, although ∼30% of mice showed no histological evidence of disease. Although liver infiltration was less substantial in OX40het and CD30hetFoxP3KO mice, infiltrates were clearly much greater than FoxP3KOdKO mice (Fig. 2 B).
The hallmark of pathogenic human autoimmune antibodies is their high titer and affinity. These autoantibodies also occur in FoxP3KO mice, for example against stomach mouse tissue (Fig. 2 C). Stomach antibodies were also clearly evident in FoxP3KOOX40het and FoxP3KOCD30het mice but at minimal low titers in FoxP3KOdKO mice (Fig. 2 C) even when old (not depicted), consistent with our previously reported observations that both CD30 and OX40 are required to generate high-affinity antibodies (Gaspal et al., 2005). Autoantibodies and tissue infiltrates were not observed in FoxP3+dKOhet or FoxP3+dKO mice (unpublished data).
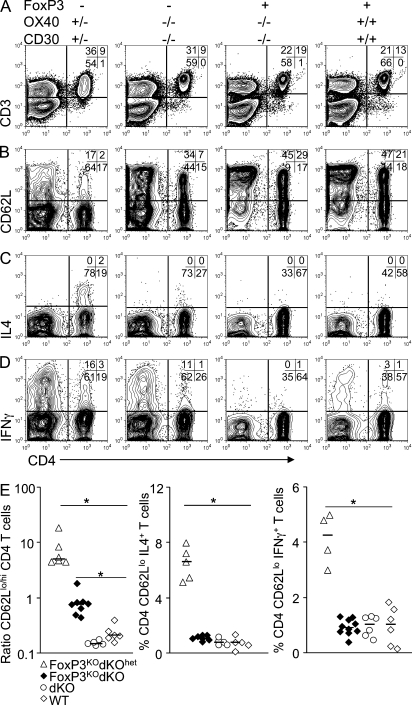
Compared with normal mice, FoxP3KOdKOhet, FoxP3KOOX40het, and FoxP3KOCD30het mice exhibited marked lymphadenopathy, and this was significantly but not completely attenuated in FoxP3KOdKO mice (Fig. S1, E and F). Naive CD4 T cells reside exclusively within secondary lymphoid organs like spleen and lymph node (Reinhardt et al., 2001). In FoxP3-deficient human disease, autoimmunity is associated with immunodeficiency within the naive CD4 and also CD8 T cell compartments in lymph nodes and spleen (Costa-Carvalho et al., 2008). Compared with normal mice, FoxP3KO mice had grossly abnormal architecture, with destruction of white pulp areas of both lymph node and spleen (Fig. 2, D and E), and this was associated with gross depletion of CD4+CD62Lhi naive T cells (Fig. 3 A and Fig. S2). In contrast, in FoxP3KOdKO mice the spleen and lymph node architecture was normal, with well organized B and T cell areas (Fig. 2, D and E). Although there was still marked skewing of CD4 T cells to effector/memory phenotype CD62Llo cells, a significant proportion of CD4 T cells were CD62Lhi naive cells. To a lesser extent, naive CD8 T cells were also reduced in FoxP3KO mice but were restored in FoxP3KOdKO mice cells (Fig. 3).
Figure 3.
Activation status and cytokine expression from ∼4–5-wk-old FoxP3KO mice with intact and deficient OX40 and CD30 signals. For cytokine expression, splenocytes from FoxP3KOdKOhet, FoxP3KOdKO, dKO (FoxP3+, OX40KO CD30KO), and WT mice were activated on anti-CD3–coated (5 µg/ml) plates in the presence of 0.5 µg/ml anti-CD28 antibody. 4 h later, CD62Llo CD3+ T cells were analyzed for intracellular IL4 and IFN-γ. (A) Lymphocyte gated cells. (B) Expression of CD62L on CD3+CD4+CD8+ gated T cells. Expression of IL4 (C) or IFN-γ (D) in CD62LloCD3+CD4+CD8+ gated T cells. (E) Representative FACS data from the different group of mice. The horizontal bars show the median (*, P < 0.002). Data are representative of four separate experiments.
CD4 T cells are activated but fail to express IL4 and IFN-γ in FoxP3KOdKO mice
Our previous studies have shown that dKO CD4 T cells proliferate just as well as their WT counterparts both in vitro and in vivo (Gaspal et al., 2005), can be induced to express both Th1 and Th2 cytokines under appropriate conditions of stimulation in vivo (Kim et al., 2005), but fail to be sustained as effector/memory cells (Gaspal et al., 2005, 2008). As reported previously by others, FoxP3KO mice have an increased fraction of CD4+CD62LloCD44hi effector/memory cells that express more IL4 and IFN-γ than FoxP3+ mice (Fig. 3 B and Fig. S2). Compared with FoxP3KOdKOhet mice, expression of IL4 and IFN-γ in activated FoxP3KOdKO CD4 T cells was substantially reduced and little different from both WT and dKO mice sufficient in FoxP3 (Fig. 3).
In contrast to CD4 T cells, CD8 T cells from FoxP3KOdKO mice were not only highly activated but expressed high levels of IFN-γ compared with FoxP3-sufficient controls. CD8 T cells are implicated in the immunopathology of autoimmune diseases. Therefore, the failure of FoxP3KOdKO CD8 T cells to cause disease suggests that CD8 immune-mediated damage in autoimmune diseases depends on OX40 and CD30 signals to CD4 T cells, or alternatively the potential CD8-mediated damage is much less than for CD4 T cells, confirming previous findings (Blair et al., 1994).
To test directly whether the defect in FoxP3KOdKO CD4 T cells was a result of decreased survival, we transferred neonatal thymocytes, which have been previously shown to transfer disease (Godfrey et al., 1994), from FoxP3KOdKOhet or FoxP3KOdKO mice into RAGKO recipients. RAGKO mice that received FoxP3KOdKOhet thymocytes developed disease at ∼3 wk (Fig. S3 A). Both CD4 (Fig. S3 B) and CD8 (Fig. S3 C) T cells were activated (CD44hiCD62Llo) and a high proportion of both CD4 and CD8 T cells expressed IFN-γ (Fig. S3, B and C). RAGKO mice injected with FoxP3KOdKO or FoxP3+dKOhet thymocytes did not get sick. These mice were culled after 10 wk and their T lymphocytes analyzed. Although all the CD4 T cells were CD44hiCD62Llo (Fig. S3 B), far fewer CD4 cells (P < 0.05) were recovered from mice that received FoxP3KOdKO thymocytes compared with FoxP3KOdKOhet or FoxP3+dKOhet thymocytes, and very few of these cells expressed IFN-γ (Fig. S3 D; P < 0.05). CD8 T cell numbers were also reduced, but the reduction was much less than for CD4 T cells, although the expression of IFN-γ was significantly less (P < 0.05; Fig. S3 D).
Blocking antibodies to OX40L and CD30L abrogate disease mediated by FoxP3KO T cells
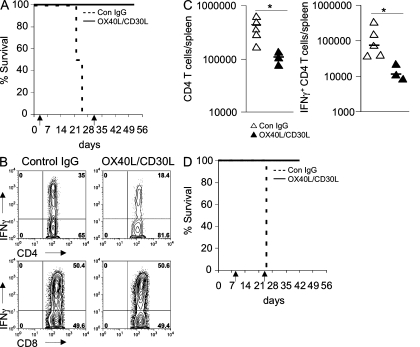
To exclude effects of OX40 and CD30 on T cell development and to test whether in vivo blockade of OX40 and CD30 signals might be effective in treating autoimmune disease, 107 FoxP3KOdKOhet thymocytes were transferred into RAGKO mice. After transfer, mice received either twice weekly injections of blocking mAbs to OX40 and CD30 ligands (Akiba et al., 2000) or control rat IgG (see Materials and methods). Mice that received control rat IgG developed signs of FoxP3KO disease at ∼3 wk after transfer, whereas mice treated with blocking mAbs looked healthy at this time and remained healthy for several weeks even after blocking mAbs were stopped (Fig. 4 A). FACS analysis of these mice sacrificed at the same time as sick mice showed that they had reduced numbers of CD4+ T cells, and much reduced expression of IFN-γ, similar to that observed for FoxP3KO dKO thymocytes transfers (Fig. 4, B and C). Analysis of liver tissue in mice injected with blocking mAbs also showed that T cell infiltrates were much reduced (unpublished data). We do not think these mAbs are simply depleting cells that express these molecules, as lymphoid tissue inducer cells that express high levels of OX40 and CD30 ligands (Kim et al., 2003) are not depleted by these mAbs (unpublished data).
Figure 4.
Blocking mAbs to OX40L and CD30L prevent FoxP3 autoimmune disease. RAGKO mice were transferred with 107 FoxP3KO thymocytes and treated with biweekly injections of either 1 mg of control rat IgG or 0.5 mg OX40L and 0.5 mg CD30L mAbs. (A) Kaplan-Meier survival curve (four mice in each group). (B) IFN-γ cytokine production analysis of gated CD3+ T cells 3 wk after transfer. (C) Number of CD4+ T cells and IFN-γ+ CD44+ CD4+ T cells in the spleen 3 wk after transfer. The horizontal bars show the median (*, P < 0.02). (D) Kaplan-Meier survival curve when the antibody treatment was delayed for 8 d. Arrows indicate when mAb therapy was started and when it was stopped. Data are representative of two separate experiments.
To exclude effects of OX40 and CD30 on T cell activation, treatment with blocking mAbs was delayed for 8 d to allow FoxP3KO T cells to be fully activated. At this time point after transfer, FACS analysis of splenocytes showed that all transferred CD4 T cells were CD44hi and most were CD62Llo (Fig. S4). Injection of blocking mAbs from this time point again prevented disease onset in remaining RAGKO mice that received FoxP3KO thymocytes (Fig. 4 D).
We have previously shown that OX40 and CD30 signals are essential for the generation of CD4 memory antibody responses in mice (Gaspal et al., 2005). In this paper, we show that these signals, particularly the former, are required for the immunopathology driven by Th1 and Th2 FoxP3-deficient autoimmune effector CD4 T cells. The absence of lethal disease with minimal tissue infiltration in FoxP3KO mice lacking OX40 and CD30 demonstrates that these signals are crucial for effector CD4 T cell–driven autoimmune tissue damage. Molecular phylogeny shows that the TNF ligands for OX40 and CD30 evolved late (Glenney and Wiens, 2007), as orthologs of these genes are present only in mammals and birds (www.ensembl.org), which like mammals can develop spontaneous autoimmune disease (Wick et al., 2006). In contrast, there is little or no evidence that lower vertebrates lacking these genes spontaneously develop clinically relevant autoimmunity. In addition to suppressing autoimmunity, FoxP3-dependent Treg cells prevent inflammatory responses to food and commensal microbiota in the gut (Izcue et al., 2009). However, in the absence of OX40 and CD30, there is no evidence of gut inflammation in the absence of Treg cells.
Therapeutic trials of anti-OX40 ligand mAbs are already in human clinical trials for asthma (Clinical Trials NCT00983658). This study highlights the crucial importance of both OX40 and CD30 cells in mediating tissue damage in human autoimmune diseases mediated by pathogenic CD4 T cells and suggests that strategies that block both pathways would act synergistically to prevent this damage occurring. In support of this, we showed that injection of blocking antibodies to OX40 and CD30 ligands could also prevent disease onset in an adoptive transfer model of FoxP3KO disease, emphasizing the potential therapeutic role in human autoimmune disease.
MATERIALS AND METHODS
Mice.
All experiments on animals were approved by Birmingham University local ethical panel review and UK Home office guidelines. Animals were bred in accordance with home office guidelines at the University of Birmingham, Biomedical Services Unit. Heterozygous FoxP3het females were provided by A. Rudensky (Howard Hughes Medical Institute and Immunology Program, Memorial Sloan-Kettering Cancer Center, New York, NY) and were crossed with CD30KOOX40KO (dKO) or mice deficient in OX40 (OX40KO) or CD30 (CD30KO).
Thymocyte transfer.
Approximately 10 × 106 FoxP3KO thymocytes from newborn (day 2–5) male FoxP3KO mice were injected i.v. into RAGKO mice to transfer FoxP3KO disease.
Blocking antibody injection.
Blocking mAbs against mouse OX40L (clone RM134L) and CD30L (clone RM153) were prepared as described previously (Akiba et al., 2000). Control rat IgG was purchased from Sigma-Aldrich. Mice were twice weekly injected with either 0.5 mg anti-OX40L and 0.5 mg anti-CD30L mAbs or 1 mg of control IgG.
Immunofluorescence microscopy.
5-µM frozen tissue cryostat sections were stained with fluorescent antibodies as described previously (Kim et al., 2003). Liver sections were pretreated with avidin/biotin blocking reagents (Sigma-Aldrich). Primary anti–mouse antibodies used were: anti-B220 biotin (clone RA3-6B2; eBioscience), anti-CD3 FITC and biotin (145-2C11; BD), anti-CD4 Alexa Fluor 647 (GK1.5; eBioscience), anti-CD11c biotin (N418; eBioscience), anti-IgM rhodamine red (Jackson ImmunoResearch Laboratories, Inc.), CD4–Alexa Fluor 647 (clone L3T4), and anti-FoxP3 FITC (FJK-16s). Biotinylated Abs were detected with streptavidin–Alexa Fluor 555 or streptavidin–Alexa Fluor 647 (Invitrogen). FITC-conjugated Abs were detected using rabbit anti-FITC Abs (Invitrogen) and then goat anti–rabbit FITC Abs (SouthernBiotech). IgG autoantibodies were detected by adding sera (diluted 1/40 in 1% BSA/PBS) to RAGKO stomach sections followed by goat anti–mouse IgG Alexa Fluor 594 (Invitrogen). Sections were counterstained with DAPI (Invitrogen) and mounted using DABCO (Sigma-Aldrich).
FACS and intracellular cytokine staining.
Splenocytes prepared from the indicated mice were restimulated in vitro for 4 h at 37°C in the presence of GolgiStop (BD) on plates coated with 5 µg/ml anti-CD3 Ab and 0.5 µg/ml of soluble anti-CD28 Ab (BD). Cells were then surface stained with anti-CD3 FITC, CD62L PE, CD8 PE Texas red, CD4 PE-Cy5.5, and CD44 PECy7 (EBioscience) and then fixed and permeabilized with Cytofix/Cytoperm Plus (BD) according to the manufacturer’s instructions. Intracellular cytokine expression was revealed by staining permeabilized cells with APC-conjugated mAb to IFN-γ and IL4 (BD).
Statistics.
Mann-Whitney nonparametric statistics were used throughout to analyze the significance of differences between groups of mice.
Online supplemental material.
Fig. S1 shows confocal micrographs of cryostat tissue sections from Foxp3KOdKO mice aged 6 mo. Fig. S2 shows CD4 T cell number and activation status in FoxP3KOdKO mice. Fig. S3 shows that RAGKO mice injected with FoxP3KOdKO thymocytes do not develop disease. Fig. S4 shows activation status of FoxP3KO CD4 T cells 8 d after transfer into RAGKO hosts compared with CD4+ T cells from WT C57BL/6 spleen. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20101484/DC1.
Acknowledgments
We are grateful to Dr. Alexander Rudensky for permission to use FoxP3-deficient mice.
This work was supported by a Program Grant from the Wellcome Trust to P.J.L. Lane and G. Anderson.
The authors have no competing financial interests.
References
- Akiba H., Miyahira Y., Atsuta M., Takeda K., Nohara C., Futagawa T., Matsuda H., Aoki T., Yagita H., Okumura K. 2000. Critical contribution of OX40 ligand to T helper cell type 2 differentiation in experimental leishmaniasis. J. Exp. Med. 191:375–380 10.1084/jem.191.2.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C.L., Christie J., Ramsdell F., Brunkow M.E., Ferguson P.J., Whitesell L., Kelly T.E., Saulsbury F.T., Chance P.F., Ochs H.D. 2001. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 27:20–21 10.1038/83713 [DOI] [PubMed] [Google Scholar]
- Blair P.J., Bultman S.J., Haas J.C., Rouse B.T., Wilkinson J.E., Godfrey V.L. 1994. CD4+CD8- T cells are the effector cells in disease pathogenesis in the scurfy (sf) mouse. J. Immunol. 153:3764–3774 [PubMed] [Google Scholar]
- Brunkow M.E., Jeffery E.W., Hjerrild K.A., Paeper B., Clark L.B., Yasayko S.A., Wilkinson J.E., Galas D., Ziegler S.F., Ramsdell F. 2001. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 27:68–73 10.1038/83784 [DOI] [PubMed] [Google Scholar]
- Costa-Carvalho B.T., de Moraes-Pinto M.I., de Almeida L.C., de Seixas Alves M.T., Maia R.P., de Souza R.L., Barreto M., Lourenço L., Vicente A.M., Coutinho A., Carneiro-Sampaio M. 2008. A remarkable depletion of both naïve CD4+ and CD8+ with high proportion of memory T cells in an IPEX infant with a FOXP3 mutation in the forkhead domain. Scand. J. Immunol. 68:85–91 10.1111/j.1365-3083.2008.02055.x [DOI] [PubMed] [Google Scholar]
- Fontenot J.D., Gavin M.A., Rudensky A.Y. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330–336 10.1038/ni904 [DOI] [PubMed] [Google Scholar]
- Gaspal F., Bekiaris V., Kim M.Y., Withers D.R., Bobat S., MacLennan I.C., Anderson G., Lane P.J., Cunningham A.F. 2008. Critical synergy of CD30 and OX40 signals in CD4 T cell homeostasis and Th1 immunity to Salmonella. J. Immunol. 180:2824–2829 [DOI] [PubMed] [Google Scholar]
- Gaspal F.M., Kim M.Y., McConnell F.M., Raykundalia C., Bekiaris V., Lane P.J. 2005. Mice deficient in OX40 and CD30 signals lack memory antibody responses because of deficient CD4 T cell memory. J. Immunol. 174:3891–3896 [DOI] [PubMed] [Google Scholar]
- Glenney G.W., Wiens G.D. 2007. Early diversification of the TNF superfamily in teleosts: genomic characterization and expression analysis. J. Immunol. 178:7955–7973 [DOI] [PubMed] [Google Scholar]
- Godfrey V.L., Rouse B.T., Wilkinson J.E. 1994. Transplantation of T cell-mediated, lymphoreticular disease from the scurfy (sf) mouse. Am. J. Pathol. 145:281–286 [PMC free article] [PubMed] [Google Scholar]
- Izcue A., Coombes J.L., Powrie F. 2009. Regulatory lymphocytes and intestinal inflammation. Annu. Rev. Immunol. 27:313–338 10.1146/annurev.immunol.021908.132657 [DOI] [PubMed] [Google Scholar]
- Khattri R., Cox T., Yasayko S.A., Ramsdell F. 2003. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4:337–342 10.1038/ni909 [DOI] [PubMed] [Google Scholar]
- Kim M.Y., Gaspal F.M., Wiggett H.E., McConnell F.M., Gulbranson-Judge A., Raykundalia C., Walker L.S., Goodall M.D., Lane P.J. 2003. CD4(+)CD3(-) accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity. 18:643–654 10.1016/S1074-7613(03)00110-9 [DOI] [PubMed] [Google Scholar]
- Kim M.Y., Bekiaris V., McConnell F.M., Gaspal F.M., Raykundalia C., Lane P.J. 2005. OX40 signals during priming on dendritic cells inhibit CD4 T cell proliferation: IL-4 switches off OX40 signals enabling rapid proliferation of Th2 effectors. J. Immunol. 174:1433–1437 [DOI] [PubMed] [Google Scholar]
- Reinhardt R.L., Khoruts A., Merica R., Zell T., Jenkins M.K. 2001. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 410:101–105 10.1038/35065111 [DOI] [PubMed] [Google Scholar]
- Singh N., Chandler P.R., Seki Y., Baban B., Takezaki M., Kahler D.J., Munn D.H., Larsen C.P., Mellor A.L., Iwashima M. 2007. Role of CD28 in fatal autoimmune disorder in scurfy mice. Blood. 110:1199–1206 10.1182/blood-2006-10-054585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick G., Andersson L., Hala K., Gershwin M.E., Selmi C., Erf G.F., Lamont S.J., Sgonc R. 2006. Avian models with spontaneous autoimmune diseases. Adv. Immunol. 92:71–117 10.1016/S0065-2776(06)92002-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildin R.S., Ramsdell F., Peake J., Faravelli F., Casanova J.L., Buist N., Levy-Lahad E., Mazzella M., Goulet O., Perroni L., et al. 2001. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 27:18–20 10.1038/83707 [DOI] [PubMed] [Google Scholar]
- Withers D.R., Jaensson E., Gaspal F., McConnell F.M., Eksteen B., Anderson G., Agace W.W., Lane P.J. 2009. The survival of memory CD4+ T cells within the gut lamina propria requires OX40 and CD30 signals. J. Immunol. 183:5079–5084 10.4049/jimmunol.0901514 [DOI] [PMC free article] [PubMed] [Google Scholar]